Abstract
Influenza infection is a major clinical problem and Echinacea purpurea, a widely consumed botanical product, is purported to alter the course of respiratory infections including influenza. Mice infected with WSN influenza A and treated with E. purpurea polysaccharide extract had less weight loss than untreated mice but similar pulmonary viral titers. Echinacea-treated mice had lower systemic and pulmonary KC and IL-10 levels and lower systemic IFN-γ levels following influenza infection. These suggest that E. purpurea alters the clinical course of influenza infection in mice through modulation of cytokines and not direct antiviral activity.
Keywords: Influenza, Echinacea, Cytokines, Mice
1. Introduction
Influenza causes significant morbidity and mortality worldwide. Emerging influenza strains and drug resistance patterns pose major public health challenges [1-3]. Current influenza control is focused on prevention through vaccination, and requires novel vaccine development on an annual basis. Improved understanding of the immune response to influenza, and agents that alter this response, will inform influenza treatment and prevention.
One agent purported to alter the course of respiratory viruses is Echinacea purpurea. Echinacea is the most widely consumed botanical product in the United States [4], but its immunomodulatory properties are unclear. Factors contributing to this lack of clarity include the heterogeneity of species, plant parts (e.g., aerial (non-root) versus root), and methods of preparation. In addition, biochemical data describing the active components of echinacea are limited [5]. At least three species of echinacea (purpurea, angustifolia, and pallida) are widely available, and many of these appear to have different immunomodulatory potential based on cytokine stimulatory properties [6,7].
E. purpurea has been evaluated as an adjuvant in cancer vaccines and minimal effect was detected [8]. However, effects of echinacea on respiratory virus immune response and influenza vaccine response have been reported [7]. Direct antiviral effects of an ethanol extract of E. purpurea aerial and root components, containing mainly alkylamides and caffeic acid derivatives, have also been recently described [9]. Clarifying the antiviral and immunomodulatory properties of E. purpurea during influenza is clinically relevant due to (1) the wide consumption of echinacea, (2) potential for a recurrent influenza pandemic, and (3) the demand for novel agents to optimize influenza vaccination and treatment. Such agents include dose sparing compounds that could be combined with treatment regimens and/or vaccines (i.e., adjuvants). In this study we have evaluated the effect of E. purpurea aerial polysaccharide extract on clinical, viral and cytokine response to influenza infection in a live mouse model.
2. Materials and methods
2.1. Mice
Female C57BL6 mice were purchased from Jackson Laboratories, Maine. Animal care and experiments were performed in accordance with institutional guidelines and with the approval of the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center. All mice were 6–8 weeks old with average weights between 15 and 20 g. Mice that lost more than 30% of total body weight were sacrificed.
2.2. Infection
Influenza A/WSN/33 (H1N1) strain was kindly provided by the laboratory of Dr. Peter Palese (Mt. Sinai Medical Center, New York, NY). All virus used for described experiments came from a single viral preparation batch prepared in MDCK cells. Mice were infected intranasally with 500 PFU of WSN33 in 30 μl 1× PBS while anesthetized with isoflurane. For determination of viral titers lung samples were homogenized in PBS, then supernatants were frozen at −80 °C. Test for viable virus by plaque forming assay was performed in MDCK cells [10]. Separate experiments for day 6 viral titers were performed four times, for day seven, three times, for days 3 and 4 twice each, and for days 2 and 5, once each.
2.3. Clinical monitoring
All mice were weighed daily and the percentage of weight change was plotted over time.
2.4. Echinacea
2.4.1. Echinacea preparation
E. purpurea aerial (non-root) extract [8] was provided by Gaia Herbs (Brevard, NC) via the botanical core facility at Memorial Sloan Kettering Cancer Center. The crop was harvested in a company owned farm. Voucher specimen (HK40433) was deposited at the Hong Kong Herbarium of the Agricultural & Fishery Conservation. E. purpurea aerial part including leaves, stems and flower heads were homogenized in water and the liquid phase was collected by hydraulic press. After filtration through a 100 μm screen, the sample was precipitated using 20% aqueous ethanol and filtered through a 10 μm screen. The filtrate was further precipitated under 70% aqueous ethanol and the solid sediments were collected, lyophilized and dissolved in deionized water, which was then passed through a G-50 column. The resulting elution was designated echinacea neutral and weak acidic polysaccharide (subsequently referred to as echinacea) and was freeze-dried to powder form. The preparation contained <0.5 endotoxin units (EU) of lipopolysaccharide (LPS) as per certificate of analysis.
2.4.2. Echinacea administration
Phosphate buffer solution (Invitrogen, Carlsbad, CA) was used to reconstitute sample at stock concentration of 100 mg/ml and incubated at 37 °C in a water bath for 2 h. The suspension was filtered using sterile 0.45 μm PVDF syringe driven filter units (Millipore, Bedford, MA). Filtered preparation was stored for a maximum of 10 days, at 4 °C, prior to use. The dose of echinacea used in all experiments was 10 mg (100 μl of stock solution) administered to mice daily by gavage until sacrifice or for a maximum of five consecutive days starting on the day of infection (day 0), immediately following infection.
2.5. Cytokine analyses
Cytokines tested were IL-1β, IL-2, IL-4, IL-5, KC (hu IL-8), IL-10, IL-12, IFN γ, TNF α. Cytokine levels in lung homogenate and serum were assessed using multiplex ELISA (Mesoscale Discovery, Mouse TH1/TH2 9-Plex) according to manufacturer’s instructions. Samples were run in duplicate. Samples with intraassay (duplicate) coefficient of variation <30% were included in analyses. Day 3 serum evaluation included 10–11 mice per infection group (treated vs. untreated), from 3 separate experiments, run on two separate MSD plates. Day 7 evaluation included 6–7 mice per infection group, from 2 separate experiments, run on 2 separate MSD plates. For lung, day 3 included 10–11 mice per infection group, from 3 experiments, run on one MSD plate. Day 7 included 9–10 mice per infection group, from 3 experiments, run on 2 separate MSD plates.
2.6. Statistics
2.6.1. Weight analyses
We compared the weight loss in each group from the day of infection until 7 days post-infection using a repeated measures model. This model was used because mice were followed longitudinally, with weight measurements taken daily. Briefly, standard regression models assume that all observations are independent. As multiple weight measurements from the same mice are not independent, we adjusted for the correlation within subjects. In order to test whether the association between time from infection and weight loss differed significantly between treated and untreated mice, we included an interaction term between time from infection and echinacea treatment in the model. For illustration, we plotted the mean percent weight loss for each group over time.
2.6.2. Viral titers
Viral titers were log transformed and compared between groups (untreated versus treated) using the Student’s t-test.
2.6.3. Cytokine profile
The Mann–Whitney test was used to compare cytokine profiles among the different treatment groups. Cytokine analyses compared (1) whether infection with influenza, in the absence of treatment, was associated with changes in various cytokine levels, and (2) whether treatment with echinacea during influenza infection was associated with differences in cytokine levels compared to infection without treatment. Box plots were created to illustrate the differences in cytokine levels; shaded regions represent the interquartile range (IQR), capped whiskers represent the upper and lower adjacent values (the highest value within 1.5 times the IQR of the upper or lower bound of the IQR) and circles above or below the whiskers designate outside values.
For all statistical analyses a p value of less than 0.05 was considered significant.
3. Results
3.1. Echinacea-treated mice had better clinical outcomes than untreated mice after influenza infection
To study the effect of echinacea administration on the clinical course of influenza, we intranasally infected mice with 500 PFU influenza virus then treated mice with either echinacea or PBS daily for 5 days following infection. As a surrogate marker for disease severity we used percentage weight change compared to baseline weight (day 0). In total, 59 mice were followed for weight loss: 24 infected, untreated (Flu(+)/Ech(−)) mice, 16 infected, treated (Flu(+)/Ech(+)) mice, 9 uninfected, treated (Flu(−)/Ech(+)) mice and 10 uninfected, untreated (Flu(−)/Ech(−)), or null, mice. Non-influenza-infected mice, both treated and untreated with echinacea, gained a small amount of weight over the 7-day study period. Flu(−)/Ech(−) mice gained a mean of 0.07 g/day [95% CI: 0.04, 0.1] and Flu(−)/Ech(+) mice gained 0.05 g/day [95% CI: 0.02, 0.07]. As expected, influenza-infected mice tended to lose weight; however, weight loss appeared to be attenuated with echinacea treatment. Infected, untreated (Flu(+)/Ech(−)) mice lost 0.57 g/day [95% CI: 0.50, 0.63] and infected, treated (Flu(+)/Ech(+)) mice lost 0.27 g/day [95% CI: 0.20, 0.33]. Decreased weight loss in treated versus untreated mice was statistically significant on repeated measures analysis (p < 0.001). Fig. 1 illustrates the mean percent weight loss in each group over time. In summary, uninfected ((Flu(−)/Ech(−)) and (Flu(−)/Ech(+))) mice have relatively constant weight whereas infected groups both lost weight following influenza infection; weight loss was greater in untreated (Flu(+)/Ech(−)) compared to treated (Flu(+)/Ech(+)) groups.
Fig. 1.
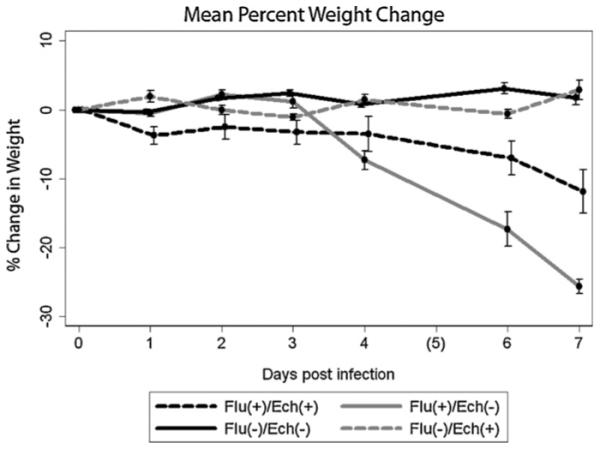
Mean percent weight change. Infected treated (Flu(+)/Ech(+)) mice lost less weight than infected untreated (Flu(+)/Ech(−)) mice (p < 0.001 determined by repeated measures). The y-axis represents the mean percent weight change. The x-axis represents days following influenza infection (day of infection was day 0). Dashed black line represents Flu(+)/Ech(+) mice, solid gray line represents Flu(+)/Ech(−) mice, solid black line represents Flu(−)/Ech(−) mice, dashed gray line represents Flu(−)/Ech(+) mice. Error bars show standard error.
3.2. Echinacea treatment did not alter lung viral titers
To assess the impact of echinacea treatment on viral replication, we measured viral titers in lung homogenates of treated and untreated mice on days 2, 3, 4, 5, 6 and 7 post-infection. Fig. 2 shows mean viral titers for the different treatment groups at each time-point examined, from a representative experiment. Compared to untreated mice, echinacea-treated mice had similar viral titers on all days (p > 0.3 for all days).
Fig. 2.
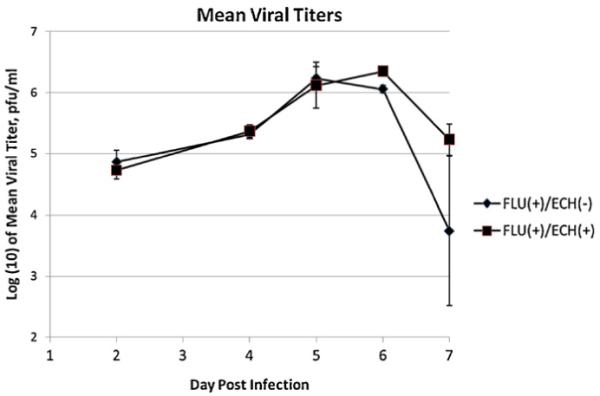
Mean viral titers. Mean viral titers, in log (10) transformed units, from day 2 to 7 following influenza infection. The y-axis represents log base 10 of the viral titer. The x-axis represents days following infection. The figure shows a representative of four experiments. Bars show standard error.
3.3. Echinacea-treated mice had lower KC, IFNγ, and IL-10 cytokines than untreated mice
To determine whether echinacea in the absence of influenza infection had any cytokine altering properties, mice were fed echinacea and bled on day 3 then sacrificed on day 7 for serum and lung cytokine analyses. In the absence of influenza infection, mice treated with echinacea had similar serum and lung cytokine profiles compared to untreated mice (Fig. 3, Table 1).
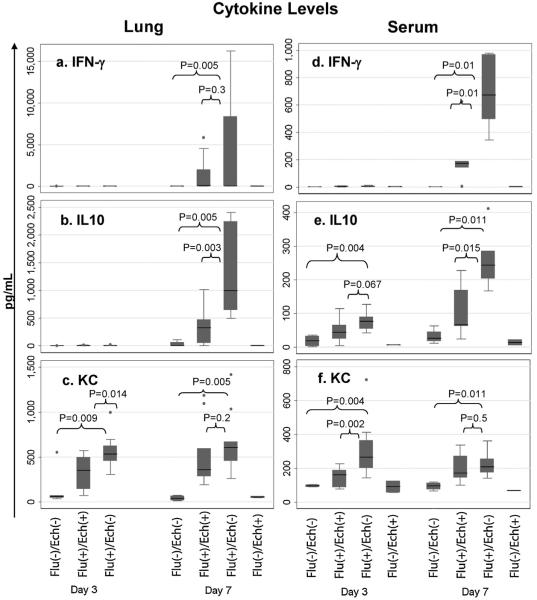
Fig. 3.
Levels of IFN γ, IL-10 and KC in Lung and Serum in Treated versus Untreated Mice. On day 7 echinacea-treated mice had significantly less IFN γ in serum and less IL-10 in lung and serum. On day 3 echinacea-treated mice had less KC in serum and lung. The y-axis indicates total amount of cytokine detected in pg/ml. The x-axis indicates treatment group and time following infection. (a)–(c) Show lung homogenate levels, (d)–(f) show serum levels. Shaded regions represent the interquartile range (IQR), capped whiskers represent the upper and lower adjacent values (the highest value within 1.5 times the IQR of the upper or lower bound of the IQR) and circles above or below the whiskers designate outside values.
Table 1.
Effect of echinacea on serum and lung cytokines
| Echinacea only median (IQR) (pg/ml) | Null median (IQR) (pg/ml) | P value null vs. Untreated | Untreated median (IQR) (pg/ml) | P value untreated vs. treated | Treated median (IQR) (pg/ml) | |
|---|---|---|---|---|---|---|
| IFN γ | ||||||
| Serum day 3 | 0.34 (0.33, 0.34) | 0.34(0.0,0.96) | 0.006 | 4.54 (1.87, 7.81) | 0.2 | 3.04 (0.38, 5.31) |
| Serum day 7 | 0.9 (0.53, 1.36) | 0 (0, 2) | 0.01 | 673 (497, 969) | 0.01 | 173 (143, 188) |
| Lung day 3 | - | 0.00(0.00,0.0) | 0.025 | 2.21 (1.11, 3.42) | 0.9 | 2.21 (0.31, 3.86) |
| Lung day 7 | 0.06 (0, 0.13) | 1.5 (0.0, 5.0) | 0.005 | 78.2(52.7,8354) | 0.3 | 92.9 (6.9,2004) |
| IL-10 | ||||||
| Serum day 3 | 6.2 (5.5, 6.8) | 18.2(3.2,32.6) | 0.004 | 76.2 (54.1, 89.0) | 0.067 | 44.1 (24.6, 64.8) |
| Serum day 7 | 13.1 (4.3, 21.8) | 26 (18, 45) | 0.011 | 243 (204, 286) | 0.015 | 66 (63, 168) |
| Lung day 3 | - | 0.00 (0.0, 0.0) | 0.16 | 0.00 (0.00, 8.67) | 0.6 | 0.00 (0.00, 5.40) |
| Lung day 7 | 4.6 (0, 9.3) | 2 (0, 57) | 0.005 | 992 (645, 2237) | 0.003 | 327 (55, 473) |
| KC | ||||||
| Serum day 3 | 90 (56, 124) | 95 (89, 102) | 0.004 | 265 (201, 364) | 0.002 | 160 (88, 188) |
| Serum day 7 | 66 (65, 68) | 94 (74, 112) | 0.011 | 208 (174, 254) | 0.5 | 170 (143, 271) |
| Lung day 3 | - | 60 (48, 70) | 0.009 | 533 (456, 623) | 0.014 | 349 (146, 493) |
| Lung day 7 | 55 (47, 64) | 39 (16, 67) | 0.005 | 603 (458, 669) | 0.2 | 360 (286, 592) |
| IL-12 | ||||||
| Serum day 3 | 691 (519, 811) | 861(831, 964) | 0.013 | 1321(1021,1494) | 0.12 | 1024 (864,1318) |
| Serum day 7 | 1296(1289,1303) | 865(678,1176) | 0.5 | 988 (699, 1694) | 0.8 | 1229 (743,1629) |
| Lung day 3 | - | 254 (186,379) | 0.012 | 1669(1391,1949) | 0.045 | 1342 (470,1630) |
| Lung day 7 | 317 (246, 388) | 213 (98, 421) | 0.005 | 1992 (936, 2355) | 0.4 | 1167 (999,2099) |
| IL-5 | ||||||
| Serum day 3 | 3.69 (1.90, 5.48) | 3.2 (2.4, 3.2) | 0.014 | 8.5 (4.4, 9.9) | 0.4 | 5.9 (4.7, 6.6) |
| Serum day 7 | 7.61 (7.37, 7.85) | 6.7 (5.5, 10.6) | 0.088 | 15.8 (9.5, 28.5) | 0.025 | 43.0 (19.6, 61.6) |
| Lung day 3 | - | 0.05(0.0,0.27) | 0.019 | 7.63(4.78, 11.57) | 0.021 | 2.57 (1.41, 6.56) |
| Lung day 7 | 0.54 (0.41, 0.68) | 1 (0, 3) | 0.007 | 108 (10, 171) | 0.7 | 139 (21, 284) |
| TNFα | ||||||
| Serum day 3 | 3.58 (1.78, 5.38) | 58(26.6,65.6) | 0.4 | 30.2 (0.0, 59.4) | 0.4 | 46.0 (33.0, 56.1) |
| Serum day 7 | 23.0 (22.8, 23.3) | 12.4 (0,34.9) | 0.5 | 13.0 (0.1, 29.7) | 0.6 | 32.5 (0.0, 53.9) |
| Lung day 3 | - | 0.00 (0, 3.28) | 0.7 | 0.00 (0.00, 0.0) | 0.075 | 1.18 (0.00, 6.2) |
| Lung day 7 | 1.15 (0, 2.29) | 0.0 (0.0, 1.2) | 0.03 | 81.3 (7.9, 140.3) | 0.17 | 17.3 (8.0, 38.7) |
Cytokine levels for uninfected, treated mice (Flu(−)/Ech(+)), uninfected, untreated mice (Flu(−)/Ech(−)), infected, untreated mice (Flu(+)/Ech(−)), and infected, treated mice (Flu(+)/Ech(+)). Median and interquartile range for each group is presented, in pg/ml. Influenza effect: p values comparing null to untreated mice are presented in the column adjoining these two groups. Echinacea effect: p values comparing untreated to treated mice are presented in the column adjoining these two groups. Summary of levels for each cytokine, shown separately by tissue (serum or lung), number of days after infection (D3 or D7) and treatment group (echinacea only (Flu(−)/Ech(+)), null (Flu(−)/Ech(−)), untreated (Flu(+)/Ech(−)), and treated (Flu(+)/Ech(+)). All values are medians (IQR).
To begin to understand the immunomodulatory effect of echinacea we measured a panel of serum and pulmonary cytokines known to play a role in immunity against influenza infection. Cytokines measured were IFN γ, TNF α, IL-1β, IL-2, IL-4, IL-5, KC (human IL-8), IL-10, IL-12 (total). To verify that our influenza model was consistent with prior studies, we compared influenza-infected mice (Flu(+)/Ech(−)) to null mice (Flu(−)/Ech(−)). On day 3, infected mice had increased IFNγ, IL-12, and KC in serum and lung (Table 1). IL-10 was increased in serum only; IL-5 was increased in lung. On day 7, infected mice had increased IFNγ, IL-10, and KC in serum and lung. In addition IL-5, IL-12 and TNF-α were increased in the lung (p ≤ 0.03 for all, Table 1).
We next examined whether echinacea altered cytokine response during influenza infection.
On day 3, echinacea-treated (Flu(+)/Ech(+)) mice had significantly lower KC, IL-5, and IL-12 in lung and lower KC in serum, compared to untreated (Flu(+)/Ech(−)) mice (Table 1). On day 7, treated mice had lower IL-10 and IFNγ in serum, and lower IL-10 in lung, compared to untreated mice (Table 1). The cytokines for which greatest percentage decrease was observed during treatment were day 7 serum IFN γ (74%), serum IL-10 (73%), and lung IL-10 (67%), followed by day 3 serum KC (40%) and lung KC (35%). Fig. 3 summarizes the decreases in IFNγ, IL-10 and KC in treated versus untreated mice. To a lesser extent, day 3 lung IL-12 was decreased in treated mice (20%). IL-5 was also significantly decreased in lung on day 3, but absolute cytokine values for all groups were under 10 pg/ml. Levels of IL-1β, IL-2, IL-4, and TNFα were similar between treated and untreated mice.
4. Discussion
Influenza is a contagious, acute respiratory disease caused by infection of the host respiratory tract mucosa by an influenza virus. Influenza accounts for more than 30,000 deaths and a cost of 87 billion US$ annually in the United States alone. The severity of illness during influenza infection depends on virus-host interactions. Severity is a function of direct virus-induced cytopathology as well as host innate and adaptive immune responses. Optimal immunity against influenza is multilayered and redundant, as high degrees of protection are often observed in models in which major components of the immune system, such as CD4 and CD8 T cells or B cells, are compromised or absent [11].
Consumers frequently use herbal remedies for amelioration of symptoms of influenza although this approach has not been validated in clinical trials [12]. E. purpurea has become the most popular commercial herbal preparation in North America and Europe [6]. Echinacea is frequently used by consumers and complementary/alternative medicine practitioners as an immune stimulant to augment responses against respiratory infection and particularly influenza. Recent in vitro studies show that E. purpurea combined root and aerial ethanol extract inhibits influenza virus entry [9] but there is limited data related to in vivo effects of E. purpurea.
Mouse models of influenza A virus infection provide a well-established experimental system to study immune response to influenza infection. Weight loss is a validated surrogate marker of severity of illness and predictor of mortality [13-19]. To characterize a potential immunomodulatory effect of echinacea we have used an established mouse model of live H1N1 influenza A infection. In our model, clinical effects (weight loss) [13], lung viral titers [14], and cytokine levels [15-19] were consistent with influenza effects described in the literature. These findings confirm that in our hands the model is a valid platform for evaluation of echinacea effects on influenza infection.
The E. purpurea aerial (non-root) extract that we are using contains significant amounts of polysaccharides and little to none of other known immunomodulatory components (alkylamides, cichoric acid and cynarin). Our compound was not further purified as our aim was to identify effects relevant to commercially available (heterogeneous) compounds [8]. Of note, our compound contains <0.5 EU LPS. Although trace amounts of LPS could be present in our preparation, it seems unlikely that LPS contributed to the observed results because echinacea inhibited, rather than enhanced, inflammatory response. We cannot exclude however an early desensitization effect of LPS [20].
Treatment with neutral and weak acidic polysaccharide extract of E. purpurea for 5 days, starting on the day of influenza infection, resulted in less severe illness as measured by weight loss. An effect on the clinical severity of influenza may be related to a direct antiviral effect, an immunomodulatory effect, or a combination of the two. Our preparation contains polysaccharides, which are known to possess immunostimulatory properties, and does not contain alkylamides that are thought to be responsible for antiviral activity in echinacea preparations. Using our echinacea extract, there was no significant difference in viral titers observed with echinacea treatment. This most likely indicates that attenuated weight loss was due to modulation of the immune response, rather than an antiviral effect. These findings complement the recent observation that echinacea has maximal antiviral when combined directly with virus prior to infection, although the preparation used for that study included root components and ours did not [9].
To begin to understand the pathways involved in the observed clinical benefit, we performed cytokine analyses. Influenza infection is known to upregulate a number of cytokines. In our hands influenza infection induced a similar profile to that published by other groups, including elevation of IFN γ, TNF α, IL-1β, IL-5, KC, IL-10 and IL-12 [15-19,21]. Cytokine profiles in our study showed several significant differences between treated and untreated mice. Day three lung and serum KC were significantly decreased in treated versus untreated mice. Day seven lung and serum IL-10 as well as serum IFN γ were decreased in treated versus untreated mice.
The trend toward cytokine inhibition detected in our study may be a reflection of antiviral activity of our compound, since influenza is known to induce IL-10, IFN γ, and KC. This seems unlikely because lung viral titers show no antiviral effect by our echinacea extract. Alternatively, echinacea may have an immunomodulatory effect. Inhibition of IL-10 and IFN-γ by E. purpurea is relevant given the recent finding [19] that IL-10 expression impedes Th17 response during influenza and is associated with decreased survival in lethal dose influenza. This study showed that the major source of IL-10 during influenza infection is Foxp3 negative CD4 T cell effectors in the lung which coproduce IFN γ. It is possible that E. purpurea inhibits such influenza specific CD4 cells, leading to decreased immune mediated pathology and better clinical outcome. Of note, blocking IL-10 increased morbidity in a separate study [22], though there are many possible explanations for this discrepancy [19].
Decreased serum IFN γ detected in our study could also be consistent with inhibition of NK T cells, as both of IFN γ and IL-10 are upregulated by these cells in influenza infection [15]. KC inhibition detected in our study could be related to a direct antiviral effect of E. purpurea or a reflection of enhanced type 1 interferon signaling [20]. Again, our data argues for an immunomodulatory effect. It is important to note that KC inhibition has been associated with increased mortality due to post-influenza bacterial pneumonia [21]. However, KC effects were no longer apparent by day 7, the typical onset time of post-influenza bacterial pneumonia. Direct analysis of echinacea effects on influenza and cellular recruitment during influenza, as well as post-influenza bacterial infections, will be needed to clarify the mechanism of echinacea related cytokine changes.
In summary, our results indicate that E. purpurea aerial (non-root) polysaccharide extract does not have evident antiviral effect but modifies influenza-related (1) clinical course and (2) immune response, as measured by cytokines, with decreased pulmonary and systemic KC (human IL-8) early in influenza infection then decreased pulmonary and systemic IL-10 and systemic IFN-γ later in the course of infection. It is interesting to note that, in vitro, root-containing extract from the same echinacea species and a similar preparation method did have antiviral activity, reinforcing the likely unique characteristics of distinct plant parts [9]. It is possible that some form of the extract described in this study could be clinically useful for (1) influenza treatment or (2) prophylaxis against post-viral bacterial pneumonia, which is possibly mediated by elevated IL-10 following influenza [23]. However, the observed KC inhibitory effects could worsen post-influenza pneumonia, so further studies will be required to evaluate the effect of echinacea in this setting. A limitation of our study is that we evaluated a single dosing regimen of echinacea. Further studies are needed to establish the clinically relevant dose in humans. As clinical challenges related to influenza continue to escalate, further, systematic evaluation of this compound may be timely.
Acknowledgements
The authors thank Nana Mensah and Nii Konii significant technical assistance and Dr. Peter Palese for providing influenza A/WSN/33 (H1N1). Support for this study was provided by a Pilot Award from the Weill Cornell Medical College Clinical and Translational Science Center, NIH Award Number UL1 RR024996 (GP, DF, XL), T32 AI 007613 (DF), Grant 1R21A1073926-01A2 (MS) and Stony-Wold Herbert Fund Grant 0266-0907 (MS) and also by National Center for Complementary & Alternative Medicine Award Number P50AT002779 (GP, EK, WX, BC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Clinical and Translational Science Center, National Center for Complementary & Alternative Medicine or the National Institutes of Health.
References
- [1].Garten R, Davis C, Russell C, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Puthavathana P, Sangsiriwut K, Korkusol A, Pooruk P, Auewarakul P, Pittayawanganon C, et al. Avian influenza virus (H5N1) in human, Laos. Emerg Infect Dis. 2009;15:127–9. doi: 10.3201/eid1501.080524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention (CDC) Update: influenza activity—United States, September 28, 2008–January 31, 2009. MMWR. 2009;58:115. [PubMed] [Google Scholar]
- [4].Bardia A, Nisly NL, Zimmerman MB, Gryzlak B, Wallace RB. Use of herbs among adults based on evidence-based indications: findings from the National Health Interview Survey. Mayo Clin Proc. 2007;82:561–6. doi: 10.4065/82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Woelkart, Bauer The role of alkamides as an active principle of echinacea. Planta Med. 2007;73:615–23. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- [6].Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (E. angustifolia, E. pallida, E. purpurea): a review of their chemistry, pharmacology, and clinical properties. J Pharm Pharmacol. 2005;57:929–54. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- [7].Senchina D, Wu L, Flinn G, Konopka del N, McCoy JA, Widrlechner MP, et al. Year and a half old, dried Echinacea roots retain cytokine modulating capabilities in an in vitro human older adult model of influenza vaccination. Planta Med. 2006;72:1207–15. doi: 10.1055/s-2006-947254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ragupathi G, Yeung K, Leung P, Lee M, Lau CB, Vickers A, et al. Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine. 2008;26:4860–5. doi: 10.1016/j.vaccine.2008.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pleschka S, Stein M, Schoop R, Hudson J. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian Influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV) Virol J. 2009;6:197. doi: 10.1186/1743-422X-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tobita K, Suguira A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975;162:9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- [11].Brown D, Roman E, Swain S. CD4 T cell responses to influenza infection. Semin Immunol. 2004;16:171–7. doi: 10.1016/j.smim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- [12].Turner R, Bauer R, Woelkart K, Hulsey T, Gangemi J. An evaluation of Echinachea angustifolia in experimental rhinovirus infections. N Eng J Med. 2005;353:341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- [13].Verhoeven D, Teijaro JR, Farber DL. Pulse-oximetry accurately predicts lung pathology and the immune response during influenza infection. Virology. 2009;390:150–6. doi: 10.1016/j.virol.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mendel DB, Tai CY, Escarpe PA. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–6. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Szyszko E, Brokstad K, Cox R, Hovden A, Madhun A, Haaheim L. Impact of influenza vaccine formulation with a detailed analysis of the cytokine response. Scand J Immunol. 2006;64:467–75. doi: 10.1111/j.1365-3083.2006.01805.x. [DOI] [PubMed] [Google Scholar]
- [16].Ho L, Denney L, Luhn K, Teoh D, Clelland C, McMichael A. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol. 2008;38:1913–22. doi: 10.1002/eji.200738017. [DOI] [PubMed] [Google Scholar]
- [17].Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Graham M, Braciale V, Braciale T. Influenza virus specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–82. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McKinstry K, Strutt T, Buck A, Curtis J, Dibble J, Huston G, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–63. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Scott M, Liu S, Shapiro R, Vodovotz Y, Billiar T. Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism. Hepatology. 2009;49:1695–708. doi: 10.1002/hep.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shahangian A, Chow E, Tian X, Kang J, Ghaffari A, Liu S, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–20. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–84. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van der Sluijs K, van Elden L, Nijhuis M, Schuurman R, Pater J, Florquin S, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]