Abstract
The role of classic morphogens such as Sonic hedgehog (Shh) as axon guidance cues has been reported in a variety of vertebrate organisms (Charron and Tessier-Lavigne, 2005). In this work, we provide the first evidence that Xenopus sonic hedgehog (Xshh) signaling is involved in guiding retinal ganglion cell (RGC) axons along the optic tract. Xshh is expressed in the brain during retinal axon extension, adjacent to these axons in the ventral diencephalon. Retinal axons themselves express Patched 1 and Smoothened co-receptors during RGC axon growth. Blocking Shh signaling causes abnormal ventral pathfinding, and targeting errors at the optic tectum. Misexpression of exogenous N-Shh peptide in vivo also causes pathfinding errors. Retinal axons grown in culture respond to N-Shh in a dose-dependent manner, either by decreasing extension at lower concentrations, or retracting axons in the presence of higher doses. These data suggest that Shh signaling is required for normal RGC axon pathfinding and tectal targeting in the developing visual system of Xenopus. We propose that Shh serves as a ventral optic tract repellent that helps to define the caudal boundary for retinal axons in the diencephalon, and that this signaling is also required for initial target recognition at the optic tectum.
Keywords: Xenopus axon guidance, retinal ganglion cells, retinotectal pathfinding, sonic hedgehog, optic tract, diencephalon
Introduction
Billions of neurons form precise connections throughout the vertebrate nervous system. In order to establish these connections, the axons of neuronal cells must find their way to their appropriate targets during neural development, yet the selective pathways that are used to achieve such brain wiring are not fully understood. Cues that regulate axon guidance are numerous and varied. They include those that are diffusible or anchored, those that act over long or short ranges, and those that are repulsive, attractive, and/or adhesive to extending axons (Yamamoto et al., 2003, Chilton, 2006 and Erskine and Herrera, 2007). In addition to identifying axon guidance cues and their corresponding receptors, the intracellular mechanisms that are involved in axon outgrowth, pathfinding and targeting are also being studied. Guidance cues are believed to control axon behavior through cytoskeletal rearrangements, which can direct axonal growth cone dynamics and steering (Dent and Gertler, 2003, Farrar and Spencer, 2008, Lowery and Van Vactor, 2009, and Bashaw and Klein, 2010).
The retinotectal pathway of Xenopus laevis has served as an important model for axon guidance. Much of the neurogenesis and neuroanatomy of Xenopus retinal ganglion cells (RGCs) has been well described, and these cells create the sole link between the eye and the brain, that allows for visual perception (Holt, 1989, Harris and Holt, 1990, and Dingwell et al., 2000). The stereotypic path that RGC axons take from the retina to the midbrain has also been well characterized. Xenopus, in particular, has been a model organism of choice for retinal axon guidance studies because of the accessibility of the RGC axons along the entire length of the visual pathway, and the relative ease with which Xenopus embryos can be obtained and manipulated (Chien and Harris, 1994). Retinal axons travel through quite diverse microenvironments during visual system development. They first extend within the retina, to the optic disc and nerve head, forming an axon bundle, the optic nerve, as they exit the eye, beginning at embryonic stage 28 in Xenopus. By stage 32, these axons prepare to innervate the contralateral side of the brain by crossing the embryonic midline, thus forming the optic chiasm during contralateral wiring. Retinal axons will next travel along the lateral surface of the neuroepithelium in the diencephalon, forming the optic tract, before turning caudally (at stage 35/36), towards their final target at the end of the tract, the optic tectum, at stage 39/40. Throughout this entire journey, RGC growth cones will survey their environments to find the appropriate molecular cues that will guide their movements, and lead them to the appropriate final targets for subsequent synaptogenesis in the optic tectum.
Recent evidence has suggested that classic morphogens, such as Wnts, TGFβ/BMP family members, and Hedgehogs may all serve as axon guidance cues for a variety of axons, in different organisms (Charron and Tessier-Lavigne, 2005, Sanchez-Camacho et al., 2005, and Zou and Lyuksyutova, 2007). Several groups have provided increasing evidence that Sonic hedgehog (Shh) is an important axon guidance cue throughout vertebrate neural development. In chicks, Shh is important for pathfinding within the retina (Kolpak et al., 2005) and at the optic chiasm (Trousse et al., 2001). In mice, Shh functions during intraretinal RGC extension (Sanchez-Camacho and Bovolenta, 2008), and in guidance at the optic chiasm (Hao et al., 2007 and Sanchez-Camacho and Bovolenta, 2008). Shh is also used by commissural axons of the mouse spinal cord, to promote midline axon guidance, in conjunction with Netrin-1 (Charron et al., 2003) and through Boc receptors (Okada et al., 2006). Post-commissural axon guidance along the spinal longitudinal axis also utilizes Shh signals (Bourikas et al., 2005). In rat commissural axons, non-canonical Shh signaling via the Src family of kinases has been described as a mechanism for axons to bypass transcription, and allow for rapid responses to Shh guidance cues in growth cones (Yam et al., 2009).
Overall, there is growing evidence that Shh is an important guidance cue for different types of axons. In the visual system, as described above, Shh is reported to be a chemoattractant and repellent in the retina, and a repellent for retinal axons at the optic chiasm. Beyond these points along the visual pathway, retinal axons must still travel a relatively long distance (approximately 430 μm in Xenopus, Harris et al., 1985) to reach their final target, yet guidance cues along the optic tract are not well characterized. Previous studies have shown that Xenopus retinal axons rely on directional cues that are stable and local to the optic tract (Harris, 1989, Taylor, 1990 and Cornel and Holt, 1992) and molecules such as heparan sulfates (Walz et al., 1997 and Irie et al., 2002) and matrix metalloproteinases (Webber et al., 2002, Hehr et al., 2005 and Chen et al., 2007) have been implicated in this process. The role of morphogens such as Shh in the diencephalon remains unknown. Therefore, we were interested in examining whether Hedgehog signaling is involved in retinal axon guidance at regions after midline crossing, when RGC axons project from the ventral to dorsal ends of the developing diencephalon. In this work, we describe a novel function for Sonic hedgehog as a guidance cue for retinal axons along the optic tract in Xenopus. We also show that Hedgehog signaling is involved in optic tectum target recognition.
Results
Xshh is expressed adjacent to the optic tract during RGC axon guidance
To begin to examine the role of Shh in retinal axon guidance in the diencephalon, we first characterized the expression pattern of Xshh during Xenopus RGC axon extension, using whole mount in situ hybridization with a DIG-labeled riboprobe that specifically recognizes Xshh mRNA. We found strong expression in the anterior regions of stage 32-39/40 embryos, when RGC axons are traveling the optic tract. In dissected brains from these stained embryos, Xshh expression was evident in a distinct pattern along the caudal and ventral-most regions of the diencephalon. Between stages 32 and 34, we observed a broad band of Xshh at the floorplate and ventral end of the developing brain (Figures 1A and 1B). By stage 38, this expression became slightly more refined, with a narrow stripe of Xshh seen from the ventral end of the diencephalon (including the floorplate), to the caudal bend region, about half way across the dorsal-ventral axis of this tissue (Figure 1C). While Xshh expression was quite strong towards the caudal side of these brains, there was no appreciable staining in the rostral and dorsal most regions during these stages. In transverse sections of stage 37/38 embryos, at the level of the diencephalon, we also observed robust Xshh staining in the ventral floorplate region of the neural tube, as well as in the notochord (Figure 1D). After crossing the embryonic midline to form the optic chiasm, retinal axons will continue to travel superficially along the neuroepithelium, where they are in close proximity to this ventral Xshh expression.
Figure 1. Xshh expression in the developing brain.
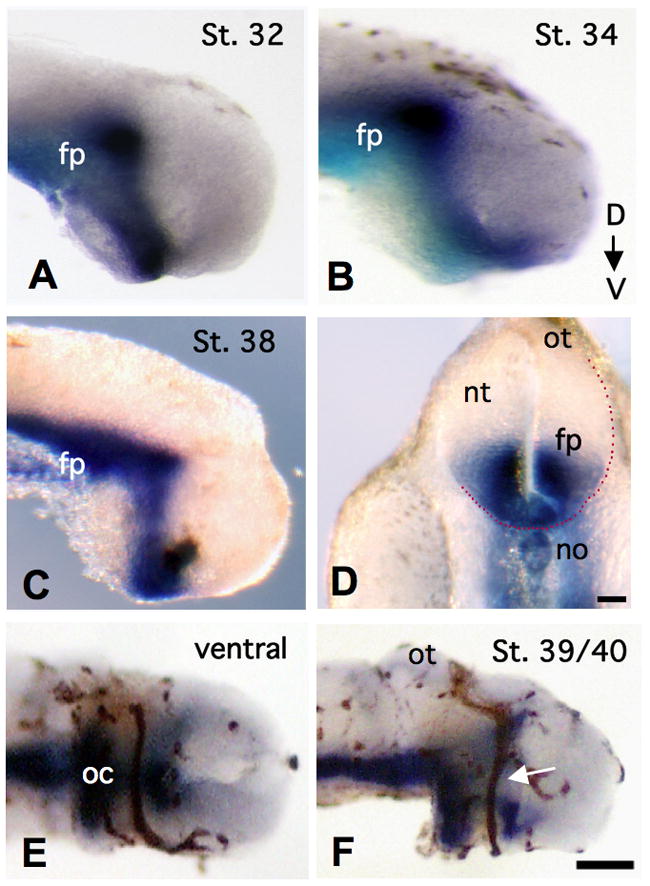
Panels A – C show lateral views of Xshh mRNA expression (blue) in stage 32, stage 34 and stage 38 embryonic brains, respectively. In D, Xshh is found at the level of the midbrain in a transverse section of a stage 38 Xenopus embryo. Dotted red line denotes the presumptive location of the optic tract. A ventral view of HRP-labeled retinal axons (brown), along with Xshh expression (blue) in a stage 38 brain is shown in E, at the optic chiasm. A lateral view of the same co-labeled brain, along the optic tract, is pictured in F, with the white arrow at RGC axons, midway across the diencephalon. Unless otherwise noted, in all appropriate figures, dorsal is to the top. (floor plate = fp, notochord = no, neural tube = nt, optic chiasm = oc, and optic tectum = ot, scale bar in D = 100 μm, and in F = 40 μm)
Next, we examined the expression of Xshh, relative to HRP labeled RGC axons, as they project across the optic tract to the tectum, using double labeling of stage 39/40 Xenopus embryonic brain tissue, as described in Campbell et al., 2001. With this approach, we were able to visualize the precise trajectory of retinal axons, with a surrounding region of strong Xshh expression. In a ventral view of RGC axons at the optic chiasm, we found that these axons extended through a “tunnel” of Xshh (Figure 1E). This expression pattern was quite similar to that previously described for chick RGCs at the optic chiasm (Trousse et al., 2001). A lateral view of this double-labeled brain shows that as RGC axons moved along the optic tract, they did so adjacent to a strong region of Xshh expression (Figure 1F), located just caudal to the optic tract. This Xshh expression diminished near the mid-diencephalic bend and was not detected along the dorsal end of the tract, nor near the target optic tectum by stage 39/40. From these findings, it appears that Xshh mRNA is made during RGC axon extension, and is most prominent in a region immediately adjacent to the ventral optic tract.
Ptc1 and Smo are expressed in RGCs
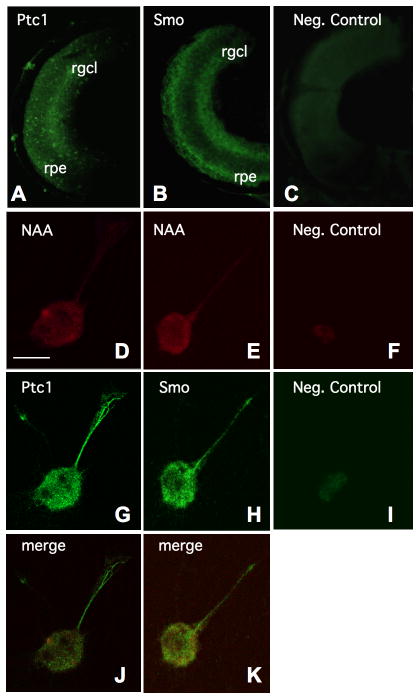
We next characterized the expression of two known Shh co-receptors, Patched 1 (Ptc1) and Smoothened (Smo), in Xenopus retinal tissue, and in cultured retinal explants. In cross sections of eyes taken from stage 39 embryos, we observed Ptc1 expression in the retinal ganglion cell layer, as well as more faint staining in the developing retinal pigment epithelium (RPE) (Figure 2A). We also found strong Smo expression in the retinal ganglion cell layer of stage 39 retinas (Figure 2B). Smo was present more uniformly throughout the RPE, with stronger expression than Ptc1, and well above any nonspecific background seen in the negative control (Figure 2C). Our findings are consistent with the X-ptc1 and X-smo mRNA expression patterns previously reported for stage 40–41 retinas (Perron et al., 2003).
Figure 2. XPtc1 and XSmo co-receptor expression in RGCs.
Ptc1 and Smo expression are observed in the RGC layer and the retinal pigment epithelium (RPE) of sectioned retinas from stage 39 embryos, in A and B, respectively, as compared to a negative control in C. In D and E, individual cultured RGCs are identified by neurofilament associated antigen (NAA) immunoreactivity. These retinal cells are also shown to express both Ptc1 (in G), and Smo (in H). No appreciable staining is detected in negative controls, as shown in F and I. (retinal ganglion cell layer = rgcl, retinal pigment epithelium = rpe, scale bar in D = 10μm)
To determine Ptc1 and Smo expression in individual RGC axons, we prepared cultured explants of stage 30–32 eyebuds, and then performed immunocytochemistry with the appropriate antibodies. First, we confirmed the identity of the cultured cells as RGCs using an anti-neurofilament associated antigen (NAA) antibody (Figures 2D–2F), as previously described by Hocking et al., 2008. NAA immunoreactivity was evident in the cell bodies and axons of these retinal explants. We then found that both Ptc1 (Figure 2G) and Smo (Figure 2H) were strongly expressed in these RGCs, throughout the cell bodies and extended retinal axons, as compared to the negative controls in Figure 2F and 2I, respectively. Merged images of NAA and Ptc1, or Smo labeled cells (Figures 2J and 2K) highlight that these cultured RGCs express both Shh receptors. We also found that Ptc1 and Smo co-receptors could be localized to cultured retinal axon growth cones, within lamellopodia and filopodial tips (Supplementary Figure 1).
Our Ptc1 and Smo expression data show that the appropriate receptors are expressed in a spatiotemporal pattern that is consistent with a role for Shh signaling in RGC axon guidance in Xenopus. Ptc1 and Smo may be utilized by the growth cones of retinal axons to receive Shh signals and trigger downstream intracellular responses that allow for proper outgrowth and pathfinding. Collectively, the results of these ligand and receptor expression studies show that RGC axons come into contact with Xshh as they travel along the optic tract in the developing diencephalon, and also, that they express Ptc1 and Smo receptors during retinal axon extension, suggesting that a Shh-mediated signaling pathway may directly influence RGC axon guidance in Xenopus.
Cyclopamine disrupts normal RGC axon guidance in the diencephalon
In order to determine whether Shh signaling is required for normal RGC axon extension along the Xenopus optic tract between stages 30–40, we disrupted this signaling pathway using the drug cyclopamine, which has been shown to bind and inhibit signal transduction at the level of the co-receptor, Smo (Chen et al., 2002). After bath application of 20 μM cyclopamine (or a structurally similar analogue, jervine, which also blocks Shh signaling) to stage 30 embryos, we found distinct abnormalities related to post-chiasm retinal axon guidance, observed once treated samples had reached stage 39/40. These abnormal phenotypes included a widening of RGC projections along the optic tract, retinal axon defasiculation, axonal projection into the neuroepithelium, and tectal targeting errors, with results reproducible over a range of cyclopamine concentrations (from 20 μM to 100 μM). They also occurred using either cyclopamine or jervine, with similar concentrations tested. Controls were either untreated, or treated with tomatidine, an inactive analogue of cyclopamine and jervine (Berman et al., 2003), and they exhibited normal RGC axon guidance.
With disruption of Shh signaling, RGC axons formed a consistently widened pathway as they enter the brain at the ventral optic tract, as compared to control embryos (Figures 3A and 3B). Optic tract widening appears to be due (at least in part) to an unraveling of retinal axon tracts, such that individual axons could be viewed separately in the diencephalon, away from their normal bundled pathway. This apparent defasiculation of retinal axons caused noticeable gaps between axons in the diencephalon (Figure 3C). After cyclopamine treatment, retinal axons also traveled off their typical pathway by diving into the neuroepithelial tissue. While we found retinal axons in close proximity to the pigmented epidermal surface in control brain samples (Figure 3D), those sections taken from cyclopamine treated brains revealed retinal axons projecting inwards, through the diencephalic neuroepithelium and much closer to the ventricular surface of the brain (Figure 3E). The variety of ventral tract abnormalities that occurred with drug inhibition all indicate that Shh is required for proper RGC axon pathfinding in the diencephalon.
Figure 3. Abnormal RGC axon extension after Shh disruption.
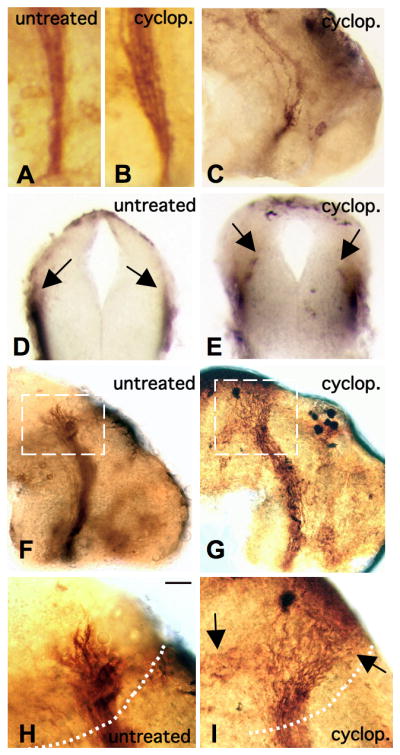
Changes in the width of retinal projections at the ventral optic tract in an untreated embryo in A, and a cyclopamine treated sample in B, are shown. Cyclopamine treatment also causes defasiculation of retinal axons, as shown in C. In D, the transverse section of a control sample reveals normal RGC axonal projections (arrows) just below the lateral surface neuroepidermis. After cyclopamine treatment, as shown in E, RGC axons project inwards (arrows). In F, a lateral view of a control sample shows normal RGC axon extension along the entire optic tract. After cyclopamine treatment, as in G, there is spreading of retinal axons at the tectum. A closer view of the tectum in an untreated control, in H, versus a cyclopamine treated embryo, in I, shows abnormal targeting (arrows). (The tectal boundary is outlined by the dotted white lines in H, and I and the scale bar in H = 10 μm)
Surprisingly, cyclopamine treatment also had affects on RGC axon extension at the dorsal end of the optic tract, near the optic tectum. After Hh function blocking, retinal axons continued to extend, but failed to recognize the target optic tectum, in approximately 30% of the cases observed. While RGC axons did extend towards the tectum, they did not follow a normal trajectory upon entry into the tectal region. After drug treatment, we observed that retinal axons had spread out along the tectal boundary, rather than enter the tectum in a fairly localized manner, as observed in the untreated control embryos (Figures 3F versus 3G). In a few instances, retinal axons continued to extend dorsally, towards the midline and opposite side of the brain. Others appeared to extend more ventrally - growing away from their tectal target and back towards the floorplate (Figures 3H and 3I). The results of these inhibition studies suggest a role for Shh signaling in retinal axon guidance along the entire length of the optic tract, from the brain entry point, to the dorsal optic tectum.
We used two different sets of measurements to quantify the optic tract width abnormalities, which we observed following Shh signal inhibition. First, we measured the width of the retinal axons at five distinct points along the ventral portion of the optic tract (Figure 4A), in jervine treated experimental and tomatidine control samples. We found that there were statistically significant changes in axon projection width, where the average widths of the retinal axons treated with jervine were greater than those of the control samples, at all five points along the ventral tract (within 1.96 standard errors of the mean, 95% certainty, Figure 4B). This tract widening represents an inability of RGC axons to maintain their progression along the normal pathway in the absence of Shh signaling. To determine whether the widened optic tracts found in the jervine treated embryos were due to retinal axon projection into more caudal areas of the diencephalon, we made additional measurements to quantify the distance between the caudal-most side of the retinal axon tract and a standardized reference line drawn from the brain entry point to the midbrain/hindbrain border. These measurements showed that the distance between the reference line and the caudal edge of the retinal axons decreased significantly in experimental samples, compared to controls, indicating that there are more caudally wandering RGC axons when Shh signaling is blocked (Figure 4C).
Figure 4. Quantitative analysis of RGC axon errors after Shh disruption.
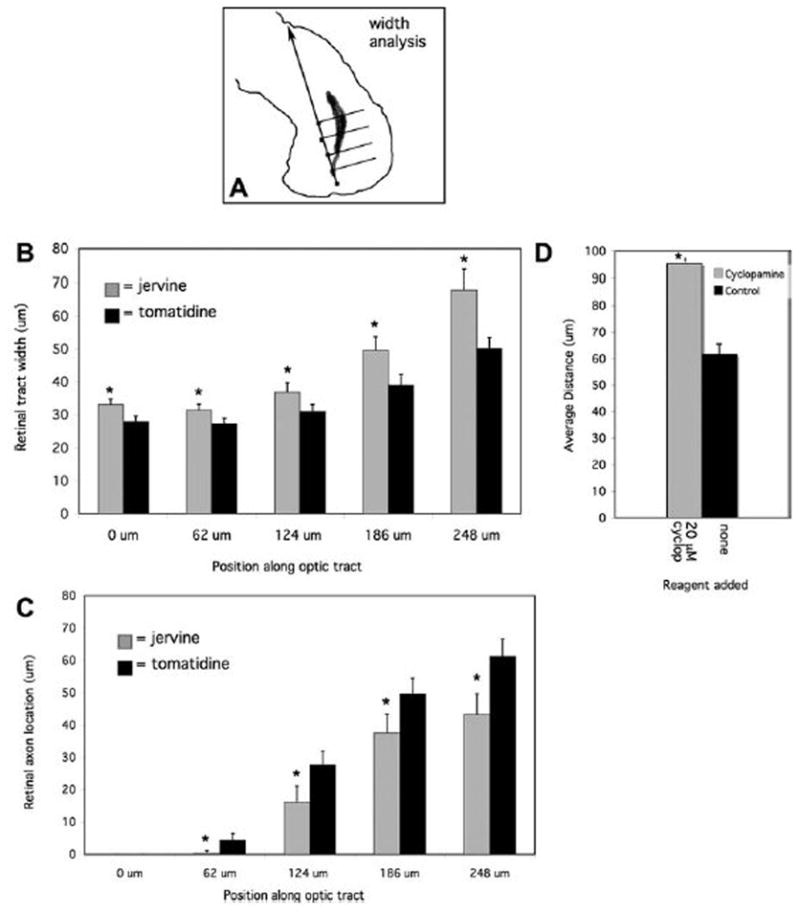
The width of the ventral portion of the optic tract was measured at five set intervals (boxes) along a standardized line from the brain entry point to the target tectum, as depicted in A. Measurements of the width of the retinal axon tract are plotted versus tract position along the reference line in B. In C, the distance between the caudal side of the retinal axons and the reference line is shown for the five intervals described above. The average distance that the RGC axons extend from the neuroepithelial surface is shown in D. (In each case, the error bars represent the standard error of the mean; *p < 0.01 (unpaired, two-tailed Student t test). For graphs B and C, n=30 in each case, and in D, n=36 for cyclopamine treated and n=24 for controls)
To further characterize the observed neuroepithelial depth abnormalities, we analyzed transverse sections through the diencephalon after drug treatments and RGC axon labeling with an anti-neurofilament antibody. From these sections, we measured the distance that the retinal axons traveled, relative to the surface of the neuroepithelium, in control and drug treated brains. While the average distance from the surface for control RGC axons was 61.6 μm (corresponding to a normally superficial trajectory), this average was significantly higher at 95.2 μm, after Shh inhibition (Figure 4D). The abnormal depth phenotype suggests that Shh signals are also involved in limiting retinal axon projections to the surface of the diencephalic neuroepithelium.
To confirm that Shh signaling was inhibited by our drug treatments, we used in situ hybridization for Xgli1, a gene known to be upregulated in response to Shh in Xenopus. After bath application of cyclopamine (or jervine), we found decreased Xgli1 in stage 38 embryos, indicating a disruption in the Shh pathway (Supplementary Figures 2A and 2B). We also showed that cyclopamine treatments did not appear to disrupt the normal development of the neuroepithelium and optic tract, which could lead to abnormal RGC axon pathfinding in this region, by comparing the expression of three different neural patterning markers - the transcription factor, Islet-1, the neural cell adhesion molecule, NCAM, and the neurotransmitter, GABA - in treated and untreated embryos, as described previously by Chen et al., 2007. After immunocyto-chemistry with antibodies against these marker proteins, we found no discernable differences in staining, in transverse sections of the treated and control samples (Supplementary Figure 2C–2J). Therefore, it is likely that the abnormal phenotypes we described are due to the loss of Shh signal, rather than the result of any indirect affects of a gross disruption of normal neuroepithelial development. Our inhibition studies all suggest that Shh is required for normal retinal pathfinding along the ventral optic tract, potentially forming a repulsive barrier in this region, which helps to define the boundaries of the optic tract.
Ectopic Shh signal causes abnormal RGC axon pathfinding in vivo
The potential role of Shh as a guidance cue for retinal axons in Xenopus was also tested through protein misexpression studies, in which we introduced exogenous Shh signal to the extending RGC axons in vivo, using acrylic beads pre-soaked in 20 ng/ml of N-Shh peptide, a 180 amino acid N-terminal signaling domain. In separate experiments, we implanted these N-Shh-soaked beads subepidermally at two ectopic locations within stage 30 Xenopus embryonic brains, either: (1) at the caudal bend of the optic tract, or (2) just posterior to the optic tectum. We implanted control embryos with beads pre-soaked in 20 ng/ml of BSA, to ensure that neither the introduction of excess protein, nor the bead implantation itself, could disrupt normal development, and to verify that any abnormal RGC axon extension was specifically due to N-Shh addition.
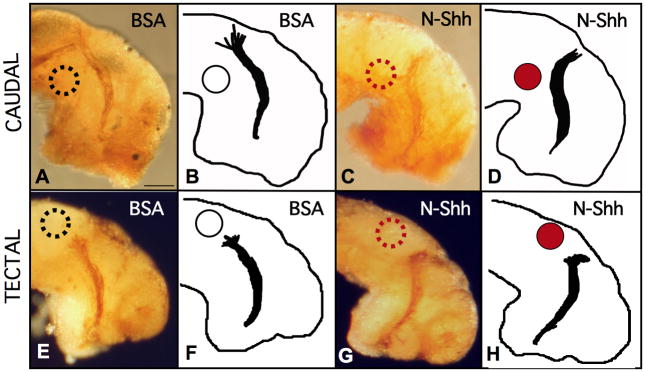
In the first set of experiments, we implanted N-Shh-soaked, or BSA-soaked beads, at the caudal bend of the optic tract. Under these conditions, we found that retinal axons turned away from the ectopic source of Shh signal. Rather than continuing towards their normal target when confronted with ectopic N-Shh at the caudal bend of the optic tract, retinal axons were deflected in the opposite direction. Compared to BSA controls, caudally implanted N-Shh beads repelled retinal axons rostrally (Figures 5A–5D). Instead of continuing towards the tectum, in 47% of these samples, retinal axons either turned away from the tectal target, extending off the normal pathway completely, or they stopped before entering the tectum. We did not observe such abnormal RGC axon extension in the majority of control embryos, where 82% of samples extended RGC axons to the tectum normally.
Figure 5. Ectopic N-Shh deflects RGC axons in vivo.
The results of bead implantation studies are shown with HRP labeled axons (A,C,E,G) & tracings (B,D,F,H). In A and B, a BSA-soaked control bead (white) was placed caudal to the ventral tract, and resulted in normal extension to the optic tectum. C and D show the results of a caudal N-Shh soaked (red) bead implant. In E and F, a BSA bead placed just behind the tectum, supports normal RGC axon extension to this target. In G and H, N-Shh appears to prevent RGC axons from entering the tectal region. (Scale bar in A = 50 μm and Table 1. summarizes the results of these studies)
In a separate set of experiments, we also placed N-Shh-soaked or BSA-soaked beads just behind the tectum, to create an ectopic source of Shh signal at the dorsal end of the optic tract. BSA at the tectum did not appear to prevent normal RGC axon guidance to this target site, with 73% of BSA samples extending towards the bead, and into the tectum normally (Figures 5E and 5F). This was not the case with N-Shh beads. Ectopic tectal N-Shh caused RGC axons to stop extending just outside of the target tectum in 39% of the cases observed. In another 5% of these samples, RGC axons were deflected away from the bead and the tectum (Figures 5G and 5H). Both caudal and tectal N-Shh beads caused disruptions of normal RGC axon guidance along the optic tract (see Table 1 for summary), providing strong evidence that Shh signal can influence Xenopus retinal axon pathfinding in vivo.
Table 1.
Summary of In vivo N-Shh Studies.
| Conditions | Bead Location | Extends toward bead | Extends away from bead | Extension stopped |
|---|---|---|---|---|
| BSA soaked | Caudal to tract | 9/11 (82%) | 1/11 (9%) | 1/11 (9%) |
| N-Shh soaked | Caudal to tract | 9/17 (53%) | 7/17 (41%) | 1/17 (6%) |
| BSA soaked | At tectum | 11/15 (73%) | 0/15 (0%) | 4/15 (27%) |
| N-Shh soaked | At tectum | 10/18 (56%) | 1/18 (5%) | 7/18 (39%) |
Retinal axons show decreased outgrowth in the presence of Shh
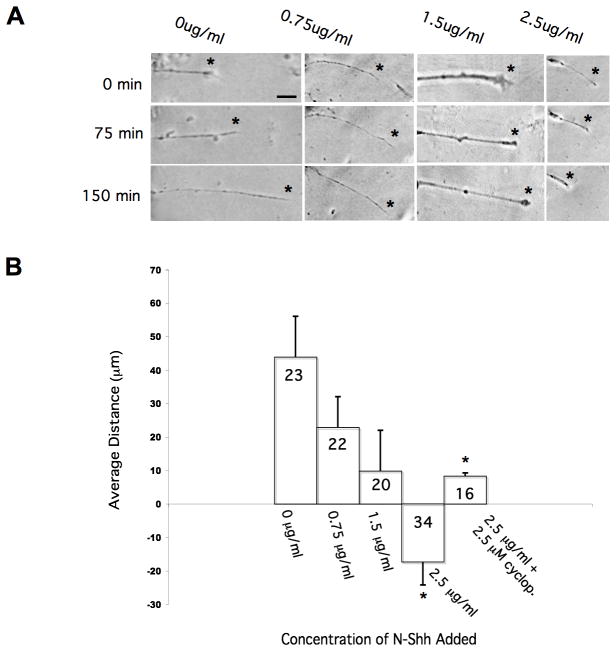
To examine the affects of Shh signaling on individual RGC axons, we grew retinal explants in culture, and determined axonal behavior, with and without the addition of N-Shh peptide. After overnight culturing, retinal axons were visualized at a starting time point, t=0. After 75 minutes (t=75), another image was taken of the same axon. In a subset of samples, we added varying concentrations of N-Shh (from 0.75 μg/ml to 2.5 μg/ml) to the culture media at t=75, followed by an additional incubation of 75 minutes. After this time, we recorded a final image, t=150. In the controls, retinal axon images were taken at t=0, t=75 and t=150 minutes, without any additions to the cultures. We also used NAA immunoreactivity to confirm the presence of RGC axons in these in vitro assays. For those control retinal axons grown in culture media only, we found that they extended appreciably over the 150 minutes observed, with an average distance traveled of 43.9 μm (Figure 6A, column one). This outgrowth changed with the addition of N-Shh peptide. In the presence of 0.75 μg/ml of N-Shh, retinal axons extended an average distance of 22.9 μm (Figure 6A, column two). Retinal axon outgrowth decreased further with an increased concentration of N-Shh, with 1.5 μg/ml supporting an average axon distance traveled of just 9.8 μm (Figure 6A, column three). We found that axon extension turned to retraction with the highest concentration of N-Shh tested. When 2.5 μg/ml of N-Shh was added to the retinal cultures, we found an average axon distance traveled of −17.3 μm (Figure 6A, column four). To verify that this repulsive response was due to high concentrations of N-Shh, we also tested neurite behavior in the presence of 2.5μg/ml N-Shh plus 2.5 μM cyclopamine. Under these conditions, the average distance traveled was 8.31 μm, indicating a significant change from axon retraction, to extension, once Hh signaling was blocked. Our results for these growth response assays are summarized in Figure 6B, and they suggest that retinal axons can respond differentially to Shh signal, in a dose-dependent manner. At high concentrations of Shh signal, RGC axons are repelled, while lower concentrations of Shh support axon extension.
Figure 6. Retinal explant neurites respond to N-Shh signals in vitro.
In A, the first column of panels shows the behavior of an individual RGC axon over 150 minutes in culture, in the absence of Shh signal. In the middle two columns, increasing amounts of N-Shh were added, with 0.75 and 1.5 μg/ml used, respectively. In the last column, the highest concentration of N-Shh, at 2.5 μg/ml, was added to the explant cultures. The graph in B summarizes these results, showing dose-dependent retraction of RGC axons in N-Shh. In each panel, asterisks indicate the location of the axon tip. (Scale bar in A = 10 μm, and in B, n values shown in graph bars; *p <0.01 (unpaired, two-tailed Student t test, comparing the average distance of axon extension in control versus N-Shh containing conditions).
Discussion
Little is known about post-chiasmatic retinal axon guidance along the optic tract in the diencephalon, and at the target optic tectum. Here, we provide the first evidence that Shh plays a role in retinal pathfinding at distal regions of the developing visual system in Xenopus. First, our expression studies show that Xshh mRNA is distributed in a pattern quite similar to that reported for chick and mouse at the optic chiasm, with strong expression on either side of the route taken by RGC axons as they cross the ventral midline (Trousse et al., 2001 and Hao et al., 2007). This spatiotemporal expression pattern suggests that Xenopus RGC axons, like those of chicks and mice, use a conserved mechanism, where Shh is a chemorepellent that guides retinal axons at the optic chiasm during contralateral wiring of the visual system. We also find strong Xshh expression along the ventral optic tract, immediately adjacent to the RGC axons, from the brain entry point to the caudal bend of the tract, in the mid-diencephalon between stages 32 – 40. During these stages of RGC axon extension in Xenopus, both Ptc1 and Smo are expressed in RGC cell bodies in the retina, and also in retinal axons and their growth cones. Thus, it appears that Shh may influence retinal axon guidance beyond the optic chiasm, in the developing diencephalon.
To examine Shh’s function along the optic tract, we performed various inhibition studies to disrupt Shh signaling, after RGC axons have successful crossed the optic chiasm. In vivo function blocking with cyclopamine (or jervine) reveals that Shh is required for normal RGC pathfinding at the optic tract in Xenopus. Without Shh signaling, retinal axons travel off the ventral tract and into more caudal regions of the diencephalon, ultimately extending farther away from the target tectum. Our data show that tract widening occurs in regions caudal to the normal projection path of RGC axons, such that aberrant axons are found in regions of high Xshh expression, when Shh signaling is blocked. We also show that Shh is required for the superficial localization of the optic tract at the surface of the neuroepithelium. In vivo misexpression studies provide further evidence for Shh function during RGC axon guidance in the diencephalon. Ectopic expression of N-Shh peptide at the caudal bend of the optic tract introduces high levels of Shh signal near this region, and under such conditions, retinal axons are deflected off the normal pathway, and away from the target optic tectum. These results suggest that Shh may serve as a repellent cue, which helps to define visual pathway boundaries along the optic tract.
Shh signaling also appears to function at the dorsal end of the optic tract during target recognition. Our data are consistent with the idea that retinal axons may use Shh to identify their target and to stop extending at the tectum. As shown in the inhibition studies, in the absence of normal Shh signaling, retinal axons can branch at the tectal border, moving both ventrally and dorsally, and extending past the normal stopping point in the tectum itself. After ectopic misexpression of N-Shh behind the tectum, retinal axons also fail to enter the target normally. Instead, they stop extending at the tectal border, or in a few cases, extend away from the tectum all together. These results show that Shh can repel Xenopus retinal axons in vivo and also, that this signaling pathway may be involved in normal tectal target recognition.
Previous studies have identified other guidance cues near the optic tract in the developing Xenopus brain, including Sema3A, which is expressed in the telencephalon between stages 34 and 41 (Campbell et al., 2001) and Slit1, which is also found in the telencephalon, near the mid-diencephalic turn (Atkinson-Leadbeater et al., 2010). Our work here shows that Xshh is expressed in the diencephalon at these same stages, in a pattern that is complementary to Sema3A and Slit1. Therefore, Shh may work in conjunction with other repellent guidance cues, to establish the optic pathway in the developing brain. Along the caudal side of the optic tract, Shh restricts retinal projections, while the rostral expression of other important chemorepellents, such as Sema3A and Slit1, prohibit projections into the telencephalon. This model could explain the relatively mild phenotypes that we observed with cyclopamine treatment, with respect to tract widening and defasiculation, since we have inhibited only one set of repellents with this approach. Although these retinal axons extended abnormally, we found no cases where they grew into the telencephalic regions of the brain, suggesting that multiple repellents may help to define the path of retinal axons as they form the optic tract. Since some of the same chemorepellents have been identified in other vertebrate embryonic brains, including Slits in rats (Ringstedt et al., 2000), zebrafish (Hutson and Chien, 2002) and mice (Thompson et al., 2006), and chondroitin sulfate proteoglycans in chicks (Ichijo and Kawabata, 2001), similar mechanisms may be utilized to help wire the visual systems of these organisms, as well.
The target recognition errors at the optic tectum were somewhat unexpected, since we found little detectable Xshh mRNA at the tectal end of the tract. There are several possible explanations for these results. A previous report on Shh’s role as a retinal axon guidance cue showed that Shh can serve as an attractant at low concentrations, or a repellent at high concentrations in the retina (Kolpak et al., 2005). Accordingly, a similar mechanism could be at work further along the visual pathway. As RGC axons enter the optic tract, they encounter caudal and rostral repellents that promote axon fasciculation, and restrict projections to the optic tract. As these axons extend more dorsally, there is less Shh, which may help to promote bending towards the tectal target and preparation for target recognition. Thus, our targeting errors could be due to an inability of retinal axons to sense lowered amounts of Shh protein near the optic tectum, which they would normally use to identify their target site. To specifically test the sensitivity of Xenopus retinal axons to different concentrations of Shh, we performed in vitro growth response assays using cultured retinal explants, exposed to varying amounts of N-Shh peptide. We found a dose-dependent response to N-Shh, with increasing concentrations of N-Shh causing decreased retinal axon projection. These results show that Xenopus RGC axons do have the ability to respond differentially to different concentrations of Shh signal. In our growth response assays, higher levels of Shh caused axon retraction, while lower doses supported retinal axon extension, as was previously reported for chick axonal extension within the retina (Kolpak et al., 2005). Although Xshh mRNA expression was not apparent near the optic tectum, Shh protein has been shown to diffuse several cell diameters away from its source of synthesis (Yang et al., 1997, Zeng et al., 2001 and Goetz et al., 2002). Therefore, it is possible that Shh protein is present in low levels at the dorsal-most regions of the optic tract, having diffused from the ventral cells of the diencephalon. Alternatively, retinal axons may utilize a cell autonomous source of Shh to mediate target recognition at the tectum, since the RGC axons themselves have been reported to express and secrete Shh (Traiffort et al., 2001 and Sanchez-Camacho and Bovolenta, 2008).
Another intriguing possibility is that a different Hh ligand, (or another signal which works through the Smo receptor), is responsible for target recognition at the optic tectum. There are three known Hh homologues in Xenopus – sonic (Xshh), banded (Xbhh), and cephalic (Xchh), and these three genes have distinct neural expression patterns in developing embryos, as described by Ekker et al., 1995. Since Xchh appears to be expressed almost exclusively in the hindbrain, just posterior to the optic tectum during the stages of RGC axon extension in the diencephalon (Perron et al., 2003), this ligand is a good candidate for a retinal axon targeting cue in Xenopus. Experiments using approaches that can separately test the functions of each Xhh homologue (including morpholinos designed against Xhhs) are on going in our lab, to help clarify the roles of Hh signaling during retinal axon guidance, and to directly test this hypothesis. In the future, it will also be important to examine the combined functions of Hedgehogs, plus other guidance cues, since molecules such as Sema3A (Campbell et al., 2001), Slits (Piper et al., 2006) and netrin-1 (Shewan et al., 2002) are all expressed near the optic tectum in Xenopus.
Based on the findings presented in this work, we propose the following model for Shh function during RGC axon pathfinding in the diencephalon in Xenopus (Figure 7). Our results suggest that RGC axons use Shh signals as guidance cues to project along the optic tract, and perhaps, to recognize their target region at the optic tectum. High levels of Shh along the ventral portion of the tract repel RGC axons away from the caudal regions of the diencephalon, and define a caudal boundary for retinal axons traveling along the optic tract. Shh also serves as a chemorepellent that limits retinal axon projections to the surface of the diencephalic neuroepithelium. At the dorsal end of the tract, lower levels of Shh (or other Hh ligands, such as Cephalic hedgehog) may help to guide retinal axons towards the optic tectum, and also aide in precise target recognition and entry into this region. Our work provides the first evidence that Shh is required for normal RGC axon pathfinding along the optic tract, and identifies Shh as one of the few known molecules involved in diencephalic axon guidance. We have also uncovered what appears to be a novel role for Smo-dependent Hh signaling during optic tectum targeting, providing new insights into the potential guidance mechanisms that function during the establishment of the distal visual pathway.
Figure 7. A proposed model for Shh function during Xenopus RGC axon optic tract projection.
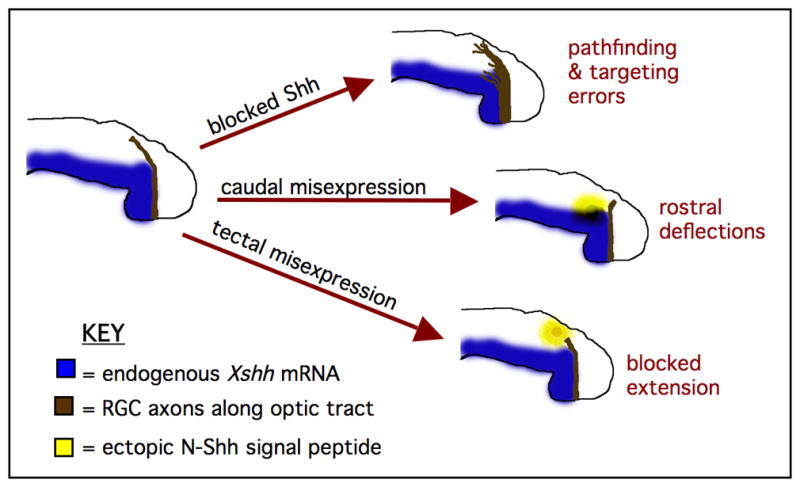
The results of our studies suggest that Xenopus RGC axons use Shh as a guidance cue to extend along the optic tract and to recognize their target. High levels of Shh may repel RGC axons along the ventral optic tract and limit their projections to the surface of the diencephalon, while lower Shh levels (along with other factors) may help to guide retinal axons towards the optic tectum and aide in initial target recognition.
Experimental Procedures
Animals
Embryos were obtained either through mating adult Xenopus laevis frogs (from Xenopus Express, Brooksville, FL, USA), or in vitro fertilization of eggs from females stimulated with human chorionic gonadotropin (Carolina Biologicals and Sigma Aldrich). Embryos were raised in 0.1X Marc’s Modified Ringers solution (MMR, 1 mM NaCl, 0.2 mM KCl, 0.2 mM CaCl2, 0.1 mM MgSO4, 0.5 mM HEPES, pH 7.8) and staged according to a standard guide (Nieuwkoop and Faber, 1994). Rates of development were controlled through incubations at 22°C, 16°C and 14°C. For most studies, embryos were used between stages 30–40, when retinal axons are extending along the optic tract in the diencephalon.
Whole Mount In situ Hybridization
Digoxigenin (DIG)-labeled sense and antisense RNA probes were made against Xshh and Xgli1 (Lofstrand Services, LLC), using DNA plasmids generously provided by A. Carrasco, S. Ekker and M. Perron. Labeled probes were introduced into MEMFA fixed whole embryos or dissected brain tissue using standard protocols (Sive, Grainger and Harland, 2000). Briefly, fixed embryos (or brains) were rehydrated into PTw (1X PBS and 0.1% Tween-20), Proteinase K treated to permeablize, washed in 0.1 M triethanolamine and acetic anhydride, refixed in 4% paraformaldehyde and hybridized with 1 μg/ml of labeled probe. Additional stringency washes in SSC, incubation in an RNase cocktail, 1X MAB washes, blocking in BMB Blocking Reagent and detection with an anti-DIG-AP antibody and BM purple substrate all followed (reagents from Roche Applied Science). Staining was visualized using digital microscopy with a Nikon SMZ800 stereoscope. Transverse sectioning, and microdissection of brain tissue were done manually.
Immunocytochemistry
Whole mount immunocytochemistry was performed on 4% paraformaldehyde fixed brain tissue from stage 38–40 embryos, with PBS and PBST (PBS with 0.1% BSA and 0.5% Triton-X 100) washes, blocking in 5% goat serum, incubation in primary antibodies: 1:50 anti-NCAM, 1:80 anti-Islet-1, (from DSHB), and 1:3000 anti-GABA (Sigma Aldrich), with 1:1000 goat-anti-mouse Alexa 546 or goat-anti-rabbit Alexa 488 secondary antibodies (Molecular Probes). For immunofluorescent staining of retinal explants, these samples (made as described below) were fixed in 4% paraformaldehyde and washed in 1X PBS, blocked in 5% goat serum in 0.4% Triton X-100 and incubated in primary antibodies: 1:200 anti-Smo (Santa Cruz Biotechnology), 1:200 anti-Ptc1 (Abbiotec), or 1:100 anti-NAA (3A10, DSHB). Samples were washed in 1X PBS following primary antibody incubation, then treated with 1:400 goat anti-rabbit Alexa 488 or goat-anti-mouse Alexa 546 secondary antibodies (Molecular Probes). Samples were viewed and imaged using confocal microscopy. Immunhistochemistry on slides with cross sections (35 μm thickness, made with a Leica VT1000 Vibratome) of retinas from stage 38–40 embryos was also performed, as described above, using 1:100 anti-Ptc1 and 1:100 anti-Smo antibodies.
Retinal Ganglion Cell Axon Labeling
Horseradish peroxidase (HRP) was used for anterograde filling of retinal axons through the eye cavity, and then enzymatic detection was performed using a diaminobenzidine (DAB) substrate to locate the position of the RGC axons along the optic tract (Cornel and Holt, 1992). Stage 38–40 embryos were anaesthetized using 0.05% MS-222 (tricaine methane sulfonate, Carolina Biologicals) in 1X MMR. Embryos were then immobilized in agarose-coated dishes with micropins, skin overlying one eye was removed and HRP crystals (Peroxidase Type VI-A from Horseradish, Sigma Aldrich) dissolved in 1% lysophosphatidycholine were placed into the exposed eye cavity. Following HRP introduction, embryos were transferred to a recovery dish containing 1X MMR and incubated for 25-minutes, to ensure adequate time for transport of HRP along axons. Embryos were then fixed in 1% glutaraldehyde, brain tissue was dissected manually and washed in PBT. Axons were then visualized using the HRP substrate DAB (Sigma Aldrich). Immunocytochemistry with an anti-neurofilament (NF) antibody (1:100, Zymed) was also performed to label RGC axons in some experiments, as noted.
Function Blocking Drug Treatments
Stage 30 embryos were treated, via bath application, with: (1) 20 μM cyclopamine (Toronto Research Chemicals), or an analogue, jervine (EMD Chemicals, Inc.), in 0.1X MBS for Shh signal blocking, or (2) 0.1X MBS or 20 μM tomatidine for controls. Tomatidine is another chemical analogue that is structurally similar to cyclopamine and jervine, but it does not inhibit Hedgehog signaling, so it serves as an additional control in these studies. After drug treatment, embryos were grown to stage 39/40 in MBS, RGCs labeled, then visualized for guidance errors, using either anterograde HRP labeling, or an anti-NF antibody to view RGC axons, as described above.
Axon Projection Analysis
To quantify abnormal axonal projections along the optic tract, the following analyses were done on samples labeled using an anti-NF antibody. For retinal axon tract width quantification, a reference line from the brain entry point to the mid-brain/hindbrain boundary was drawn and standardized to a normal length of 620 μm in the NIH software program JIMage. At five distinct points along this reference line (at 62 μm increments), the distance from the caudal point of the retinal axon tract to the reference line was measured and standardized. Additionally, the total width of the RGC axon tracts was also measured and standardized. Data from 30 samples in each group (n=30 tracts) and the mean of these measurements were taken. The tissue depth traveled by RGC axons was also examined in transverse sections of stage 38 control and cyclopamine treated brains by measuring their distance from the neuroepithelial surface on each side of the diencephalon, using Adobe Photoshop software. The average distance was then calculated and comparisons between control (n=24) and cyclopamine treated samples (n=36) were made. For quantification of changes in axon length in retinal explant in vitro assays with N-Shh peptide, images of single axons were recorded at various time points (t=0, t=75 and t=150 minutes) using digital microscopy with a Nikon Eclipse TE2000-S inverted microscope and NIS Elements software. Then, the length of each axon was measured using Adobe Photoshop and compared, relative to untreated controls, as an average distance traveled.
Bead Implantations
Heparin-acrylic beads (Sigma Aldrich) were washed in 1X MMR, then soaked in N-Shh peptide (R&D Systems, Inc.) or BSA control (Sigma Aldrich) at a concentration of 20 ng/μl. Stage 30–32 embryos were anesthetized with 0.05% MS-222 and pinned to agarose-coated dishes, then soaked beads placed subepidermally, to hold each bead in place at specified locations (caudal to the optic tract, or just posterior to the optic tectum), using watchmaker’s forceps. Embryos were grown to approximately stage 39/40 in 0.1X MMR, followed by anterograde labeling of RGC axons with HRP, as described previously. Embryos with missing or displaced beads were discarded from these studies.
In vitro RGC Culturing
Retinal explants from stage 30 embryos were plated onto laminin, poly-L-Lysine coated culture slides (BD Biosciences) and grown in a culture media of 60% L-15 medium, 10% Fetal Calf Serum, 2% Protein Extract (from stage 28–32 embryos), 2% gentamicin and 2% fungizone (Webber et al., 2002). Explants were typically incubated for 24 – 48 hours at 22°C in a laminar flow tissue culture hood, under aseptic conditions. For in vitro growth cone response assays, varying concentrations of N-Shh peptide, (from 0.75–2.5 μg/ml) were added directly to the culture media after selecting individual axons in single field of view. Following two incubation periods of 75 minutes each, the same retinal explants were visualized with Nikon Eclipse TE2000-S inverted microscope and images were taken using digital microscopy and the NIS Elements software program.
Supplementary Material
In A, retinal growth cone expression of Ptc1 protein is detected in the lamellopodia and at the tips of an RGC axon (arrow), from cultured retinal explants. Expression of Smo protein is also found in RGC axon growth cone lamellopodia and at tips, as shown in B. (Scale bar in A = 10 μm)
A change in Xgli1 expression is observed in control, A, versus cyclopamine treated, B, embryos. The expression of the neural patterning markers Islet-1 (C and D), NCAM (E and F) and GABA (G and H), in 40 μm transverse sections of cyclopamine treated and untreated brains from stage 39 embryos is shown. Panels I and J reveal background staining in negative controls, to which no primary antibodies were added.
Acknowledgments
Grant sponsor: NIH Award #K01 NS052551
The authors are grateful for the excellent technical expertise and mentorship of J. Raper, D. Kessler and L. Pineda-Salgado. We also acknowledge R. Hoang and C. Jones for their help with manuscript preparation, and A. Stout at the University of Pennsylvania School of Medicine Cell and Developmental Biology Microscopy Facility for confocal microscopy assistance. Finally, we thank other former Haverford College undergraduate students (B. Wendel, R. Nehmer and J. Slawson) and N. Bullock for their contributions. This work was supported by NIH Award #K01 NS052551.
References
- Atkinson-Leadbeater K, Bertolesi G, Hehr C, Webber C, Cechmanek P, McFarlane S. Dynamic expression of axon guidance cues for optic tract development is controlled by fibroblast growth factor signaling. J Neurosci. 2010;30(2):685–693. doi: 10.1523/JNEUROSCI.4165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashaw G, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D, Karha S, Maitra A, Montes de Oca R, Gerstenblith M, Briggs K, Parker A, Shimada Y, Eshelman J, Watkins D, Beachy P. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardó M, Stoeckli E. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nature Neurosci. 2005;8(3):297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- Campbell D, Regan A, Lopez J, Tannahill D, Harris C, Holt C. Semaphorin 3A elicits stage-dependent collapse, turning and branching in Xenopus retinal growth cones. J Neurosci. 2001;21(21):8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon A, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- Chen J, Taipale J, Cooper MK, Beachy P. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hehr C, Atkinson-Leadbeater K, Hocking J, McFarlane S. Targeting of retinal axons requires the metalloproteinase ADAM10. J Neurosci. 2007;27(31):8448–56. doi: 10.1523/JNEUROSCI.1841-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C, Harris WA. Axonal guidance from retina to tectum in embryonic Xenopus. Curr Top Dev Biol. 1994;29:135–167. doi: 10.1016/s0070-2153(08)60549-9. [DOI] [PubMed] [Google Scholar]
- Chilton J. Molecular mechanisms of axon guidance. Dev Biol. 2006;292:13–24. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Cornel E, Holt C. Precocious pathfinding: retinal axons can navigate in an axonless brain. Neuron. 1992;9(6):1001–1011. doi: 10.1016/0896-6273(92)90061-h. [DOI] [PubMed] [Google Scholar]
- Dent E, Gertler F. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dingwell K, Holt C, Harris WA. The multiple decisions made by growth cones of RGCs as they navigate from the retina to the tectum in Xenopus embryos. J Neurobiol. 2000;44(2):246–259. [PubMed] [Google Scholar]
- Ekker S, McGrew L, Lai C, Lee J, von Kessler D, Moon R, Beachy P. Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis. Development. 1995;121:2337–2347. doi: 10.1242/dev.121.8.2337. [DOI] [PubMed] [Google Scholar]
- Erskine L, Herrera E. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308(1):1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Farrar N, Spencer G. Pursuing a ‘turning point’ in growth cone research. Dev Biol. 2008;318:102–111. doi: 10.1016/j.ydbio.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Goetz J, Suber L, Zeng X, Robbins D. Sonic Hedgehog as a mediator of long-range signaling. Bioessays. 2002;24(2):157–65. doi: 10.1002/bies.10056. [DOI] [PubMed] [Google Scholar]
- Hao Y, Wang J, Chan C, Chan S. Disruption of Sonic Hedgehog signaling affects axon routing in the mouse optic chiasm. Neuroembryology Aging. 2007;4:76–84. [Google Scholar]
- Harris WA, Holt C, Smith T, Gallenson N. Growth cones of developing retinal cells in vivo, on culture surfaces, and in collagen matrices. J Neurosci Res. 1985;13:101–122. doi: 10.1002/jnr.490130108. [DOI] [PubMed] [Google Scholar]
- Harris WA. Local positional cues in the neuroepithelium guide retinal axons in embryonic Xenopus brain. Nature. 1989;339:218–221. doi: 10.1038/339218a0. [DOI] [PubMed] [Google Scholar]
- Harris WA, Holt CE. Early events in the embryogenesis of the vertebrate visual system: cellular determination and pathfinding. Annu Rev Neurosci. 1990;13:155–169. doi: 10.1146/annurev.ne.13.030190.001103. [DOI] [PubMed] [Google Scholar]
- Hehr C, Hocking J, McFarlane S. Matrix metalloproteinases are required for retinal ganglion cell axon guidance at select decision points. Development. 2005;132(15):3371–3379. doi: 10.1242/dev.01908. [DOI] [PubMed] [Google Scholar]
- Hocking J, Hehr C, Chang R, Johnston J, McFarlane S. TGFβ ligands promote the initiation of retinal ganglion cell dendrites in vitro and in vivo. Mol Cell Neurosci. 2008;37:247–260. doi: 10.1016/j.mcn.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Holt C. A single-cell analysis of early retinal ganglion cell differentiation in Xenopus: from soma to axon tip. J Neurosci. 1989;9:3123–3145. doi: 10.1523/JNEUROSCI.09-09-03123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson L, Chien C. Pathfinding and error correction by retinal axons: the role of astray/robo2. Neuron. 2002;33:205–217. doi: 10.1016/s0896-6273(01)00579-7. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Kawabata I. Roles of the telencephalic cells and their chondroitin sulfate proteoglycans in delimiting an anterior border of the retinal pathway. J Neurosci. 2001;21:9304–9314. doi: 10.1523/JNEUROSCI.21-23-09304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie A, Yates E, Turnbull J, Holt C. Specific heparan sulfate structures involved in retinal axon targeting. Development. 2002;129:61–70. doi: 10.1242/dev.129.1.61. [DOI] [PubMed] [Google Scholar]
- Kolpak A, Zhang J, Bao Z. Sonic hedgehog has a dual effect on the growth of retinal ganglion axons depending on its concentration. J Neurosci. 2005;25(13):3432–3441. doi: 10.1523/JNEUROSCI.4938-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery L, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nature Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop and Faber. Normal Table of Xenopus laevis (Daudin) Garland Publishing Inc; New York: 1994. [Google Scholar]
- Okada A, Charron F, Morin S, Shin D, Wong K, Fabre P, Tessier-Lavigne M, McConnell S. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Perron M, Boy S, Amato MA, Viczian A, Koebernick K, Pieler T, Harris W. A novel function for Hedgehog signaling in retinal pigment epithelium differentiation. Development. 2003;130:1565–77. doi: 10.1242/dev.00391. [DOI] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung K, Cogill E, Holt C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, O’Leary DD. Slit inhibition of retinal axon growth and its role in retinal axon pathfinding and innervation patterns in the diencephalon. J Neurosci. 2000;20:4983–4991. doi: 10.1523/JNEUROSCI.20-13-04983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Camacho C, Rodriguez J, Ruiz M, Trousse F, Bovolenta P. Morphogens as growth cone signaling molecules. Brain Res Rev. 2005;49:242–252. doi: 10.1016/j.brainresrev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Sánchez-Camacho C, Bovolenta P. Autonomous and non-autonomous Shh signalling mediate the in vivo growth and guidance of mouse retinal ganglion cell axons. Development. 2008;135:3531–3541. doi: 10.1242/dev.023663. [DOI] [PubMed] [Google Scholar]
- Shewan D, Dwivedy A, Anderson R, Holt C. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nature Neurosci. 2002;5(10):955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- Sive Hl, Grainger RM, Harland RM. Early development of Xenopus laevis: a laboratory manual. Cold Spring Harbor: CSHL Press; 2000. [Google Scholar]
- Taylor J. The directed growth of retinal axons towards surgically transposed tecta in Xenopus; an examination of homing behaviour by retinal ganglion cell axons. Development. 1990;108(1):147–58. doi: 10.1242/dev.108.1.147. [DOI] [PubMed] [Google Scholar]
- Thompson H, Barker D, Camand O, Erskine L. Slits contribute to the guidance of retinal ganglion cell axons in the mammalian optic tract. Dev Biol. 2006;296:476–484. doi: 10.1016/j.ydbio.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Moya K, Faure H, Hassig R, Ruat M. High expression and anterograde axonal transport of aminoterminal sonic hedgehog in the adult hamster brain. Eur J of Neurosci. 2001;14:839–850. doi: 10.1046/j.0953-816x.2001.01708.x. [DOI] [PubMed] [Google Scholar]
- Trousse F, Marti E, Gruss P, Torres M, Bovolenta P. Control of retinal ganglion cell axon growth: a new role for Sonic hedgehog. Development. 2001;128:3927–3936. doi: 10.1242/dev.128.20.3927. [DOI] [PubMed] [Google Scholar]
- Walz A, McFarlane S, Brickman Y, Nurcombe V, Bartlett P, Holt C. Essential role of heparan sulfates in axon navigation and targeting in the developing visual system. Development. 1997;124:2421–2430. doi: 10.1242/dev.124.12.2421. [DOI] [PubMed] [Google Scholar]
- Webber C, Hocking J, Yong V, Stange C, McFarlane S. Metalloproteases and Guidance of Retinal Axons in the Developing Visual System. J Neurosci. 2002;22(18):8091–8100. doi: 10.1523/JNEUROSCI.22-18-08091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam P, Langlois S, Morin S, Charron F. Sonic Hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tamada A, Murakami F. Wiring of the brain by a range of guidance cues. Prog Neurobiol. 2003;68:393–407. doi: 10.1016/s0301-0082(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Drossopoulou G, Chuang P, Duprez D, Marti E, Bumcrot D, Vargesson N, Clarke J, Niswander L, McMahon A, Tickle C. Relationship between dose, distance and time in Sonic Hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development. 1997;124(21):4393–404. doi: 10.1242/dev.124.21.4393. [DOI] [PubMed] [Google Scholar]
- Zeng X, Goetz J, Suber L, Scott W, Schreiner C, Jr, Robbins D. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411(6838):716–20. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- Zou Y, Lyuksyutova A. Morphogens as conserved axon guidance cues. Curr Opin Neurobiol. 2007;17:22–28. doi: 10.1016/j.conb.2007.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In A, retinal growth cone expression of Ptc1 protein is detected in the lamellopodia and at the tips of an RGC axon (arrow), from cultured retinal explants. Expression of Smo protein is also found in RGC axon growth cone lamellopodia and at tips, as shown in B. (Scale bar in A = 10 μm)
A change in Xgli1 expression is observed in control, A, versus cyclopamine treated, B, embryos. The expression of the neural patterning markers Islet-1 (C and D), NCAM (E and F) and GABA (G and H), in 40 μm transverse sections of cyclopamine treated and untreated brains from stage 39 embryos is shown. Panels I and J reveal background staining in negative controls, to which no primary antibodies were added.