Abstract
The Suzuki-Miyaura cross-coupling of sterically hindered and electron-rich ortho,ortho’-substituted aryl halides with potassium vinyltrifluoroborate utilizing microwave irradiation has been conducted while adjusting solvent ratio, irradiation time, and catalyst loading to find optimal conditions. Coupling of benzyl 3,5-bis(benzyloxy)-4-bromobenzoate leads to a mixture of the desired styrene derivative, and the reduced product. 4-Bromo-1,3,5-trimethoxybenzene, methyl 4-bromo-3,5-dimethoxybenzoate, and mesitylene bromide were also coupled to test the breadth and scope of this methodology. Of these substrates tested only 4-bromo-1,3,5-trimethoxybenzene was not vinylated successfully, which is believed to be due to the electron rich nature of this system.
Keywords: Suzuki-Miyaura, Potassium vinyltrifluoroborate, Styrenes, Palladium catalysis, Hindered aryl bromides
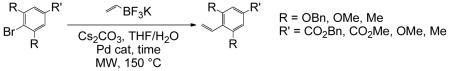
Substituted styrenes (typically assembled using a transition metal-mediated cross-coupling) are useful intermediates in both the formation of new polymeric material and in the formation of specialty chemicals.1 Mild vinylation of aryl bromides and/or iodides using trivinylcyclotriboroxane,1 vinylmagnesium bromide,2 vinyltrimethylsilane,3 and vinylpolysiloxanes4 has been observed. N-Heterocyclic carbenes bearing palladium (II) catalysts have demonstrated high catalytic activity capable of coupling unactivated aryl chlorides.5 Aryl bromides and activated aryl chlorides also undergo mild palladium-catalyzed couplings at room temperature with alkenes utilizing dicyclohexylmethylamine as a base.6 Potassium vinyltrifluoroborate is a stable alternative that circumvents the limitations of vinylboronic acids, and derivatives of vinylboronic esters.7-10 Molander first reported the Suzuki-Miyaura cross-coupling vinylation of aryl halides with potassium vinyltrifluoroborate in 2002;8 other examples have been reported in the literature since.10-16 However, most of the literature is dominated by Suzuki-Miyaura type couplings involving boronic acids or their derivatives.5,6,12-21 This paper investigates the Suzuki-Miyaura cross-coupling reaction between various sterically hindered and electron-rich arenes including benzyl 3,5-bis(benzyloxy)-4-bromobenzoate (1, Scheme 1) with potassium vinyltrifluoroborate (2) with the assistance of microwave irradiation.
Scheme 1.
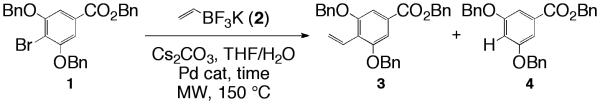
Microwave-enhanced vinylation of benzyl 3,5-bis(benzyloxy)-4-bromobenzoate (1).
Scheme 1 illustrates the first reaction of interest, and the two possible products of the cross-coupling reaction; the desired vinylated product, benzyl 3,5-bis(benzyloxy)-4-vinylbenzoate (3), and reduced product, benzyl 3,5-bis(benzyloxy)benzoate (4). As with conventional conditions14 the reduced product was observed under microwave conditions, but only in trace amounts (less than 1% of the total product distribution). Product ratios were determined, if necessary, using 1H NMR integration of the crude reaction mixture using the methylene hydrogens from the benzyl ethers of the starting material 1, as well as for the vinyl product 3; chemical shifts of 5.18 ppm (s, 4H) and 5.14 ppm (s, 4H), respectively. The amount of reduced product 4, if any, was determined by integration of the triplet at 6.80 ppm (t, J = 2.4 Hz, 1H).
Initially, we focused on optimizing the vinylation of benzyl 3,5-bis(benzyloxy)-4-bromobenzoate (1) (a compound that has been of interest for this lab towards the synthesis of derivatives of the antibiotic cytosporone E14) using 9 mol % of the palladium catalyst, [PdCl2(dppf)CH2Cl2], 5 equivalents of potassium vinyltrifluoroborate (2), 3 equivalents of cesium carbonate, and 0.051 M solution of THF/H2O (10:1), with respect to 1, at 150 °C (entries 1-8, Table 1). For all the reactions that gave the desired product, 3, an average yield of 51% was observed. Entries 5-7 demonstrate that time frames longer than 20 min did not increase the yield above 57%. It is also apparent from Entries 1-4 that at least 20 min is needed to ensure completion of the reaction. This is a significant improvement from the 3-4 days required for the conventional process and a decrease in the formation of the reduced product 4.14 Interestingly, the average yield was observed to be lower if the times were divided (avg. 47%) despite that many entries had the same overall irradiation times. (i.e. Entries 1 vs. 5, 20 min; Entries 2 and 3 vs. 6, 30 min.)
Table 1.
Effect of irradiation time on benzyl 3,5-bis(benzloxy)-4-bromobenzoate (1)
| Entrya | Time | 1:3:4b | Yield of 3c |
|---|---|---|---|
| 1 | 10 min × 2 | 0:1:trace | 45 |
| 2 | 10 min × 3 | 0:1:trace | 49 |
| 3 | 15 min × 2 | 0:1:trace | 48 |
| 4 | 15 min | 11:50:1 | n/a |
| 5 | 20 min | 0:1:trace | 57 |
| 6 | 30 min | 0:1:trace | 57 |
| 7 | 45 min | 0:1:trace | 56 |
| 8 | 60 min | 0:1:trace | 56 |
9 mol % PdCl2(dppf)CH2Cl2, THF:H2O (10:1) at 0.051 M, 5 equiv of 2, 3 equiv Cs2CO3, 150 °C.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
Entries 9 and 10, shown in Table 2, were designed to emulate the conventional reaction, which required two additions of 9 mol % PdCl2(dppf)CH2Cl2 catalyst.14 In both cases the reactions went to completion, but the yields were similar to entries 1-3 in Table 1, where irradiation times were split to allow for the second addition of catalyst (avg. 47%). This suggested that only one addition of catalyst (and only 9 mol%) was sufficient for the reaction. (These reactions were monitored by TLC in between catalyst loading and revealed that nearly all of the starting material, 1, was consumed at the point of second addition.)
Table 2.
Effect of doubling the catalyst loading from 9 mol % to 18 mol % with benzyl 3,5-bis(benzloxy)-4-bromobenzoate (1)
18 mol % PdCl2(dppf)CH2Cl2 (added in two equal 9 mol% portions), THF:H2O (10:1) at 0.051 M, 5 eq. of 2, 3 eq. C2CO3, 150 °C.
Isolated yield.
Determined by 1H NMR integration of the crude mixture.
Further optimization of the conditions in this cross-coupling reaction probed the use of a variety of Pd (II) catalysts including: [PdCl2(dppf)CH2Cl2], Pd(OAc)2 and PdCl2] and phosphine ligands, where warranted, [X-Phos, RuPhos, and PPh3] (entries 11-17, Table 3). A few reactions (entries 15-17, Table 3) included the addition of tetrabutylammonium iodide, which has been shown to increase the nucleophilicity of the potassium organotrifluoroborates,14,22 but it had no positive effect with our substrate. The only reaction that yielded only the desired product was Entry 12, which employed PdCl2 and X-Phos.23 However, these reagents were added as 2 mol % and 6 mol % respectively in two additions over 60 min (for a total of 4 mol % of PdCl2, and 12 mol % of X-Phos), yielding only 29% yield of 3. Utilizing PPh3 or RuPhos (Entries 13 and 14) also gave a poor product distribution, and these conditions mirror Molander’s conditions for sterically hindered ortho,ortho’-substituted electron-rich aryl halides.12 These results, along with previous results, necessitated the employment of the original catalyst PdCl2(dppf)CH2Cl2 versus PdCl2, X-Phos for success (Tables 1, 2 and 3).12,22,24
Table 3.
Effect of catalyst and additives on the yield of benzyl 4-bromo-3,5-bis(benzloxy)benzoate (3)
| Entrya | Catalystb | Mole % | Time | 1:3:4c | Yield of 3d |
|---|---|---|---|---|---|
| 11 | PdCl2, X-Phos | 2,6 | 30 min | 2:1:trace | n/a |
| 12 | PdCl2, X-Phos | (2,6) × 2 | 30 min × 2 | 0:1:trace | 29 |
| 13 | PdCl2, PPh3 | 2,6 | 30 min | 2.3:1:trace | n/a |
| 14 | PdCl2, RuPhos | 2,6 | 30 min | 1.6:1:0 | n/a |
| 15b | Pd(OAc)2, dppb | 5,5 | 30 min | 0:3:1 | n/a |
| 16b | PdCl2(dppf)CH2Cl2 | 9 | 60 min | 1:3:1 | n/a |
| 17b | PdCl2(dppf)CH2Cl2 | 9 | 90 min | 0:5:1 | n/a |
THF:H2O (10:1) at 0.051 M, 5 equiv of 2, 3 equiv Cs2CO3, 150 °C.
Employed Bu4NI.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
The effect of solvent molarity for the reaction was also reinvestigated (Table 4, Entries 18-20), while keeping the solvent ratio of THF:H2O (10:1).14 The optimum concentration was still determined to be the 0.051 M in comparison to entries 5 and 10 (Tables 1 and 2), under microwave conditions, but it was found that the coupling could tolerate concentrations up to a 0.091 M solution with a slight drop in yield (entry 19, Table 4).
Table 4.
Toleration of vinylation reaction to changes in solvent molarity with benzyl 3,5-bis-(benzloxy)-4-bromobenzoate (1)
| Entrya | Time | Molarity | 1:3:4b | Yield of 3c |
|---|---|---|---|---|
| 18 | 30 min | 0.027 M | 7:15:1 | n/a |
| 19 | 30 min | 0.091 M | 0:1:trace | 44 |
| 20 | 30 min | 0.18 | 0:9:1 | n/a |
9 mol % PdCl2(dppf)CH2Cl2, THF:H2O (10:1) at 0.051 M, 5 equiv of 2, 3 equiv Cs2CO3, 150 °C for 30 min
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
During optimization of the conventional reaction12 it was found that the reaction needed 5 equivalents of potassium vinyltrifluoroborate (2); therefore, this variable was studied again with the microwave reaction (Entries 21-22, Table 5). 2 was varied from 1.1 to 10 equivalents where it was found that 5 equivalents still gave the best overall yield (57%; Entry 5 & 6, Table 1). The use of 10 equivalents was an interesting result because when the reaction is under conventional control the use of an extreme excess of 2 increases the yield to 87%.14 Notably, under microwave conditions the yield decreased to 44% (Entry 22, Table 5).
Table 5.
The effect of varying equivalents of potassium vinyltrifluoroborate with benzyl 3,5-bis-(benzloxy)-4-bromobenzoate (1)
9 mol % PdCl2(dppf)CH2Cl2, THF:H2O (10:1) at 0.051 M, 3 equiv Cs2CO3, 150 °C for 30 min.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
Although the reaction worked moderately well with 9 mol % of the catalyst PdCl2(dppf)CH2Cl2 (Table 1, Entries 5 and 6), decreasing the catalyst loading was still desired. To directly explore this variable the catalyst loading was sequentially adjusted from 9 mol % to 3 mol %, (Entries 23-25, Table 6). It was found that employing 3 mol % or 7 mol % of the catalyst resulted in incomplete reactions or lowered yields (Entries 23 and 25, Table 6). Using 7 mol % of the referred catalyst was experimentally similar to using 9 mol % of the catalyst, giving a 50% yield when compared to Table 1 (Entries 5 and 6). Using 5 mol % of the catalyst PdCl2(dppf)CH2Cl2 produced the desired product 3 with a 77% yield (Table 6, Entry 24). This factor not only increased the yield, but further confirmed that only one addition of catalyst was needed and the presence of the reduced product 4 was also not seen via 1H NMR.
Table 6.
Effect of molar percent of PdCl2(dppf)CH2Cl2 catalyst loading
PdCl2(dppf)CH2Cl2, THF:H2O (10:1) at 0.051 M, 3 equiv Cs2CO3, 150 °C for 30 min.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
Solvent systems have been a particular point of interest in cross-coupling reactions; particularly when microwave irradiation is employed due to the need for a solvent with an appreciable dielectric constant.19 A variety of solvents and solvent mixtures have been employed with superb results in cross-coupling reactions.5,6,12,14,21,22,24,25 We chose to investigate the effects of changing the solvent ratios of THF and H2O and irradiation time with respect to the optimized conditions: 5 mole % of PdCl2(dppf)CH2Cl2, 0.051 M solvent concentration, 5 equiv of 2 (CH2=CHBF3K), and irradiation at 150 °C; THF:H2O ratios were varied from 11:1 to 9:1 irradiation times of 30 and 20 min were employed, respectively (Tables 7 and 8).
Table 7.
Effect of different solvent ratios at 30 minutes of irradiation time
5 mol % PdCl2(dppf)CH2Cl2, THF:H2O at 0.051 M, 3 eq. Cs2CO3, 150 °C for 30 min.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
Table 8.
Effects of different solvent ratios at 20 minutes of irradiation time
5 mol % PdCl2(dppf)CH2Cl2, THF:H2O at 0.051 M, 3 equiv Cs2CO3, 150 °C for 20 min.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
Decreasing the water concentration of the solvent system reduced, which is demonstrated by Entry 26 of Table 7, affording a modest yield of 3 (61% yield), with no reduced product observed. Decreasing the amount of H2O in the (THF:H2O) mixture was discontinued after these results due to the reduced yield compared to the yield previously observed for a (10:1) ratio (Entry 24, Table 6). These screenings also revealed that the 10:1 (THF:H2O) conditions at 20 min and 30 min yielded identical (Entry 24 in Table 6, and Entry 27 in Table 8), acceptable results of 77% yield of 3. (Interestingly, similar reactivity occurred in Table 1, Entries 5 and 7; where 20 and 30 min irradiation times yielded 57% of 3.) Ultimately, the 9:1 (THF:H2O) mixture was the most successful, affording 3 with similar yields again at 20 and 30 min irradiation times with 93% and 89%, (Entry 29 in Table 8, and Entry 27 in Table 7 respectively).12 Increasing the reaction time for the 9:1 mixture did not result in any improved results (not shown).
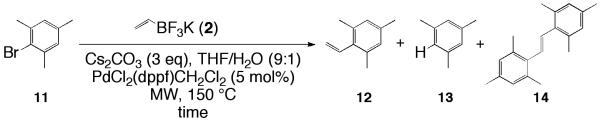
With the optimized reaction conditions (being 5 mol% PdCl2(dppf)CH2Cl2, 5 equiv of potassium vinyltrifluoroborate, 3 equiv cesium carbonate, 0.051 M THF/H2O (9:1), and heating at 150 °C for 20 min) for 3 in hand we studied the applicability and scope of our method with other activated, ortho,ortho’-hindered aryl halides; 1-bromo-2,4,6-trimethoxybenzene 5 (Scheme 2), methyl 3,5-dimethoxy-4-bromobenzoate 8 (Scheme 3), and 2-bromomesitylene 11 (Scheme 4).
Scheme 2.
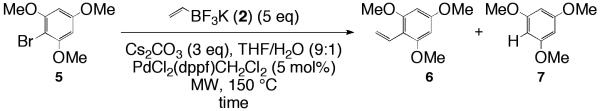
Attempted vinylation of 2-bromo-1,3,5-trimethoxybenzene (5) employing optimized conditions.
Scheme 3.
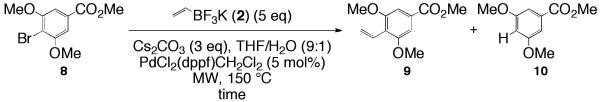
Vinylation of methyl 4-bromo-3,5-dimethoxybenzoate (8).
Scheme 4.
Vinylation of mesitylene bromide (11).
Vinylation attempts of 2-bromo-1,3,5-trimethoxhybenzene 5 with our optimized (Scheme 2) conditions yielded only starting material. Regardless of the time employed, (Entries 30-32, Table 9). The strongly activated ring is believed to account for this outcome, as has been noted in the literature.12,14,20,21 It should be noted here for the reader that although our methodology was unproductive, Fu and Nolan’s pre-mentioned vinylation methods appear to be a more amenable to substrates involving extremely electron rich and sterically hindered aryl halides.5,6
Table 9.
Vinylation attempts with 2-bromo-1,3,5-trimethoxybenzene (5)
5 mol % PdCl2(dppf)CH2Cl2, THF:H2O (9:1) at 0.051 M, 3 equiv Cs2CO3, 150 °C.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
The vinylation of methyl 4-bromo-3,5-dimethoxybenzoate 8 (see Scheme 3) was found to be strongly time dependent as the time was increased from 20 minutes to 30 min. Entries 33 and 34 demonstrate this observation (44% to 82%; respectively, Table 10). Experiments running longer than 30 min did not show any increase in yield. Both substrates 5 and 8 are similar in that they both have the ortho,ortho’-hindering alkoxy groups but differ in the functional group that is para to the bromo substituent. The success of substrate 8 is believed to be attributed to the presence of the electron-withdrawing methyl ester present in the molecule as seen with substrate 3.
Table 10.
Vinylation results with methyl 4-bromo-3,5-dimethoxybenzoate (8)
5 mol % PdCl2(dppf)CH2Cl2, THF:H2O (9:1) at 0.051 M, 3 equiv Cs2CO3, 150 °C.
Determined by 1H NMR integration of the crude mixture.
Isolated yield.
Mesitylene bromide (11) was chosen for its three moderately activating methyl groups and the ortho,ortho’ hindrance of the bromo substituent (Scheme 4). The vinylation of mesitylene bromide 11 (Scheme 4) was treated similarly to the 1,3,5-trimethoxybenzene 5 due to their electron rich nature. Hence reaction times were begun at 30 min and ranged to 60 min (Entries 35-37, Table 11). Entry 36 showed a 67% yield of a (1:5.1:0:0) mixture of 11, 12, 13, and 14 after irradiation for 45 min with no stilbene side product observed. Extending irradiation times increased the vinylated product yield (80%), but also introduced the Heck product 14 (see Entry 37). This was an improvement over the conventional conditions, which converted substrate 11 almost exclusively to the stilbene product 14.14 It should also be noted that Denmark and Butler have recently reported superior results (99% yield) with substrate 11 via polyvinylsiloxanes.4b Buchwald has also modestly coupled 11 (using conventional conditions) with a 83% yield of 12 with no side products.24
Table 11.
Vinylation of mesitylene bromide (11)
| Entrya | Time | 11:12:13:14b | Yield of 12b |
|---|---|---|---|
| 35 | 30 min | 1:4:0:0 | 37 |
| 36 | 45 min | 1:5.1:0:0 | 67 |
| 37 | 60 min | 1:9:0:1 | 80 |
5 mol % PdCl2(dppf)CH2Cl2, THF:H2O (9:1) at 0.051 M, 3 equiv Cs2CO3, 150 °C.
Yields and ratios determined by n-dodecane internal standard and 1H NMR integration.
In conclusion, microwave assistance (compared to the conventional conditions)14 for this cross-coupling reaction has reduced the amount of catalyst loading by over three-fold from 18 mol % to 5 mol %, it has demonstrated a dramatic decrease in reaction time from 3-4 days to 20 minutes (nearly 300 times faster), and it has increased the yield from 73% to 93% for benzyl ester 1. This investigation also revealed that the reaction methodology is more tolerant to solvent ratios with all substrates employed than previously believed and that proper catalyst loading and correct irradiation time can help eliminate the production of the reduced product. It has also been shown that the vinylation with potassium vinyltrifluoroborate is best accomplished with ortho,ortho’-substituted aryl halides with at least one electron-withdrawing group present thus reducing the electron-rich nature of the aromatic halide (which is common to the Suzuki-Miyaura reaction in general).
Experimental
Typical Suzuki-Miyaura coupling conditions
A dry 10 mL Pyrex tube (from CEM) fitted with an airtight rubber septa was charged with a stir bar, potassium vinyltrifluoroborate (2) (133 mg, 0.995 mmol), cesium carbonate (194 mg, 0.597 mmol), PdCl2(dppf)CH2Cl2 (7.2 mg, 0.0099 mmol), and benzyl 3,5-bis(benzyloxy)-4-bromobenzoate (1) (100 mg, 0.199 mmol) was flushed with argon. Degassed THF (3.87 mL) and degassed deionized H2O (0.430 mL) were then added. The rubber septum was replaced with a septum cap from CEM to give a sealed system. The resulting rust-red colored solution was placed in a CEM Discover microwave unit and allowed to react for 30 minutes at 150 °C. The resulting brown solution was diluted with 7 mL of H2O and extracted with Et2O (10 mL × 3). The combined organic layers were then washed with 1 M HCl (10 mL) and brine (10 mL). The clear, yellow solution was dried over MgSO4 and filtered. The solvent was removed under reduced pressure using a rotary evaporator. The red-brown residue was purified using flash chromatography (3% EtOAc/5% CHCl3/Hexanes) to yield benzyl 3,5-bis(benzyloxy)-4-vinylbenzoate 3 as a colorless solid in 93% yield (76 mg). mp = 102-103 °C. 1H NMR (CDCl3: 300 MHz): δ 7.44-7.28 (m, 17H), 7.08 (dd, J = 18.1 and 12.2 Hz, 1H), 6.25 (dd, J = 18.0 and 2.5 Hz, 1H), 5.52 (dd, J = 12.2 and 2.6 Hz, 1H), 5.34 (s, 2H), 5.12 (s, 4H). 13C NMR (CDCl3; 300 MHz): 166.2, 157.6, 136.8, 136.2, 129.5, 128.8, 128.7, 128.4, 128.3, 128.2, 127.7, 127.0, 121.6, 120.4, 106.9, 71.0, 67.0. IR (solid): υ = 3063, 3026, 1708, 1623, 1567, 1120, 1114 cm−1.
Typical CEM Discover microwave settings
Power: 150 W, Ramp time: 02:00 min, Hold time 30:00 min, Temperature: 150 °C, with stirring. The pressure feedback from the CEM Discover instrument ranged from 150 psi to 175 psi.
Acknowledgments
The authors thank the NIH Grant Number P20 RR-016461 from the National Center for Research Resources NIH SC-INBRE grant, ACS PRF (Type G 39541-GB1), and the College of Charleston Department of Chemistry and Biochemistry for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Kerins F, O’Shea DF. J. Org. Chem. 2002;67:4968–4971. doi: 10.1021/jo020074o. [DOI] [PubMed] [Google Scholar]
- (2).Bumagin NA, Luzikova EV. J. Organomet. Chem. 1997;532:271–273. [Google Scholar]
- (3).Jeffery T. Tetrahedron Lett. 1999;40:1673–1676. [Google Scholar]
- (4).a) Denmark SE, Wang Z. J. Organomet. Chem. 2001;624:372–375. [Google Scholar]; b) Denmark SE, Butler CR. Org. Lett. 2006;8:63–66. doi: 10.1021/ol052517r. [DOI] [PubMed] [Google Scholar]
- (5).a) Navarro O, Kaur H, Mahjoor P, Nolan SP. J. Org. Chem. 2004;69:3173–3180. doi: 10.1021/jo035834p. [DOI] [PubMed] [Google Scholar]; b) Marion N, Navarro O, Edwin SD, Scott NM, Nolan SP. J. Am. Chem. Soc. 2006;128:4101–4111. doi: 10.1021/ja057704z. [DOI] [PubMed] [Google Scholar]
- (6).Littke AF, Fu GC. J. Am. Chem. Soc. 2001;123:6989–7000. doi: 10.1021/ja010988c. [DOI] [PubMed] [Google Scholar]
- (7).Vedejs E, Chapman RW, Fields SC, Lin S, Schrimf MR. J. Org. Chem. 1995;60:3020–3027. [Google Scholar]
- (8).a) Darses S, Michaud G, Benet J-P. Tetrahedron Lett. 1998;39:5045–5048. [Google Scholar]; b) Molander GA, Rivero MR. Org. Lett. 2002;4:107–109. doi: 10.1021/ol0169729. [DOI] [PubMed] [Google Scholar]
- (9).a) Matteson DS. J. Am. Chem. Soc. 1960;82:4228–33. [Google Scholar]; b) Onak T. Organoborane Chemistry. Academic Press; New York: 1975. [Google Scholar]
- (10).Molander GA, Figueroa R. Aldrichimi. Acta. 2005;38:49–56. [Google Scholar]
- (11).Molander GA, Bernardi CR. J. Org. Chem. 2002;67:8424–8429. doi: 10.1021/jo026236y. [DOI] [PubMed] [Google Scholar]
- (12).Molander GA, Brown AR. J. Org. Chem. 2006;71:9681–9686. doi: 10.1021/jo0617013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).a) Joucla L, Cusati G, Pinel C, Djakovitch L. Tetrahedron Lett. 2008;49:4738–4741. [Google Scholar]; b) Caneque T, Cuadro AM, Alvarez-Builla J, Vaquero JJ. Tetradedron Lett. 2009;50:1419–1422. [Google Scholar]
- (14).Carter RR, Wyatt JK. Tetrahedron Lett. 2006;47:6091–6094. b) Using conventional conditions this reaction needed 3-4 days at 150 °C to proceed to completion and required 18 mol % (added in two equimolar catalytic portions) of the palladium catalyst [PdCl2(dppf)CH2Cl2] along with 5 equiv of potassium vinyltrifluoroborate to attain a 73% isolated yield.
- (15).a) Kappe CO. Angew. Chem., Int. Ed. 2004;43:6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]; b) Kappe CO, Dallinger D. Nat. Rev. Drug Discov. 2006;5:51–63. doi: 10.1038/nrd1926. [DOI] [PubMed] [Google Scholar]; c) Nilsson P, Olofsson K, Larhed M. Top. Curr. Chem. 2006;266:126–134. [Google Scholar]
- (16).a) Harker RL, Crouch DR. Synthesis. 2007:25–27. [Google Scholar]; b) Arvela RK, Leadbeater NE, Mack TL, Kormos CM. Tetrahedron Lett. 2005;47:217–220. [Google Scholar]
- (17).a) Kabalka GW, Zhou L-L, Naravane A. Lett. Org. Chem. 2007;4:325–328. [Google Scholar]; b) Kabalka GW, Al-Masum M. Tetrahedron Lett. 2005;46:6329–6331. [Google Scholar]
- (18).Kabalka GW, Al-Masum M, Mereddy AR, Dadush E. Tetrahedron Lett. 2006;47:1133–1136. [Google Scholar]
- (19).Kabalka GW, Dadush E, Al-Masum M. Tetrahedron Lett. 2006;47:7459–7461. [Google Scholar]
- (20).Singh BK, Cavalluzzo C, De Maeyer M, Debyser Z, Parmar VS, Van der Eycken E. Eur. J. Org. Chem. 2009:4589–4592. [Google Scholar]
- (21).a) Billingsly KL, Andersom KW, Buchwald SL. Angew. Chem. Int. Ed. 2006;45:3484–3488. doi: 10.1002/anie.200600493. [DOI] [PubMed] [Google Scholar]; b) Barden TE, Buchwald SA. Org. Lett. 2004;6:2649–2652. doi: 10.1021/ol0491686. [DOI] [PubMed] [Google Scholar]
- (22).Batey RA, Quach TD. Tetrahedron Lett. 2001;42:9099–9103. [Google Scholar]
- (23).Molander GA, Biolatto B. Org. Lett. 2002;4:1867–1870. doi: 10.1021/ol025845p. [DOI] [PubMed] [Google Scholar]
- (24).Billingsley KL, Anderson KW, Buchwald SL. Angew. Chem., Int. Ed. 2006;45:3484–3488. doi: 10.1002/anie.200600493. [DOI] [PubMed] [Google Scholar]
- (25).a) Molander GA, Jean-Gerrard J. Org. Chem. 2009;74:1297–1303. doi: 10.1021/jo802453m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Molander GA, Canturk BL. J. Org. Chem. 2009;74:973–980. doi: 10.1021/jo802590b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dreher SD, Lim S-E, Sandrock DL, Molander GA. J. Org. Chem. 2009;74:3626–3631. doi: 10.1021/jo900152n. [DOI] [PMC free article] [PubMed] [Google Scholar]