Abstract
Many clinical studies have documented favourable effects (reduced morbidity and mortality) of beta-adrenoceptor (β-AR) antagonists, such as carvedilol, metoprolol, propranolol, atenolol and bisoprolol, in congestive heart failure. These agents attenuate the effects of sympathetic activation during the development of heart failure, prevent ventricular remodelling and improve cardiac function. Because β-AR blockers are known to exert negative inotropic action, the mechanisms responsible for their beneficial effects in heart failure have been a subject of debate. While attenuation of changes in β-AR cyclic AMP-mediated signal transduction in heart failure is considered to be responsible for the beneficial effects of β-AR antagonists, other mechanisms such as the effects of these agents on subcellular remodelling, oxidative stress, apoptosis and defect in calcium handling, are equally important in preventing cardiac alterations in the failing heart. Moreover, β-AR antagonists are not a homogeneous group of drugs because they differ in their pharmacokinetics and pharmacodynamics, in addition to the selective and nonselective nature of their actions on β-AR. Various β-AR blocking agents have been shown to possess different ancillary properties and produce effects that are independent of β-AR. In fact, different β-AR antagonists have been observed to lower the elevated levels of plasma catecholamines in heart failure. Thus, the beneficial effects of β-AR antagonists are not only elicited through their interaction with mediated β-AR signal transduction sites in the myocardium, but other mechanisms may also contribute to their favourable actions in heart failure.
Keywords: β-adrenoceptor antagonists, Atenolol, Carvedilol, Heart failure, Metoprolol, Propranolol
The sympathetic nervous system (SNS) regulates cardiac function through the release of noradrenaline and subsequent activation of beta-adrenergic receptors (β-AR) and, to some extent, alpha-adrenergic receptors (α-AR) (1). It is now well known that the SNS is activated during the development of congestive heart failure (CHF), and its prolonged activation results in the change in the size and shape of the heart (ventricular remodelling) and progression of cardiac dysfunction (2). Accordingly, some agents with β-AR-blocking activity were developed for the treatment of heart failure; however, their use was discontinued because these agents were found to exert negative inotropic action on the heart (3). In spite of some reservations and a great deal of caution, recent years have witnessed a renewed interest in the use of β-AR antagonists for the treatment of CHF, and there is a surge in the development of these drugs. It is, therefore, considered worthwhile to discuss the results of some clinical studies showing beneficial effects of β-AR-blocking agents in CHF. We also plan to highlight the β-AR signal transduction mechanisms, which become defective during the course of development of CHF.
β-AR signal transduction pathways and β-AR antagonists
Under physiological conditions, the SNS remains in a resting state and exerts no influence on heart function (4). However, in the event of heart failure, the SNS is activated and initially helps to maintain cardiac function by increasing inotropic support (5), but prolonged activation of the SNS causes ventricular remodelling and progression of heart failure (6). Three types of β-AR (β1, β2 and β3) and one type of α-AR (α1) are expressed in the human heart and play a role in the regulation of cardiac function. β1-AR and β2-AR are coupled via stimulatory G protein to the effector enzyme, adenylyl cyclase, which converts ATP to cyclic AMP (cAMP). cAMP acts as a second messenger and produces inotropic, chronotropic and growth-promoting effects through protein kinase A (PKA) via phosphorylation of different proteins (7). PKA phosphorylates voltage-dependent L-type calcium channels, resulting in an increase in Ca2+ influx into cardiomyocytes during action potential and, thus, is responsible for the inotropic effect (8,9). However, phosphorylation of phospholamban and troponin I induces Ca2+ uptake into the sarcoplasmic reticulum (SR) and reduces the affinity of Ca2+ to troponin C, resulting in a lusitropic effect (10). β1-AR and β2-AR are also responsible for positive chronotropic, dromotropic and bathmotropic effects (10). However, there is evidence that the effects mediated by β1-AR are also regulated by mechanisms independent of PKA (11). It was observed that apoptosis of cardiomyocytes can be caused by stimulation of β1-AR, leading to an increase in intracellular Ca2+ concentration and activation of Ca2+ calmodulin-dependent protein kinase II (11). This theory was further supported by a study (12) that demonstrated that inhibition of Ca2+ calmodulin-dependent protein kinase II results in more effective inhibition of apoptosis compared with inhibition of calpain, calcineurin/PP2B or death-associated protein kinase.
β2-AR are involved in proliferation of cardiomyocytes. One study (13) documented that activation of β2-AR and subsequent increase in cAMP caused proliferation of cardiac fibroblasts in adult rats. However, another study (14) conducted on human cardiac fibroblast reported that an increase in cAMP and mitogen-activated protein kinase activation did not induce proliferation of cardiac fibroblast. Furthermore, the increased proliferation of cardiac fibroblast by β2-AR was shown to be caused by the secretion of heat-sensitive growth factors (14). On the other hand, β2-AR stimulation has been reported to prevent cardiac apoptosis because of their coupling with inhibitory G (Gi) proteins and inhibition of the adenylyl cyclase-cAMP-PKA pathway (15). The other pathway through which β2-AR contribute to the antiapoptosis effect is the activation of phosphatidylinositol 3-kinase by β2-AR Gi coupling. Phosphatidylinositol 3-kinase stimulates protein kinase B and results in attenuating concurrent β2-AR stimulatory G protein-mediated activation of PKA and decreasing apoptosis (16). It is evident from the above discussion that β2-AR stimulation may cause cardiac hypertrophy and may protect the myocardium from the apoptotic effects of prolonged activation of the SNS. Such observations need to be taken into consideration when prescribing β-AR antagonists in heart failure.
It is suggested that β3-AR are coupled to adenylyl cyclase via Gi proteins and, thus, their negative inotropic action is considered to be elicited by changing the adenylyl cyclase activity (6). These receptors have been suggested to neutralize the inotropic, chronotropic and dromotropic effects of β1-AR and β2-AR (6). This action is particularly significant in pathological states associated with adrenergic hyperactivity because β3-AR receptors are stimulated at higher concentrations of catecholamines than β1-AR and β2-AR (17,18). The effects of β3-AR are also mediated through the activation of endothelial nitric oxide (NO), which results in increased production of cyclic GMP (cGMP) and activity of cGMP-dependent protein kinase (17,18). The activation of cGMP-dependent protein kinase stimulates phosphodiesterase and results in myocardial relaxation (19,20). Apart from their action on the myocardium, β3-AR stimulation produces coronary and peripheral vasodilation (17). However, β3-AR stimulation can have detrimental effects in end-stage heart failure due to their negative inotropic action (21). Because β3-AR are stimulated only in the hyperadrenergic state and cause negative inotropism, β-AR antagonists can be useful in these settings.
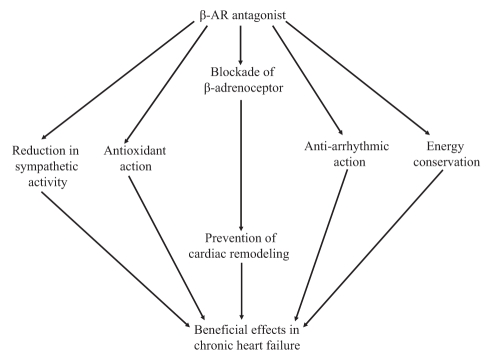
AR antagonists are classified into three categories, namely, α-AR antagonists, β-AR antagonists and mixed antagonists (22,23). The major AR antagonists and their receptor selectivity are listed in Table 1. The α1-AR are coupled via the Gq protein to the effector enzyme phospholipase C, which may produce diacylglycerol and result in the activation of protein kinase C (23). It is suggested that β-AR antagonists are further divided into three types depending on their antiadrenergic profile and presence of ancillary properties. The first-generation compounds are nonselective and block both β1-AR and β2-AR with equal affinity; the main prototypes of this group are propranolol and timolol. The second-generation compounds are cardioselective agents that block β1-AR with greater affinity than β2-AR; two agents in this group are metoprolol, which is 75 times more selective for β1-AR compared with β2-AR, and bisoprolol, which is 120 times more selective. The third-generation compounds include carvedilol and bucindolol, which block both β1-AR and β2-AR with almost equal affinity and have some additional properties that differentiate them from the first-generation compounds. Carvedilol blocks α1-AR and, in addition, has antioxidant action. Bucindolol has vasodilatory action and shows intrinsic sympathomimetic activity (24). Thus, it is evident that AR antagonists may affect cardiac function by acting on cardiac and noncardiac sites through specific and nonspecific AR-mediated signal transduction mechanisms or on modification of other cellular events (Figure 1).
TABLE 1.
Adrenergic antagonists and their receptor selectivity
| Adrenergic antagonist | Receptor selectivity | |
|---|---|---|
| Alpha (α)-antagonist | Prazosin, doxazosin, tamsulosin | α1 |
| Phenoxybenzamine | α1 > α2 | |
| Phentolamine | α1 and α2 | |
| Yohimbine | α2 | |
| Beta (β)-antagonist | Metoprolol | β1 |
| Atenolol | β1 | |
| Acebutalol | β1 | |
| Betaxolol | β1 | |
| Esmolol | β1 | |
| Propranolol | β1 and β2 | |
| Carteolol | β1 and β2 | |
| Timolol | β1 and β2 | |
| Butoxamine | β1 | |
| Mixed antagonist | Labetalol | β1, β2 and α1 |
| Carvedilol | β1, β2 and α1 |
Figure 1).
Pathways through which beta-adrenergic receptor (β-AR) antagonists exert beneficial effects in chronic heart failure. Apart from their action on β-AR, they result in the reduction of sympathetic activity by acting directly on prereceptor nerve endings. In addition, they preserve energy and have antioxidant and antiarrhythmic actions; the latter is responsible for beneficial effects in heart failure
It is important to know the effects of sustained adrenergic drive on the failing heart to understand the mechanism by which long-term β-AR antagonist therapy produces beneficial results in heart failure. In healthy hearts, approximately 60% to 80% of the β-AR expressed are β1, 20% to 40% are β2 and approximately 2% are β3 (6). It has been documented that in failing hearts, β1-AR undergoes selective downregulation and the ratio of β1:β2 changes from 77:23 to 60:38 (25). Because α1-AR are upregulated in heart failure, this changes the AR profile from predominantly β1 to a mixed profile with a β1:β2:α1 ratio of 2:1:1 (26,27). It should be noted that β-AR-mediated signal transduction depends on the stage of heart failure and is differently regulated in the left and right ventricles. While the signal transduction parameters are depressed after eight to 24 weeks of myocardial infarction (MI) in the left ventricle, the right ventricle shows increased signal transduction at eight weeks and depressed signal transduction at 24 weeks (28). These differential changes may be due to differences in the type and stage of hypertrophy because β1-AR expression was increased in the hypertrophied heart due to volume overload, but was unaltered at the early stage of heart failure due to pressure overload (29). However, in the late stages of both types of hypertrophy, there is a downregulation of β-AR. Accordingly, it appears that in earlier compensated stages of heart failure, β-AR are upregulated or unchanged but, at later decompensated stages, they are downregulated. Apart from changes in the expression of β-AR, G protein levels play a critical role in the pathogenesis of heart failure. It has been documented that there are increased levels of Gi proteins in the myocardium of patients with heart failure (30). Another theory for the alterations in the β-AR signal transduction is the change in the affinity of the receptors. A decrease in the affinity of β-AR was reported in CHF (31). However, a subsequent study (27) showed no change in the affinity. Nonetheless, it is becoming clear that alterations in the β-AR and G proteins depend on the stage of heart failure and various stressful stimuli.
In an interesting experiment, transgenic mice with heart-specific overexpression of β1-AR were studied; these mice showed increased contractility of the heart at a young age, but developed cardiac hypertrophy, which was followed by progressive heart failure. The results suggested that overexpression of β1-AR leads to initial improvement of heart function and ultimately results in heart failure (32). In a similar study (33), it was reported that overexpression of alpha-stimulatory G protein over the life of a transgenic mouse resulted in cardiomyocyte degeneration and replacement fibrosis as well as hypertrophy of the remaining cells; these changes were responsible for heart failure. It has also been shown that stimulation of β1-AR increases apoptosis through the cAMP-dependent pathway, while β2-AR stimulation inhibits apoptosis via Gi coupling (34). However, a high level of overexpression of β2-AR ultimately results in systolic dysfunction and cardiomyopathy (35). These data indicate that chronic AR activation, which is a compensatory mechanism at initial stages, may exert harmful effects in chronic stages. It is known that in heart failure, β-AR signal transduction is reduced because of desensitization of β-AR and changes in Gi protein as well as expression of adenylyl cyclase enzyme (22). Because a substantial signalling capacity remains despite significant loss of signal transduction at the end stage of heart failure (22), it was suggested that decreased signal transduction is an adaptive mechanism to prevent progression of heart failure, and any therapy that supplements this intrinsic mechanism would be beneficial. It has been documented that a state of protracted sympathetic hyperactivity in chronic heart failure occurs, and this hyperactivity is inversely proportional to the left ventricular ejection fraction (36). Furthermore, it has been indicated that this hyperactivity is not in response to a decrease in arterial pressure, but due to an impairment of reflexes from heart receptors that inhibit efferent sympathetic activity (36).
Mechanisms of beneficial effects of β1-AR antagonists in heart failure
Although attenuation of changes in β-AR-mediated signal transduction in failing heart contribute to the beneficial effects of β-AR antagonists in heart failure, it appears that some other mechanisms are equally responsible. It was suggested that several benefits of β-AR antagonists were due to reduction in the heart rate. A study (37) was conducted to investigate whether metoprolol and a pure heart rate-reducing agent, ivabradine, had similar effects on cardiac hemodynamics, ventricular remodelling and Ca2+ handling in MI-induced heart failure in rats. It was found that although ivabradine had beneficial effects on cardiac hemodynamics, metoprolol had additional benefits in preventing left ventricular dilation and hypertrophy. Metoprolol was associated with increased contractility of isolated cardiomyocytes and better Ca2+ handling in the post-MI rat heart (37). Such observations indicated that some other mechanism apart from the action on AR must be responsible for the effects of metoprololol.
While comparing the effects of different β-AR antagonists on rat heart sarcolemmal (SL) Ca2+ transport activities (38), it was found that propranolol and oxyprenolol had biphasic actions; the lower concentrations were stimulatory while higher concentrations were inhibitory. In addition, pindolol was stimulatory, while acebutolol had no effect on SL Ca2+ transport (38). Those observations suggest that the SL membrane is the site of action of β-AR antagonists; however, the exact role of SL Ca2+ pump activity in heart failure is not clear. On the other hand, SR dysfunction due to abnormalities in SR proteins was indicated to play a major role in heart failure, and the genes expressing SR Ca2+ pump, Ca2+-release channels and other regulatory proteins could be potential targets for the treatment of heart failure (39). Moreover, improvement of human heart muscle function due to β-AR antagonists (carvedilol, metoprolol and atenolol) was associated with restoration of normal SR ryanodine receptor (RyR2) channel activity (40). Similar effects on SR Ca2+ leak from RyR2 were seen with propranolol (41). Furthermore, metoprolol was reported to improve cardiac function by preventing alterations in Ca2+ cycling proteins, such as RyR2, and the ratio of SERCA2a and Na+-Ca2+ exchanger in addition to increasing Ca2+ transients in the failing heart (42). However, carvedilol treatment was found to result in a more significant improvement of SERCA expression than metoprolol (43). Carvedilol has been proven to directly inhibit L-type Ca2+ current similar to Ca2+ antagonists and, thus, decrease the influx of Ca2+ in cardiomyocytes (44). Because one of the mechanisms associated with the progression of left ventricular hypertrophy and heart failure is catecholamine-induced Ca2+ overload and PKA activation, carvedilol was shown to decrease Ca2+ load by depressing the L-type Ca2+ currents (45). It was also demonstrated in a study (46) using SR vesicles isolated from the left ventricle that carvedilol improved intracellular Ca2+ handling in heart failure by correcting defective interdomain interaction within the RyR2, thereby improving cardiac function. The expression of SERCA messenger RNA (mRNA) and protein, which is downregulated after MI, is restored with low-dose carvedilol treatment (47). The antioxidant property of carvedilol plays a crucial role in restoring the expression of SERCA mRNA and protein (48). These findings support the view that restoration of Ca2+ pump and Ca2+-release channel functions is one of the possible explanations for beneficial effects of β-AR antagonists in failing hearts (40).
It is now well known that activation of the SNS results in the release of noradrenaline from the sympathetic nerve endings and adrenaline from the adrenal medulla and, thus, the elevated levels of plasma catecholamines are associated with ventricular remodelling and heart failure (6,49). Noradrenaline exerts its effects by acting on AR in the heart where the type of receptor plays an important role; β1-AR is involved in noradrenaline-induced cardiac apoptosis (50) whereas β2-AR is known to prevent this (51). It was also found that noradrenaline caused apoptosis in rat cardiomyocytes through downregulation of Bcl-2 and activation of caspase-2 pathways (51); caspase inhibitors were observed to prevent noradrenaline-induced cardiac apoptosis. In rats with MI-induced heart failure, both low and high doses of atenolol and propranolol attenuated cardiac dysfunction and depressed the MI-induced increase in adrenaline; the increased noradrenaline levels due to MI were lowered by high doses of these agents but were unaffected by low doses (52). It was observed in patients with chronic heart failure that atenolol improved ventricular function and clinical status without affecting the plasma levels of noradrenaline (53). On the other hand, a nonselective β-AR antagonist, propranolol, was reported to cause a reduction in noradrenaline spillover in heart failure patients (54). Likewise, carvedilol caused a significant decrease in cardiac and systemic noradrenaline spillover in patients with heart failure, whereas metoprolol failed to produce such changes (55). Carvedilol, unlike atenolol, significantly blunted the increase in plasma noradrenaline during exercise and this effect was attributed to the blockade of presynaptic β2-AR (56). While the different β-AR antagonists depressed the release of noradrenaline due to their effects on preterminal nerve endings (57), vagal stimulation was found to improve long-term survival of rats with chronic heart failure (58). Moreover, in rats with adriamycin-induced heart failure, it was seen that carvedilol treatment resulted in the upregulation of muscarinic cholinergic receptors in the endocardial tissues of the left ventricle (59). Thus, it appears that the antiadrenergic effects independent of β-AR may be another pathway through which β-AR antagonists prevent the progression of heart failure (Figure 1).
Apart from their action at the cellular and molecular level, β-AR antagonists were shown to produce favourable changes in heart failure by acting on myofibrils (60). It was observed in a study (60) conducted on heart failure in rats that atenolol and propranolol treatment attenuated MI-induced depression in myofibrillar Ca2+-stimulated ATPase activity and phosphorylated the cardiac troponin I protein. The MI-induced decrease in the α-myosin heavy chain (MHC) and the increase in β-MHC proteins were also attenuated by both propranolol and atenolol at low and high doses (60); the changes in gene expression for α-MHC and β-MHC were not attenuated with low-dose propranolol (60). Similar effects on myofibrillar function were also observed with metoprolol and carvedilol (61). These studies support the view that both selective and nonselective β-AR antagonists exert beneficial effects by their effects on contractile and regulatory proteins in heart failure.
It was first reported that propranolol exerted antioxidant properties independent of β-AR blockade and this mechanism was considered to be responsible for the protection against SL lipid peroxidation (62,63). In one such study (64) on cultured human coronary artery endothelial cells, it was found that adrenaline-induced apoptosis was associated with the activation of Fas-Fas ligand and caspase-3 signal transduction pathway, and carvedilol was more effective than atenolol in attenuating these effects of adrenaline because of its antioxidant property. Effects of treatment with carvedilol, metoprolol and metoprolol plus bunazocin (selective α1-AR antagonist) were investigated on experimental MI in rat hearts (65). In this study, carvedilol showed a greater antioxidant activity, attenuation of inflammatory mediators and activation of nuclear factor-kappaB. Furthermore, addition of bunazocin to metoprolol did not add to the effects of metoprolol alone (65). Another study (66) demonstrated that oxidative stress-induced apoptosis was independent of α-AR and β-AR, and carvedilol treatment delayed the process; this effect was not seen with other β-AR antagonists such as metoprolol, propranolol and atenolol. However, N-acetyl-L-cysteine and the combination of N-acetyl-L-cysteine and propranolol showed antioxidant activity similar to carvedilol (66). The effects of carvedilol and its hydroxylated analogue on apoptosis were tested in an experimental MI model of the rat heart. It was observed that although carvedilol treatment prevented apoptosis, its hydroxylated analogue did not; these data support the view that carvedilol has antiapoptotic effects independent of β-AR antagonism (67). In another study (68), the effects of carvedilol on abnormality of L-type Ca2+ current induced by oxygen-free radical in a single guinea pig cardiomyocyte were examined; this drug treatment resulted in the reduction of this defect. Carvedilol, unlike metoprolol, was found to inhibit reactive oxygen species because its molecular structure favours redox recycling (69,70). While metoprolol was observed to produce beneficial effects on ventricular remodelling by improving Ca2+ handling only, carvedilol improved cardiac redox state and Ca2+ handling, thus highlighting the contribution of its antioxidant action (71). Carvedilol inhibited mitochondrial oxygen consumption and superoxide production during Ca2+ overload in isolated heart mitochondria (69). From these observations, it appears that carvedilol has a more potent antioxidant effect than any other β-AR antagonist, and this property is not only involved in preventing catecholamine-induced apoptosis but also abnormalities in Ca2+ handling. However, it has been reported that metoprolol also exerted antioxidant properties similar to carvedilol and, thus, attenuated ventricular remodelling (72). Such conflicting results appear to be due to differences in doses of different β-AR antagonists used in various studies.
Carvedilol has been shown to possess several ancillary properties that are responsible for the improvement of heart failure independent from the upregulation of β-AR (73). In addition to the antioxidant effect, carvedilol was found to improve endothelial dysfunction because it increased NO levels and upregulated NO synthase 3 mRNA (74) in rats with streptozotocin-induced diabetes. Other β-AR antagonists, such as propranolol and metoprolol, inhibited the synthesis and release of endothelin (75). In a rat heart model of isoproterenol-induced hypertrophy, it was observed that carvedilol produced better effects on cAMP production and cardiac hypertrophy, as well as reduced left ventricular weight without affecting the heart rate and blood pressure compared with metoprolol (76). It should also be noted that ventricular arrhythmias are the major cause of morbidity in heart failure and the increase of transmural heterogeneity of ventricular repolarization plays an important role in causing these arrhythmias (77). Carvedilol decreases the transmural heterogeneity of ventricular repolarizations due to its direct electrophysiological property rather than its effects on ventricular remodelling (78). Another mechanism through which carvedilol prevents apoptosis was demonstrated in a study (79) in which the expression of autophagic-, antiapoptotic- and apoptotic-related proteins were studied in rat hearts with MI induced by coronary artery ligation. It was observed that antiapoptosis-related proteins increased in response to upregulation of autophagy by carvedilol treatment. Carvedilol, unlike metoprolol, was also found to reduce the effect of β-AR antagonists on the increased expression of β3-AR in chronic heart failure (80). Another mechanism responsible for beneficial effects of metoprolol in attenuating ventricular remodelling is related to decreasing cardiomyocyte loss through apoptosis. It was found that the number of nuclear DNA defragmentation events in cardiomyocytes, which is a marker of apoptosis, was lower in dogs who experienced heart failure and were treated with metoprolol (81). Because β1-AR stimulation due to adrenergic activation in heart failure increases the expression of proapoptotic protein Bxl-X(S), metoprolol has been shown to attenuate the expression of this proapoptotic protein indicating this pathway is one of the mechanisms responsible for its beneficial effect in heart failure (82). In a study (83) conducted on MI-induced heart failure in rats, it was observed that apoptosis was attenuated by β2-AR antagonists to a greater extent than by the β1-AR antagonists.
Although it is not fully understood how the blockade of β-AR pathway increases contractility of the heart and improves cardiac function in heart failure, some investigators have attributed these effects to the prevention of the β-AR signal transduction abnormalities and subsequent retardation of ventricular remodelling. β-AR antagonists, such as metoprolol, improve the efficiency of the AR signalling pathway by restoring the down-regulated receptors and, thus, increasing their numbers. On the other hand, carvedilol showed no effect on the number of AR receptors but inhibited receptor kinase and, thus, improved the efficiency of β-AR signalling pathway (7,22).
Propranolol was found to be ineffective in post-MI rats who experienced heart failure in terms of improvement in left ventricular remodelling, systolic function or intracellular Ca2+ handling (84). However, it was observed that long-term treatment with propranolol failed to attenuate cardiac hypertrophy, but abolished the oxidative stress in a rat model of heart failure in addition to a reduction in the sensitivity to catecholamine-induced arrhythmias (85).
In another study (86), the effects of β-AR antagonists in the brain were investigated to examine whether their actions contributed to beneficial effects in heart failure. It was found that chronic intracerebroventricular administration of metoprolol resulted in slowing of the progression of ventricular remodelling in MI-induced heart failure in rats.
Metoprolol was also seen to attenuate the myocardial expression of tumour necrosis factor-alpha and interleukin-1β in rats with MI-induced heart failure, thus revealing another mechanism through which β-AR antagonists may be helpful in chronic heart failure (87). On the other hand, nebivolol has been shown to prevent hydroxyl radical-induced contractile dysfunction through a direct effect on myofilaments and by preserving the function of SR (88). Thus, there appears to be several mechanisms that could be responsible for the benefits of β-AR antagonists in heart failure (Figure 1).
In some experiments, the effects of carvedilol treatment on cardiac function, ventricular remodelling and the adrenergic system were compared with that of metoprolol (89). It was observed that carvedilol and metoprolol produced the same degree of β-AR blockade as evidenced by changes in heart rate; however, carvedilol produced greater improvement in ventricular function in the failing heart. It was also found that carvedilol, unlike metoprolol, lowered the coronary sinus noradrenaline levels. In addition, metoprolol increased cardiac β-AR density, whereas carvedilol showed no effect on cardiac β-AR expression (89). The superiority of carvedilol over metoprolol in the treatment of heart failure was also reported by other investigators (90). In another study on the post-MI rat heart, carvedilol prevented left ventricular remodelling with respect to volume expansion and segmental hypertrophy in a dose-dependent manner, whereas metoprolol prevented left ventricular dilation without any effect on cardiac hypertrophy (91). Furthermore, carvedilol significantly reduced myocardial collagen in the noninfarcted myocardium in MI-induced heart failure in rats, whereas metoprolol had no effect (92). Although metoprolol attenuated postinfarct ventricular remodelling by blocking β1-AR, it did not improve myocardial energy metabolism and function (93). When the effects of atenolol were compared with metoprolol in dogs with microembolization-induced heart failure, it was found that atenolol prevented the decrease in ejection fraction, whereas metoprolol increased the ejection fraction, indicating the superiority of metoprolol over atenolol (94). The effects of long-term therapy with metoprolol on ventricular remodelling and progression of heart failure were also examined in dogs with heart failure. It was observed that treatment with metoprolol resulted in a 46% reduction in replacement fibrosis, 54% reduction in interstitial fibrosis and 20% reduction in cardiac hypertrophy (95). The data from these studies suggest that various β-AR antagonists may prevent ventricular remodelling, but the mechanisms of their beneficial effect in heart failure seem to be different from each other.
Clinical trials of β-AR antagonists in heart failure
Analysis of some studies (96) conducted in the mid-1970s showed favourable effects of β-AR antagonists on left ventricular function and clinical symptoms in heart failure (96). These trials revealed that β-AR antagonist therapy in heart failure improved the ejection fraction, and reduced the mortality and hospitalization time (97,98). A meta-analysis (99) that included 22 trials and 10,135 patients with heart failure (New York Heart Association [NYHA] class II and III) demonstrated the impact of β-antagonist therapy. The data revealed that there were 624 deaths among 4862 patients who received placebo, while only 444 deaths were reported among 5273 patients who received β-AR antagonists. The best estimate of the benefits is 3.8 lives saved and four fewer hospitalizations per 100 patients treated for one year. The majority of the studies in this metaanalysis were conducted on metoprolol and carvedilol; however, some studies used bisoprolol, bucindolol and nebivolol. The major clinical studies conducted on various β-AR antagonists (Table 2) are discussed in the following section (100–109).
TABLE 2.
Results of the major clinical studies of beta (β)-adrenoceptor antagonists in chronic heart failure
| Agent | Receptor selectivity | Effects | Reference |
|---|---|---|---|
| Metoprolol | β1 | Improved systolic function between one and three months, regression of left ventricular mass and change in shape from spherical to normal elliptical | (100) |
| Metoprolol | β1 | Decreased mortality (RR reduction of 34%) | MERIT-HF (101) |
| Carvedilol | Nonselective | 5.7% more reduction in mortality compared with metoprolol tartrate | COMET (102) |
| Carvedilol | Nonselective | Reduced hospitalizations and mortality | (103) |
| Carvedilol | Nonselective | Reduced all-cause mortality, cardiovascular mortality and nonfatal myocardial infarction | CAPRICORN (104) |
| Carvedilol | Nonselective | Improved clinical status and reduced mortality, well tolerated even in patients with very low systolic blood pressures (85 mmHg to 100 mmHg) | COPERNICUS (105) |
| Atenolol | β1 | Improved survival rates but lower than metoprolol | (106) |
| Bisoprolol | β1 | Reduced mortality independent of severity of heart failure | CIBIS-II (107) |
| Nebivolol | β1 | Reduced mortality | SENIORS (108) |
| Propranolol | Nonselective | Reduced mortality in patients with ejection fraction of 40% or greater | (109) |
CAPRICORN The Carvedilol Post Infarct Survival Control in Left Ventricular Dysfunction; CIBIS-II The Cardiac Insufficiency Bisoprolol Study II; COMET Carvedilol Or Metoprolol European Trial; COPERNICUS Carvedilol Prospective Randomized Cumulative Survival; MERIT-HF Metoprolol CR/XL Randomized Intervention Trial in Heart Failure; SENIORS Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with heart failure
Metoprolol
A study (100) was performed to examine the time course of improvement of cardiac function in patients with dilated cardiomyopathy when administered metoprolol. It was observed that after an initial decline in ventricular function, there was an improvement between one and three months of therapy. It was also observed that there was regression of a left ventricular mass and change in the shape from spherical to normal elliptical by 18 months of therapy (100). A large randomized trial (Metoprolol CR/XL Randomized Intervention Trial in Heart Failure [MERIT-HF; 101]) was designed to assess whether the improvement in the cardiac hemodynamics and function produced by metoprolol were associated with a reduction in mortality and morbidity in heart failure. This study was conducted at 313 sites in the United States and Europe; 3991 patients with chronic heart failure (NYHA class II to IV) and ejection fraction of 40% or less, and well maintained on standard therapy were included. On randomization, 1990 patients received metoprolol and 2001 patients received placebo. Metoprolol succinate starting from either 12.5 mg/day in NYHA class III and IV, or 25 mg/day in NYHA class II and titrated six to eight weeks up to a target dose of 200 mg/day was given to patients in this study group. It was observed that all-cause mortality was 7.2% in the treatment group and 11% in the placebo group; there was a RR reduction of 34%. Moreover, RR reductions were 38% for cardiovascular deaths, 41% for sudden deaths and 49% for deaths due to worsening heart failure. Although the study was planned for three years, it had to be stopped at the halfway point because of the mortality benefits in the treatment group (101). In another study (Carvedilol Or Metoprolol European Trial [COMET; 102]), the effects of carvedilol on mortality in heart failure were compared with those of metoprolol. Patients with chronic heart failure with NYHA class II to IV and an ejection fraction of 35% receiving treatment with angiotensin-converting enzyme (ACE) inhibitors and diuretics were included in the study. In this trial, 3029 patients were randomly assigned to receive either metoprolol (metoprolol tartrate, target dose 50 mg twice daily) or carvedilol (target dose 25 mg twice daily). It was observed that all-cause mortality was 34% for carvedilol and 40% for metoprolol. The absolute reduction in the mortality rate over five years was 5.7% (102).
Carvedilol
As it became clear that β-AR antagonists produced hemodynamic and symptomatic improvement in heart failure, a study (103) was planned to investigate the effects of carvedilol on mortality and morbidity in patients with chronic heart failure. In this study, 1094 patients with mild, moderate or severe heart failure with an ejection fraction of 35% or less were enrolled; 696 patients received carvedilol and 398 patients received placebo. The standard therapy comprising digoxin, diuretics and ACE inhibitors was continued, and the patients were observed for six months for hospitalizations and deaths related to cardiovascular causes. It was found that there was a reduction in the risk of death and hospitalizations with carvedilol treatment (103). Another study (The Carvedilol Post Infarct Survival Control in Left Ventricular Dysfunction [COPERNICUS; 104]) was designed to investigate the effects of carvedilol on morbidity and mortality in patients with post-MI heart failure. In this multicentre randomized trial (104), 1959 patients treated for MI and left ventricular ejection fraction of 40% or less were randomly assigned to receive either carvedilol (6.25 mg at start and gradually increased to 25 mg twice a day) or placebo. It was observed that cardiovascular mortality, nonfatal MI and all-cause mortality were lower in the carvedilol group (104). Clinicians were apprehensive of using carvedilol in high-risk heart failure patients with low blood pressure, fearing that it would interfere with the homeostatic action of the sympathetic nervous system and would result in dizziness, hypotension and worsening of heart failure. Thus, a multicentre study (COPERNICUS; 105]) was designed to investigate the influence of pretreatment systolic blood pressure on the efficacy and safety of carvedilol in patients with chronic heart failure (105). Patients with severe heart failure having dyspnea or fatigue at rest or on minimal exertion for two or more months and an ejection fraction of less than 25% despite treatment were enrolled. Unlike other studies, this study even included patients with a very low systolic blood pressure (85 mmHg to 100 mmHg). Patients were randomly assigned in a double-blinded fashion to receive either carvedilol (3.125 mg initially to be titrated to a target dose of 25 mg twice daily) or placebo. Results showed that carvedilol not only improved the clinical status of patients, but also resulted in reduction of mortality and hospitalizations. It was also found that carvedilol was well tolerated in patients with low blood pressure; these patients had the greatest need for treatment with carvedilol (105).
Atenolol
Atenolol has also been shown to be beneficial in heart failure. In a study by Celic et al (106), 150 patients on standard treatment with NYHA class II and III and an ejection fraction of 40% or less were randomly assigned to three groups, namely metoprolol, atenolol and a control group. The cumulative survival rate for patients treated with metoprolol was 88% and for those treated with atenolol, the survival rate was 78%; while for the control group, the survival rate was just 48%. Although atenolol showed a favourable effect on patient survival in heart failure, metoprolol was more effective (106). However, in a cohort study (110) of high-risk patients with heart failure, it was found that the adjusted risk of hospitalization for heart failure was not significantly different in patients receiving atenolol, carvedilol or short-acting metoprolol tartrate (110).
Bisoprolol
A multicentre, double-blinded, randomized controlled clinical trial (The Cardiac Insufficiency Bisoprolol Study II [CIBIS-II; 107]), including 2647 symptomatic patients with NYHA class III or IV and an ejection fraction of 35% or less receiving standard therapy with diuretics and ACE inhibitors, was conducted to investigate the efficacy of bisoprolol in decreasing all-cause mortality in patients with chronic heart failure. Patients were randomly assigned to receive bisoprolol 1.25 mg or placebo – the drug dose being gradually increased to 10 mg per day. The study had to be stopped after the second interim analysis because the bisoprolol-treated group showed a significant reduction in the mortality rate; these effects were independent of the severity or cause of heart failure. It was also found that bisoprolol reduced mortality in patients with heart failure at all tolerated dose levels and its withdrawal increased mortality (111). The benefits seen with bisoprolol treatment in terms of all-cause mortality were the same as with enalapril, but bisoprolol treatment showed fewer sudden deaths; however, more episodes of worsening of heart failure were seen with bisoprolol treatment (112).
Nebivolol and propranolol
Nebivolol is the most selective β1-AR antagonist having vasodilator properties without any action on α-AR (113). It stimulates β3-AR resulting in NO production, not only in the vascular system but also in the myocardium; thus having a favourable effect on heart failure (114). The clinical effects of this drug in terms of mortality were investigated in a randomized trial (Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with heart failure [SENIORS; 108]) including 2128 patients 70 years of age or older with heart failure regardless of the ejection fraction. It was found that the all-cause mortality rate was lower in the nebivolol-treated group (108). In older patients with post-MI heart failure and an ejection fraction of 40% or greater on diuretics and ACE inhibitors, propranolol treatment resulted in a reduction in mortality and improvement in left ventricular ejection fraction (109).
CONCLUSION
The paradox of β-AR antagonist use in heart failure has aroused such an interest in contemporary investigators that many hypotheses have been proposed to explain the intricate mechanisms of these agents. From the foregoing discussion of the hypotheses, it is clear that β-AR antagonists not only benefit heart failure through AR but also through other pathways. In the chronic stages of heart failure β1-AR are downregulated and the affinities of the receptors are also decreased. These changes attenuate the systolic function, but at the same time prevent β1-AR-mediated apoptosis. Moreover, the decreased affinity of these receptors and downregulation is an intrinsic adaptive mechanism to decrease the workload and energy consumption of the heart. This leads us to the question, ‘How do β-AR antagonists help in this scenario?’ These agents not only result in supplementing to this adaptive mechanism, but also protect the heart in the event of sudden deteriorations, which are associated with increased sympathetic stimulation. Moreover, in the hyperadrenergic state, β3-AR are stimulated and result in a decrease of systolic function, which can be detrimental in end-stage heart failure. This can be effectively attenuated by nonselective β-AR antagonists. Because β2-AR stimulation is considered to be beneficial in heart failure, blockade of these receptors by some β-blockers can be seen to exert detrimental effects in the failing heart. Apart from the action on AR, these agents improve Ca2+ handling by their effects on SL L-type Ca2+channels and SR RyR2. In addition to exerting antiapoptotic effects mediated by their antioxidant action, β-AR antagonists have direct action on myofibrils and various regulatory proteins in cardiomyocytes. Antiadrenergic action independent of receptors is another pathway through which these agents attenuate the effects of noradrenaline on the heart.
Various β-AR antagonists differ in their selectivity for receptors and ancillary properties. There is evidence to suggest that the nonselective β-antagonist, carvedilol, has the most potent antioxidant action, improves Ca2+ handling and exerts prereceptor antiadrenergic effects. Clinical studies have also documented the superiority of carvedilol in comparison with other β-AR antagonists. There were some reservations about the COMET study that metoprolol tartrate was used instead of metoprolol succinate to compare with carvedilol. However, it is clear from the overall experimental data and clinical studies that carvedilol produces the maximum benefits in chronic heart failure and is well tolerated even in patients with low blood pressure. Other agents, such as metoprolol and bisoprolol, are also very effective in preventing ventricular remodelling. However, more work needs to be performed on setting guidelines for these agents in different stages of heart failure and with different etiologies of heart failure. Particularly, the ancillary properties of β-AR antagonists should be kept in mind while prescribing these agents. Furthermore, the noncardiac effects of β-AR antagonists including those on sympathetic nerves and endothelium may play a critical role in inducing beneficial actions of these agents in heart failure.
Acknowledgments
This study was supported by a grant from the Canadian Institutes of Health Research. The infrastructural support for this work was provided by the St Boniface Hospital Research Foundation (Winnipeg, Manitoba).
REFERENCES
- 1.Dhalla NS, Wang X, Sethi R, Das PK, Beamish RE. β-adrenergic linked signal transduction mechanisms in failing hearts. Heart Fail Rev. 1997;2:55–65. [Google Scholar]
- 2.Gheorghiade M, Benatar D, Konstam MA, Stoukides CA, Bonow RO. Pharmacotherapy for systolic dysfunction: A review of randomized clinical trials. Am J Cardiol. 1997;80:14–27. doi: 10.1016/s0002-9149(97)00816-3. [DOI] [PubMed] [Google Scholar]
- 3.Frishman WH. Beta-adrenergic blockers: A 50-year historical perspective. Am J Therap. 2008;15:565–76. doi: 10.1097/MJT.0b013e318188bdca. [DOI] [PubMed] [Google Scholar]
- 4.Spann JF, Sonnenblick EH, Cooper T, Chidsey CA, Willman VL, Braunwald E. Cardiac norepinephrine stores and the contractile state of heart muscle. Circ Res. 1966;19:317–25. doi: 10.1161/01.res.19.2.317. [DOI] [PubMed] [Google Scholar]
- 5.Swedberg K, Viquerat C, Rouleau JL, et al. Comparison of myocardial catecholamine balance in chronic congestive heart failure and in angina pectoris without failure. Am J Cardiol. 1984;54:783–6. doi: 10.1016/s0002-9149(84)80208-8. [DOI] [PubMed] [Google Scholar]
- 6.Adameova A, Abdellatif Y, Dhalla NS. Role of excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can J Physiol Pharmacol. 2009;87:493–514. doi: 10.1139/y09-042. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol. 1997;80:26–40. doi: 10.1016/s0002-9149(97)00846-1. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the β2 subunit of L-type voltage-dependent calcium channels. Biochemistry. 1999;10:10361–70. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- 9.Castellano M, Bohm M. The cardiac β-adrenoceptor-mediated signaling pathway and its alterations in hypertensive heart disease. Hypertension. 1997;29:715–22. doi: 10.1161/01.hyp.29.3.715. [DOI] [PubMed] [Google Scholar]
- 10.Opie L. Receptors and signal transduction. In: Opie L, editor. The Heart: Physiology, from Cell to Circulation. Philadelphia: Lippincott, Williams and Wilkins; 2001. pp. 173–207. [Google Scholar]
- 11.Zhu WZ, Wang SQ, Chakir K, et al. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–25. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olofsson MH, Havelka AM, Brnjic S, Shoshan MC, Linder S. Charting calcium-regulated apoptosis pathways using chemical biology: Role of calmodulin kinase II. BMC Chem Biol. 2008;8:2. doi: 10.1186/1472-6769-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leicht M, Greipel N, Zimmer H. Comitogenic effect of catecholamines on rat cardiac fibroblasts in culture. Cardiovasc Res. 2000;48:274–84. doi: 10.1016/s0008-6363(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 14.Turner NA, Porter KE, Smith WHT, Ball SG, White HL, Balmforth AJ. Chronic β2 adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res. 2003;57:784–92. doi: 10.1016/s0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 15.Communal C, Colucci WS. The control of cardiomyocyte apoptosis via the beta-adrenergic signaling pathways. Arch Mal Coeur Vaiss. 2005;98:236–41. [PubMed] [Google Scholar]
- 16.Santos IN, Spadari-Bratfisch RC. Stress and cardiac beta adrenoceptors. Stress. 2006;9:69–84. doi: 10.1080/10253890600771858. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier C, Leblais V, Kobzik L, et al. The negative intropic effect of beta 3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102:1377–84. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartiani L, De Paoli P, Stillitano F, et al. Functional remodeling in post-myocardial infarcted rats: Focus on beta-adrenoceptor subtypes. J Mol Cell Cardiol. 2006;40:258–66. doi: 10.1016/j.yjmcc.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Mery PF, Lohmann SM, Walter U, Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci. 1991;88:1197–201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beavo JA. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol Rev. 1995;75:725–48. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 21.Birenbaum A, Tesse A, Loyer X, et al. Involvement of β3 adrenoceptor in altered β-adrenergic response in senescent heart: Role of nitric oxide synthase 1-derived nitric oxide. Anesthesiol. 2008;109:1045–53. doi: 10.1097/ALN.0b013e31818d7e5a. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman BB. Adrenoceptor antagonist drugs. In: Katzung BG, editor. Basic & Clinical Pharmacology. New York: Lange Medical Books/McGraw-Hill; 2001. pp. 138–54. [Google Scholar]
- 23.Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 24.Satwani S, Dec GW, Narula J. β-adrenergic blockers in heart failure: Review of mechanisms of action and clinical outcomes. J Cardiovasc Pharmacol Therapeut. 2004;9:243–55. doi: 10.1177/107424840400900404. [DOI] [PubMed] [Google Scholar]
- 25.Bristow MR, Ginsburg R, Umans V, et al. Beta-1 and beta-2 adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective beta-1 receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 26.Bristow MR. Changes in myocardial and vascular receptors in heart failure. J Am Coll Cardiol. 1993;22:61–71. doi: 10.1016/0735-1097(93)90465-d. [DOI] [PubMed] [Google Scholar]
- 27.Dhalla NS, Dixon IMC, Suzuki S, Kaneko M, Kobayashi A, Beamish RE. Changes in adrenergic receptors during the development of heart failure. Mol Cell Biochem. 1992;114:91–5. doi: 10.1007/BF00240302. [DOI] [PubMed] [Google Scholar]
- 28.Sethi R, Saini HK, Wang X, Elimban V, Babick A, Dhalla NS. Differential changes in beta-adrenoceptor signal transduction in left and right ventricles of infarcted rats. Can J Physiol Pharmacol. 2006;84:747–54. doi: 10.1139/y05-150. [DOI] [PubMed] [Google Scholar]
- 29.Sethi R, Saini HK, Guo X, Wang X, Elimban V, Dhalla NS. Dependence of changes in beta-adrenoceptor signal transduction on type and stage of cardiac hypertrophy. J Appl Physiol. 2007;102:978–84. doi: 10.1152/japplphysiol.00921.2006. [DOI] [PubMed] [Google Scholar]
- 30.Bohm M, Gierschik P, Pieske B, et al. Increase of Giα in human hearts with dilated but not ischemic cardiomyopathy. Circulation. 1990;82:1249–65. doi: 10.1161/01.cir.82.4.1249. [DOI] [PubMed] [Google Scholar]
- 31.Vatner DE, Vatner SF, Fujii AM, Homcy CJ. Loss of high affinity cardiac beta adrenergic receptors in dogs with heart failure. J Clin Invest. 1985;76:2259–64. doi: 10.1172/JCI112235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta-1 adrenergic receptor transgenic mice. Proc Natl Acad Sci. 1999;96:7059–64. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwase M, Bishop SP, Uechi M, et al. Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression. Circ Res. 1996;78:517–24. doi: 10.1161/01.res.78.4.517. [DOI] [PubMed] [Google Scholar]
- 34.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta-1 and beta-2 adrenergic receptors on cardiac myocyte apoptosis: Role of pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 35.Liggett SB, Teppe NM, Lorenz JN, et al. Early and delayed consequences of beta-2 adrenergic receptor overexpression in mouse hearts: Critical role for expression level. Circulation. 2000;101:1707–14. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 36.Graham LN, Smith PA, Stoker JB, Mackintosh AF, Mary ASG. Time course of sympathetic neural hyperactivity after uncomplicated myocardial infarction. Circulation. 2002;106:793–7. doi: 10.1161/01.cir.0000025610.14665.21. [DOI] [PubMed] [Google Scholar]
- 37.Maczewski M, Mackiewicz U. Effect of metoprolol and ivabradine on left ventricular remodeling and Ca2+ handling in post-infarction rat heart. Cardiovasc Res. 2008;79:42–51. doi: 10.1093/cvr/cvn057. [DOI] [PubMed] [Google Scholar]
- 38.Dzurba A, Ganguly PK, Guerin A, Dhalla NS. Alterations in the heart sarcolemmal Ca2+ transport activity by some beta-adrenergic antagonists. Basic Res Cardiol. 1984;79:620–6. doi: 10.1007/BF01908380. [DOI] [PubMed] [Google Scholar]
- 39.Dhalla NS, Temsah RM. Sarcoplasmic reticulum and cardiac oxidative stress: An emerging target for heart disease. Expert Opin Ther Targets. 2001;5:205–17. doi: 10.1517/14728222.5.2.205. [DOI] [PubMed] [Google Scholar]
- 40.Reiken S, Wehrens XHT, Vest JA, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–66. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 41.Doi M, Yano M, Kobayashi S, et al. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation. 2002;105:1374–9. doi: 10.1161/hc1102.105270. [DOI] [PubMed] [Google Scholar]
- 42.Zou C, Liu ZH, Jiang B, et al. Effects of metoprolol on cardiac function and myocyte calcium regulatory protein expressions in rabbits with experimental heart failure. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:476–9. [PubMed] [Google Scholar]
- 43.Sun YL, Hu SJ, Wang LH, Hu Y, Zhou JY. Effect of beta-blockers on cardiac function and calcium handling in postinfarction heart failure rats. Chest. 2005;128:1812–21. doi: 10.1378/chest.128.3.1812. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J, Niwa R, Kamiya K, Toyama J, Kodama I. Carvedilol blocks the repolarizing K+ currents and the L-type Ca2+ current in rabbit ventricular myocytes. Eur J Pharmacol. 1999;376:189–201. doi: 10.1016/s0014-2999(99)00368-4. [DOI] [PubMed] [Google Scholar]
- 45.Yao A, Kohmoto O, Oyama T, et al. Characteristic effects of alpha1-beta1,2-adrenergic blocking agent, carvedilol, on [Ca2+]i ventricular myocytes compared with those of timolol and atenolol. Circ J. 2003;67:83–90. doi: 10.1253/circj.67.83. [DOI] [PubMed] [Google Scholar]
- 46.Mochizuki M, Yano M, Oda T, et al. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J Am Coll Cardiol. 2007;49:1722–32. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 47.Sun YL, Hu SJ, Wang LH, Hu Y, Zhou JY. Comparison of low and high doses of carvedilol on restoration of cardiac function and calcium-handling proteins in rat failing heart. Clin Exp Pharmacol Physiol. 2005;32:553–60. doi: 10.1111/j.1440-1681.2005.04230.x. [DOI] [PubMed] [Google Scholar]
- 48.Koitabashi N, Arai M, Tomaru K, Nagai R, Kurabayashi M. Carvedilol effectively blocks oxidative stress-mediated downregulation of sarcoplasmic reticulum Ca2+-ATPase 2 gene transcription through modification of Sp1 binding. Biochem Biophys Res Commun. 2005;328:116–24. doi: 10.1016/j.bbrc.2004.12.139. [DOI] [PubMed] [Google Scholar]
- 49.Esler M. Measurement of sympathetic nervous system activity in heart failure: The role of norepinephrine kinetics. Heart Fail Rev. 2000;5:17–25. doi: 10.1023/A:1009889922985. [DOI] [PubMed] [Google Scholar]
- 50.Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–50. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 51.Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat endothelial cells via down-regulation of Bcl-2 and activation of beta-adrenergic and caspase-2 pathways. Cardiovasc Res. 2004;61:143–51. doi: 10.1016/j.cardiores.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Machackova J, Sanganalmath SK, Barta J, Dhalla KS, Dhalla NS. Amelioration of cardiac remodeling in congestive heart failure by β-adrenoceptor blockade is associated with depression in sympathetic activity. Cardiovasc Toxicol. 2010;10:9–16. doi: 10.1007/s12012-009-9058-y. [DOI] [PubMed] [Google Scholar]
- 53.Gabrielli O, Puyo AM, De Rosa A, Armando I, Barontini M, Levin G. Atenolol improves ventricular function without changing plasma noradrenaline but decreasing plasma atrial natriuretic factor in chronic heart failure. Auton Autacoid Pharmacol. 2002;22:261–8. doi: 10.1046/j.1474-8673.2002.00266.x. [DOI] [PubMed] [Google Scholar]
- 54.Newton GE, Parker JD. Acute effects of beta-1 selective and nonselective beta-adrenergic receptor blockade on cardiac sympathetic activity in congestive heart failure. Circulation. 1996;94:353–8. doi: 10.1161/01.cir.94.3.353. [DOI] [PubMed] [Google Scholar]
- 55.Azevedo ER, Kubo T, Mak S, et al. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: Differential effects on sympathetic activity. Circulation. 2001;104:2194–9. doi: 10.1161/hc4301.098282. [DOI] [PubMed] [Google Scholar]
- 56.Herman RB, Jesudason PJ, Mustafa AM, Husain R, Choy AM, Lang CC. Differential effects of carvedilol and atenolol on plasma noradrenaline during exercise in humans. Br J Clin Pharmacol. 2003;55:134–8. doi: 10.1046/j.1365-2125.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majewski H, McCulloch MW, Rand MJ, Story DF. Adrenaline activation of pre-junctional beta-adrenoceptors in guinea-pig atria. Br J Pharmacol. 1980;71:435–44. doi: 10.1111/j.1476-5381.1980.tb10956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 59.Xu XL, Zang WJ, Lu J, et al. Effects of carvedilol on M2 receptors and cholinesterase-positive nerves in adriamycin-induced rat failing heart. Auton Neurosci. 2006;130:6–16. doi: 10.1016/j.autneu.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Machackova J, Sanganalmath SK, Elimban V, Dhalla NS. Beta-adrenergic blockade attenuates cardiac dysfunction and myofibrillar remodeling in congestive heart failure. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01015.x. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brixius K, Lu R, Boelck B, et al. Chronic treatment with carvedilol improves Ca2+- dependent ATP consumption in triton X-skinned fiber preparations of human myocardium. J Pharmacol Exp Ther. 2007;322:222–7. doi: 10.1124/jpet.106.116798. [DOI] [PubMed] [Google Scholar]
- 62.Mak TI, Weglicki WB. Protection by beta-blocking agents against free radical-mediated sarcolemmal lipid peroxidation. Circulation Res. 1988;63:262–6. doi: 10.1161/01.res.63.1.262. [DOI] [PubMed] [Google Scholar]
- 63.Kramer JH, Mak T, Freedman AM, Weglicki WB. Propranolol reduces anoxia/reoxygenation-mediated injury of adult myocytes through an anti-radical mechanism. J Mol Cell Cardiol. 1991;23:1231–44. doi: 10.1016/0022-2828(91)90081-v. [DOI] [PubMed] [Google Scholar]
- 64.Romeo F, Li D, Shi M, Mehta JL. Carvedilol prevents epinephrine-induced apoptosis in human coronary artery endothelial cells: Modulation of Fas/Fas ligand and caspase-3 pathway. Cardiovasc Res. 2000;45:788–94. doi: 10.1016/s0008-6363(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 65.Zhuang XF, Yin CQ, Wang HY, Sun NL. Distinctive effects of carvedilol in the non-infarct zone: Remodeling of the ligated rat heart linked to oxidative stress. J Int Med Res. 2009;37:1354–64. doi: 10.1177/147323000903700510. [DOI] [PubMed] [Google Scholar]
- 66.Wang R, Miura T, Harada N, et al. Pleiotropic effects of the beta-adrenoceptor blocker carvedilol on calcium regulation during oxidative stress-induced apoptosis in cardiomyocytes. J Pharmacol Exp Ther. 2006;318:45–52. doi: 10.1124/jpet.105.099903. [DOI] [PubMed] [Google Scholar]
- 67.Schwarz ER, Kersting PH, Meven DA, et al. Cardioprotection by carvedilol: Antiapoptosis is independent of beta-adrenoceptor blockage in rat heart. J Cardiovasc Pharmacol Ther. 2003;3:207–15. doi: 10.1177/107424840300800306. [DOI] [PubMed] [Google Scholar]
- 68.Liu N, Yu R, Ruan Y, Zhou Q, Pu J, Li Y. Protective effect of carvedilol on abnormality of L-type calcium current induced by oxygen free radical in cardiomyocytes. J Huazhong Univ Technolog Med Sci. 2004;24:433–6. doi: 10.1007/BF02831101. [DOI] [PubMed] [Google Scholar]
- 69.Kametani R, Miura T, Harada N, et al. Carvedilol inhibits mitochondrial oxygen consumption and superoxide production during calcium overload in isolated heart mitochondria. Circ J. 2006;70:321–6. doi: 10.1253/circj.70.321. [DOI] [PubMed] [Google Scholar]
- 70.Lysko PG, Webb CL, Gu JL, Ohlstein EH, Ruffolo RR, Yue TL. A comparison of carvedilol and metoprolol antioxidant activities in vitro. J Cardiovasc Pharmacol. 2000;2:277–81. doi: 10.1097/00005344-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 71.Bartholomeu JB, Rolim NPL, Bechara LRG, et al. Intracellular mechanisms of specific beta-adrenoceptor antagonists involved in improved cardiac function and survival in a genetic model of heart failure. J Mol Cell Cardiol. 2008;45:240–9. doi: 10.1016/j.yjmcc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 72.Kawaki K, Qin F, Shite J, Mao W, Fukuoka S, Liang S. Importance of antioxidant and antiapoptotic effects of beta-receptor blockers in heart failure therapy. Am J Physiol Heart Circ Physiol. 2004;287:1003–12. doi: 10.1152/ajpheart.00797.2003. [DOI] [PubMed] [Google Scholar]
- 73.Bohm M, Flesch M, Knorr A, et al. Beta-adrenergic signal transduction following carvedilol treatment in hypertensive cardiac hypertrophy. Cardiovasc Res. 1998;1:146–55. doi: 10.1016/s0008-6363(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 74.Fu GS, Huang H, Chen F, et al. Carvedilol ameliorates endothelial dysfunction in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2007;567:223–30. doi: 10.1016/j.ejphar.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 75.Garlichs CD, Zhang H, Mugge A, Daniel WG. Beta-blockers reduce the release and synthesis of endothelin-1 in human endothelial cells. Eur J Clin Invest. 1999;29:12–6. doi: 10.1046/j.1365-2362.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 76.Hanada K, Asari K, Saito M, Kawana J, Mita M, Ogata H. Comparison of pharmacodynamics between carvedilol and metoprolol in rats with isoproterenol-induced cardiac hypertrophy: Effects of carvedilol enantiomers. Eur J Pharmacol. 2008;589:194–200. doi: 10.1016/j.ejphar.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Xue Q, Ma J, et al. Effects of imidapril on heterogeneity of action potential and calcium current of ventricular myocytes in infracted rabbits. Acta Pharmacol Sin. 2004;25:1458–63. [PubMed] [Google Scholar]
- 78.Zhong JH, Chen XP, Yun ML, Li WJ, Chen YF, Yao Z. Low-dose carvedilol reduces transmural heterogeneity of ventricular repolarization in congestive heart failure. Acta Pharmacol Sin. 2007;28:1161–5. doi: 10.1111/j.1745-7254.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhang JL, Lu JK, Chen D, et al. Myocardial autophagy variation during acute myocardial infarction in rats: The effects of carvedilol. Chin Med J. 2009;122:2372–9. [PubMed] [Google Scholar]
- 80.Zhao Q, Wu TG, Jiang ZF, Chen GW, Lin Y, Wang LX. Effect of beta-blockers on beta-3-adrenoceptor expression in chronic heart failure. Cardiovasc Drugs Ther. 2007;21:85–90. doi: 10.1007/s10557-007-6016-4. [DOI] [PubMed] [Google Scholar]
- 81.Sabbah HN, Sharov VG, Gupta RC, Todor A, Singh V, Goldstein S. Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis in dogs with heart failure. J Am Coll Cardiol. 2000;36:1698–705. doi: 10.1016/s0735-1097(00)00913-x. [DOI] [PubMed] [Google Scholar]
- 82.Prabhu SD, Wang G, Luo J, Gu Y, Ping P, Chandrasekar B. Beta-adrenergic receptor blockade modulates Bcl-X(S) expression and reduces apoptosis in failing myocardium. J Mol Cell Cardiol. 2003;35:483–93. doi: 10.1016/s0022-2828(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 83.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–90. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 84.Litwin SE, Katz SE, Morgan JP, Douglas PS. Effects of propranolol treatment on left ventricular function and intracellular calcium regulation in rats with postinfarction heart failure. Br J Pharmacol. 1999;127:1671–9. doi: 10.1038/sj.bjp.0702701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mansuy P, Mougenot N, Ramirez-Gil JF, et al. Effects of prolonged propranolol treatment on left ventricular remodeling and oxidative stress after myocardial infarction in rats. J Cardiovasc Pharmacol. 2000;35:806–13. doi: 10.1097/00005344-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 86.Gourine A, Bondar SI, Spyer KM, Gourine AV. Beneficial effect of the central nervous system beta-adrenoceptor blockade on the failing heart. Circ Res. 2008;102:633–6. doi: 10.1161/CIRCRESAHA.107.165183. [DOI] [PubMed] [Google Scholar]
- 87.Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. Beta-adrenergic blockade in developing heart failure: Effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000;101:2103–9. doi: 10.1161/01.cir.101.17.2103. [DOI] [PubMed] [Google Scholar]
- 88.Janssen PM, Zeitz O, Rahman A, Hasenfuss G. Protective role of nebivolol in hydroxyl radical induced injury. J Cardiovasc Pharmacol. 2001;38:17–23. doi: 10.1097/00005344-200112003-00004. [DOI] [PubMed] [Google Scholar]
- 89.Gilbert EM, Abraham WT, Olsen S, et al. Comparative hemodynamic, left ventricular functional and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94:2817–25. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- 90.Doggrell SA. Carvedilol versus other beta-blockers in heart failure. Expert Opin Invest Drugs. 2001;10:971–80. doi: 10.1517/13543784.10.5.971. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y, Tang Y, Ruan Y, et al. Comparison of metoprolol with low, middle and high doses of carvedilol in prevention of post-infarction left ventricular remodeling in rats. Jpn Heart J. 2003;44:979–88. doi: 10.1536/jhj.44.979. [DOI] [PubMed] [Google Scholar]
- 92.Wei S, Chow LT, Sanderson JE. Effect of carvedilol in comparison with metoprolol on myocardial infarction collagen postinfarction. J Am Coll Cardiol. 2000;36:276–81. doi: 10.1016/s0735-1097(00)00671-9. [DOI] [PubMed] [Google Scholar]
- 93.Omerovic E, Bollano E, Soussi B, Waagstein F. Selective beta1-blockade attenuates post-infarct remodeling without improvement in myocardial energy metabolism and function in rats with heart failure. Eur J Heart Fail. 2003;5:725–32. doi: 10.1016/s1388-9842(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 94.Zaca V, Rastogi S, Mishra S, et al. Atenolol is inferior to metoprolol in improving left ventricular function and preventing ventricular remodeling in dogs with heart failure. Cardiology. 2009;112:294–302. doi: 10.1159/000159123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morita H, Suzuki G, Mishima T, et al. Effects of long-term monotherapy with metoprolol CR/XL on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Cardiovasc Drugs Ther. 2002;16:443–9. doi: 10.1023/a:1022142620189. [DOI] [PubMed] [Google Scholar]
- 96.Terrovitis JV, Anastasiou-Nana MI, Nanas JN. Out-patient management of chronic heart failure. Expert Opin Pharmacother. 2005;6:1857–81. doi: 10.1517/14656566.6.11.1857. [DOI] [PubMed] [Google Scholar]
- 97.Heidenreich PA, Lee TT, Massie BM. Effect of beta-blockade on mortality in patients with heart failure: A meta-analysis of randomized clinical trials. J Am Coll Cardiol. 1997;30:27–34. doi: 10.1016/s0735-1097(97)00104-6. [DOI] [PubMed] [Google Scholar]
- 98.Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of beta-adrenergic blockade in chronic heart failure. A meta-analysis of double-blind, placebo-controlled, randomized trial. Circulation. 1998;98:1184–91. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- 99.Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med. 2001;134:550–60. doi: 10.7326/0003-4819-134-7-200104030-00008. [DOI] [PubMed] [Google Scholar]
- 100.Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154–61. doi: 10.1016/0735-1097(94)00543-y. [DOI] [PubMed] [Google Scholar]
- 101.MERIT-HF Study Group Effects of controlled-release metoprolol on mortality, hospitalizations, and well-being in patients with heart failure: The metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF) JAMA. 2008;283:1295–302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 102.Poole-Wilson PA, Swedberg K, Cleland JGF, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol European trial (COMET): Randomized controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 103.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Eng J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 104.The CAPRICORN Investigators Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomized trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 105.Rouleau JL, Roecker EB, Tendera M, et al. Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure. J Am Coll Cardiol. 2004;43:1423–9. doi: 10.1016/j.jacc.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 106.Celic V, Pencic B, Dekleva M, Dimkovic S, Kocijancic M. Metoprolol and atenolol in mild-to-moderate chronic heart failure: Comparative study. Srp Arh Celok Lek. 2005;133:242–7. doi: 10.2298/sarh0506242c. [DOI] [PubMed] [Google Scholar]
- 107.CIBIS-II group The cardiac insufficiency bisoprolol study II (CIBIS-II): A randomized trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 108.Flather MD, Shibata MC, Coats AJ, et al. Randomization trial to determine the effect of nebivolol on mortality and cardiovascular hospital admissions in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 109.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–9. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]
- 110.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of beta-adrenergic antagonists (Atenolol, Metoprolol tartrate, Carvedilol) on risk of rehospitalization in adults with heart failure. Am J Cardiol. 2007;100:690–6. doi: 10.1016/j.amjcard.2007.03.084. [DOI] [PubMed] [Google Scholar]
- 111.Simon T, Mary-Krause M, Funck-Brentano C, Lechat P, Jaillon P. Bisoprolol dose-response relationship in patients with congestive heart failure: A subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS II) Eur Heart J. 2003;24:552–9. doi: 10.1016/s0195-668x(02)00743-1. [DOI] [PubMed] [Google Scholar]
- 112.Dobre D, van Veldhuisen DJ, Goulder MA, Krum H, Willenheimer R. Clinical effects of initial six months monotherapy with bisoprolol versus enalapril in the treatment of patients with mild to moderate chronic heart failure. Data from the CIBIS III trial. Cardiovasc Drugs Ther. 2008;22:399–405. doi: 10.1007/s10557-008-6116-9. [DOI] [PubMed] [Google Scholar]
- 113.Ritter JM. Nebivolol: Endothelium-mediating vasodilating effect. J Cardiovasc Pharmacol. 2001;38:13–6. doi: 10.1097/00005344-200112003-00003. [DOI] [PubMed] [Google Scholar]
- 114.Maffei A, Di Pardo A, Carangi R, et al. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension. 2007;50:652–6. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]