Abstract
Holoprosencephaly (HPE) is the most common malformation of the forebrain, resulting from a failure to completely septate the left and right hemispheres at the rostral end of the neural tube. Because of the tissue interactions that drive head development, these forebrain defects are typically accompanied by midline deficiencies of craniofacial structures. Early events in setting up tissue precursors of the head, as well as later interactions between these tissues, are critical for normal head formation. Defects in either process can result in HPE. Signaling by Bone Morphogenetic Proteins (BMPs), a family of secreted cytokines, generally plays negative roles in early stages of head formation, and thus must be attenuated in multiple contexts to ensure proper forebrain and craniofacial development. Chordin and Noggin are endogenous, extracellular antagonists of BMP signaling that promote the normal organization of the forebrain and face. Mouse mutants with reduced levels of both factors display mutant phenotypes remarkably analogous to the range of malformations seen in human HPE sequence. Chordin and Noggin function in part by antagonizing the inhibitory effects of BMP signaling on the Sonic hedgehog and Nodal pathways, genetic lesions in each being associated with human HPE. Study of Chordin;Noggin mutant mice is helping us to understand the molecular, cellular and genetic pathogenesis of HPE and associated malformations.
Keywords: holoprosencephaly, BMP, BMP antagonist, Chordin, Noggin, mouse, forebrain
INTRODUCTION
Development of the head is a complex process, and is consequently highly susceptible to disruption. Improper regulation of the events of head formation leads to a multitude of defects, with a high rate of mortality and morbidity [reviewed by Cohen, 2002]. Perhaps the most clinically significant of the congenital malformations resulting from early failures of head development is holoprosencephaly (HPE), in which the ventral forebrain is incompletely septated into hemispheres (OMIM 236100). These forebrain defects are typically associated with variable midline craniofacial abnormalities, such as cyclopia, proboscis, and cleft palate. Even the forebrain defects present in mild cases of clinical holoprosencephaly can be associated with mental deficiency [Wallis and Muenke, 2000].
It is thus of pragmatic interest to understand the mechanisms employed in the embryo that establish and pattern the mammalian brain and face. In this review, we summarize findings from the mouse model system that elucidate the molecular embryology of normal head formation and holoprosencephaly. We focus on the relevant functions of Bone Morphogenetic Protein (BMP) signaling and its attenuation, highlighting the roles of the BMP antagonists Chordin (Chrd) and Noggin (Nog). When genetic dosage of the Chrd and Nog genes is reduced, variable HPE can result (Figure 1). The telencephalon of the early forebrain shows defects in septum formation, such that septation into hemispheres is incomplete. This is accompanied by variable craniofacial deficits. The mechanistic basis of these defects is complex, but reveals insights useful in understanding human HPE.
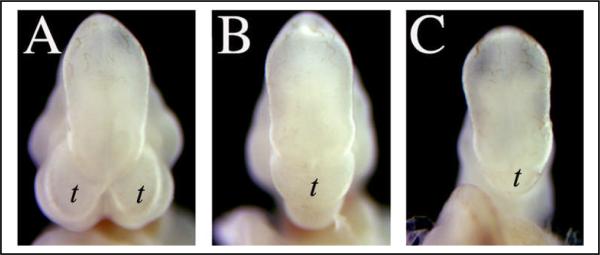
Figure 1. Reduced gene dosage of the BMP antagonists Chordin and Noggin can result in variable holoprosencephaly.
(A) At the ninth day of embryonic gestation in the mouse (E9.5), the forebrain is clearly septated ventrally into telencephalic vesicles (t) on either side of the midline. (B) In some Chrd−/−;Nog+/− embryos, only a single medial telencephalic vesicle occurs. (C) In the most severely afflicted Chrd−/−;Nog+/− embryos, the single telencephalic vesicle can be truncated or even absent.
TISSUE INTERACTIONS ARE CRITICAL FOR EARLY ROSTRAL DEVELOPMENT IN THE MAMMALIAN EMBRYO
The key developmental defects that result in HPE occur in the fourth week of human gestation. The first signs of head development occur shortly after gastrulation has formed the three germ layers (ectoderm, mesoderm and endoderm) from the epiblast, the ovoid sheet of cells that gives rise to all embryonic tissues. Early signaling interactions specify that a small part of the ectoderm has the normal fate of becoming forebrain. Once presumptive forebrain tissue has been induced, further signaling interactions between the rostral neural ectoderm and adjacent tissues dictate the future pattern of regionalized identity within the forebrain. Concomitant with patterning, the presumptive forebrain undergoes extensive morphogenesis (changes in size and shape). In addition to rapid growth by proliferation, the rostral neural plate folds into a tube, so that medial becomes ventral and lateral becomes dorsal.
The most significant source of the signals regulating early forebrain development is the anterior primitive streak and its derivatives. The anterior primitive streak is the most interior terminus of the slit-like zone where the three germ layers are formed by cells delaminating from the primitive ectoderm. It gives rise to the anterior foregut endoderm and the axial mesendoderm, the midline tissue underlying the rostral neural plate. The significance of this region for head development was identified in amphibian embryos by Hans Spemann, leading to his Nobel Prize of 1935. With his colleagues, he discovered that the clump of tissue where the axial mesendoderm forms is able to induce and organize brain development in naïve tissue, such as ventral epidermis. This tissue consequently has been known as the Spemann organizer, or gastrula organizer, and is conserved in all vertebrates. In the mouse, rostral derivatives of the gastrula organizer are essential for correct induction and maintenance of the forebrain [Dufort et al., 1998; Klingensmith et al., 1999; Liu et al., 1999; Shawlot et al., 1999]. However, the mouse gastrula organizer (the node) is unable to induce forebrain structures on its own [Tam and Steiner, 1999]. Thus, the gastrula organizer and its derivatives are necessary but not sufficient for forebrain induction and early patterning.
In mammalian embryos, cues from the extraembryonic endoderm that transiently overlies the future forebrain neurectoderm are also important for its development. This tissue is known as the anterior visceral endoderm (AVE). Surgical ablation of the AVE results in truncation of the forebrain [Thomas and Beddington, 1996], while presence of the AVE even without the gastrula organizer can trigger some markers of putative forebrain fate [Ding et al., 1998]. The AVE is characterized by expression of several transcription factors including Lim-1, Foxa2, Otx2, Hex, and Hesx1. While many of these factors are important in later development of the brain, ablation of these genes specifically in extra-embryonic tissues reveals an earlier role of the AVE in head induction [Beddington and Robertson, 1998, 1999]. Nevertheless, transplantation of the AVE is not capable of inducing neural tissue in vivo [Tam and Steiner, 1999]. Mammalian head induction is therefore dependent upon synergistic cues from both the AVE and the rostral tissues produced by the gastrula organizer [Shawlot et al, 1999]. A current model is that the AVE creates a labile or permissive condition for forebrain induction, a fate then conferred to the rostral ectoderm by signals from the organizer and its derivatives (Fig 2).
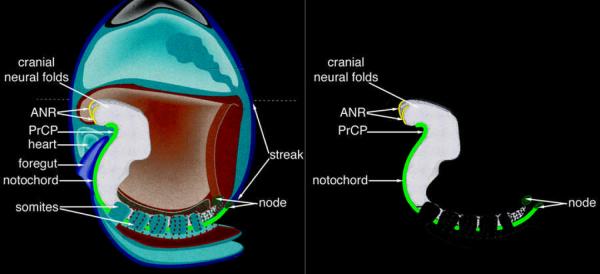
Figure 2. Interdependence of the gastrula organizer and anterior visceral endoderm in mouse forebrain initiation.
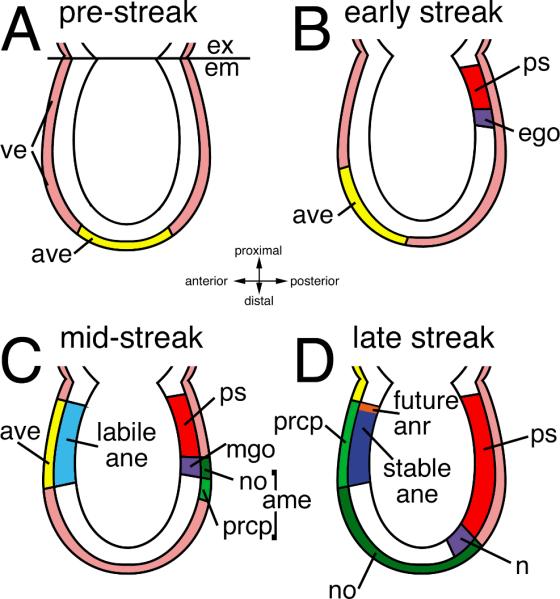
Highly schematized depiction of early axis formation in mouse during pre-streak through late streak stages. Anterior visceral endoderm (ave, yellow) specific gene expression is initiated at the distal tip of the egg cylinder in the pre-streak embryo (A). This region rotates toward the presumptive anterior pole as the early primitive streak (ps, red) is forming at the posterior pole during the early streak stage (B). The anterior extreme of the primitive streak is molecularly distinct and marks the formation of the early gastrula organizer (ego, purple). At mid-streak stage (C), the ave has induced a labile anterior neural ectoderm (ane) character (light blue) in the adjacent anterior epiblast. In the posterior end of the embryo, the rostral axial mesendoderm (AME, in shades of green) is formed from the midgastrula organizer (mgo, purple), and is comprised of the prechordal plate (prcp, light green) and the notochord (no, dark green). At the late streak stage, the definitive node (n, purple) is apparent at the distal tip of the primitive streak. The prcp has displaced the midline ave to the extraembryonic region, and now lies adjacent to the presumptive anterior neural ectoderm. This interaction stabilizes anterior character (dark blue). The anterior neural ridge (anr, orange) forms at the neural-surface ectoderm junction and provides trophic factors. Other abbreviations: ve, visceral endoderm; ex (pink), extra-embryonic region; em, embryonic tissues.
Development of the head is organized around the closed rostral ends of two tubes: the ectodermal neural tube, and the endodermal gut tube. Mesoderm lies between. With head mesenchyme present laterally, axial mesoderm runs along the midline (Fig 3). Underlying the ventral midline of the forebrain is the prechordal plate (PrCP), a tissue with both mesodermal and endodermal characteristics. It is a critical source of signals for ventral patterning of the forebrain, particularly Sonic hedgehog [Shimura and Rubenstein, 1997; Chiang et al., 1996]. At the junction between the rostralmost limit of the neurectoderm and the surface ectoderm is anterior neural ridge (ANR). It is important for telencephalic growth, via secretion of growth factors such as Fgf8 [Rubenstein et al., 1998]. The foregut endoderm is also essential for patterning and growth of the forebrain [Martinez-Barbera et al., 1999; Withington et al., 2001].
Figure 3. Key signaling sources for forebrain development in the neurula.
The left image shows in diagrammatic form the tissues of the neurulating embryo, at the 5 somite stage. At the right, most tissues have been subtracted from the diagram to highlight the location of the notochord (green), prechordal plate (PrCP) (green), and anterior neural ridge (ANR; yellow) realtive to the cranial neural folds. Rostral neurectoderm is shown in white. The prechordal plate, anterior neural ridge, foregut endoderm and notochord are all signaling sources for early brain development. Left panel adapted from Hogan et al. [1994].
Meanwhile, cranial neural crest cells (NCCs), a migratory population that derives from the dorsal neural tube, colonize the rostral structures created by the juxtapositions of germ layers and contribute to head mesenchyme. Interactions between these tissues lead to the development of the tissues and organs of the head, neck and upper thorax. In mouse, cranial NCCs are formed from the dorsal aspect of the hindbrain, midbrain, and forebrain, and migrate toward the eye and frontonasal process, and into the rostral branchial arches [Serbedzija et al., 1992] (Fig 4). Neural crest migration from the mouse neural tube is first observed at the 5-somite stage in the rostral hindbrain, and is complete by the 16-somite stage. Among their many roles in craniofacial development, NCC descendents contribute much of the tissue of the face, including the mandible, maxilla and frontonasal mass. Signals such as Shh from the axial mesendoderm and foregut endoderm are critical for the survival and patterning of the craniofacial NCCs.
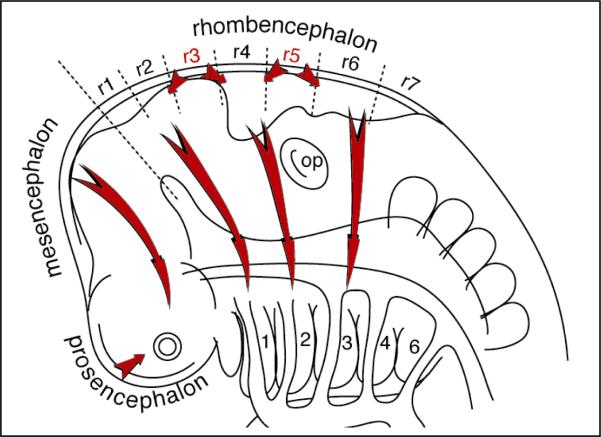
Figure 4. Origin and migration of cranial neural crest cells.
Cranial neural crest cells delaminate from the dorsal forebrain, midbrain and hindbrain in the mouse. Cells streaming from the dorsal forebrain (prosencephalon) migrate ventro-laterally, and cells from the midbrain (mesencephalon) migrate rostro-laterally to surround the anterior neural tube and eye (arrows). Derivatives of the cranial NCCs form much of the tissue mass of the face. In the hindbrain (rhombencephalon), separate streams of cells from rhombomeres r1 and r2, r4, and r6 enter the first, second, and third branchial arches, respectively (labeled 1,2,3).
Holoprosencephaly can result from a primary failure in some of these processes. In most cases, it likely stems from deficient signals from the PrCP. This in turn could be caused either because PrCP tissue is deficient, or because the PrCP signals are reduced despite normal morphology of the tissue. In either case, ventralizing signals to the forebrain and survival signals to the NCC would likely be insufficient. Another known cause of HPE, at least in mouse models, is reduced production of anterior primitive streak derivatives as a result of signaling imbalances during gastrulation or shortly thereafter. The result is that the forebrain, NCC and other rostral tissues are not provided sufficient developmental signals necessary for their patterning, growth or survival. Finally, defects in the target tissues of these signals could potentially underlie the forebrain or craniofacial malformations associated with HPE. Examples are the inability to respond to a patterning or trophic signal either in the forebrain neurectoderm or in the migrating cranial NCC would be comparable to lack of the signal itself.
BMP ANTAGONISM IS CRITICAL FOR EARLY HEAD DEVELOPMENT
The molecular basis of the ability of Spemann's organizer to induce forebrain and other head structures has been the topic of intensive studies, resulting in the identification of key genes. The Chordin (Chd in frog and Chrd in mouse) and Noggin (Nog) genes were identified via differential screening of dorsalized Xenopus cDNA libraries [Sasai et al., 1994; Smith and Harland, 1992]. These genes encode proteins with no sequence homology to each other, yet both were identified based upon similar embryological activity: the ability to dorsalize mesoderm in Xenopus tissues. Additionally, each induces neural tissue in naive ectoderm. Chordin is an approximately 120 kDa protein which contains four cysteine-rich domains, while Noggin is approximately 26 kDa. Chordin and Noggin each contain signal sequences, suggesting that they are secreted proteins. Both are candidates for endogenous organizer activity because each is expressed in the organizer, is able to induce partial secondary axis formation by RNA injection, and is induced by organizer-specific transcription factors.
Chordin and Noggin interact antagonistically with the Bone Morphogenetic Protein (BMP) family, a subset of the TGF□ superfamily of signaling molecules. In Xenopus mRNA microinjection experiments, the neuralizing influences of Chordin and Noggin are inhibited by co-injection of BMP4 mRNA [Sasai et al., 1995]. Experiments in Drosophila and Xenopus indicate that each factor acts solely via antagonizing the BMPs. Following the purification of Chordin and Noggin proteins, it was determined that each binds with nanomolar affinity to BMPs [Piccolo et al., 1996; Zimmermann et al., 1996]. In short, neither acts through its own receptor, but rather exerts influence in the extracellular space by binding to Bone Morphogenetic Proteins and sequestering them in an inactive complex (Fig 4). Chordin and Noggin have the highest affinity for BMP4, but also antagonize BMP2, and to a lesser extent, BMP7. Though the affinity of Noggin for BMP4 is nearly 10-fold greater than Chordin for BMP4, Noggin is required at a 10-fold greater concentration for induction of neural tissue in vivo [Harland and Gerhart, 1997; Piccolo et al., 1996; Zimmermann et al., 1996]. Thus the biological response to Chordin and Noggin may depend on factors other than their in vitro affinities.
This implies that BMP activity opposes the initiation of forebrain and neural gene expression. In frog experiments, many kinds of results support the view that while BMP activity promotes surface ectoderm development, it inhibits neural ectoderm fate. For example, injection of mRNA encoding a truncated, dominant negative BMP receptor resulted in the induction of neural tissue from naïve ectodermal “animal cap” explants [Sasai et al., 1995]. Simultaneous elimination in frog embryos of Chd, Nog and Follistatin, a more general TGF-beta antagonist, via morpholino antisense oligonucleotides precludes neural ectoderm formation [Khokha et al., 2005]. Thus, BMP activity inhibits anterior neurectoderm development. A key molecular role of the Spemann's organizer, critical for its ability to induce forebrain development, is antagonism of BMP signaling.
Given that the gastrula organizer and the genes that encode its activities are conserved in mammals, findings from Xenopus raise the issue of whether there are also negative roles for BMPs in early mammalian forebrain development. The Chrd and Nog genes in mouse are co-expressed in the gastrula organizer [Klingensmith et al., 1999; Bachiller et al., 2000] and in axial mesendoderm, except for the most rostral portion of the PrCP [Anderson et al., 2002]. In mouse naïve ectoderm explants, recombinant BMP inhibits the expression of forebrain genes from the earliest stages of forebrain specification up through the headfold stage, when forebrain morphogenesis is beginning; in contrast, the organizer and its derivatives, as well as the AVE, promote forebrain specification and maintenance [Yang and Klingensmith, 2006]. Moreover, this study found that recombinant Chrd and Nog proteins directly promote forebrain gene expression in naïve ectoderm, and that the organizer and its derivatives are sources of BMP antagonism that suppress the inhibitory effects of BMP on forebrain induction. Consistent with this evidence from explant studies that BMP signaling inhibits formation of forebrain precursors within the naïve epiblast, ablation of BMP receptor 1A in the early embryo resulted in a greatly enlarged presumptive forebrain at the expense of more posterior neural domains [Davis et al., 2004].
CHORDIN; NOGGIN MUTANTS RECAPITULATE HUMAN HOLOPROSENCEPHALY SEQUENCE
HPE is a phenotypically heterogeneous disorder characterized by failure of anterior midline growth and patterning, and associated with specific malformations of the face involving the loss of midline structures. The incidence of holoprosencephaly in human has been estimated as high as 1:250 total embryos, including abortuses, and 1:10,000 live births [Wallis and Muenke, 2000]. The spectrum of craniofacial phenotypes includes complete absence of the eyes or cyclopic eye together with the formation of a central primitive nasal proboscis in the most severe cases. In more mild cases, defects include cebocephaly (single nostril), clefting of the lip and palate, hypotelorism (closely spaced eyes), and absence of medial teeth. Classic examples are shown in Figure 6A–D.
Figure 6. Chrd;Nog mutants recapitulate human holoprosencephaly sequence.
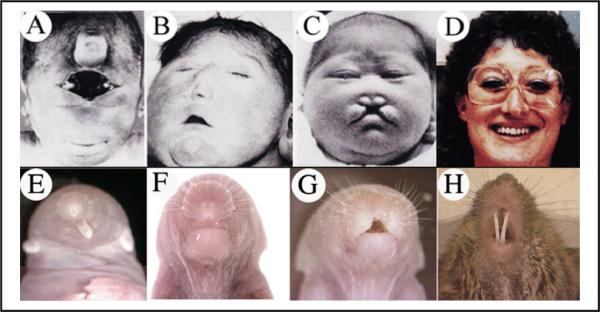
Panels A–D, adapted from a figure by Roessler et al., [1996], illustrate the spectrum of human HPE sequence. In a very severe form (A), a partially fused eye field (synopthalmia) is accompanied by craniofacial defects such as a proboscis located above the eye field, with a single nostril. Panel B shows cebocephaly, with a hallmark single nostril. An absence of midline tissue causes the oral clefting and small nose seen in Panel C. A mild form of HPE (D) allows viability but is accompanied by modest midline craniofacial deficits, resulting in narrowly spaced eyes (hypotelorism) and single central incisor in the upper jaw. Panels E–H show mice of the genotype Chrd−/−;Nog+/− mirroring precisely the craniofacial defects in the humans above. In E, note the proboscis with a single nostril. There is a single medial eye just under the skin below the proboscis, not visible in this image. In F, a narrow snout has a single nostril. The image in panel G shows oral clefting and minimal nose structures. The mildest craniofacial phenotypes, as in H, are viable. The teeth seen are the lower jaw incisors; the upper incisors are completely absent.
Variable HPE results from reduction of Chrd and Nog gene dosage in mice. In the complete absence of both genes, severe HPE is invariant, but is accompanied by severe axial development phenotypes throughout the body plan [Bachiller et al., 2000]. However, in animals of the genotype Chrd−/−;Nog+/−, phenotypes are restricted to the head. Most such animals appear to be normal, but among those with head malformations, the craniofacial phenotypic series observed is a striking reflection of the range of human presentations [Anderson et al., 2002]. Figure 6E–H shows examples of Chrd−/−;Nog+/− animals, paired with their corresponding human phenotype. In both species, most individuals with significant malformations are neonatal lethal, due to both their craniofacial and neural deficits. Interestingly, among those illustrated, only those with the mildest midline defects (involving absence of upper incisors) are viable as adults (Figs 6D,H).
In human HPE, the severity of the brain defect is frequently correlated with specific craniofacial malformations, which has spawned the adage “the face predicts the brain.” This is very much the case in Chrd−/−;Nog+/− mice as well, in a manner that reflects the human correlations. Figure 7 illustrates this in human and mouse neonates. Both were born at Duke Univ. Medical Center around the same time, with a craniofacial presentation of cebocephaly (looking very similar to the individuals in Figs 6B and F). Both have alobar holoprosencephaly due to a complete lack of the septum that should create the hemispheres of the forebrain. Both died a day or so after birth.
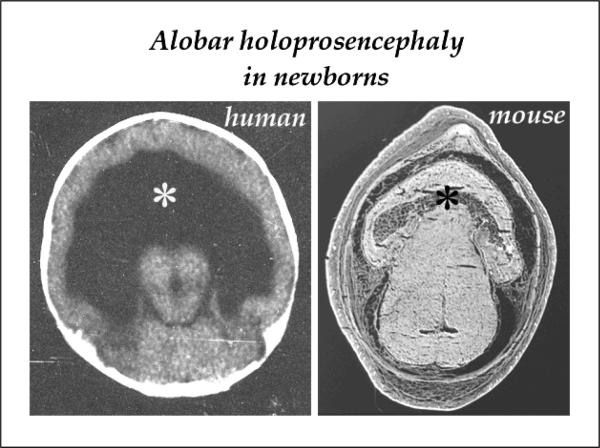
Figure 7. Alobar holoprosencephaly in human and mouse neonates.
Virtual and actual histological sections are shown through the brains of similarly afflicted human and mouse newborns, respectively, at approx 1 day of age. The human infant was in the Duke Neonatal Intensive Care Unit (NICU), while the mouse pup was in the University Vivarium. Both had a single telencephalic lobe due to a complete lack of the septum (asterisks) that would normally create the bilateral hemispheres.
MULTIPLE MECHANISMS FOR BMP ANTAGONISM IN GENERATING HPE IN MICE
Regionalization of the forebrain is directed by localized centers of inductive signaling that influence regionalized cell growth and fate determination. Shh is expressed in the prechordal midline, and is essential for the specification of the rostral ventral midline of the □diencephalon and the midline of the face [Chiang et al., 1996; Shimamura and Rubenstein, 1997]. Fgf8 is expressed in the anterior neural ridge, and promotes growth and specification of the rostral most neural plate [Meyers et al., 1998; Shimamura and Rubenstein, 1997]. We found that Chordin and Noggin expression in both of these regional forebrain organizers protects them from the influences of adjacent sources of BMPs [Anderson et al., 2002]. Specifically, we observed that increased BMP signaling represses the expression of Shh and Fgf8, in the rostral ventral neural midline, and the anterior neural ridge, respectively. One outcome of this is HPE, but in more severe embryos there were also rostral truncations. In the most severe cases, virtually the entire “face” and forebrain is absent (e.g. Fig 5E).
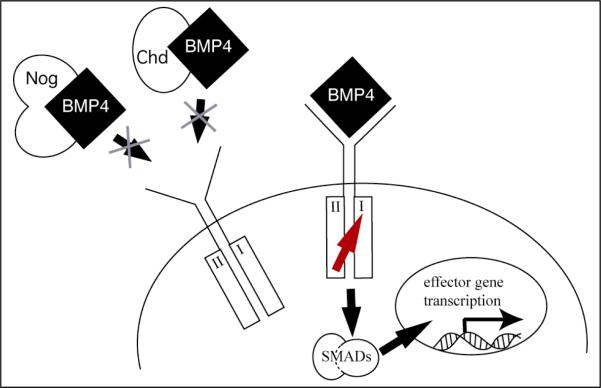
Figure 5. Paradigm of extracellular BMP antagonism.
BMP hetero- or homodimers bind cognate hetero-multimeric receptors at the cell surface. This results in phosphorylation of the type I receptor by the type II receptor (arrow) and signal transduction through the SMAD family of transcriptional activators to evoke changes in target gene expression. Chordin and Noggin bind BMPs in the extracellular space and prevent activation of receptors. As a result, these BMP antagonists act permissively, allowing presumptive target cells to execute a default program elicited by a deficit of BMP signal transduction.
In addition, the expression boundaries of Chordin and Noggin in the axial mesendoderm, when compared to specific markers of the prechordal plate, define novel subregions within the prechordal plate. This is important in light of ablation studies that have shown that the posterior region of the AME is essential for the maintenance of the rostral portion [Camus et al., 2000]. In sum, the Anderson et al. [2002] study demonstrated the importance of endogenous BMP antagonism in the function of axial gastrula organizer derivatives. Furthermore, it implicated the organizer and its derivatives in the pathogenesis of HPE. The same molecular mechanism (BMP vs. BMP antagonism) is evoked in other rostral patterning centers besides the axial mesendoderm (e.g. the ANR) during patterning of the head; therefore these may be coordinately regulated.
We have also observed that Chordin and Noggin are necessary for the contribution of the cranial neural crest to the developing head. In their absence, phenotypes such as micrognathia or agnathia can occur [Stottmann et al., 2001]. This is a result of ectopic BMP activity in the NCC environment. Early studies of neural crest induction revealed that the epidermis adjacent to the dorsal part of the neural tube was a potent source of inductive factors [Selleck and Bronner-Fraser, 1995]. BMPs can mimic the effect of the epidermis [Liem et al., 1995]. BMPs are expressed in the surface ectoderm adjacent to the neural plate, and are later expressed within the dorsal part of the neural tube, the site of neural crest formation, and in target tissues of the NCCs. BMP antagonists are also expressed at the neural plate boundary, and later in the dorsal neural tube and dorsal foregut. Nog is also expressed in migrating NCCs themselves [Stottmann et al., 2001]. Ironically, reduction in BMP antagonism by loss of Chrd and Nog alleles increases the induction and delamination of neural crest in the dorsal neural tube, but decreases NCC survival as it migrates to its target tissues [Anderson et al., 2006]. The apparent requirement for BMP antagonism during the migration of neural crest cells raises the possibility that Chordin and Noggin secreted from the neural crest cells may affect non-crest cells in the migratory environment. Reciprocally, the environment could be affecting the neural crest cells. These are avenues for future research.
Many of the genes that have been associated with HPE in humans or mice are expressed in or around the organizer. To understand their functions relative to each other, we have generated several double mutants to see if a moderate deficiency in two different pathways can produce HPE and associated craniofacial phenotypes (when neither deficiency alone causes such defects). Several of these mutant combinations resulted in HPE, and molecular analysis revealed a common loss of Shh expression in the rostral midline (our unpublished data). However, the proper localization of Shh activity at the rostral midline requires several steps. (1) The PrCP must be specified at the organizer and subsequently displaced to the rostral midline by morphogenetic mechanisms, (2) the character and continuity of the AME must be maintained, and (3) Shh must be sufficiently expressed even once the PrCP is appropriately positioned. Defects in any of these steps may lead to the common loss of Shh at the rostral ventral midline, and HPE. Our analysis has revealed a role for BMP antagonism in each of these processes.
A strong genetic interaction occurred between mutations in Chrd or Nog and mutations in Nodal signaling, resulting in often severe HPE. These and subsequent studies have demonstrated that modulation of BMP signaling is essential for correct Nodal signaling in the gastrula [Yang et al., submitted]. Importantly, Nodal is not expressed in the early head or adjacent tissues. The observed HPE results from deficient production of anterior primitive streak derivatives in gastrulation.
ADDITIONAL EVIDENCE FOR THE RELEVANCE OF BMP SIGNALING TO HOLOPROSENCEPHALY
Several other mouse mutants provide further demonstrations that inappropriate BMP signaling can lead to HPE. Twisted gastrulation (Twsg1) is another BMP signaling regulator, which forms a ternary complex with Chrd and BMP. Twsg1 mutants further support the relevance of BMP signaling in the mouse forebrain development, although its function as an antagonist or agonist is still controversial [Petryk et al., 2004; Zakin and DeRobertis, 2004]. Consistent with the affected Chrd−/−;Nog+/− embryos, affected Twsg1 mutants also show a decrease in Shh and Fgf8 expression from the PrCP and anterior neural ridge respectively, resulting in ventral forebrain and midline craniofacial defects. Megalin is a low-density lipoprotein receptor-related protein (LRP2) with many domains of expression and activity in many signaling pathways, including BMP. It is expressed in the neuroepithelium of the early embryo, and its absence results in HPE phenotypes [Spoelgen et al., 2005]. These embryos resemble Chrd−/−;Nog+/− mutants, with decreased Shh expression in the ventral telencephalon. This decrease appears to result from a failure in uptake and degradation of Bmp4 by Megalin's endocytic activity in the developing forebrain. The ectopic Bmp4 activity would then repress local Shh expression, exactly analogous to the situation in which the BMP antagonists are reduced [Anderson et al., 2002].
Early cues necessary for head induction are inhibition of BMP signals, but also Wnt signals, another type of receptor-mediated intercellular signaling ligands. Interestingly, the gene encoding the Wnt antagonist, Dickkopf1 (Dkk1) genetically interacts with Nog in early head development. Although neither Dkk1+/− nor Nog+/− embryos exhibit HPE phenotype on their own, some Dkk1+/−;Nog+/− embryos display a wide spectrum of rostral truncations accompanied with loss of the Fgf8 and Shh-dependent homeobox gene Nkx2.1 expression in the forebrain [Barrantes et al., 2003].
Another type of HPE, a middle inter-hemispheric (MIH) variant, manifests dorsal telencephalic midline defects without obvious craniofacial deformities. The roof plate (RP) of the neural tube is a key signaling center for dorsal forebrain patterning, expressing multiple BMPs [Cheng et al., 2006]. Mice lacking BMP receptors specifically in the telencephalon show the MIH phenotype, as a result of missing the choroid plexus and the cortical hem [Fernandes et al., 2007], two structures along the dorsal midline of the telencephalon. Moreover, most ventral gene expression and patterning domains are maintained in these mutants. Interestingly, ectopic Shh signaling in the dorsal telencephalon causes loss of BMP signaling from the dorsal telencephalic midline, resulting in MIH [Huang et al., 2007]. This further illustrates the mutually antagonistic relationship between BMP and Shh signaling in early neural patterning.
IMPLICATIONS OF MURINE STUDIES OF BMP ANTAGONISTS FOR HOLOPROSENCEPHALY PATHOGENESIS IN HUMANS
At present, mutations in nine or so genes arguably involved in the Shh and Nodal signaling pathways have been identified in patients with HPE. Mutations in a few other genes have been identified as well, but collectively cases with mutations in any of these genes are a small minority of the overall sample set. This suggests other genes involved in the malformation are yet to be identified in human HPE patients. One of the most obvious and important questions resulting from the study of HPE in Chrd;Nog mutant mice is whether BMP antagonists are responsible for any instances of HPE in humans. While CHORDIN and NOGGIN do not map to any HPE loci, it remains possible that they represent novel loci, or act as modifiers of the identified HPE loci. Interestingly, TWSG1, another BMP antagonist, has been linked with a defined HPE locus, HPE4. Human genomic DNA samples derived from individuals affected with HPE should be assessed for mutations in BMP antagonists and other components of the BMP signaling pathway.
Based on our double and triple mutant analysis, we would suggest that BMP antagonist mutations might be sensitizing alleles for known HPE loci. This would seem to be especially true for HPE loci that are associated with Nodal signaling [Yang et al., submitted]. We envision that the presence of a loss-of-function mutation in CHORDIN, NOGGIN or TWSG1 might make the consequences of a Nodal pathway mutation more severe. Thus, among human HPE cases with Nodal pathway mutants, perhaps the more strongly affected individuals also harbor BMP antagonist mutations.
Finally, our results and those of other BMP studies highlight the need to consider that there are multiple, distinct embryological causes of HPE phenotypes. They involve (1) failures in the creation of axial mesendoderm due to gastrulation signaling imbalances; (2) deficient signaling from axial midline derivatives; (3) local failures in forebrain signaling or signal reception; or (4) deficient NCC contribution to craniofacial structures. This suggests that the range of potential mutations and mechanisms considered in human HPE studies should be expanded to better understand this malformation syndrome.
ACKNOWLEDGMENTS
We gratefully acknowledge funding from the NIH (NIDCR and NICHD) for support of this research.
Biographies
Author biographies
John Klingensmith, PhD, is an Associate Professor of Cell Biology and Pediatrics at Duke University School of Medicine. He obtained his doctoral degree at Harvard Medical School working in Drosophila developmental genetics, and did postdoctoral work in Toronto on mouse embryology and genetics.
Maiko Matsui, BA, is a graduate student in Cell Biology at Duke University. She obtained her undergraduate degree from Kyoto University, and has a long standing interest in the mechanisms of craniofacial and brain malformations.
Yu-Ping Yang, PhD, is now a postdoctoral fellow in Cell and Developmental Biology at Vanderbilt University. She did her doctoral research on the molecular mechanisms of early forebrain development at Duke University.
Ryan M. Anderson, PhD, is currently a postdoctoral fellow working in zebrafish development at UCSF. He did his graduate work in the developmental genetics of mouse holoprosencephaly at Duke University.
REFERENCES
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Stottmann RW, Choi M, Klingensmith J. Endogenous BMP antagonists regulate mammalian neural crest generation and survival. Dev Dynamics. 2006;235:2507–2520. doi: 10.1002/dvdy.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Barrantes IDB, Davidson G, Grone HJ, Westphal H, Niehrs C. Dkk1 and noggin cooperate in mammalian head induction. Genes and Dev. 2003;17:2239–2244. doi: 10.1101/gad.269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Anterior patterning in the mouse. Trends in Genetics. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Camus A, Davidson BP, Billiards S, Khoo P, Rivera-Perez JA, Wakamiya M, Behringer RR, Tam PP. The morphogenetic role of midline mesendoderm and ectoderm in the development of the forebrain and the midbrain of the mouse embryo. Development. 2000;127:1799–1813. doi: 10.1242/dev.127.9.1799. [DOI] [PubMed] [Google Scholar]
- Cheng X, Hsu C, Currle DS, Hu JS, Barkovich AJ, Monuki E. Central roles of the roof plate in telencephalic development and holoprosencephaly. Journal of Neuroscience. 2006;26:7640–7649. doi: 10.1523/JNEUROSCI.0714-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Perspectives on holoprosencephaly: Part I. Epidemiology, genetics, and syndromology. Teratology. 1989;40:211–35. doi: 10.1002/tera.1420400304. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP Receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Dufort D, Schwartz L, Harpal K, Rossant J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development. 1998;125:3015–3025. doi: 10.1242/dev.125.16.3015. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Gutin G, Alcorn H, McConnell SK, Hebert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann's organizer. Ann Rev of Cell and Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Huang X, Litingtung Y, Chiang C. Ectopic sonic hedgehog signaling impairs telencephalic dorsal midline development: implication for human holoprosencephaly. Human Molecular Genetics. 2007;16:1454–1468. doi: 10.1093/hmg/ddm096. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Ang SL, Bachiller D, Rossant J. Neural induction and patterning in the mouse in the absence of the node and its derivatives. Dev Biol. 1999;216:535–549. doi: 10.1006/dbio.1999.9525. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–243345. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O'Connor MB. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev Biol. 2004;267:374–386. doi: 10.1016/j.ydbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Beachy PA. Patterning of the embryonic forebrain. Curr Opin Neurobiol. 1998;8:18–26. doi: 10.1016/s0959-4388(98)80004-4. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Ann Rev of Neurosci. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer- specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;377:757. doi: 10.1038/377757a0. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Wakamiya M, Kwan KM, Kania A, Jessell TM, Behringer RR. Lim1 is required in both primitive streak-derived tissues and visceral endoderm for head formation in the mouse. Development. 1999;126:4925–4932. doi: 10.1242/dev.126.22.4925. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JLR. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–40. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Spoelgen R, Hammes A, Anzenberger U, Zechner D, Anderson OM, Jerchow B, Willnow TE. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–414. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol. 2001;240:457–473. doi: 10.1006/dbio.2001.0479. [DOI] [PubMed] [Google Scholar]
- Tam PP, Steiner KA. Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development. 1999;126:5171–5179. doi: 10.1242/dev.126.22.5171. [DOI] [PubMed] [Google Scholar]
- Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol. 1996;6:1487–1496. doi: 10.1016/s0960-9822(96)00753-1. [DOI] [PubMed] [Google Scholar]
- Wallis D, Muenke M. Mutations in holoprosencephaly. Hum Mutat. 2000;16:99–108. doi: 10.1002/1098-1004(200008)16:2<99::AID-HUMU2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Withington S, Beddington R, Cooke J. Foregut endoderm is required at head process stages for anteriormost neural patterning in chick. Development. 2001;128:309–320. doi: 10.1242/dev.128.3.309. [DOI] [PubMed] [Google Scholar]
- Yang Y, Klingensmith J. Roles of organizer factors and BMP antagonism in mammalian forebrain establishment. Dev Biol. 2006;296:458–475. doi: 10.1016/j.ydbio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Zakin L, De Robertis EM. Inactivation of mouse Twisted gastrulation reveals its role in promoting Bmp4 activity during forebrain development. Development. 2004;131:413–424. doi: 10.1242/dev.00946. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar J, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein-4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]