Abstract
Although hyperglycemia is one factor that determines the outcome of myocardial ischemic insult, it is still not clear whether it is causally related to decreased ischemic tolerance in diabetic patients. In contrast to clinical and epidemiological studies demonstrating a higher risk of cardiovascular disorders in diabetic patients, experimental data are not unequivocal and suggest that, aside from higher myocardial vulnerability, diabetes mellitus may be associated with the triggering of adaptive processes leading to paradoxically lower susceptibility to ischemia. It has been proposed that this phenomenon shares some molecular pathways with short-term preconditioning and other forms of endogenous protection against ischemia/reperfusion injury in the nondiseased heart. The present article reviews some controversial findings of enhanced resistance to ischemia in the diabetic heart that stem from experimental studies in different models of myocardial ischemia/reperfusion injury. Specifically, it addresses the issue of potential mechanisms of increased resistance to ischemia in an experimental model of streptozotocin-induced diabetes, particularly with respect to the role of reactive oxygen species, hyperglycemia as one of the stress factors, and cell-signalling mechanisms mediated by ‘prosurvival’ cascades of protein kinases in relation to the mechanisms of classical ischemic preconditioning. Finally, mechanisms involved in the suppression of protection in the diabetic myocardium including the effect of concomitant pathology, such as hypercholesterolemia, are discussed.
Keywords: Adaptation, Cell signalling, Diabetic heart, Myocardial ischemia
SUSCEPTIBILITY TO ISCHEMIA/REPERFUSION INJURY IN THE DIABETIC HEART: CONTROVERSIES IN EXPERIMENTAL STUDIES
Diabetes mellitus is associated with a higher risk of congestive heart failure and ischemic heart disease including myocardial infarction, which has been described in various clinical and epidemiological studies (1,2). Deterioration of heart function and rhythm disorders that can develop in diabetic patients even in the absence of coronary artery disease, are common manifestations of diabetic cardiomyopathy associated with impaired homeostasis of Na+, Ca2+ and K+ in cardiomyocytes. Development of diabetes leads to oxidative stress (3,4) and defects in the sarcolemmal and sarcoplasmic reticular membranes of cells, as well as to alterations in the function of ion transport systems (eg, Na+/K+- and Ca2+-ATPase, Na+/H+ and Na+/Ca2+ exchangers, and Ca2+ channels) resulting in abnormal handling of Na+ and Ca2+ in the diabetic myocardium, thereby possibly compromising its tolerance to ischemia (5–9).
However, in contrast to human studies, experimental data are not unequivocal, suggesting that diabetic hearts are not uniform in their susceptibility to ischemic injury, which may not only be increased, but also unchanged or even decreased (10,11). One of the main reasons for the differences between clinical and experimental studies may be the contribution of other pathologies in diabetic patients such as hypertension, hypercholesterolemia (HCH), microvascular dysfunction, neuropathy and nephropathy, as well as treatment with antidiabetic drugs, particularly, sulfonylureas that block the preconditioning (PC) effect during myocardial ischemia by preventing the opening of K(ATP) channels (11–14). These circumstances may determine the overall outcome of myocardial response to ischemia in diabetic patients.
First, paradoxical results showing better recovery of contractile function in the hearts of diabetic rats subjected to ischemia/reperfusion (I/R) were reported by Tani and Neely (15). Later, Liu et al (16) showed that acute streptozotocin (STZ)-induced diabetes reduced the size of infarction measured by tetrazolium staining by nearly 70% in a rat model of regional I/R. Several mechanisms have been proposed to explain the discrepancies in experimental studies. It was suggested that differences among the duration, severity and the type of diabetes, and different study protocols testing the effect of ischemia alone or ischemia and subsequent reperfusion, as well as different end points accounted for the conflicting results. Further explanations such as severity of ischemic protocol (ie, global zero flow versus low-flow ischemia), the presence of the metabolic substrate in the perfusion medium and the differences in the experimental models used in the studies (open-chest animals or isolated hearts) should also be taken into consideration. Finally, species differences and the distribution of coronary collaterals and myocardial perfusion may contribute to the variability of the results (10,17).
Thus, studies of infarct size indicate that diabetic dogs develop larger infarcts than normal dogs (18), whereas in diabetic rabbits and rats, infarct size tends to be smaller (16,19). Similarly, susceptibility to arrhythmias in the hearts of diabetic rats has been found to be both enhanced and reduced (20,21). Functional recovery on reperfusion in diabetic rats has been reported to be improved in the early stage and impaired in the later phase of the disease (22).
Significant alterations in heart function occur in the chronic (nine weeks) phase of STZ-induced diabetes (increased ratio of heart weight to body weight, decreased heart rate and coronary flow, and lower rates of contraction and relaxation, along with reduced myocardial levels of high energy phosphates and morphological deteriorations in the endothelial capillary cells as a manifestation of developing diabetic cardiomyopathy). Surprisingly, these diabetic hearts were more resistant to Ca2+ overload after transient depletion of Ca2+ followed by its repletion: 85% of the diabetic hearts survived Ca2+ paradox-induced injury in contrast to 0% survival in nondiabetic hearts (23). In these surviving hearts, not only was restoration of electrical activity and coronary flow almost completely achieved, an improved recovery of contractile function that reached 75% of the baseline values was also observed. Moreover, recovery of systolic function was accompanied by a remarkable attenuation of irreversible contracture and diastolic dysfunction, which was associated with a tremendous rise in left ventricular end-diastolic pressure in the nondiabetic controls as a consequence of Ca2+ overload of cardiomyocytes. Importantly, the concentration of ATP and total adenine nucleotides in the diabetic hearts that survived Ca2+ paradox injury was significantly higher than in the nondiabetic controls (24). The latter was accompanied by superior preservation of the cell ultrastructure demonstrated by the absence of hypercontracted and ruptured myofibrils, and by the maintenance of sarcolemmal and mitochondrial integrity (25).
Our in vivo studies in open-chest anesthetized rats with acute (one week) STZ-induced diabetes (26) clearly demonstrated the differences in the susceptibility of the diabetic hearts to ischemia-induced ventricular arrhythmias and irreversible myocardial damage. The size of the myocardial infarct (IS) (as a percentage of the area at risk [AR]) in the diabetic rats reached only 67% of that in the nondiabetic controls, whereas the incidence and severity of ventricular arrhythmias were not influenced by the diabetic state. The latter was also confirmed in other in vivo studies (27,28) showing that the incidence of severe ventricular arrhythmias during left anterior descending coronary artery occlusion and reperfusion, such as ventricular tachycardia (VT) and fibrillation (VF), was similar in the acutely diabetic hearts and in the nondiabetic controls.
Our studies performed on Langendorff-perfused rat hearts in the same model of acute STZ-induced diabetes (29) also demonstrated that despite the development of cardiomyopathy in the early stage of the disease (demonstrated by bradycardia and reduced rates of contraction and relaxation, as well as by decreased pressure-rate product – an index of cardiac contractile performance) only 42% of these hearts exhibited VT, of which 16% had short episodes of VF and no sustained VF (SVF). This was in contrast to 100%, 70% and 36% of nondiabetic hearts that demonstrated VT, VF and SVF, respectively (P<0.05). Moreover, this antiarrhythmic protection was maintained even in the chronic (eight weeks) phase of the disease, and although VT occurred in almost all of the diabetic hearts, the total duration of both VT and VF was substantially shorter than in the respective controls (30).
Furthermore, in chronically diabetic rats, antiarrhythmic protection was observed not only in isolated heart preparations, but also in the diabetic animals in vivo, in which the ectopic activity was also lower, the incidence of VT was reduced by more than two-fold compared with the respective controls and lethal SVF was completely abolished. In contrast, anti-infarction protection in these animals was attenuated compared with the acute phase of the disease, indicating a dichotomy in the susceptibility of the diabetic hearts to ischemia-induced ventricular arrhythmias and irreversible injury in relation to the duration of the diabetic state (26).
Collectively, these results support a view of somewhat lower sensitivity of the diabetic rat heart to I/R injury in both stages of the disease, although with different manifestations of an enhanced ischemic tolerance.
Mechanisms of lower sensitivity to I/R injury in the diabetic heart
Metabolic alterations in the diabetic heart involve impaired metabolism of carbohydrates including glucose transport and utilization (31). The role of glycogen metabolism during ischemia is still a matter of debate. On one hand, anaerobic glycolysis provides a major source of ATP production, but on the other hand, it also results in the increased accumulation of acidic glycolytic metabolites that decrease intracellular pH and contribute to the severity of injury. It is suggested that processes related to the alterations in glucose metabolism and to the regulation of intracellular pH are responsible for the reduced sensitivity to ischemia under certain experimental conditions (6,8,10,11). For example, in contrast to less severe models using low flow or moderate ischemia (with lower degree of acidosis due to the partial washout of lactate), in a setting of severe zero-flow ischemia, decreased glycolytic flux may be actually beneficial and attenuate the production of glycolytic products and H+ load. Under conditions of global I/R associated with Na+ and Ca2+ gain, a lower rate of glycolysis due to inhibition of phosphofructokinase activity – the rate-limiting step in glycolysis – by enhanced levels of fatty acids (32) and decreased activity of Na+/H+-exchanger collectively or separately, may result in a lower gain of Na+ during ischemia and, thus, reduced influx of Ca2+ via Na+/Ca2+ exchange during early reperfusion (6,8,10).
Furthermore, it was found that in the acutely diabetic rat heart, the mitochondrial mechanisms of energy transfer can adapt to the conditions of increased energy demands due to augmented calcium transients and that the latter may also account for its reduced susceptibility to injury (33).
Antiarrhythmic mechanisms in the diabetic myocardium
Regarding the mechanisms of arrhythmogenesis, it is known that outward potassium currents are reduced to a different extent in the epi- and endocardial layers of the diabetic myocardium (34), thus attenuating dispersion of refractoriness as a substrate for re-entry arrhythmias occurring during ischemia. Moreover, K(ATP) channels in diabetic cardiomyocytes have been found to be open and significantly more sensitive at higher levels of ATP (35).
Activation of K(ATP) channels has generally been considered to be one of the mechanisms of cardioprotection (36), which includes protection against arrhythmias related to triggered activity caused by enhanced Ca2+ influx (37). Moreover, sarcolemmal K(ATP) channels appeared to be involved in antiarrhythmic protection in the rabbit (38) and canine (39) myocardium. In addition, resistance to arrhythmias caused by high calcium in the hearts of diabetic rats can be explained by the alterations in the properties of L-type Ca2+ channels and reduced Ca2+ influx (7).
Moreover, accumulation of lactate during ischemia is substantially lower in the diabetic myocardium and does not reach the same level as in nondiabetic hearts (29,30). Thus, a decreased production of acid metabolites and subsequent reduced local acidosis as a source of alterations in the cell membrane currents, and electrophysiological changes promoting arrhythmogenesis (40) may contribute to the suppression of arrhythmias occurring during ischemia in diabetic hearts. In addition, lower susceptibility to reperfusion-induced arrhythmias in the hearts of diabetic rats is supported by the observation of an increased resistance to calcium overload in the diabetic myocardium (41).
Diabetes as an alternative form of an adaptive PC-like phenomenon
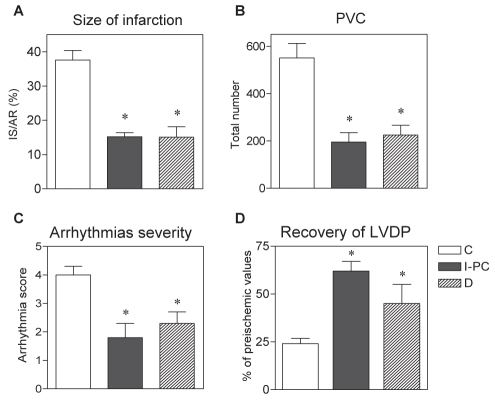
Figure 1 summarizes the cardioprotective effects observed in Langendorff-perfused hearts of acutely diabetic rats compared with the same effects in the hearts of nondiabetic animals subjected to one episode of I/R (ischemic PC [I-PC]) before sustained ischemia (27,42–44). Our studies revealed that both acute diabetes and I-PC in nondiabetic hearts resulted in similarly smaller infarctions (infarct size/area at risk reduced by 60%) (Figure 1A), lower vulnerability to ischemia-induced severe ventricular arrhythmias (Figures 1B and 1C) demonstrated by a significantly decreased total number of premature ventricular complexes (Figure 1B) and a reduced severity of arrhythmias (lower arrhythmia score [Figure 1C]). In addition, postischemic recovery of left ventricular function (left ventricular developed pressure) after global I/R was also significantly improved in both diabetic hearts and nondiabetic I-PC hearts (Figure 1D).
Figure 1).
Effect of acute (one-week) streptozotocin-induced diabetes (D) and ischemic preconditioning (I-PC) in the nondiabetic myocardium on the size of myocardial infarction (IS) (A), number of premature ventricular complexes (PVC) (B), severity of ischemia-induced ventricular arrhythmias (C) and postischemic recovery of left ventricular developed pressure (LVDP) (D) in Langendorff-perfused rat hearts. IS is expressed as a percentage of the area at risk (AR). LVDP recovery is expressed as a percentage of preischemic values. Data are presented as mean ± SEM from 10 to 12 hearts per group. *P<0.05 versus nondiabetic control (C) group
Potential mechanisms of PC-like protection in the diabetic myocardium are summarized in Table 1. Altered metabolism of glucose has been proposed to play a role in cardioprotective mechanisms in the diabetic myocardium based on the finding that inhibition of glucose uptake by cells can mimic I-PC and salvage normal myocardium (45), whereas restoration of glycogen stores depleted by I-PC leads to a loss of myocardial protection (46). Moreover, high glucose (25 mM) treatment has been shown to render normal cardiomyocytes resistant to chronic hypoxia-induced apoptosis and necrosis by preventing the accumulation of Ca2+ during hypoxia (47). Importantly, high concentrations of glucose rendered cardiomyocytes resistant to apoptosis in the absence of insulin (48). Furthermore, it has been demonstrated that enhanced glucose represents a stressful stimulus that is able to trigger an adaptive response in the diabetic heart (49).
TABLE 1.
Potential mechanisms of preconditioning-like protection in the diabetic myocardium: Relevance to ischemic preconditioning
| Alterations in glucose metabolism (45), high glucose acting as a preconditioning mimetic in nondiabetic myocytes (47,48) |
| Abnormal calcium handling – calcium preconditioning (50) |
| Increased production of reactive oxygen species (4,56) coupled with higher levels of antioxidants (42,49) |
| Increased activation of protein kinase C epsilon (64,65) |
| Increased mitogen-activated protein kinase activity, enhanced activation of extracellular signal-regulated kinases 1 and 2 (51,52) |
| Increased phosphoinositide 3-kinase/Akt activity, endothelial nitric oxide synthase activity (48,53,54) |
| Increased hypoxia-inducible factor 1-alpha, vascular endothelial growth factor; decreased inflammation and fibrosis (55) |
Increased resistance to ischemia in experimental models of diabetes can also be associated with alterations in intracellular calcium signalling, consistent with the findings demonstrating that increased calcium concentrations can induce PC-like protection in the normal heart (50).
Furthermore, research has demonstrated a higher activity of prosurvival protein kinases in acutely diabetic myocardium (51–54). In addition, several other protective mechanisms such as reduction in the levels of proinflammatory cytokines increase in the cell survival factors (hypoxia inducible factor 1-alpha and vascular endothelial growth factor) and angiogenesis, and reduced fibrosis, have been found to be activated in the acute phase of STZ-induced diabetes (55).
Thus, greater tolerance to ischemic injury observed in the diabetic heart can be considered an alternative form of intrinsic cardioprotection analogous to that induced by I-PC in the normal heart or by adaptation to chronic myocardial hypoxia when numerous metabolic stimuli, particularly those related to oxidative stress and increased reactive oxygen species (ROS) production and to intracellular calcium signalling can trigger protection against acute I/R injury.
The role of free radicals and the pro-oxidative/antioxidative state in the diabetic heart
Kakkar et al (56) demonstrated that although increased generation of ROS can be detected in the very early stage of STZ-induced diabetes, the latter was also accompanied by an increased level of antioxidative enzymes (eg, catalase, superoxide dismutase and glutathione peroxidase). Enhanced levels of endogenous antioxidants may, thus, be considered to be a manifestation of the adaptive response in the rat diabetic myocardium (57) induced by increased ROS similar to that demonstrated in nondiabetic hearts exposed to I-PC (43,58).
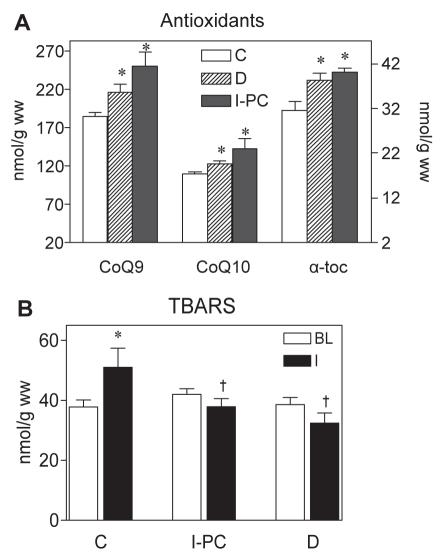
Increased generation of ROS coupled with higher activities of antioxidant enzymes and concentration of antioxidants in models of experimental diabetes have been reported by several authors (49,56). In a recent study, we found that one week after the induction of diabetes in a rat model of STZ-induced diabetes (42), a decreased susceptibility to ventricular arrhythmias manifested by a lower total number of PVCs and shorter duration of VT episodes during 30 min of ischemia was associated with significantly elevated levels of antioxidants such as coenzyme Q10 and, in particular, coenzyme Q9 in the left ventricular myocardium of diabetic animals. In addition, the levels of alpha-tocopherol were enhanced by 22.5% in the diabetic hearts. Similar increases were also observed in the myocardium of the nondiabetic rats preconditioned with one episode of I/R (Figure 2A). In the study by Chen et al (49), higher resistance of the diabetic hearts to I/R injury was associated with an increased antioxidant capacity of the myocardium already in the acute phase of STZ-induced diabetes with severe hyperglycemia (blood glucose level greater than 20 mM), or when the hearts of normal animals were perfused with a similarly high concentration of glucose.
Figure 2).
Pro-oxidative/antioxidative effects in the myocardium of diabetic rats and in the hearts of normal rats preconditioned with one cycle of ischemia/reperfusion (I-PC) (5 min each). A Effects of acute diabetes mellitus (D) and I-PC on the tissue levels of antioxidants determined by high-performance liquid chromatography. α-toc alpha-tocopherol; CoQ9 Coenzyme Q9; CoQ10 Coenzyme Q10. Data are presented as mean ± SEM of eight to 10 hearts per group. *P<0.05 versus nondiabetic control (C) hearts. B Myocardial concentration of thiobarbituric acid reactive substances (TBARS) at baseline (BL) and after test ischemia (I) in the nonpreconditioned controls, hearts preconditioned with I-PC and hearts from one-week diabetic rats. Data are presented as mean ± SEM of six to eight hearts per group. *P<0.05 versus BL; †P<0.05 versus nondiabetic controls
The results of our study also highlighted an important aspect related to the antioxidant treatment of diabetics. While treatment with the antioxidant N-acetyl-L-cysteine and the nitric oxide (NO) synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) conferred effective protection against ischemia-induced arrhythmias, both interventions were not effective in the diabetic rat heart and did not confer any additional myocardial protection during I/R (42). Thus, it is possible that changes in the pro-oxidant/antioxidant state modify a response of the diabetic heart to antioxidant treatment. The latter may result in a reduced effectiveness of N-acetyl-L-cysteine to further suppress arrhythmias in the diabetic myocardium. Similarly, pretreatment with L-NAME that was found to be cardioprotective and reduced the extent of I/R injury in the normal nonpreconditioned hearts (59), was antiarrhythmic only in the nondiabetic hearts (42). Conversely, because L-NAME suppressed increased ischemic tolerance in the acutely preconditioned nondiabetic myocardium (59), its antiarrhythmic effect may be also attenuated under conditions of the diabetic state.
Thus, it is conceivable that both cardioprotection conferred by I-PC in the healthy heart and increased resistance to ischemia in the diabetic myocardium share similar molecular protective pathways such as ROS and NO signalling. These data are supported by results of the study by Chen et al (49) who demonstrated that, in the presence of L-NAME, reperfusion arrhythmias were increased in the diabetic heart, suggesting that NO is involved in the mechanisms of protection in the diabetic myocardium and, more importantly, that the efficacy of antiradical interventions may differ in the nonadapted and adapted myocardium.
The role of ROS-mediated signalling in the mechanisms of higher ischemic tolerance of the diabetic myocardium has been demonstrated in experiments performed in the hearts of acutely diabetic rats, which showed an increased resistance to calcium overload despite markedly impaired biophysical properties (decreased fluidity) of the sarcolemmal membrane due to the processes of ROS formation and nonenzymatic glycation of membrane proteins (24). Moreover, reversal of these deleterious effects of ROS on protein glycation by treatment with resorcylidene aminoguanidine attenuated not only the changes in membrane properties and its enhanced rigidity, but abolished calcium tolerance in diabetic hearts, indicating that a certain amount of ROS formation is required to trigger adaptive processes in the diabetic myocardium (41). In addition, moderately increased myocardial production of ROS during the PC procedure (43) is likely to play a role in myocardial preservation through the attenuation of ROS levels during the subsequent phase of sustained ischemia (60). Similar to the preconditioned myocardium in diabetic hearts, cardioprotective effects during ischemia were associated with lower production of ROS (reduced thiobarbituric acid reactive substance levels) than in nondiabetic rat myocardium (Figure 2B).
Thus, it appears that radicals play a dual role in the heart. Apart from their damaging role in the nonadapted myocardium, they may act as signalling molecules involved in protective mechanisms of both short-term cardioprotection and long-lasting cardiac adaptation (59,61). These findings further address the issue of the effectiveness of antiradical interventions in the intact nonadapted (ie, unstressed) myocardium and in the myocardium that has shifted to a defensive phenotype in response to stress.
The role of protein kinases in cardioprotection of the diabetic myocardium
It has been found that calcium-induced protection against I/R injury is associated with the translocation of protein kinase C (PKC) (50). Moreover, hyperglycemia is a powerful stimulus for PKC activation and is known to occur in the diabetic myocardium even in the early phase of the disease (62). In addition, translocation of PKC has been shown to mediate cardioprotection in the hearts of STZ-induced diabetic rats (63), whereas PKC inhibition abolishes the increased resistance to I/R injury in diabetic hearts (64). Furthermore, it was shown that the activation of cardiac PKC epsilon in genetically engineered mice mediates antiapoptotic effects and leads to the preservation of left ventricular pump function, which promotes the survival phenotype and inhibits the negative inotropic properties of chronic hyperglycemia (65).
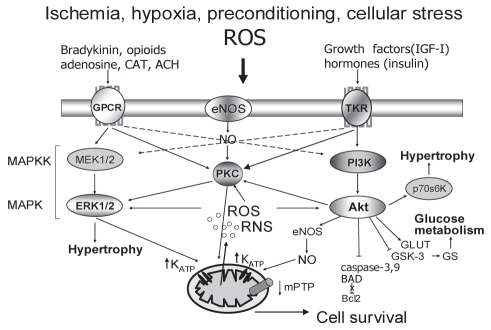
Extracellular signal-regulated kinases (ERK) 1/2 and phosphoinositide 3-kinase (PI3K)/Akt cascades, which are both involved in hypertrophic growth, cross-talk at different levels of cell transduction and converge on the same mitochondrial target proteins, have generally become regarded as the so-called ‘survival’ cascades involved in the mechanisms of the acute heart rescue (66–68). Figure 3 is a schematic representation of these two potentially regulatory cascades and their interaction in cardioprotection in the normal heart.
Figure 3).
Schematic representation of molecular signalling mediated by ‘survival’ cascades of protein kinases. ACH Acetylcholine; Akt Akt kinase; BAD Proapoptotic protein; Bcl-2 Antiapoptotic protein; CAT Catecholamines; eNOS Endothelial nitric oxide [NO] synthase; ERK1/2 Extracellular signal-regulated cascade of mitogen-activated protein kinases [MAPK]; GLUT Glucose transporter; GPCR G protein-coupled receptor; GS Glycogen synthase; GSK-3 GS kinase-3β; IGF-I Insulin growth factor; KATP ATP-sensitive K+ channel; MAPKK Mitogen-activated protein kinase kinase; MEK1/2 Upstream regulator of MAPK; mPTP Mitochondrial permeability transition pore; p70s6K Ribosomal S6 kinase; PI3K Phosphoinositide-3 kinase; PKC Protein kinase C; RNS Reactive nitrogen species; ROS Reactive oxygen species; TKR Tyrosine kinase receptor
Consistent with studies demonstrating an important role of protein kinase signalling in cardioprotection in the diabetic myocardium (53,64), we detected a nearly threefold increase in the levels of phosphorylated ERK1/2 in diabetic hearts in the acute (one week) phase that correlated with reduced infarct size (51), suggesting a potential positive role of this cascade in the response of the diabetic heart to an acute ischemic challenge. In concert, the study by Xu et al (52) clearly demonstrated that short- and long-term duration of hyperglycemia exert opposite influences on intracellular signalling mediated by the ERK1/2 pathway, which correlated with the size of the myocardial infarct and the intensity of apoptotic processes. While in the acute phase of STZ-induced diabetes, increased ERK1/2 activity, reduced infarct size and smaller number of dead myocytes were observed. These protective effects were suppressed according to the duration of the disease.
Because the PI3K/Akt cascade plays an important role in cell survival mechanisms and in the regulation of glucose metabolism in the normal heart (68), it could be expected to be involved in the changes in ischemic tolerance in diabetic patients. Accordingly, it was reported by Ma et al (53) that PI3K/Akt and endothelial NO synthase become transiently activated in the early stage of diabetes, thereby leading to a subsequent increase in NO formation, which may protect the heart from I/R injury.
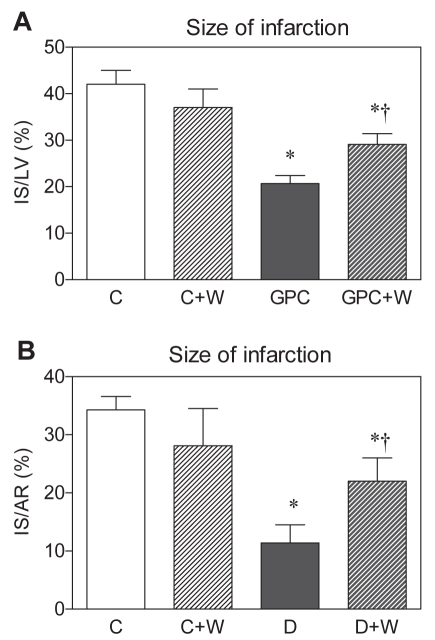
To elucidate the role of PI3K/Akt in the PC-like effect of high glucose (22 mM) in the normal heart (glucose PC) and in response to I/R injury in the diabetic heart (blood glucose greater than 20 mM) in lethal injury, the PI3K/Akt inhibitor wortmannin was administered at a concentration (100 nM) that suppressed PC-induced infarct size limitation in normal hearts (44). While infarct size was not affected by wortmannin in the control groups, cardioprotection was abolished in both glucose preconditioned (Figure 4A) and diabetic (Figure 4B) groups by treatment with this compound, indicating that activation of the PI3K/Akt cascade is involved in the mechanisms of anti-infarct protection triggered by high glucose.
Figure 4).
Effect of the phosphoinositide-3 kinase inhibitor wortmannin (W; 100 nM) on the size of myocardial infarction in Langendorff-perfused rat hearts preconditioned with high glucose (GPC) (A) and in the hearts of acutely (one-week) diabetic (D) rats (B). Data are presented as mean ± SEM of eight to 10 hearts per group. *P<0.05 versus controls (C); †P<0.05 versus GPC or D. AR Area at risk; IS Infarct size; LV Left ventricle
Therefore, similar to the nondiabetic heart (Figure 3), it appears that in diabetic hearts, many molecular mechanisms can be responsible for the activation of endogenous cardioprotection, at least in the early phase of the disease.
Effect of PC in the diabetic heart
Whether the diseased myocardium can be preconditioned similarly to a healthy one is still a matter of discussion. Some authors consider PC to be a ‘healthy heart’ phenomenon, and it is well documented that enhanced resistance to I/R injury in the normal preconditioned myocardium may be abolished by concurrent pathological conditions such as HCH (69).
In this respect, of major concern may also be hypoglycemic therapy with oral antidiabetic agents, particularly sulphonylurea drugs (eg, glibenclamide) in humans, because as a consequence of their action, K(ATP) inhibition (and suppression of clinical manifestations of PC under conditions of myocardial ischemia) may be one of the causes of higher mortality in diabetic patients (12,14,70). Accordingly, Loubani et al (13) explained a failure to precondition human cardiomyocytes by the treatment of patients with oral hypoglycemics. Furthermore, Hassouna et al (71) reported that PC with ischemia, phenylephrine, adenosine or diazoxide failed to protect diabetic myocardium. However, activation of PKC or p38-mitogen-activated protein kinase was still protective. These authors suggested that mitochondrial dysfunction in the diabetic myocardium, possibly dysfunctional mitochondrial K(ATP) channels, leads to the inability to respond to PC.
In contrast, using other experimental models, several studies have demonstrated that under certain conditions, the diabetic myocardium can be preconditioned, albeit with a stronger PC stimulus. In other words, increased threshold for cardioprotection in the diabetic myocardium requires higher intensity PC. Thus, Tsang et al (54) reported that only three cycles of I-PC significantly reduced the size of infarction in diabetic rats compared with the efficiency of one cycle of I-PC in nondiabetic animals. Cardioprotective effects correlated with significant elevation in Akt phosphorylation after only three cycles of I-PC in diabetic animals, whereas similar changes in Akt activity were detected in the hearts of nondiabetic animals after only one cycle of I-PC. The latter indicates that the diabetic heart still maintains the potential to be preconditioned; however, to achieve PC protection of the diabetic myocardium, it is necessary to increase the I-PC stimulus to reach the threshold for cardioprotection and a critical level of Akt phosphorylation to confer a protective effect.
Accordingly, it was shown in our study of the isolated rat heart (29) that one cycle of I-PC did not confer any additional antiarrhythmic protection or further reduction in the accumulation of lactate during ischemia of acutely diabetic hearts compared with nonpreconditioned hearts. It appears that because the diabetic myocardium appears to be already ‘preconditioned’, the latter may mask the effect of any further protective interventions.
In agreement with our results, Galagudza et al (72) recently reported that rats with alloxan-induced insulin-dependent diabetes mellitus also exhibited smaller infarct size and lower susceptibility to ventricular tachyarrhythmias. However, the same procedure using one cycle of I-PC was ineffective in the diabetic animals – different from the healthy rats – thus supporting the existence of an endogenous cardioprotective phenotype (metabolic PC) in experimental diabetes.
It is also possible that due to induction of the adaptive mechanisms, the diabetic state increases the threshold for cardioprotection and, thus, a stronger PC stimulus is required to achieve this threshold than in the nonadapted myocardium.
Loss of endogenous cardioprotection in the diabetic heart
Duration of the diabetic state markedly determines myocardial response to I/R. It was shown in our studies (29,30) that an increased resistance to ischemia-induced arrhythmias in the acutely diabetic myocardium can be attenuated in the later phase of the disease, with more severe manifestations of the diabetic state and development of diabetic cardiomyopathy. Thus, in the chronic (eight-week) phase of the disease, the severity of arrhythmias in isolated rat hearts was higher than in the acute phase because the incidence of severe ventricular arrhythmias, such as VT and VF, was increased compared with diabetes in the acute phase (ie, one week). Moreover, anti-infarct protection in these animals was blunted compared with anti-infarct protection in the acute phase of the disease (26). Xu et al (52) also demonstrated that in the chronic phase of STZ-induced diabetes, antiinfarct protection was lost, and that this effect was associated with a decrease in ERK1/2 phosphorylation compared with time-matched nondiabetic controls, indicating that long-term duration of hyperglycemia exerts a negative influence on ischemic myocardial injury that may depend on an ERK1/2-mediated intracellular signalling pathway.
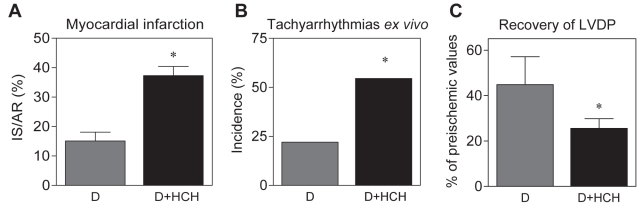
Similar to the loss of protection against I/R injury rendered by I-PC in the nondiabetic heart that was abrogated by an additional pathological condition (69), HCH induced by a high-cholesterol fatty diet appeared to be one of the reasons for the loss of anti-infarct protection in the acutely diabetic Langendorff-perfused rat hearts in our study (Figure 5A). Furthermore, in this ‘double-disease’ model, we observed that in vivo, HCH exacerbated severe ventricular arrhythmias in open-chest animals subjected to left anterior descending artery occlusion (27,28), tachyarrhythmias in isolated hearts (Figure 5B), and impaired postischemic recovery of left ventricular function in diabetic hearts subjected to global ischemia and reperfusion (Figure 5C). This suggests that the combination of diabetes and other pathologies also blunts endogenous cardioprotection in the diabetic heart.
Figure 5).
Effect of hypercholesterolemia (HCH) induced by high-cholesterol fatty diet in acutely diabetic (D) rats on the size of myocardial infarct (IS) (A), ventricular tachyarrhythmias (B) and recovery of left ventricular developed pressure (LVDP) (C) in isolated rat hearts. Data are presented as mean ± SEM of 10 to 12 hearts per group. *P<0.05 versus diabetic hearts. AR Area at risk
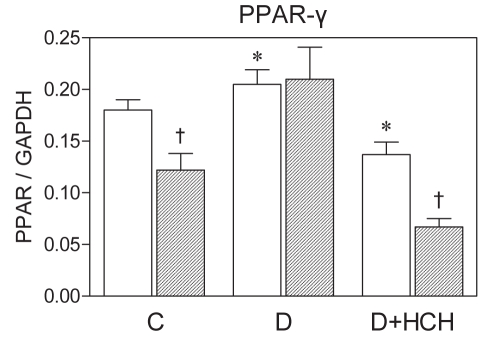
Although peroxisome proliferator-activated receptors (PPAR) as key transcriptional regulators of lipid metabolism, energy production (73) and inflammation (74) have been suggested to play an important role in susceptibility to myocardial I/R injury in the normal heart (75), their role in cardioprotection, particularly in pathological models such as diabetes and HCH, is not fully understood. To clarify this issue, we explored a potential link between a response to I/R in the hearts of both diabetic and diabetic HCH rats, and changes in cardiac PPAR gene expression. Enhanced resistance to I/R injury in the diabetic hearts was associated with preservation of baseline messenger RNA levels of PPAR-γ on I/R – in contrast to its marked downregulation in nondiabetic controls. Concurrent HCH attenuated both cardioprotection and changes in PPAR-γ levels in diabetic animals (Figure 6). These findings indicate that PPAR activation may be involved in the adaptive cardioprotective mechanisms activated in the diabetic myocardium in the acute phase to counteract metabolic disorders or, on the other hand, that loss of cardioprotection is potentially related to the downregulation of PPAR promoting proinflammatory effects. Thus, PPAR may represent an important therapeutic target in the management of ischemic heart disease in diabetic patients.
Figure 6).
Expression of peroxisome proliferator-activated receptor (PPAR-γ) messenger RNA in the hearts of control (C), diabetic (D) and diabetic-hypercholesterolemic (D+HCH) rats at baseline (open bars) and after 30 min of global ischemia and 2 h reperfusion (filled bars). Data are presented as mean ± SEM of at least four hearts per group. *P<0.05 versus baseline in controls; †P<0.05 versus baseline. GAPDH Glyceraldehyde-3 phosphate dehydrogenase
CONCLUSION
Experimentally induced diabetes mellitus may trigger adaptive processes in the myocardium in, at least, the early stage of the disease that are analogous to short-term cardioprotection in the nondiseased preconditioned myocardium. Similar to classical I-PC, the PC-like effect of high glucose against lethal myocardial injury is mediated via activation of the PI3K/Akt cascade. Response to I/R in the diabetic heart may be attenuated according to the duration of the diabetic state and by concomitant pathology such as HCH, and in both conditions is associated with changes in gene expression of PPAR, suggesting an important role of PPAR in I/R injury in pathologically altered myocardium.
Footnotes
FUNDING: Supported by grants VEGA SR 2/0173/08, 1/0620/10, APVV-LPP-0393-09, APVV 0538-07 and GSRT 5190/2005-759.
REFERENCES
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors. The Framingham Study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Dubrey SW, Reaveley DR, Seed M, et al. Risk factors for cardiovascular disease in IDDM. A study of identical twins. Diabetes. 1994;43:831–5. doi: 10.2337/diab.43.6.831. [DOI] [PubMed] [Google Scholar]
- 3.Singal PK, Bello-Klein A, Farahmand F, Sandhawalia V. Oxidative stress and functional deficit in diabetic cardiomyopathy. Adv Exp Med Biol. 2001;498:213–20. doi: 10.1007/978-1-4615-1321-6_27. [DOI] [PubMed] [Google Scholar]
- 4.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: A review. J Biol Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 5.Dhalla NS, Liu X, Panagia V, Takeda A. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40:239–47. doi: 10.1016/s0008-6363(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 6.Khandoudi A, Bernard M, Cozzone P, Feuvray D. Intracellular pH and role of Na+/H+ exchange during ischemia and reperfusion of normal and diabetic rat hearts. Cardiovasc Res. 1990;24:873–8. doi: 10.1093/cvr/24.11.873. [DOI] [PubMed] [Google Scholar]
- 7.Lee SL, Ostadalova I, Kolar F, Dhalla NS. Alterations in Ca2+ channels during the development of diabetic cardiomyopathy. Mol Cell Biochem. 1992;109:173–9. doi: 10.1007/BF00229773. [DOI] [PubMed] [Google Scholar]
- 8.Pierce GN, Ramjiawan B, Dhalla NS, Ferrari R. Na+/H+ exchange in cardiac sarcolemmal vesicles isolated from diabetic rats. Am J Physiol. 1990;258:H255–E261. doi: 10.1152/ajpheart.1990.258.1.H255. [DOI] [PubMed] [Google Scholar]
- 9.Anzawa R, Bernard M, Tamareille S, et al. Intracellular sodium increase and susceptibility to ischaemia in hearts from type 2 diabetic db/db mice. Diabetologia. 2006;49:598–606. doi: 10.1007/s00125-005-0091-5. [DOI] [PubMed] [Google Scholar]
- 10.Feuvray D, Lopaschuk GD. Controversies on the sensitivity of the diabetic heart to ischemic injury: The sensitivity of the diabetic heart to ischemic injury is decreased. Cardiovasc Res. 1997;34:113–20. doi: 10.1016/s0008-6363(97)00037-0. [DOI] [PubMed] [Google Scholar]
- 11.Galinanes M, Fowler AG. Role of clinical pathologies in myocardial injury following ischaemia and reperfusion. Cardiovasc Res. 2004;61:512–21. doi: 10.1016/j.cardiores.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Brady PA, Terzic A. The sulfonylurea controversy: More questions from the heart. J Am Coll Cardiol. 1998;31:950–6. doi: 10.1016/s0735-1097(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 13.Loubani M, Fowler A, Standen NB, Galinanes M. The effect of gliclazide and glibenclamide on preconditioning of the human myocardium. Eur J Pharmacol. 2005;515:142–9. doi: 10.1016/j.ejphar.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Fisman EZ, Motro M, Tenenbaum A. Non-insulin antidiabetic therapy in cardiac patients: Current problems and future prospects. Adv Cardiol. 2008;45:154–70. doi: 10.1159/000115193. [DOI] [PubMed] [Google Scholar]
- 15.Tani M, Neely JR. Hearts from diabetic rats are more resistant to in vitro ischemia: Possible role of altered Ca2+ metabolism. Circ Res. 1988;62:931–40. doi: 10.1161/01.res.62.5.931. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Thornton JD, Cohen MV, Downey JM, Schaffer SW. Streptozotocin-induced non-insulin dependent diabetes protects the heart from infarction. Circulation. 1993;88:1273–8. doi: 10.1161/01.cir.88.3.1273. [DOI] [PubMed] [Google Scholar]
- 17.Paulson D. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res. 1997;34:104–12. doi: 10.1016/s0008-6363(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 18.Forrat R, Sebbag L, Wiensperger A, Guidollet J, Delaye J, De Lorgeril M. Acute myocardial infarction in diabetic dogs with experimental diabetes. Cardiovasc Res. 1993;27:1908–12. doi: 10.1093/cvr/27.11.1908. [DOI] [PubMed] [Google Scholar]
- 19.Hadour G, Ferrera R, Sebbag L, Forrat R, Delaye J, De Lorgeril M. Improved myocardial tolerance to ischemia in the diabetic rabbit. J Mol Cell Cardiol. 1998;30:1869–75. doi: 10.1006/jmcc.1998.0751. [DOI] [PubMed] [Google Scholar]
- 20.Hekimian G, Khandoudi N, Feuvray D, Beigelman PM. Abnormal cardiac rhythm in diabetic rats. Life Sci. 1985;37:547–51. doi: 10.1016/0024-3205(85)90467-9. [DOI] [PubMed] [Google Scholar]
- 21.Kusama Y, Hearse DJ, Avkiran M. Diabetes and susceptibility to reperfusion-induced ventricular arrhythmias. J Mol Cell Cardiol. 1992;24:411–21. doi: 10.1016/0022-2828(92)93195-p. [DOI] [PubMed] [Google Scholar]
- 22.Tosaki A, Engelman DT, Engelman RM, Das DK. The evolution of diabetic response to ischemia/reperfusion and preconditioning in isolated working rat hearts. Cardiovasc Res. 1996;31:526–36. [PubMed] [Google Scholar]
- 23.Ravingerová T, Styk J, Pancza D, et al. Diabetic cardiomyopathy in rats: Alleviation of myocardial dysfunction caused by Ca2+ overload. Diabetes Res Clin Pract. 1996;(Suppl 31):S105–S112. doi: 10.1016/0168-8227(96)01237-5. [DOI] [PubMed] [Google Scholar]
- 24.Ziegelhoffer A, Ravingerova T, Styk J, et al. Mechanisms that may be involved in calcium tolerance of the diabetic heart. Mol Cell Biochem. 1997;176:191–8. [PubMed] [Google Scholar]
- 25.Tribulová N, Ravingerová T, Volkovová K, et al. Resistance of diabetic rat hearts to Ca overload-related injury. Histochemical and ultrastructural study. Diabetes Res Clin Pract. 1996;31:S113–22. doi: 10.1016/0168-8227(96)01238-7. [DOI] [PubMed] [Google Scholar]
- 26.Ravingerová T, Neckár̆ J, Kolá̆ F. Ischemic tolerance of rat hearts in acute and chronic phases of experimental diabetes. Mol Cell Biochem. 2003;249:167–74. doi: 10.1023/a:1024751109196. [DOI] [PubMed] [Google Scholar]
- 27.Adameová A, Kuželová M, Andelová E, et al. Hypercholesterolemia abrogates an increased resistance of diabetic rat hearts to ischemia-reperfusion injury. Mol Cell Biochem. 2007a;295:129–36. doi: 10.1007/s11010-006-9282-8. [DOI] [PubMed] [Google Scholar]
- 28.Adameová A, Ravingerová T, Švec P, Fáberová V, Kuželová M. The myocardial infarct size-limiting and antiarrhythmic effects of acyl-CoA: Cholesterol acyltransferase inhibitor VULM 1457 protect the hearts of diabetic-hypercholesterolaemic rats against ischaemia/reperfusion injury both in vitro and in vivo. Eur J Pharmacol. 2007b;576:114–21. doi: 10.1016/j.ejphar.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 29.Ravingerova T, Stetka R, Volkovova K, Pancza D, Dzurba A, Ziegelhöffer A, Styk J. Acute diabetes modulates response to ischemia in isolated rat heart. Mol Cell Biochem. 2000;210:143–51. doi: 10.1023/a:1007129708262. [DOI] [PubMed] [Google Scholar]
- 30.Ravingerova T, Neckar J, Kolar F, et al. Ventricular arrhythmias following coronary artery occlusion in rats: Is the diabetic heart less or more sensitive to ischaemia? Basic Res Cardiol. 2001;96:160–8. doi: 10.1007/s003950170066. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues B, Cam MC, McNeill JH. Myocardial substrate metabolism: Implications for diabetic cardiomyopathy. J Mol Cell Cardiol. 1995;27:169–79. doi: 10.1016/s0022-2828(08)80016-8. [DOI] [PubMed] [Google Scholar]
- 32.Opie LH. Myocardial ischemia, reperfusion and cytoprotection. Rev Port Cardiol. 1996;15:703–8. [PubMed] [Google Scholar]
- 33.Ziegelhöffer A, Ravingerová T, Waczuliková I, et al. Energy transfer in acute diabetic rat hearts: Adaptation to increased energy demands due to augmented calcium transients. Ann NY Acad Sci. 2002;967:463–8. [PubMed] [Google Scholar]
- 34.Shimoni Y, Severson D, Giles W. Thyroid status and diabetes modulate regional differences in potassium currents in rat ventricle. J Physiol (Lond) 1995;488:673–88. doi: 10.1113/jphysiol.1995.sp020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JM, Wahler GM. ATP-sensitive potassium channels are altered in ventricular myocytes from diabetic rats. Mol Cell Biochem. 1996;158:43–51. doi: 10.1007/BF00225881. [DOI] [PubMed] [Google Scholar]
- 36.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 37.Spinelli W, Sorota S, Siegal M, Hoffman BF. Antiarrhythmic actions of the ATP-regulated K+ current activated by pinacidil. Circ Res. 1991;68:1127–37. doi: 10.1161/01.res.68.4.1127. [DOI] [PubMed] [Google Scholar]
- 38.Tan HL, Mazon P, Verberne HJ, et al. Ischemic preconditioning delays ischemia induced cellular electrical uncoupling in rabbit myocardium by activation of ATP sensitive potassium channels. Cardiovasc Res. 1993;27:644–51. doi: 10.1093/cvr/27.4.644. [DOI] [PubMed] [Google Scholar]
- 39.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channel prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–33. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 40.Cascio WE, Johnson TA, Gettes LS. Electrophysiologic changes in ischemic ventricular myocardium: I. Influence of ionic, metabolic and energetic changes. J Cardiovasc Electrophysiol. 1995;11:1039–62. doi: 10.1111/j.1540-8167.1995.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 41.Ziegelhoffer A, Styk J, Ravingerová T, et al. Prevention of processes coupled with free radical formation prevents also the development of calcium resistance in the diabetic heart. Life Sci. 1999;18/19:1999–2003. doi: 10.1016/s0024-3205(99)00464-6. [DOI] [PubMed] [Google Scholar]
- 42.Matejikova J, Kucharska J, Pancza D, Ravingerova T. The effect of antioxidant treatment and NOS inhibition on the incidence of ischaemia-induced arrhythmias in the diabetic rat heart. Physiol Res. 2008;57:S55–60. doi: 10.33549/physiolres.931552. [DOI] [PubMed] [Google Scholar]
- 43.Matejíková J, Kucharská J, Pintérová M, Pancza D, Ravingerová T. Protection against ischemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: Involvement of mitochondrial K(ATP) channels and reactive oxygen species. Physiol Res. 2009;58:9–19. doi: 10.33549/physiolres.931317. [DOI] [PubMed] [Google Scholar]
- 44.Ravingerová T, Matejíková J, Pancza D, Kolá̆ F. Reduced susceptibility to ischemia-induced arrhythmias in the preconditioned rat heart is independent of PI3-kinase/Akt. Physiol Res. 2009;58:443–7. doi: 10.33549/physiolres.931743. [DOI] [PubMed] [Google Scholar]
- 45.Goto M, Tsuchida A, Liu Y, Cohen MV, Downey JM. Transient inhibition of glucose uptake mimics ischemic preconditioning by salvaging ischemic myocardium in the rabbit heart. J Mol Cell Cardiol. 1995;27:1883–94. doi: 10.1016/0022-2828(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 46.Wolfe CL, Sievers RE, Visseren FLJ, Donnelly TJ. Loss of myocardial protection after preconditioning correlates with the time course of glycogen recovery within the preconditioned segment. Circulation. 1993;87:881–92. doi: 10.1161/01.cir.87.3.881. [DOI] [PubMed] [Google Scholar]
- 47.Schaffer SW, Croft CB, Solodushko V. Cardioprotective effect of chronic hyperglycemia: Effect on hypoxia-induced apoptosis and necrosis. Am J Physiol Heart Circ Physiol. 2000;278:H1948–H1954. doi: 10.1152/ajpheart.2000.278.6.H1948. [DOI] [PubMed] [Google Scholar]
- 48.Ricci C, Jong CJ, Schaffer SW. Proapoptotic and antiapoptotic effects of hyperglycemia: Role of insulin signaling. Can J Physiol Pharmacol. 2008;86:166–72. doi: 10.1139/Y08-021. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Shen WL, Wang XH, et al. Paradoxically enhanced heart tolerance to ischaemia in type 1 diabetes and role of increased osmolarity. Clin Exp Pharmacol Physiol. 2006;33:910–6. doi: 10.1111/j.1440-1681.2006.04463.x. [DOI] [PubMed] [Google Scholar]
- 50.Meldrum DR, Cleveland JC, Jr, Sheridan BC, Rowland RT, Banerjee A, Harken AH. Cardiac preconditioning with calcium: Clinically accessible myocardial protection. J Thorac Cardiovasc Surg. 1996;112:778–86. doi: 10.1016/S0022-5223(96)70065-X. [DOI] [PubMed] [Google Scholar]
- 51.Strnisková M, Barancik M, Neckar J, Ravingerova T. Mitogen-activated protein kinases in the acute diabetic myocardium. Mol Cell Biochem. 2003;249:59–65. [PubMed] [Google Scholar]
- 52.Xu G, Takashi E, Kudo M, Ishiwata T, Naito Z. Contradictory effects of short- and long-term hyperglycemias on ischemic injury of myocardium via intracellular signaling pathway. Exp Mol Pathol. 2004;76:57–65. doi: 10.1016/j.yexmp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Ma G, Al-Shabrawey M, Johnson JA, et al. Protection against myocardial ischemia/reperfusion injury by short-term diabetes: Enhancement of VEGF formation, capillary density, and activation of cell survival signaling. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:415–27. doi: 10.1007/s00210-006-0102-1. [DOI] [PubMed] [Google Scholar]
- 54.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: The importance of Akt phosphorylation. Diabetes. 2005;54:2360–4. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 55.Malfitano C, Alba Loureiro TC, Rodrigues B, et al. Hyperglycaemia protects the heart after myocardial infarction: Aspects of programmed cell survival and cell death. Eur J Heart Fail. 2010;12:659–67. doi: 10.1093/eurjhf/hfq053. [DOI] [PubMed] [Google Scholar]
- 56.Kakkar R, Mantha SV, Kalra J, Prasad K. Time course study of oxidative stress in aorta and heart of diabetic rat. Clin Sci (Lond) 1996;91:441–8. doi: 10.1042/cs0910441. [DOI] [PubMed] [Google Scholar]
- 57.Kucharská J, Gvozdjaková A, Štefek M, Sotníková R, Sumbalova Z. Adaptive changes of antioxidant status in development of experimental diabetes. Bratisl Lek Listy. 2001;102:515–9. [PubMed] [Google Scholar]
- 58.Das DK, Prasad MR, Lu D, Jones RM. Preconditioning of heart by repeated stunning. Adaptive modification of antioxidative defense system. Cell Mol Bio. 1992;38:739–49. [PubMed] [Google Scholar]
- 59.Andelová E, Barteková M, Pancza D, Styk J, Ravingerová T. The role of NO in ischemia/reperfusion injury in isolated rat heart. Gen Physiol Biophys. 2005;24:411–26. [PubMed] [Google Scholar]
- 60.Halestrap AP, Clarke SJ. Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–31. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolár F, Jezková J, Balková P, et al. Role of oxidative stress in PKC-delta upregulation and cardioprotection induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2007;292:H224–30. doi: 10.1152/ajpheart.00689.2006. [DOI] [PubMed] [Google Scholar]
- 62.Malhotra A, Reich D, Reich D, et al. Experimental diabetes is associated with functional activation of protein kinase C and phosphorylation of troponin I in the heart, which are prevented by angiotensin II receptor blockade. Circ Res. 1997;81:1027–33. doi: 10.1161/01.res.81.6.1027. [DOI] [PubMed] [Google Scholar]
- 63.Moon CH, Jung JS, Lee SH, Baik EJ. Protein kinase C inhibitors abolish the increased resistance of diabetic rat heart to ischemia-reperfusion injury. Jpn J Physiol. 1999;49:409–15. doi: 10.2170/jjphysiol.49.409. [DOI] [PubMed] [Google Scholar]
- 64.Ooie T, Takahashi N, Nawata T, et al. Ischemia-induced translocation of protein kinase C-epsilon mediates cardioprotection in the streptozotocin-induced diabetic rat. Circ J. 2003;67:955–61. doi: 10.1253/circj.67.955. [DOI] [PubMed] [Google Scholar]
- 65.Malhotra A, Begley R, Kang BP, et al. PKC epsilon-dependent survival signals in diabetic hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1343–50. doi: 10.1152/ajpheart.01200.2004. [DOI] [PubMed] [Google Scholar]
- 66.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–53. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 67.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–8. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 68.Murphy E, Steenbergen C. Preconditioning: The mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 69.Giricz Z, Lalu MM, Csonka C, Bencsik P, Schulz R, Ferdinandy P. Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: Role of matrix metalloproteinase-2 inhibition. J Pharmacol Exp Ther. 2006;316:154–61. doi: 10.1124/jpet.105.091140. [DOI] [PubMed] [Google Scholar]
- 70.Tomai F, Crea F, Gaspardone A, et al. Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Circulation. 1994;90:700–5. doi: 10.1161/01.cir.90.2.700. [DOI] [PubMed] [Google Scholar]
- 71.Hassouna A, Loubani M, Matata BM, Fowler A, Standen NB, Galiñanes M. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc Res. 2006;69:450–8. doi: 10.1016/j.cardiores.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Galagudza MM, Nekrasova MK, Syrenskii AV, Nifontov EM. Resistance of the myocardium to ischemia and the efficacy of ischemic preconditioning in experimental diabetes mellitus. Neurosci Behav Physiol. 2007;37:489–93. doi: 10.1007/s11055-007-0040-5. [DOI] [PubMed] [Google Scholar]
- 73.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–78. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 74.Smeets PJH, Planavila A, van der Vusse GJ, van Bilsen M. Peroxisome proliferator-activated receptors and inflammation: Take it to heart. Acta Physiol. 2007;191:171–88. doi: 10.1111/j.1748-1716.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- 75.Ravingerová T, Adameová A, Kelly T, et al. Changes in PPAR gene expression and myocardial tolerance to ischaemia: Relevance to pleiotropic effects of statins. Can J Physiol Pharmacol. 2009;87:1028–36. doi: 10.1139/Y09-071. [DOI] [PubMed] [Google Scholar]