Abstract
The present study was undertaken to characterize and review the changes in energy metabolism in rat myocardium in response to chronic exhaustive exercise. It was shown that a treadmill exercise program applied for six weeks led the rats into a state characterized by decreased performance, loss of body weight and enhanced muscle catabolism, indicating development of overtraining syndrome. Electron microscopy revealed disintegration of the cardiomyocyte structure, cellular swelling and appearance of peroxisomes. Respirometric assessment of mitochondria in saponin-permeabilized cells in situ revealed a decreased rate of oxidative phosphorylation (OXPHOS) due to diminished control over it by ADP and impaired functional coupling of adenylate kinase to OXPHOS. In parallel, reduced tissue content of cytochrome c was observed, which could limit the maximal rate of OXPHOS. The results are discussed with respect to relationships between the volume of work and corresponding energy metabolism. It is concluded that overtraining syndrome is not restricted to skeletal muscle but can affect cardiac muscle as well.
Keywords: Mitochondria, Overtraining, Oxidative phosphorylation, Rat myocardium
It is generally acknowledged that regularly performed endurance exercise has many health benefits including improvement of skeletal and cardiac muscle function in normal and diseased states. The underlying mechanisms include increased protein synthesis leading to muscle hypertrophy, altered isoenzyme profile of contractile proteins, and stimulation of biogenesis and functional parameters of mitochondria (1–12). However, exercising is not always favourable; it can also damage muscle cells. According to the contemporary concept, any successful training program aimed at increasing physical performance should achieve supercompensation, ie, development of increased muscle function following a single exercise stimulus (bout). For this purpose, the intensity of the exercise bout must increase stepwise and the periods of rest between the bouts must be sufficiently long to ensure regeneration of the muscle functions but short enough not to cause regression of supercompensation (13). Thus, every advantageous training program comprises a component of repetitive overloading, which in the case of lack of recovery may produce undesired effects such as the absence of performance improvement, chronic fatigue and muscle damage due to the destruction of myofibrils and atrophy of muscle fibres – all of which result in overtraining syndrome (14). In fact, these unfavourable changes take place rather frequently and explain why many athletes, although undergoing high-volume training, do not improve their performance. It has been suggested that training volume, rather than intensity, may be the major factor contributing to the development of overtraining syndrome (15). Thus, the main challenge is to select the optimal regime for exercising in terms of volume, intensity and periods between exercise to avoid damaging effects in muscle.
Studies in skeletal muscles have suggested a potential role of mitochondrial impairment in outcomes of overtraining. Acute exhaustive exercise (EE) stimulates generation of reactive oxygen species (ROS), which damage muscle cells, being side products of oxidative phosphorylation (OXPHOS) due to univalent reduction of molecular oxygen by electrons leaking from the respiratory chain (16–19). Mitochondria are the source and targets of ROS because complexes I and III of the electron transport chain are the main sites of mitochondrial superoxide production (20,21); ROS, in turn, induce damage of the respiratory chain (22). The magnitude of changes in exercise-induced ROS likely depends on the muscle type (23). Mitochondrial impairment is strongly linked to the development of cellular Ca2+ overload, which, besides increased ROS, is another condition underlying exercise-induced muscle fibre injury. Suppressed function of Ca2+ ATPase of the sarcoplasmic reticulum (SR) or sarcolemma compromises the removal of Ca2+ and thereby elevates the cytosolic Ca2+ concentration. This process eventually activates degradation of structural and contractile proteins and membrane phospholipids via Ca2+- dependent proteolytic and phospholipolytic pathways (24). At the same time, because Ca2+ overload results in uptake of Ca2+ into mitochondria (25), excessive accumulation of that ion in the matrix reduces mitochondrial capacity to synthesize ATP and causes direct damage of mitochondrial membranes.
The data regarding the effects of exercise on myocardial performance and mitochondrial structure and function are scarce and controversial. Early studies have revealed that a single bout of rigorous exercise leads to mitochondrial swelling and cristae disruption in cardiomyocytes (26). However, Ji and Mitchell (27) reported an increase in state 3 respiration in cardiac mitochondria after an acute (single) bout of maximal exercise. Repeated bouts of rigorous exercise (training) are known to depress state 3 and 4 respiration rates and increase mitochondrial susceptibility to oxidative stress (28). Terblanche et al (29) found that endurance training combined with an EE bout led to reduction in palmitoylcarnitine oxidation in rat heart tissue. In contrast, according to Kemi et al (30), aerobic exercise training exerted no effect on mitochondrial biogenesis and mitochondrial enzyme activities in normal rat heart tissue. Yet another group of studies documented positive effects of training on mitochondria. Among those studies, Sun et al (31) reported that adaptation of rat heart tissue to high-intensity swimming training is associated with upregulation of proteins participating in mitochondrial OXPHOS. Bo et al (32) reported increases in mitochondrial ATP synthase activity, P/O ratio (the ratio of added ADP to the amount of oxygen used in the process of complete phosphorylation of ADP), respiratory control ratio and manganese superoxide dismutase activity after treadmill training. The latter results concurred with data reported by Ascensão et al (33), which showed increased state 3 respiration in trained heart mitochondria. Furthermore, a concept has been put forward that training through regular exercise can induce a specific cardioprotective phenotype that enables mitochondria to resist anoxia/reoxygenization impairment (33) and apoptotic stimuli (34,35), or restore the aerobic capacity and energy transfer system in failing heart tissue (30). However, these data of beneficial effects of training are opposed by reports of repeated exhaustive training suppressing the mitochondrial capacity to accumulate Ca2+ (36) and exercise training accelerating EE-induced apoptosis in the left ventricle (37).
The reasons for conflicting data regarding the effects of exercise on cardiac mitochondria are unclear. Differences in type (eg, swimming versus treadmill running), volume and regularity of training (single exercise bout versus repeated exercises) may play a role. Unfortunately, the exercise protocols published so far seldom report the volume of work performed, which makes comparison of training programs difficult in terms of their influence on muscle and the organism as a whole. An important limitation of many studies is also that the function of mitochondria was assessed after their isolation from the muscle. The isolation procedure disrupts normal intracellular interactions of mitochondria with surrounding structures and may disintegrate the outer membrane. Another problem is that the mitochondrial preparation isolated is not representive of the whole cellular pool of organelles. Last but not least, assessment of isolated mitochondria provides no cue regarding the functional interaction of mitochondria and ATPases via specialized energy transfer pathways. These problems can be avoided by applying techniques that enable assessment of relationships of mitochondria with other structures in situ in permeabilized cells. By using this approach, it was established that in correspondence with muscle type-specific localization of mitochondria, regulation of mitochondrial function by ADP also differs strongly in vitro and in situ, depending on muscle type-specific peculiarities. It has been shown that, in cardiac cells, the sarcomeric and SR ATPases form functional complexes with adjacent mitochondria termed intracellular energy units (ICEUs) (38,39). Within the ICEUs, the energy produced by mitochondria is transferred to CaMgATPases mainly by creatine kinase (CK) and adenylate kinase (AK) (38,39). It has been shown that the function of ICEUs is strongly influenced by myocardial contraction, probably because the structural changes of myofibrils are transmitted to mitochondria, thereby modifying localized restrictions of the diffusion of adenine nucleotides at the outer membrane level (40). Also, it has been suggested that the ICEUs may serve as targets in many disorders (41–45), with a major conclusion that all conditions that affect the structure of cardiac cells may be associated with impairment of ICEUs.
The present study was undertaken to characterize and review energy metabolism in the cardiac cells of rats, under the conditions of a precisely defined overtraining exercise program, with special attention paid to in situ changes in mitochondrial localization, parameters of OXPHOS, function of the respiratory chain, coupling of mitochondrial kinases to OXPHOS and activities of enzymes participating in the intracellular energy transfer.
METHODS
Chemicals
The reduced forms of NADH, pyruvate and saponin were purchased from SERVA Electrophoresis GMBH (Germany); potassium phosphate (K2HPO4 and KH2PO4) was purchased from Calbiochem (USA); and all other reagents were purchased from Sigma-Aldrich (USA).
Animal model of exercise training
Animal experiments were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 80-23, revised 1978). The experiments were approved by the Ethical Committee for the Use of Animals in Research of the University of Tartu (Tartu, Estonia).
Male Wistar rats from the National Laboratory Animal Centre of Kuopio (Finland) were used in the present experiment. All the animals were housed under identical environmental conditions in polycarbonate type III cages at 21°C, two rats per cage, on a 12 h light/dark cycle. They received food (SDS-RM1 [C] 3/8, Witham, UK) and water ad libitum. Rats were weighed at the beginning and end of the experimental period. The rats (initial group) were randomly distributed between two groups – control rats and overtrained rats. For the latter group, a single animal model was applied to induce chronic physical exhaustion (overtraining) by treadmill running for six weeks. After a brief five-day acclimatization period that consisted of treadmill running for 5 min to 10 min, rats were subjected to running on a horizontal treadmill at a speed of 35 m/min seven days per week. The training volume was stepwise increased, so that the overall exercise time reached 2 h 20 min per day in the fourth week. When animals were unable to maintain the running speed, the mean exercise time was reduced to 1 h 50 min per day in the fifth week and to 1 h 10 min per day in the sixth week. Work per week and physical working capacity (PWC) were estimated and expressed in kJ. PWC was estimated 24 h after the last training session (recovery period), in which the rats were subjected to running on the horizontal treadmill at 35 m/min until exhaustion. The volume of work done was calculated according to Kaasik et al (46). Of all the animals entering the training program, 33% were unable to maintain the exercise activity within the given workload; therefore, only 67% of animals finished the training program. The 24-week-old rats were anesthetized with ethyl ether and sacrificed 24 h after completion of the last exercise bout.
Estimation of 3-methylhistidine in urine
The urine 3-methylhistidine (3-MeHis) level was determined (according to reference 47) to monitor protein degradation (see Calculations). Minced muscle samples were homogenized in a buffer solution (pH 7.0) containing 50 mM KCl, 10 mM K2HPO4, 1 mM EGTA, 1 mM MgCl2 and 1 mM dithiothreitol (DTT). Total muscle protein was hydrolyzed in 6 M HCl for 20 h at 110°C in vacuum-sealed flasks. HCl was removed by evaporation, and the hydrolysate was dissolved in 0.2 M pyridine to achieve a concentration of 10 mg/mL to 20 mg/mL. The urine 3-MeHis level was estimated with high-performance liquid chromatography.
Measurements of mitochondrial respiration
Mitochondrial respiration was investigated in skinned muscle fibres in which the sarcolemma was made permeable by pretreatment with saponin according to a method described earlier (48). Rat hearts were cooled in solution A containing 1.9 mM CaK2EGTA, 8.1 mM K2EGTA (free Ca2+ concentration 0.1 μM), 9.5 mM MgCl2, 0.5 mM DTT, 50 mM K-2-(N-morpholino)-ethanesulfonate (K-MES), 20 mM imidazole, 20 mM taurine, 2.5 mM Na2ATP and 15 mM phosphocreatine at a pH of 7.1 adjusted to 4°C before they were cut into halves; muscle strips 3 mm to 4 mm long and 1 mm in diameter were then prepared from the endocardium of the left ventricle. Using needles, the fibres were separated from each other, leaving only small areas of contact, and transferred into vessels containing ice-cold solution A supplemented with 50 μg/mL saponin. These vessels with muscle fibres from the myocardium in saponin-containing solution A were incubated at a mild temperature while stirring for 30 min to permeabilize the sarcolemma. Saponin-permeabilized (skinned) fibres were then washed three times in solution B containing 2.77 mM CaK2EGTA, 7.23 mM K2EGTA, 1.38 mM MgCl2, 0.5 mM DTT, 100 mM K-MES, 20 mM imidazole, 20 mM taurine and 3 mM K2HPO4 at a pH of 7.1 at 25°C, supplemented with 5 mg/mL bovine serum albumin (BSA) and 5 mM pyruvate for 10 min to completely remove all metabolites, especially trace amounts of ADP. The rates of oxygen uptake were recorded by using a high-resolution respirometer (Oroboros Oxygraph, Paar KG, Austria) or oxygraph (Rank Brothers Ltd, UK) in solution B (pH 7.1) at 25°C containing respiratory substrates and 2 mg/mL of BSA.
A multiple substrate/inhibitor titration protocol was applied to study the substrate specificity and function of the mitochondrial respiratory chain (49). After the registration of mitochondrial respiration under nonphosphorylating conditions (basal respiration rate [V0]) in the presence of 10 mM pyruvate and 2 mM malate in the oxygraph chamber (volume 2.5 mL), 2 mM ADP was added to monitor the maximum rate of NADH-linked ADP-dependent respiration; subsequently, complex I was inhibited by 10 μM of rotenone. Next, the following additions were made: 10 mM succinate to activate FADH2-linked ADP-dependent respiration, 0.1 mM atractyloside to assess respiratory control by adenine nucleotide translocase (ANT), 10 μM antimycin A to inhibit the electron flow from the complex II to cytochrome c, 0.5 mM tetramethylphenylene diamine (TMPD) with 2 mM ascorbate to activate cytochrome oxidase (COX), and 5 mM sodium azide (NaN3) to quantify COX activity as the NaN3-sensitive portion of respiration. Antimycin-sensitive respiration in the presence of atractyloside was considered to measure the excess respiration compensating the back flow of protons into the matrix (proton leak).
Coupling between OXPHOS and mitochondrial CK was estimated as follows. After the registration of basal respiration rate in skinned muscle fibres in the oxygraph chamber in 2.0 mL solution B supplemented with 10 mM pyruvate and 2 mM malate, activation of respiration by 0.1 mM ADP was recorded. Then, 20 mM creatine was added to couple the mitochondrial CK to ANT; the efficiency of coupling was expressed as the CK index (ICK) (see Calculations). Finally, 2 mM ADP was added to maximally stimulate OXPHOS. To assess coupling between OXPHOS and mitochondrial AK, after registration of basal respiration, 50 μM ATP was added to achieve the limited stimulation of mitochondria with endogenously produced ADP. The coupled reaction between mitochondrial AK (ie, AK2 isoform) and ANT was activated by 2 mM of AMP; coupling of mitochondrial AK to respiration was quantified as the AK index (IAK) (see Calculations). Finally, 2 mM ADP was added to maximally stimulate OXPHOS.
Measurement of cytochrome content
Cardiac tissues frozen at −70°C were thawed at 4°C and rapidly disrupted in 0.4 mL ice-cold 100 mM KH2PO4 buffer (pH 7.2) by an Ultra-Turrax T25 homogenizer (Janke and Kunkel, Germany) at medium speed for 30 s. Insoluble material was removed by centrifugation at 2500 g for 10 min. The supernatants obtained were centrifuged at 15,000 g for 30 min to remove myoglobin; supernatants were discarded and the sediments were rehomogenized in 0.5 mL of 100 mM KH2PO4 buffer (pH 7.2). To record the cytochrome spectra, the mitochondria in homogenate were solubilized by the addition of 5% sodium deoxycholate into the cuvette (0.3% volume/volume), then oxidized with potassium ferricyanide followed by reduction with sodium dithionite in the same cuvette (50,51). The absorbance spectra of cytochromes were recorded by scanning the samples at 535 nm to 630 nm using a dual-beam spectrophotometer (Lambda 900, ultraviolet/visible/near-infrared spectrum, PerkinElmer, USA). The cytochrome aa3 content was calculated from the difference spectrum (reduced spectrum minus oxidized spectrum) at the maximum absorption value in the range of 605 nm normalized by the absorbance of the isosbestic point at 630 nm. The cytochrome c content was determined at the maximum absorption value of 550 nm normalized by the absorbance of the isosbestic point at 540 nm. The cytochrome b content was calculated at the maximum absorption value of 562 nm normalized by the absorbance of the isosbestic point at 577 nm using the relevant extinction coefficient values (50,51).
Determination of total activities of ATPase, AK and CK
The cardiac tissue stored at −70°C was thawed at 0°C and homogenized in a solution containing 1 mM EGTA, 1 mM DTT, 5 mM MgCl2, 5 mM HEPES and 1% Triton X-100 (1:20 weight/volume) at a pH of 8.7 by an Ultra-Turrax T25 homogenizer (13,500 rpm) on ice for 30 s, followed by a 1 min period of standing on ice. This cycle of homogenization and standing was repeated twice and the homogenates were left on ice for 1 h for complete extraction of the enzymes. Activities of ATPases and AK in homogenates were measured with a Lambda 900 ultraviolet/visible/near-infrared spectrophotometer in stirring conditions in a cuvette, thermostated by a Peltier-type control unit using the coupled enzyme system of pyruvate kinase and lactate dehydrogenase. NADH oxidation was measured at 340 nm in a medium containing 20 mM Tris (pH 8.0, adjusted by 5 M HCl at 30°C), KCl 15 mM KCl, 0.3 mM DTT, 0.24 mM NADH, 0.8 mM phosphoenolpyruvate, 6 IU/mL pyruvate kinase and 3 IU/mL lactate dehydrogenase. After registration of ATPase activity in the presence of 1 mM ATP (supplemented with 0.8 mM MgCl2), 1.3 mM AMP was added to determine the AK activity as the change in NADH oxidation rate at 340 nm. Total CK activity was assayed using the coupled enzyme system of glucose-6-phosphate dehydrogenase and hexokinase by measuring NADPH production at 340 nm in a Tris-buffered medium containing 20 mM glucose, 20 mM AMP, 0.3 mM DTT, 3 mM Mg-acetate, 1 mM NADP+, 1 mM ADP, 50 mM Tris (pH 7.4), adjusted by 5 M HCl at 25°C in the presence of 2 IU/mL hexokinase and 2 IU/mL glucose-6-phosphate dehydrogenase. After addition of the aliquot (3 μL of diluted homogenate [2 μg to 3 μg protein]) and registration of the baseline activity, the CK reaction was started with 20 mM phosphocreatine and monitored as the increase in NADPH formation at 340 nm. Care was taken that the reactions measured occurred linearly in time and that the coupled enzyme systems were not limiting the overall reaction rate.
Electron microscopy
Myocardial specimens were fixed in 0.25% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and postfixed in 2% OsO4 in the same buffer. After dehydration with ethanol and acetone, the specimens were embedded in Epon 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined using a Tecnai 10 electron microscope (Philips, FEI Company, The Netherlands) at 100 kV.
Protein assay
The protein concentration was assayed according to Bradford (52) using BSA as a standard.
Calculations
The degradation rate of muscle protein was calculated as follows: 3-MeHis excretion (μmol/day × 0.75 × 100) divided by the total muscle protein (g) and expressed as percentage per day.
To estimate coupling of phosphorylation to oxidation, the respiratory control index (RCI) was calculated as the ratio of maximal ADP-activated respiration rate (VADP 2 mM) to the basal respiration rate (V0).
To assess the tightness of functional coupling between mitochondrial AK and ANT, the relative index of activation of respiration by 2 mM AMP (IAK) was calculated as IAK = (VAMP − VATP 0.05 mM)/VATP 0.05 mM, where VAMP is the AMP-activated respiration rate and VATP 0.05 mM is the ATP-activated respiration rate.
To assess the tightness of functional coupling between mitochondrial AK and ANT, the relative index of activation of respiration by 20 mM of creatine (ICK) was calculated as ICK = (VCr − VADP 0.1 mM)/VADP 0.1 mM, where VCr is the creatine-activated respiration rate and VADP 0.1 mM is the ADP-activated respiration rate.
Training volume (A) was calculated as A = m(V/t × 60 + 9.81)s, where m is body weight (kg), V is running speed (m/min), t is running time and s is running distance (m).
Statistical analysis
The data were expressed as arithmetic means with SEMs. Differences between groups were determined using Student’s t test or ANOVA where appropriate.
RESULTS AND DISCUSSION
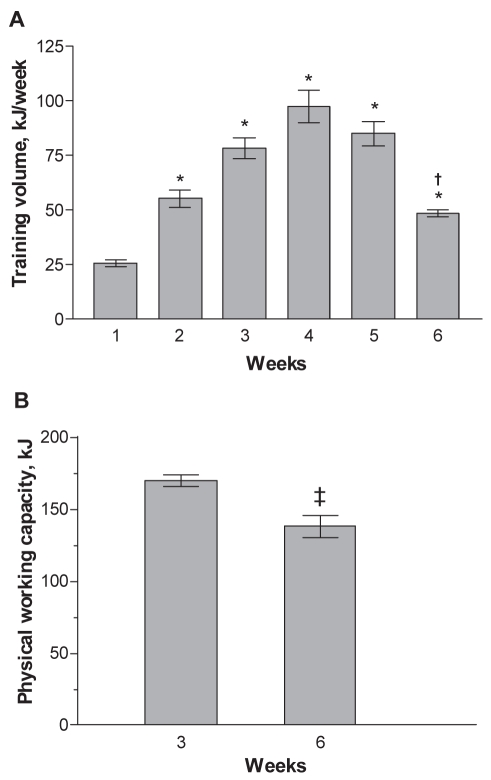
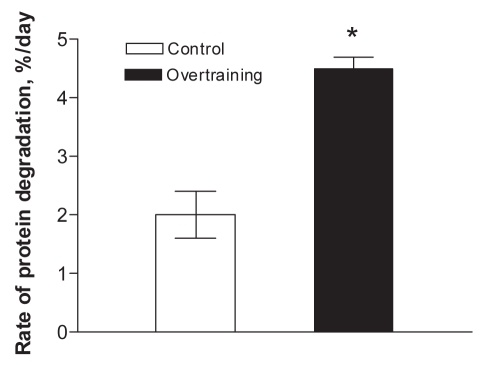
Figure 1A shows that, during the first four weeks, the training volume was stepwise increased from 26 kJ to 97 KJ. However, the rats were unable to maintain that training volume for a longer period. Therefore, during the fifth and sixth weeks, it was gradually decreased by reducing the running duration (see Methods). The PWC was found to be the highest on the third week, but decreased markedly by the sixth week (Figure 1B). This change indicated that the applied exercise protocol caused overtraining because it resulted in decreased performance ability due to fatigue and exhaustion. In a previous study (13), a similar exercise protocol elicited a marked decrease in the weight of slow-twitch oxidative (m soleus) and fast-twitch glycolytic (m plantaris and m extensor digitorum longus) muscles, parallel with decreased body weight, increased proportion of pooled excreted urinary 3-MeHis and reduced rate of myosin heavy chain (MHC) synthesis. It was concluded that overtraining led to muscle atrophy due to a prevalence of muscle catabolism over synthesis of contractile proteins, causing underlying loss of body mass of rats (13). In the present study, a marked decrease in body weight of overtrained rats was also observed, along with increased excreted 3-MeHis levels in the overtrained group compared with the control group at six-week follow-up (Table 1 and Figure 2).
Figure 1).
A Dynamics of weekly training volume expressed in kJ per week (n=8 rats). *P<0.001 compared with week 1; †P<0.001 compared with week 4. B Physical working capacity measured at a speed of 35 m/min on a horizontal treadmill and expressed in kJ (n=8 rats). ‡P<0.05 compared with control group
TABLE 1.
The effect of overtraining on heart weight (HW) and body weight (BW) of rats (n=4)
| HW, mg | BW, g | HW/BW | |
|---|---|---|---|
| Initial | 917±51 | 356±3 | 2.6±0.2 |
| Control | 1014±61 | 431±10‡ | 2.3±0.1 |
| Overtraining | 1115±20* | 309±13*¶ | 3.6±0.2†§ |
Data are presented as mean ± SEM.
P<0.05;
P<0.01;
P<0.001, statistically significant difference compared with the corresponding initial group before the training program (see the Methods section);
P<0.01;
P<0.001, statistically significant difference between controls and overtrained rats 24 h after the last exercise bout
Figure 2).
The effect of overtraining on the daily proportion of pooled excreted urinary 3-methylhistidine levels (n=8 rats). *P<0.001 compared with the control group
However, the heart weight of overtrained rats did not change compared with the control group, although it increased compared with rats initially assigned to the experiments, giving rise to an increased heart-to-body weight ratio. This finding indicates that, in contrast to skeletal muscles, the anabolic response prevails in cardiac muscle under conditions of overtraining exercise.
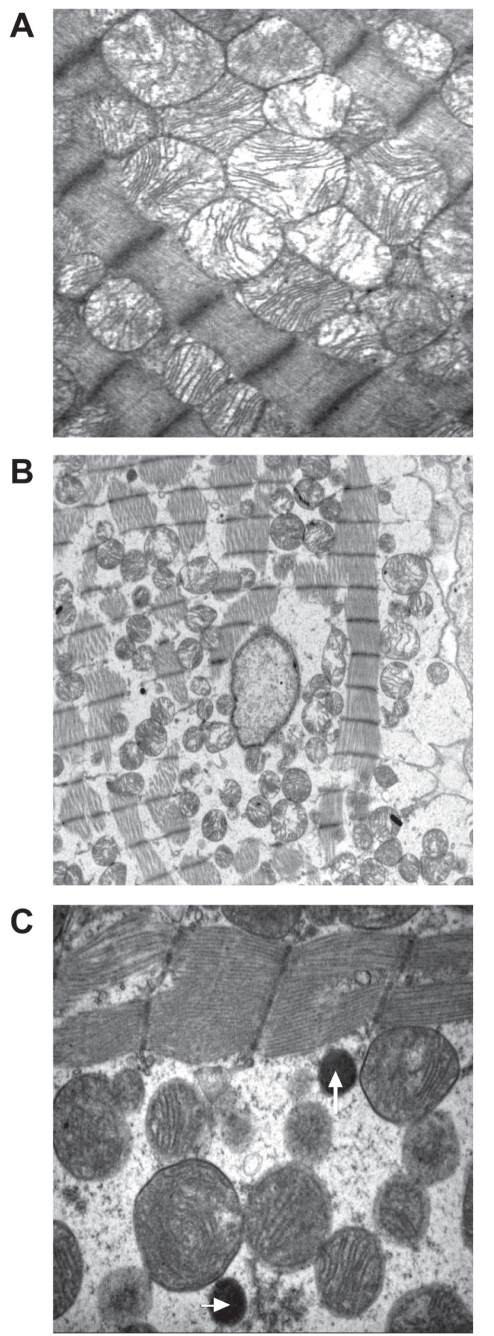
In normal heart tissue, morphological studies (53–56) revealed the remarkably precise intracellular organization of mitochondria in cardiac cells. In particular, the intermyofibrillar mitochondria are precisely positioned so they are close to the adjacent sarcomere (54–56). In the present study, electron microscopy showed that mitochondria are tightly packed between the myofibrils and exhibit intact outer and inner membranes, with distinct cristae in the control myocardium (Figure 3A). In contrast, the cardiomyocytes in the overtrained heart tissue (Figure 3B) were swollen, as indicated by wavy myofibrils and enlarged space between the cells. In some cases, the myofibrils seemed to be disrupted as well. The mitochondria appeared intact but had moved apart from the myofibrils (Figures 3B and 3C). This implied that the cytoskeletal system organizing the mitochondria relative to the myofibrils (55,57) may have disintegrated. Interestingly, among the mitochondria, rounded electron-dense dark bodies (Figure 3C) were revealed, which were not detected in the control preparations (Figure 3A). Based on several arguments, it is likely that these structures represent peroxisomes (microbodies). First, peroxisomes are rare in normal cardiomyocytes, but proliferate in response to various chemical and physical stresses including chronic alcohol consumption, high-fat diet and exercise (58–62). Second, peroxisomes contain catalase in high activities, which indicates upregulation of that enzyme in response to superoxide production, a typical process in stressed cardiomyocytes. The relationship between catalase and peroxisomes is confirmed by the observation that transgenic overexpression of catalase in mice results in a markedly increased number of peroxisomes containing immunolocalized catalase compared with wild-type cardiomyocytes (63). The observation that the peroxisomes frequently localized in the vicinity of the mitochondria suggests that these structures may interact with mitochondria to control the levels of ROS in the cytoplasm (64,65). Third, the peroxisomes are smaller in size (0.2 μm to 0.5 μm in diameter) than mitochondria (62,65), which is also relevant to structures shown in Figure 3B.
Figure 3).
Electron micrographs of control (A) and overtrained (B and C) rat myocardium. Magnification: A, ×8900; B, ×3700; and C, ×15,000. White arrows indicate the presence of peroxisomes
The structural alterations observed (Figure 3) point to the possibility that overtraining affects OXPHOS and interaction of mitochondria with ATPases in overtrained cardiac cells. To test this, the total activities of ATPases, CK and AK were assessed in homogenates of the control and overtrained cardiac muscles. However, no differences between these groups were seen; the total activities of myocardial ATPases were 261±55 μmol/min and 229±21 μmol/min per g wet weight of tissue in the control and overtrained groups, respectively. In the experimental settings, the total ATPase represented the sum of many ATPase activities, predominantly those of the MHC, SR and sarcolemmal (Na+/K+) ATPases. Because the status of each specific ATPase activity in cardiac muscle was not analyzed, it is difficult to explain why the total ATPase remained unaltered in overtraining conditions. However, some literature data helped to answer this question. In a previous study (13), the relative content of MHC-I activity decreased in fast-twitch glycolytic muscles but not in slow-twitch oxidative muscles (m soleus) in overtraining conditions. Because the heart muscle belongs to the oxidative muscle class, its myosin could be insensitive to the influences of overtraining; this would explain the unaltered total ATPase activity. In support of this assumption, heavy training exerted no effect on actomyosin ATPase in mouse cardiac muscle (66). Regarding other ATPases, a logical assumption is that increased workload is supported by enhanced ion handling in cell membranes, eg, through the changes in Na+/K+ ATPase and SR Ca2+ ATPase. Indeed, training induced expression of Na+/K+ ATPase isoforms (67). However, this process depends on the type of exercise (67) and, considering the relatively low proportion of Na+/K+ ATPase, its contribution to overall ATPase activity in cell homogenate could be negligible, thus leaving total ATPase activity unaltered. In overtrained rats, it cannot be excluded that any of the ATPases could have transiently increased and decreased to the control level by the end of training when the recordings were taken.
It is well known that vigorous endurance exercise or regular training causes increased levels of CK in athlete’s blood, indicating muscle cell injury (68). Increased CK-MB activity or mass has also been frequently documented (69–75). The latter change has been attributed to adaptation of skeletal muscle to continuous cycles of degeneration and regeneration (76), which is supported by the findings that endurance training leads to increased CK-MB levels in m vastus lateralis, in correlation with enhanced citrate synthase activity (increased capacity of OXPHOS) (77), activated expression of CK-MB in rat gastrocnemius muscle (72), and increased CK activity and CK-MB content in canine ventricle tissue (78). In contrast, Chen et al (79) reported that swimming exercise induced a loss of myocardial CK-MB into the circulation in rats due to myocardial injury, whereas Miller et al (80) found no change in CK-MB in the myocardium of dogs undergoing exercise training. The latter authors concluded that “chronic exercise training does not induce physiologically important degenerative changes in myocardium”. Considering the morphological signs of impairment in the heart (Figure 3), this conclusion seemed not to be relevant to the present study. Therefore, the CK activities and isoenzyme profile were assessed in rat hearts. The results show that the total activities of CK were similar: 796±288 μmol/min and 725±97 μmol/min per g wet weight of tissue in the control and overtrained groups, respectively. In control hearts, the isoenzyme profile (percentage of total activity) was 2.4%, 12.7%, 50.7% and 34.2% for CK-BB, CK-MB, CK-MM and mitochondrial CK, respectively, whereas in overtrained hearts the distribution was 2.8%, 11.9%, 51.6% and 33.8%, respectively. Thus, the data agree with those reported by Miller et al (80) in that exercise overtraining exerted no effect on the CK isoenzyme profile. No change was found in the myocardial activities of AK (228±16 μmol/min and 217±15 μmol/min per g wet weight of tissue in the control and overtrained groups, respectively) after overtraining.
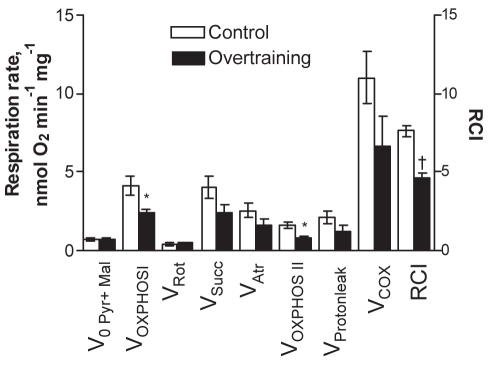
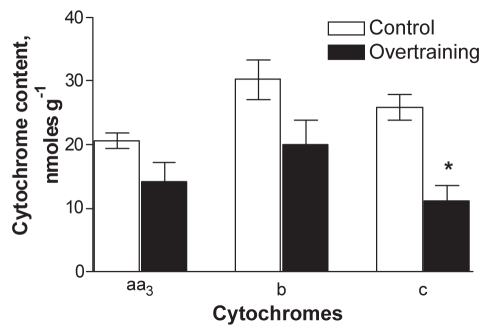
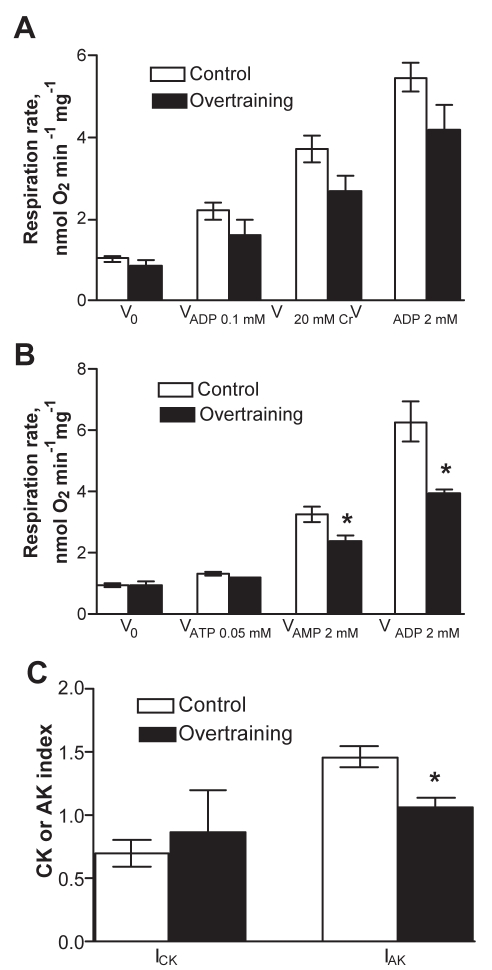
Next, the function of OXPHOS was studied in mitochondria in skinned cardiac fibres. At first, the function of different respiratory chain complexes was assessed by using a substrate/inhibitor titration protocol. The results (Figure 4) showed that the basal respiration rate (V0, in the presence of pyruvate plus malate but in the absence of ADP) was similar in the control and overtrained groups. Addition of ADP to the medium markedly stimulated the mitochondrial respiration in both groups. ADP-sensitive respiration (OXPHOS I), calculated as the difference between the respiration rates with and without ADP, was significantly lower in the overtrained group than in the control group. In addition, the RCI (ratio of respiration rates after and before ADP addition) was lower in the overtrained group (Figure 4). Thus, overtraining resulted in a reduced OXPHOS rate and attenuated control of that process by ADP in conditions of fueling of the respiratory chain through complex I. The activity of complex II-dependent respiration (state 3) was measured after successive additions of rotenone and succinate; it tended to be reduced in overtrained myocardium. The ratio of complex I- to complex II-dependent respiration was similar in both groups (ie, 1.21±0.12 and 1.36±0.29 in the control and overtrained groups, respectively), which suggests that the decreased OXPHOS rate in overtrained group was not due to specific inhibition of complex I of the respiratory chain. The subsequent dosage of atractyloside effectively inhibited respiration by blocking ANT, thus indicating preservation of the inner mitochondrial membrane in both groups. In the next phase, COX was maximally activated by combined additions of TMPD and ascorbate. Inhibition of respiration with NaN3 in these conditions enabled the calculation of COX activity, which tended to be lower in overtrained myocardium than in control myocardium. Parallel measurements of the cytochrome contents in the myocardial homogenates revealed decreased values for all cytochromes tested, with the decrease in the content of cytochrome c reaching statistical significance (Figure 5). The basal respiration values (V0) in Figures 4 and 6 did not decrease, and respiratory suppression became evident only in the presence of ADP, ie, in state 3 conditions. Moreover, it was found that overtraining resulted in a marked decrease in cytochrome c/aa3 ratio (from 1.26 in controls to 0.79 in overtrained hearts, P=0.004), which indicates the relative deficit of cytochrome c. One reason for that may be the mitochondrial loss of cytochrome c that occurs due to disruption of the outer membrane, a typical process in pathologically impaired cardiomyocytes, which also represents an initial step in the cascade of apoptotic reactions (81). Indeed, the observation that endurance training, although enhancing running performance, accelerated mitochondrial DNA deletion and apoptosis induced by superimposed EE (37), points to that mechanism. The leakiness of the outer mitochondrial membrane was tested by monitoring the effect of exogenously added cytochrome c on TMPD-dependent respiration. The results showed that cytochrome c caused variable but small increases in that parameter, without a significant difference between the control and overtrained groups. This observation could be taken to indicate that there was no overtraining-specific membrane leak and that detected permeability of the outer membrane for exogenous cytochrome c stemmed from alterations due to the preparation of the skinned fibres. However, King and Gollnick (26) observed the transitory nature of changes in mitochondrial structures (swelling with loss of cristae) because they were evident immediately after an EE bout but were reversed during the following 24 h of rest. Because the mitochondria were assessed 24 h after the last exercise bout, the absence of overtraining-specific leakiness can be attributed to restoration of the mitochondrial structure by the time of measurement. Another mechanism potentially changing the quantitative balance between the individual cytochromes may be altered mitochondrial biogenesis (see below). Whatever the reason for the relative deficit in cytochrome c, several arguments exist in favour of the assumption that it could play a specific role in controlling mitochondrial response to ADP. First, by titrating the respiratory flux with specific inhibitors in the same muscle specimen, it was frequently observed that the complex I-dependent respiration rate markedly exceeded (by 20%) the complex II-dependent rate (shown in the present study and reference 43). Second, Rossignol et al (82) showed that in heart tissue, complex I is the most important step controlling the oxygen consumption flux. This means that, in heart cells, OXPHOS is mainly controlled at the level of electron flux along the respiratory chain, makes the function of OXPHOS very sensitive to defects in the respiratory chain. Finally, Rasmussen and Rasmussen (83) showed that the values of the state 3 and respiratory control ratio (ratio of state 3 to state 4 respiration) in isolated muscle mitochondria linearly depended on the relative amount of cytochrome conservation, whereas such a relationship was not observed for state 4 respiration. Considering these data in the context of overtraining, it seems appropriate to assume that relative deficiency of cytochrome c could restrict the compensatory increase in the respiratory chain activity (respiration) in response to the consumption of proton motive force by ATP synthase that resulted in suppressed respiratory control by ADP (Figure 4). However, the reduced cytochrome c was still sufficient to maintain the low rate of state 2 respiration (V0) at equal levels in the control and overtrained groups (Figures 4 and 6).
Figure 4).
Effect of overtraining on mitochondrial respiration in situ in saponin-permeabilized fibres from rat myocardium in the presence of substrates and inhibitors specific to different complexes of the respiratory chain. V0 (Pyr+Mal) Basal respiration rate in the presence of 10 mM pyruvate and 2 mM malate; VAtr Atractyloside (Atr; 100 μM)-insensitive respiration rate; VCOX Sodium azide-sensitive portion of the respiration rate in the presence of tetramethylphenulene diamine and ascorbate; VOXPHOS I Rate of oxidative phosphorylation induced by the addition of 2 mM ADP (V0 subtracted); VOXPHOS II Rate of oxidative phosphorylation in the presence of succinate and 2 mM ADP (VOXPHOS II = VSucc − VAtr); VProton leak The difference between VAtr and respiration rate in the presence of 10 μM antimycin A; VRot Rate of complex I-dependent respiration after additon of 10 μM rotenone; VSucc Rate of complex II-dependent respiration induced by addition of 10 mM succinate (in the presence of ADP). Right ordinate: Respiratory control index (RCI) is the ratio of respiration rates after and before ADP addition, in the presence of 10 mM pyruvate and 2 mM malate. *P<0.05; †P<0.001, significantly different compared with the corresponding value in the control group (n=4)
Figure 5).
Tissue content of cytochromes in the myocardium of control and overtrained rats (n=4). *P<0.01, significantly different compared with the control group
Figure 6).
Assessment of functional coupling between mitochondrial creatine kinase (CK) (A) or mitochondrial adenylate kinase (AK) (B) and oxidative phosphorylation. Efficiency of coupling between CK or AK and the oxidative phosphorylation system was expressed as the CK index (ICK) or AK index (IAK), respectively (C). *P<0.05 significantly different compared with the control group. V0 Basal respiration rate; V20 mM Cr Creatine-activated respiration rate; VADP 0.01 mM 0.01 mM ADP-activated respiration rate; VADP 2 mM 2 mM ADP-activated respiration rate; VATP 0.05 mM ATP-activated respiration rate
The energy transfer between mitochondria and ATPase depends not only on the total activity of cellular CK or AK and their isoform distribution, but also on efficiency of coupling of kinases to OXPHOS and ATPases. Therefore, although the overall capacities of the CK- or AK-mediated energy transfer systems were not influenced by overtraining (as described above), the coupling of mitochondrial isoforms of CK and AK to ANT was tested. Figure 6A shows that for assessment of CK coupling, mitochondrial respiration was first submaximally stimulated by a small amount of ADP (0.1 mM). Thereafter, 20 mM of creatine was added. This resulted in a rapid increase in respiration because creatine switched on the functional coupling between mitochondrial CK and ANT, with local production of ADP in the CK reaction. The relative extent of that process (measured as ICK; Figure 6C) corresponded in control preparations to the extent of the process in normal rat heart tissue (48). Compared with controls, the ICK tended to decrease in overtrained cardiac specimens, but the change was insignificant. Further addition of saturating ADP stimulated the respiration maximally, due to increased diffusion of ADP from the medium (cytoplasm) to the mitochondrial intermembrane space. The extent of stimulatory effect of ADP was not statistically different between the two groups, although it again tended to be decreased in the overtrained group. To test the coupling of mitochondrial AK to ANT, the respiration was initially stimulated by the addition of a small amount of ATP (0.05 mM), which through activation of ATPases produced cytosolic ADP to stimulate OXPHOS (Figure 6B). (ADP addition was avoided in the present experiment to suppress its transformation to ATP and AMP via AK reactions.) The following addition of 2 mM AMP strongly stimulated respiration in both groups, indicating functional coupling of mitochondrial AK2 isoform with ANT, which provides local concentrations of ADP to stimulate OXPHOS (43). Interestingly, the overtrained group exhibited less stimulation of respiration (smaller IAK) than the control group, which points to decreased efficiency of functional coupling in the overtrained group. Also, the combined stimulatory effect of excess AMP and ADP was markedly lower in overtrained muscles than in control muscles. Altogether, these experiments suggested that overtraining impairs functional coupling between mitochondrial AK2 and ANT, and causes a decrease in the overall capacity of ADP-dependent OXPHOS, the latter finding confirming the results of the experiments shown in Figure 4.
Obviously, the results of the present study do not reconcile with the general concept that exercise stimulates mitochondrial biogenesis and improves their functional parameters (11,84). However, this controversy can be solved by considering that the mitochondrial adaptation to exercise highly depends on the exercise load – the latter, in turn, being a function of duration, intensity and frequency of the exercise bouts. Thus, based on current evidence, two entirely different mitochondrial responses can be outlined in relation to low/moderate exercise volume compared with EE volume. Regarding low/moderate exercise volume, it is remarkable that the mitochondrial mass in cardiac muscle increased very rapidly during a single exercise bout, so that 90 min to 120 min of exercise already led to a significant increase in individual mitochondrial area and the percentage of cytoplasm occupied by the mitochondria (54). Interestingly, the large mitochondria showed no structural impairments; no swelling or disruption of cristae was evident (54). King and Gollnick (26) suggested that if the volume of exercise increased until it caused exhaustion, mitochondrial impairment would follow. More systematically, the effect of exercise intensity was addressed recently by Pan (85), who assessed the effect of moderate-intensity exercise (ME), high-intensity exercise (HE) and EE (running on a treadmill) on volume density and numerical density of mitochondria in rat atrial myocardium. The results showed that while both ME and HE increased the volume density and numerical density of mitochondria above the control sedentary values, EE had no such effect. In parallel, the exercise volume dependency of expression of atrial natriuretic peptide, which plays an important role in regulating cardiac output, was clearly outlined: expression of atrial natriuretic peptide increased with ME and HE, but did not change after EE. These changes correlated with the morphological properties of the cells and mitochondria. Whereas in ME and HE conditions the mitochondria and other cellular structures appeared normal, in the EE condition damage of the cells became visible as shown by irregular sarcomeres, incomplete myofibrils, and swelling and flocculent mitochondria. Besides these data, Kavazis et al (34) reported that treadmill training resulted in increased levels of mitochondrial superoxide dismutases, catalase and glutathione peroxidase, along with increased levels of cytosolic heat shock protein 70 and apoptosis repressor with caspase recruitment domain and protection of mitochondria against apoptotic stimuli. The study by Sun et al (31), aimed at proteomic analysis, showed that eight-week swimming training resulted in marked upregulation of enzymes participating in OXPHOS. The endurance training load applied in the study by Kavazis et al (34) was markedly lower than the EE load used in the present study or in Pan’s study (85). In the study by Sun et al (31), the endurance training load was likely also lower because their training protocol caused absolute cardiac hypertrophy (heart weight increased in the training group over that in the sedentary control group). Altogether, these data show that, in cardiac cells, moderate-volume exercise causes increased biogenesis of mitochondria that leads to increased cellular capacity to produce ATP. This response represents an important adaptive change in cardiac muscle because it enables the cardiomyocytes to cope with increased workload and protect them from damaging effects of ROS and other death stimuli. The novel and interesting finding is that moderate-volume chronic exercise is capable of inducing a specific mitochondrial pathway of supercompensation (32). These authors showed that rats that trained according to the six-week endurance exercise protocol that induced cardiac hypertrophy and improved cardiac performance also exhibited increased ATPase synthase activity, indicating the enhanced capacity of OXPHOS to provide ATP for ATPases. More importantly, it was demonstrated that training induced a specific subset of changes that strongly modified the mitochondrial response to superimposed acute exercise bouts (AEBs) with increased intensity. The training-dependent altered response to AEBs was expressed in decreased state 4 respiration, increased ADP control over respiration, increased P/O ratio and decreased uncoupling protein-mediated respiration. Altogether, these alterations accentuate the increase in the mitochondrial efficiency in the biosynthesis of ATP. It was suggested that the underlying mechanism is likely based on modulation of the balance between mitochondrial production of ATP and ROS. It is known that ATP synthesis depends on mitochondrial membrane potential and that ROS formation depends on redox pressure, which can also be modulated via the mitochondrial membrane potential (86,87). In conditions of high ADP levels, the transmembrane proton gradient is mainly consumed for ATP synthesis, which strongly reduces ROS generation. Increased ROS levels, in turn, are known to activate uncoupling proteins, which are considered one of the powerful mechanisms for protection of mitochondria from harmful effects of superoxides (88). However, this mechanism is uneconomical because it requires decreasing the capacity of mitochondria to synthesize ATP – a high price to pay. Data reported by Bo et al (32) are important to understand how moderate-load endurance training helps to overcome this problem – it induces a more favourable pathway of protection because it stimulates expression of mitochondrial manganese superoxide dismutase and attenuates the expression of uncoupling protein 2, which enables, on one hand, better limiting of ROS production but, on the other hand, preserves the proton gradient for ATP synthesis during AEBs. Further support for the effectiveness of these mechanisms is provided by the observation that chronic ME indeed attenuates the indexes of ROS-induced stresses, as indicated by reduced ROS production, diminished thiobarbituric acid-reactive substances and protein carbonyls in cardiac mitochondria, and a negative correlation between the mitochondria protein carbonyls and COX activity (7,12,89). Thus, by virtue of the mechanisms described, the mitochondria acquire the capacity to cope with the potentially injuring stresses, but support the stresses that induce signalling systems aimed at promoting adaptation to and increased performance during the next exercise bout.
Apparently, all the beneficial mechanisms of training are lost in conditions of exhaustive high-volume exercise, which results in chronic fatigue, decreased exercise performance and disintegration of the muscle structure – all these being components of the overtraining syndrome (13,14,65,89–93). The observations that exhaustive endurance exercise applied in the present study led to altered cardiomyocyte structure and suppressed OXPHOS, in association with declining performance, strongly suggested that overtraining syndrome is not restricted to skeletal muscle but can also take place in cardiac muscle. Further studies are required to understand which mechanism is responsible for the transition from beneficial to harmful effects of exercise. One possible mechanism may be based on exercise volume-dependent alterations in the balance between fusion and fission of mitochondria. This is supported by the earlier observations that increased duration of swimming load led not only to increased mitochondrial mass but also to the appearance of a novel phenotype of mitochondria characterized by numerous invaginations, suggesting a replication phenomenon (54). Exercise was very recently shown to activate specific mechanisms of fragmentation of mitochondria by Ding et al (94), who demonstrated that an acute bout of treadmill exercise caused decreased expression of mitofusins 1 and 2 but increased expression of fission protein Fis1 genes in rat hind limb muscles. In parallel, the mitochondria functionally differed from their counterparts in control (resting) muscles because the former exhibited decreased RCI and increased state 4 respiration, not only immediately after exercise but also 24 h later (93). It would be interesting to address the role of alterations in the balance between fission/fusion in cardiac muscle in conditions of chronic exhaustive training similar to those applied in the present study. From a practical point of view, it is important to diagnose approaching physical overtraining in time to avoid excessive damage but improve performance. In this regard, Margonis et al (95) have proposed that correct and timely detection of oxidative stress may be valuable. They reported that an increase in exercise volume from high to very high strongly correlated with increases in F2-isoprostane levels in urine and reduced/oxidized glutathione ratio in blood.
Acknowledgments
This work was supported by grants from the Estonian Science Foundation (No 7117) and the Estonian Ministry of Education and Research (SF0180114As08). The authors thank Mrs Ellen Gvozdkova for her skillful technical assistance.
Footnotes
FUNDING: FNG is supported by grants from the German Federal Ministry of Trade and Commerce, project Mitoscreen Nr: IWO 072052 and the Foundation of Medical Science.
REFERENCES
- 1.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–82. [PubMed] [Google Scholar]
- 2.Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLU4. J Physiol Pharmacol. 2008;59:5–18. [PubMed] [Google Scholar]
- 3.Pereira B, Costa Rosa LFB, Safi DA, Medeiros MHG, Curi R, Bechara EJH. Superoxide dismutase, catalase, and glutathione peroxidase activities in muscle and lymphoid organs of sedentary and exercise-trained rats. Physiol Behav. 1994;56:1095–9. doi: 10.1016/0031-9384(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 4.Oh-ishi S, Kizaki T, Ookawara T, et al. Endurance training improves the resistance of rat diaphragm to exercise-induced oxidative stress. Am J Respir Crit Care Med. 1997;156:1579–85. doi: 10.1164/ajrccm.156.5.96-11035. [DOI] [PubMed] [Google Scholar]
- 5.Radák Z, Kaneko T, Tahara S, et al. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999;27:69–74. doi: 10.1016/s0891-5849(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 6.Pinho RA, Andrades ME, Oliveira MR, et al. Imbalance in SOD/CAT activities in rat skeletal muscles submitted to treadmill training exercise. Cell Biol Int. 2006;30:848–53. doi: 10.1016/j.cellbi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: Survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–11. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 8.Ventura-Clapier R. Exercise training, energy metabolism, and heart failure. Appl Physiol Nutr Metab. 2009;34:336–9. doi: 10.1139/h09-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascensão A, Magalhães JF, Soares JM, et al. Cardiac mitochondrial respiratory function and oxidative stress: The role of exercise. Int J Sports Med. 2005;26:258–67. doi: 10.1055/s-2005-837570. [DOI] [PubMed] [Google Scholar]
- 10.Tonkonogi M, Walsh B, Svensson M, Sahlin K. Mitochondrial function and antioxidative defence in human muscle: Effects of endurance training and oxidative stress. J Physiol. 2000;528:379–88. doi: 10.1111/j.1469-7793.2000.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljubicic V, Joseph A-M, Saleem A, et al. Transcriptional and posttranscriptional regulation of mitochondrial biogenesis in skeletal muscle: Effect of exercise and aging. Biochim Biophys Acta. 2010;1800:223–34. doi: 10.1016/j.bbagen.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Starnes JW, Barnes BD, Olsen M. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol. 2007;102:1793–8. doi: 10.1152/japplphysiol.00849.2006. [DOI] [PubMed] [Google Scholar]
- 13.Seene T, Kaasik P, Alev K, Pehme A, Riso EM. Composition and turnover of contractile proteins in volume-overtrained skeletal muscle. Int J Sports Med. 2004;25:438–45. doi: 10.1055/s-2004-820935. [DOI] [PubMed] [Google Scholar]
- 14.Seene T, Umnova M, Kaasik P. The exercise myopathy. In: Lehman M, et al., editors. Overload, Perfomance Incompetence, and Regeneration in Sport. New York: Kluwer Academic Plenum; 1999. pp. 119–30. [Google Scholar]
- 15.Hooper S, Mackinnon L, Howard A, et al. Markers for monitoring overtraining and recovery. Med Sci Sports Exerc. 1995;27:106–12. [PubMed] [Google Scholar]
- 16.Di Meo S, Venditti P. Mitochondria in exercise-induced oxidative stress. Biol Signals Recept. 2001;10:125–40. doi: 10.1159/000046880. [DOI] [PubMed] [Google Scholar]
- 17.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–70. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 18.Ashton T, Rowlands CC, Jones E, et al. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol Occup Physiol. 1998;77:498–502. doi: 10.1007/s004210050366. [DOI] [PubMed] [Google Scholar]
- 19.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrero A, Barja G. Localization of the site of oxygen radical generation inside the complex I of heart and nonsynaptic brain mammalian mitochondria. J Bioenerg Biomembr. 2000;32:609–15. doi: 10.1023/a:1005626712319. [DOI] [PubMed] [Google Scholar]
- 21.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–73. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 22.Molnar AM, Alves AA, Pereira-da-Silva L, Macedo DV, Dabbeni-Sala F. Evaluation by blue native polyacrylamide electrophoresis colorimetric staining of the effects of physical exercise on the activities of mitochondrial complexes in rat muscle. Braz J Med Biol Res. 2004;37:939–47. doi: 10.1590/s0100-879x2004000700001. [DOI] [PubMed] [Google Scholar]
- 23.Arslan S, Erdem S, Kilinç K, Sivri A, Tan E, Hasçelik HZ. Free radical changes in rat muscle tissue after exercise. Rheumatol Int. 2001;3:109–12. doi: 10.1007/s002960000094. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 25.Tate CA, Bonner HW, Leslie SW. Calcium uptake in skeletal muscle mitochondria. II. The effects of long-term chronic and acute exercise. Eur J Appl Physiol Occup Physiol. 1978;39:117–22. doi: 10.1007/BF00421716. [DOI] [PubMed] [Google Scholar]
- 26.King DW, Gollnick PD. Ultrastructure of rat heart and liver after exhaustive exercise. Am J Physiol. 1970;218:1150–5. doi: 10.1152/ajplegacy.1970.218.4.1150. [DOI] [PubMed] [Google Scholar]
- 27.Ji LL, Mitchell EW. Effects of adriamycin on heart mitochondrial function in rested and exercised rats. Biochem Pharmacol. 1994;47:877–85. doi: 10.1016/0006-2952(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 28.Leichtweiss SB, Leeuwenburgh C, Parmelee DJ, Fiebig R, Ji LL. Rigorous swim training impairs mitochondrial function in post-ischemic rat heart. Acta Physiol Scand. 1997;160:139–48. doi: 10.1046/j.1365-201X.1997.00138.x. [DOI] [PubMed] [Google Scholar]
- 29.Terblanche SE, Gohil K, Packer L, Henderson S, Brooks GA. The effects of endurance training and exhaustive exercise on mitochondrial enzymes in tissues of the rat (Rattus norvegicus) Comp Biochem Physiol A Mol Integr Physiol. 2001;128:889–96. doi: 10.1016/s1095-6433(00)00344-5. [DOI] [PubMed] [Google Scholar]
- 30.Kemi OJ, Høydal MA, Haram PM, et al. Exercise training restores aerobic capacity and enenrgy transfer systems in heart failure treated with losartan. Cardiovasc Res. 2007;76:91–9. doi: 10.1016/j.cardiores.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Sun B, Wang JH, Lv YY, Zhu SS, Yang J, Ma JZ. Proteomic adaptation to chronic high intensity swimming training in the rat heart. Comp Biochem Physiol. 2008;3:108–17. doi: 10.1016/j.cbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Bo H, Jiang N, Ma G, et al. Regulation of mitochondrial uncoupling respiration during exercise in rat heart: Role of reactive oxygen species (ROS) and uncoupling protein 2. Free Rad Biol Med. 2008;44:1373–81. doi: 10.1016/j.freeradbiomed.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Ascensão A, Magalhães J, Soares JMC, et al. Endurance training limits the functional alterations of heart rat mitochondria submitted to in vitro anoxia-reoxygenation. Int J Cardiol. 2006;109:169–78. doi: 10.1016/j.ijcard.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol. 2008;294:H928–35. doi: 10.1152/ajpheart.01231.2007. [DOI] [PubMed] [Google Scholar]
- 35.Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol. 2009;297:H144–52. doi: 10.1152/ajpheart.01278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce GN, Kutryk MJ, Dhalla KS, Beamish RE, Dhalla NS. Biochemical alterations in heart after exhaustive swimming in rats. J Appl Physiol. 1984;57:326–31. doi: 10.1152/jappl.1984.57.2.326. [DOI] [PubMed] [Google Scholar]
- 37.Huang C-C, Lin T-J, Chen C-C, Lin W-T. Endurance training accelerates exhaustive exercise-induced mitochondrial DNA deletion and apoptosis of left ventricle myocardium in rats. Eur J Appl Physiol. 2009;107:697–706. doi: 10.1007/s00421-009-1177-4. [DOI] [PubMed] [Google Scholar]
- 38.Seppet EK, Kaambre T, Sikk P, et al. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim Biophys Acta. 2001;504:379–395. doi: 10.1016/s0005-2728(00)00269-3. [DOI] [PubMed] [Google Scholar]
- 39.Saks VA, Kaambre T, Sikk P, et al. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–57. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anmann T, Eimre M, Kuznetsov AV, et al. Structure-function relationships in the regulation of energy transfer between mitochondria and ATPases in cardiac cells. Exp Clin Cardiol. 2006;1:189–94. [PMC free article] [PubMed] [Google Scholar]
- 41.Braun U, Paju K, Eimre M, et al. Lack of dystrophin is associated with altered integration of the mitochondria and ATPases in slow-twitch muscle cells of MDX mice. Biochim Biophys Acta. 2001;1505:258–70. doi: 10.1016/s0005-2728(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 42.Kadaja L, Kisand KE, Peet N, et al. IgG from patients with liver diseases inhibit mitochondrial respiration in permeabilized oxidative muscle cells: impaired function of intracellular energetic units. Mol Cell Biochem. 2004;256–257:291–303. doi: 10.1023/b:mcbi.0000009876.23921.e6. [DOI] [PubMed] [Google Scholar]
- 43.Seppet E, Eimre M, Peet N, et al. Compartmentation of energy metabolism in atrial myocardium of patients undergoing cardiac surgery. Mol Cell Biochem. 2005;270:49–61. doi: 10.1007/s11010-005-3780-y. [DOI] [PubMed] [Google Scholar]
- 44.Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762:164–80. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Eimre M, Puhke R, Alev K, et al. Altered mitochondrial apparent affinity for ADP and impaired function of mitochondrial creatine kinase in gluteus medius of patients with hip osteoarthritis. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1271–75. doi: 10.1152/ajpregu.00651.2005. [DOI] [PubMed] [Google Scholar]
- 46.Kaasik P, Alev K, Seene T. The effect of activity on the rat skeletal muscle contractile apparatus. Scand J Lab Anim Sci. 1996;23:35–9. [Google Scholar]
- 47.Seene T, Alev K. Effect of glucocorticoids on the turnover rate of actin and myosin heavy and light chains on different types of skeletal muscle fibres. J Steroid Biochem. 1985;22:767–71. doi: 10.1016/0022-4731(85)90284-5. [DOI] [PubMed] [Google Scholar]
- 48.Saks VA, Veksler VI, Kuznetsov AV, et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998;184:81–100. [PubMed] [Google Scholar]
- 49.Eimre M, Paju K, Pelloux S, et al. Distinct organization of energy metabolism in HL-1 cardiac cell line and cardiomyocytes. Biochim Biophys Acta. 2008;1777:514–24. doi: 10.1016/j.bbabio.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Fuller EO, Goldberg DI, Starnes JW, Sacks LM, Delivoria-Papadopoulos M. Mitochondrial respiration following acute hypoxia in the perfused rat heart. J Mol Cell Cardiol. 1985;17:71–81. doi: 10.1016/s0022-2828(85)80093-6. [DOI] [PubMed] [Google Scholar]
- 51.Maguire JJ, Davies KJA, Dallman PR, Packer L. Effects of dietary iron efficiency on iron-sulfur proteins and bioenergetic function on skeletal muscle mitochondria. Biochim Biophys Acta. 1982;679:210–20. doi: 10.1016/0005-2728(82)90292-4. [DOI] [PubMed] [Google Scholar]
- 52.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 53.Ostadal B, Schiebler TH. The development of capillaries in the rat heart: An electron microscopic study. Z Anat Entwicklungsgesh. 1971;133:288–304. [PubMed] [Google Scholar]
- 54.Laguens RP, Gómez-Dumm CLA. Fine-structure of myocardial mitochondria in rats after exercise for one-half to two hours. Circ Res. 1967;21:271–9. doi: 10.1161/01.res.21.3.271. [DOI] [PubMed] [Google Scholar]
- 55.Nozaki T, Kagaya Y, Ishide N, et al. Interaction between sarcomere and mitochondrial length in normoxic and hypoxic rat ventricular papillary muscles. Cardiovasc Pathol. 2001;10:125–32. doi: 10.1016/s1054-8807(01)00071-0. [DOI] [PubMed] [Google Scholar]
- 56.Vendelin M, Béraud N, Guerrero K, et al. Mitochondrial regular arrangement in muscle cells: A “crystal-like” pattern. Am J Physiol. 2005;288:757–67. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- 57.Capetanaki Y. Desmin cytoskeleton: A potential regulator of muscle mitochondrial behaviour and function. Trends Cardiovasc Med. 2002;12:339–48. doi: 10.1016/s1050-1738(02)00184-6. [DOI] [PubMed] [Google Scholar]
- 58.Herzog V, Fahimi HD. Identification of peroxisomes (microbodies) in mouse myocardium. J Mol Cell Cardiol. 1976;8:271–2. doi: 10.1016/0022-2828(76)90003-1. [DOI] [PubMed] [Google Scholar]
- 59.Fahimi HD, Kino M, Hicks L, Thorp KA, Abelman WH. Increased myocardial catalase in rats fed ethanol. Am J Pathol. 1979;96:373–90. [PMC free article] [PubMed] [Google Scholar]
- 60.Goldfischer S. Peroxisomes in disease. J Histochem Cytochem. 1979;27:1371–3. doi: 10.1177/27.10.390036. [DOI] [PubMed] [Google Scholar]
- 61.Zipper J. Proliferation of myocardial peroxisomes caused by several agents and conditions. J Mol Cell Cardiol. 1997;29:149–61. doi: 10.1006/jmcc.1996.0260. [DOI] [PubMed] [Google Scholar]
- 62.De Craemer D, Vamecq J, Roels F, et al. Peroxisomes in liver, heart, and kidney of mice fed a commercial fish oil preparation: Original data and review on peroxisomal changes induced by high-fat diets. J Lipid Res. 1994;35:1241–50. [PubMed] [Google Scholar]
- 63.Zhou Z, Kang YJ. Cellular and subcellular localization of catalase in the heart of transgenic mice. J Histochem Cytochem. 2000;48:585–94. doi: 10.1177/002215540004800502. [DOI] [PubMed] [Google Scholar]
- 64.McKenna O, Arnold G, Holtzman E. Microperoxisome distribution in the central nervous system of the rat. Brain Res. 1976;117:181–94. doi: 10.1016/0006-8993(76)90729-0. [DOI] [PubMed] [Google Scholar]
- 65.Hicks L, Fahimi HD. Peroxisomes (microbodies) in the myocardium of rodents and primates. A comparative ultrastructural cytochemical study. Cell Tissue Res. 1977;175:467–81. doi: 10.1007/BF00222413. [DOI] [PubMed] [Google Scholar]
- 66.Kainulainen H, Ahomäki E, Vihko V. Selected enzyme activities in mouse cardiac muscle during training and terminated training. Basic Res Cardiol. 1984;79:110–23. doi: 10.1007/BF01935813. [DOI] [PubMed] [Google Scholar]
- 67.Mohr M, Krustrup P, Nielsen JJ, et al. Effect of two different intense training regimes on skeletal muscle ion transport proteins and fatigue development. Am J Physiol. 2007;292:R1594–602. doi: 10.1152/ajpregu.00251.2006. [DOI] [PubMed] [Google Scholar]
- 68.Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007;81–82:209–30. doi: 10.1093/bmb/ldm014. [DOI] [PubMed] [Google Scholar]
- 69.Bronheimer JF, Lau F. Effects of treadmill exercise on total and myocardial creatine phosphokinase. Chest. 1981;80:146–8. doi: 10.1378/chest.80.2.146. [DOI] [PubMed] [Google Scholar]
- 70.Jaffe AS, Garfinkel BT, Ritter CS, Sobel BE. Plasma MB creatine kinase after vigorous exercise in professional athletes. Am J Cardiol. 1984;53:856–8. doi: 10.1016/0002-9149(84)90419-3. [DOI] [PubMed] [Google Scholar]
- 71.Young A. Plasma creatine kinase after the marathon – a diagnostic dilemma. Brit J Sports Med. 1984;18:269–72. doi: 10.1136/bjsm.18.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Apple FS, Billadello JJ. Expression of creatine kinase M and B mRNAs in treadmill trained rat skeletal muscle. Life Sci. 1994;55:585–92. doi: 10.1016/0024-3205(94)00484-6. [DOI] [PubMed] [Google Scholar]
- 73.Cummins P, Young A, Auckland ML, Michie CA, Stone PC, Shepstone BJ. Comparison of serum cardiac specific troponin-I with creatine kinase, creatine kinase-MB isoenzyme, tropomyosin, myoglobin and C-reactive protein release in marathon runners: Cardiac or skeletal muscle trauma? Eur J Clin Invest. 1987;17:317–24. doi: 10.1111/j.1365-2362.1987.tb02194.x. [DOI] [PubMed] [Google Scholar]
- 74.Siegel AJ, Sholar M, Yang J, Dhanak E, Lewandrowsky KB. Elevated serum cardiac markers in asymptomatic marathon runners after competition: Is the myocardium stunned? Cardiology. 1997;88:487–91. doi: 10.1159/000177396. [DOI] [PubMed] [Google Scholar]
- 75.Nie J, Tong TK, George K, Fu FH, Lin H, Shi Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners Scand. J Med Sci Sports. 2010 doi: 10.1111/j.1600-0838.2010.01096.x. (In press) [DOI] [PubMed] [Google Scholar]
- 76.Nanji AA. Serum creatine kinase isoenzymes: A review. Muscle Nerve. 1983;6:83–90. doi: 10.1002/mus.880060203. [DOI] [PubMed] [Google Scholar]
- 77.Apple FS, Tesch PA. CK and LD isoenzymes in human single muscle fibers in trained athletes. J Appl Physiol. 1989;66:2717–20. doi: 10.1152/jappl.1989.66.6.2717. [DOI] [PubMed] [Google Scholar]
- 78.Stuewe SR, Gwirtz PA, Mallet RT. Exercise training increases creatine kinase capacity in canine myocardium. Med Sci Sports Exerc. 2001;33:92–8. doi: 10.1097/00005768-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 79.Chen YJ, Serfass RC, Apple FS. Loss of myocardial CK-MB into the circulation following 3.5 hours of swimming in a rat model. Int J Sports Med. 2000;21:561–5. doi: 10.1055/s-2000-8485. [DOI] [PubMed] [Google Scholar]
- 80.Miller TD, Rogers PJ, Bauer BA, et al. Does exercise training alter myocardial creatine kinase MB isoenzyme content? Med Sci Sports Exerc. 1989;21:437–40. [PubMed] [Google Scholar]
- 81.Borutaite V. Mitochondria as decision-makers in cell death. Environ Mol Mutagen. 2010;51:406–16. doi: 10.1002/em.20564. [DOI] [PubMed] [Google Scholar]
- 82.Rossignol R, Letellier T, Malgat M, Rocher C, Mazat J-P. T issue variation in the control of oxidative phosphorylation: Implication for mitochondrial diseases. Biochem J. 2000;347:45–53. [PMC free article] [PubMed] [Google Scholar]
- 83.Rasmussen HN, Rasmussen UF. Small scale preparation of skeletal muscle mitochondria, criteria for integrity, and assays with reference to tissue function. Mol Cell Biochem. 1997;174:55–60. [PubMed] [Google Scholar]
- 84.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–72. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 85.Pan SS. Alterations of atrial natriuretic peptide in cardiomyocytes and plasma of rats after different intensity exercise. Scan J Med Sci Sports. 2008;18:346–53. doi: 10.1111/j.1600-0838.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 86.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–8. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 87.Malinska D, Kulawiak B, Kudin AP, et al. Complex III-dependent superoxide production of brain mitochondria contributes to seizure-related ROS formation. Biochim Biophys Acta. 2010;1797:1163–70. doi: 10.1016/j.bbabio.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 89.Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Rad Biol Med. 2008;44:224–9. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 90.Fry AC, Kraemer WJ. Resistance exercise overtraining and overreaching. Sports Med. 1997;23:106–29. doi: 10.2165/00007256-199723020-00004. [DOI] [PubMed] [Google Scholar]
- 91.Kreider R, Fry AC, O’Toole M. Over-training in sports: Terms, definitions, and prevalence. In: Kreider R, Fry AC, O’Toole M, editors. Over-training in Sport. Champaign: Human Kinetics; 1998. pp. 47–66. [Google Scholar]
- 92.Seene T, Umnova M, Kaasik P, Alev K, Pehme A. Overtraining injuries in athletic population. In: Tiidus PM, editor. Skeletal Muscle Damage and Repair. Windsor: Human Kinetics; 2008. pp. 173–84. [Google Scholar]
- 93.Seene T, Kaasik P, Umnova M. Structural rearrangements in contractile apparatus and resulting skeletal muscle remodelling: Effect of exercise training. J Sports Med Phys Fitness. 2009;49:410–23. [PubMed] [Google Scholar]
- 94.Ding H, Jiang N, Liu H, et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800:250–6. doi: 10.1016/j.bbagen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 95.Margonis K, Fatouros IG, Jamurtas AZ, et al. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Rad Biol Med. 2007;43:901–10. doi: 10.1016/j.freeradbiomed.2007.05.022. [DOI] [PubMed] [Google Scholar]