Abstract
Objectives
We investigated whether atorvastatin might decrease insulin sensitivity and increase ambient glycemia in hypercholesterolemic patients.
Background
Clinical trials suggest that some statin treatments might increase the incidence of diabetes despite reductions in low-density lipoprotein (LDL) cholesterol and improvement in endothelial dysfunction.
Methods
A randomized, single-blind, placebo-controlled parallel study was conducted in 44 patients taking placebo and in 42, 44, 43, and 40 patients given daily atorvastatin 10, 20, 40, and 80 mg, respectively, during a 2-month treatment period.
Results
Atorvastatin 10, 20, 40, and 80 mg significantly reduced LDL cholesterol (39%, 47%, 52%, and 56%, respectively) and apolipoprotein B levels (33%, 37%, 42%, and 46%, respectively) after 2 months of therapy when compared with either baseline (all p < 0.001 by paired t test) or placebo (p < 0.001 by analysis of variance [ANOVA]). Atorvastatin 10, 20, 40, and 80 mg significantly increased fasting plasma insulin (mean changes: 25%, 42%, 31%, and 45%, respectively) and glycated hemoglobin levels (2%, 5%, 5%, and 5%, respectively) when compared with either baseline (all p < 0.05 by paired t test) or placebo (p = 0.009 for insulin and p = 0.008 for glycated hemoglobin by ANOVA). Atorvastatin 10, 20, 40, and 80 mg decreased insulin sensitivity (1%, 3%, 3%, and 4%, respectively) when compared with either baseline (p = 0.312, p = 0.008, p < 0.001, and p = 0.008, respectively, by paired t test) or placebo (p = 0.033 by ANOVA).
Conclusions
Despite beneficial reductions in LDL cholesterol and apolipoprotein B, atorvastatin treatment resulted in significant increases in fasting insulin and glycated hemoglobin levels consistent with insulin resistance and increased ambient glycemia in hypercholesterolemic patients. (Effects of Atorvastatin on Adiponectin Levels and Insulin Sensitivity In Hypercholesterolemic Patients; NCT00745836)
Keywords: adipocytokines, glycated hemoglobin, insulin resistance, metabolic syndrome, statins
Coronary heart disease is characterized by endothelial dysfunction and insulin resistance (1,2). Statins have beneficial effects on atherosclerosis mediated by decreased low-density lipoprotein (LDL) cholesterol and improving endothelial function (3). Nevertheless, the effects of statins on insulin sensitivity are not clear.
Lipophilic statins have pleiotropic actions that might cause unfavorable metabolic effects such as reduction of insulin secretion and exacerbation of insulin resistance (4-6). Recent large-scale, randomized controlled clinical trials have raised the possibility that lipophilic statins might increase the rate of new onset diabetes (7-9). Specifically, in the HPS (Heart Protection Study), in the simvastatin group 335 subjects developed diabetes, whereas in the placebo group 293 subjects developed diabetes (hazard ratio: 1.15, 95% confidence interval [CI]: 0.98 to 1.35, p = 0.10) (7). In the ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial), the atorvastatin group developed diabetes with a hazard ratio of 1.15 (95% CI: 0.91 to 1.44) (8). In both studies, there were no significant differences between the treatment group and placebo group; however, both studies showed a trend toward an increase in new onset diabetes. In JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), rosuvastatin 20 mg significantly increased the rate of onset of new diabetes (3.0% vs. 2.4%, p = 0.01) with significant increase in glycated hemoglobin (HbA1C) (5.9% vs. 5.8%, p = 0.001) (9). Meta-analysis of randomized controlled trials suggested potential differences between individual statins, with pravastatin showing a trend toward a reduction in risk (risk ratio: 0.84; 95% CI: 0.86 to 1.49) and atorvastatin, rosuvastatin, and simvastatin together demonstrating a significant increase in risk (risk ratio: 1.14; 95% CI: 1.02 to 1.28) versus placebo (10). We hypothesized that atorvastatin, particularly at high dose, might decrease insulin sensitivity and increase ambient glycemia, HbA1C in hypercholesterolemic patients.
Methods
Study population
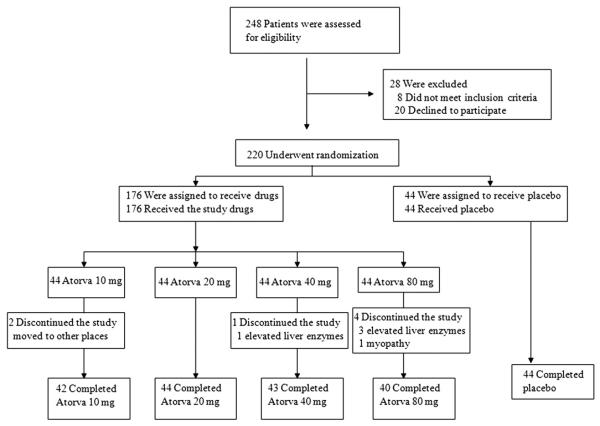
Our study was a randomized, single-blind, placebo-controlled, parallel trial in patients with hypercholesterolemia (LDL cholesterol levels ≥100 mg/dl). We recruited patients from a primary care setting in the Cardiology Department, Gil Hospital, Gachon University. Metabolic syndrome was defined according to the definition of the National Cholesterol Education Program Adult Treatment Panel III (11). Most patients were hypertensive and/or hyperlipidemic. There were some patients (n < 5) with stable angina in each group. We performed 64 multislice computed tomography scan or heart scan to help evaluate angina. We excluded patients with overt liver disease, chronic renal failure, hypothyroidism, myopathy, uncontrolled diabetes, severe hypertension, stroke, unstable angina, acute myocardial infarction, coronary revascularization within the preceding 3 months, or alcohol abuse. No patient had taken any lipid-lowering agent, hormone replacement therapy, or antioxidant vitamin supplements during the 2 months preceding our study. Activity levels of the subjects were not monitored. Clinical characteristics of these patients are summarized in Table 1. Each of 44 patients in 5 groups was randomly assigned to either placebo or atorvastatin 10, 20, 40, or 80 mg, respectively, once daily during a 2-month treatment period. Allocation concealment was achieved by using envelopes with the collaboration of a statistician. Forty-four patients taking placebo and 42, 44, 43, and 40 patients taking atorvastatin 10, 20, 40, and 80 mg, respectively, finished the study (Fig. 1). Nineteen patients taking placebo and 18, 18, 20, and 18 patients taking atorvastatin 10, 20, 40, and 80 mg, respectively, had metabolic syndrome or type 2 diabetes.
Table 1.
Baseline Characteristics of the Study Population
| Placebo (n = 44) |
Atorvastatin 10 mg (n = 42) |
Atorvastatin 20 mg (n = 44) |
Atorvastatin 40 mg (n = 43) |
Atorvastatin 80 mg (n = 40) |
|
|---|---|---|---|---|---|
| Risk factors | |||||
| Current smoking | 7 (16) | 7 (17) | 7 (16) | 8 (19) | 7 (18) |
| Metabolic syndrome | 10 (23) | 8 (19) | 9 (21) | 10 (23) | 10 (25) |
| Diabetes | 9 (21) | 10 (24) | 9 (21) | 10 (23) | 8 (20) |
| Medications | |||||
| Beta-adrenergic blockers | 10 (23) | 12 (29) | 12 (27) | 13 (30) | 11 (28) |
| Calcium-channel blockers | 5 (16) | 6 (14) | 8 (18) | 7 (16) | 6 (15) |
Values are n (%).
Figure 1. Flow Chart.
Atorva = atorvastatin.
Laboratory assays
Assays for lipids, glucose, adiponectin, high-sensitivity C-reactive protein (hsCRP), and insulin were performed as previously described (12-14), and assays for HbA1C by high-performance liquid chromatography assay (VARIANT II TURBO, BIO-RAD, Inc., Hercules, California) were performed as well. Quantitative Insulin-Sensitivity Check Index (QUICKI) was calculated as follows: QUICKI = 1/[log(insulin)+log(glucose)] (15).
Statistical analysis
Data are expressed as mean ± SD or median (range 25% to 75%). We used Student paired t or Wilcoxon signed rank test to compare values between baseline and treatment at 2 months. We used 1-way analysis of variance (ANOVA) or Kruskal-Wallis ANOVA on ranks to compare baseline or treatment effects among treatment groups. Student-Newman-Keuls multiple comparison procedures for post-hoc pair-wise comparisons were routinely used when the omnibus test was significant. An ANOVA indicates group differences, and post hoc analysis shows drug different from placebo. We calculated that 35 subjects/group would provide 80% power for detecting an absolute increase of 0.15% or greater in HbA1C between baseline and atorvastatin 10 mg, with α = 0.05 on the basis of previous studies (14). The comparison of HbA1C was prospectively designated as the primary end point of the study. A value of p < 0.05 was considered to represent statistical significance. All other end points were considered secondary. Results for secondary end points were not considered definitive, and p values for secondary end points were presented unadjusted for multiple comparisons.
Results
All patients
There were no significant differences between treatment groups for any of the baseline parameters measured (Table 2).
Table 2.
Effects of Placebo or Atorvastatin on Lipids and Endocrine Parameters in Hypercholesterolemic Patients
| Placebo (n = 44) |
Atorvastatin 10 mg (n = 42) |
Atorvastatin 20 mg (n = 44) |
Atorvastatin 40 mg (n = 43) |
Atorvastatin 80 mg (n = 40) |
Global ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | ||
| Age (yrs) | 54 ± 11 | 56 ± 10 | 58 ± 9 | 59 ± 12 | 57 ± 11 | 0.351 | |||||
| Sex (M:F) | 23:21 | 21:21 | 21:23 | 22:21 | 20:20 | ||||||
| BMI (kg/m2) | 24.8 ± 2.4 | 24.7 ± 2.3 | 24.8 ± 3.4 | 24.7 ± 3.0 | 24.9 ± 3.4 | 24.9 ± 3.3 | 25.1 ± 3.0 | 25.0 ± 3.0 | 24.9 ± 3.4 | 24.8 ± 3.3 | 0.929 |
| Body weight (kg) | 65.9 ± 8.8 | 65.8 ± 8.7 | 61.3 ± 8.7 | 61.1 ± 8.4 | 63.4 ± 9.5 | 63.2 ± 9.3 | 63.2 ± 8.0 | 63.1 ± 8.0 | 63.2 ± 7.2 | 63.0 ± 7.2 | 0.990 |
| Lipids (mg/dl) | |||||||||||
| Total cholesterol | 240 ± 32 | 228 ± 27* | 238 ± 34 | 172 ± 28‡§ | 245 ± 37 | 161 ± 33‡§ | 242 ± 31 | 144 ± 28‡§ | 253 ± 41 | 145 ± 31‡§ | <0.001 |
| Triglycerides | 172 ± 89 | 181 ± 95 | 152 ± 69 | 131 ± 50* | 157 ± 71 | 127 ± 53†§ | 179 ± 98 | 123 ± 54‡§ | 164 ± 88 | 118 ± 40‡§ | <0.001 |
| LDL cholesterol | 154 ± 29 | 145 ± 30* | 156 ± 31 | 95 ± 25‡§ | 159 ± 33 | 83 ± 29‡§ | 155 ± 27 | 73 ± 18‡§ | 169 ± 38 | 74 ± 23‡§ | <0.001 |
| Apo B | 114 ± 27 | 110 ± 21 | 115 ± 19 | 76 ± 13‡§ | 117 ± 24 | 73 ± 22‡§ | 116 ± 24 | 64 ± 14‡§ | 120 ± 23 | 64 ± 17‡§ | <0.001 |
| HDL cholesterol | 50 ± 11 | 47 ± 12 | 51 ± 12 | 50 ± 14 | 54 ± 13 | 53 ± 14 | 51 ± 12 | 46 ± 12‡ | 52 ± 11 | 47 ± 12† | 0.084 |
| Apo A-I | 140 ± 22 | 139 ± 23 | 139 ± 23 | 146 ±27* | 137 ± 20 | 145 ± 25† | 131 ± 19 | 135 ± 22 | 134 ± 20 | 141 ± 29* | 0.148 |
| Inflammation hsCRP (mg/l) | 1.00 (0.65–2.30) | 0.95 (0.60–1.70) | 0.95 (0.50–3.10) | 0.75 (0.40–1.40)* | 1.00 (0.45–2.00) | 0.70 (0.40–1.20)† | 1.00 (0.53–2.30) | 0.70 (0.43–1.38)* | 1.00 (0.65–2.00) | 0.60 (0.40–1.80) | 0.535 |
| HbA1C (%) | 5.8 ± 0.5 | 5.8 ± 0.6 | 5.8 ± 0.6 | 6.0 ± 0.6‡ | 5.9 ± 0.8 | 6.2 ± 0.9‡§ | 6.1 ± 0.8 | 6.4 ± 1.0†§ | 6.1 ± 0.8 | 6.4 ± 1.1*§ | 0.008 |
| Insulin resistance | |||||||||||
| ADP (μg/ml) | 3.3 ± 2.0 | 3.4 ± 2.0 | 2.8 ± 2.4 | 2.5 ± 2.0 | 3.1 ± 2.4 | 2.5 ± 1.8†§ | 3.4 ± 2.5 | 3.1 ± 2.5 | 3.2 ± 2.4 | 3.0 ± 2.6*§ | 0.183 |
| Insulin (μU/ml) | 7.90 ± 3.88 | 7.46 ± 2.76 | 8.12 ± 5.06 | 9.04 ± 6.91 | 8.07 ± 4.41 | 9.92 ± 6.24†§ | 8.29 ± 5.25 | 10.07 ± 5.51‡§ | 7.98 ± 6.98 | 11.07 ± 12.65†§ | 0.009 |
| Glucose (μg/dl) | 103 ± 17 | 102 ± 17 | 106 ± 18 | 106 ± 20 | 108 ± 21 | 113 ± 23 | 113 ± 24 | 116 ± 25 | 109 ± 22 | 109 ± 25 | 0.493 |
| QUICKI | 0.35 ± 0.02 | 0.35 ± 0.02 | 0.36 ± 0.04 | 0.35 ± 0.04 | 0.35 ± 0.04 | 0.34 ± 0.03†§ | 0.35 ± 0.03 | 0.34 ± 0.04‡§ | 0.36 ± 0.04 | 0.34 ± 0.03† | 0.033 |
Data are expressed as mean ± SDor median. There were no significant differences among baseline values. Global analysis of variance (ANOVA) indicates group differences. Quantitative Insulin-Sensitivity Check Index (QUICKI) = 1/[log (insulin) + log (glucose)] (15).
p < 0.05,
p < 0.01,
p < 0.001 for comparison with each baseline value;
p < 0.05 for comparison with the value after therapy with placebo.
ADP = adiponectin; Apo = apolipoprotein; BMI = body mass index; HbA1C = glycated hemoglobin A1C; HDL = high-density lipoprotein; hsCRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein.
EFFECTS ON LIPIDS
Placebo treatment resulted in slightly reduced total and LDL cholesterol levels from baseline. Atorvastatin 10, 20, 40, and 80 mg significantly reduced total cholesterol (mean changes: 28%, 34%, 40%, and 43%, respectively), triglycerides (mean changes: 2%, 10%, 22%, and 17%, respectively), LDL cholesterol (mean changes: 39%, 47%, 52%, and 56%, respectively), and apolipoprotein B levels (mean changes: 33%, 37%, 42%, and 46%, respectively) from baseline (all p < 0.001) after 2 months of administration. Importantly, these effects of atorvastatin were significantly greater than the effects of placebo (p < 0.001).
EFFECTS ON hsCRP
Placebo treatment did not significantly change hsCRP from baseline after 2 months of administration. By contrast, atorvastatin 10, 20, and 40 mg significantly reduced hsCRP from baseline (all p < 0.05) after 2 months of administration. However, these effects of atorvastatin were not significant when compared with placebo treatment (p = 0.535).
EFFECTS ON HbA1C, ADIPONECTIN, AND INSULIN RESISTANCE
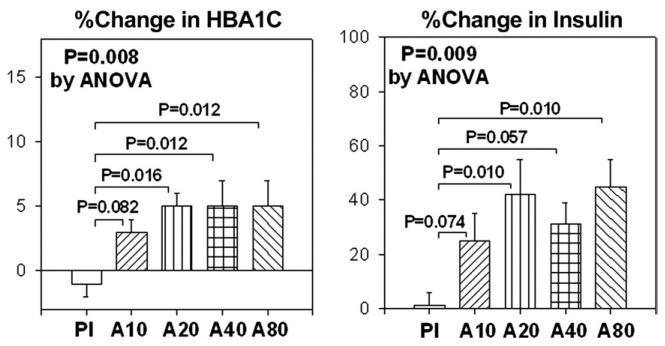
Placebo treatment did not significantly change HbA1C levels from baseline. Atorvastatin 10, 20, 40, and 80 mg significantly increased HbA1C levels (mean changes: 2%, 5%, 5%, and 5%, respectively) from baseline (all p < 0.05) after 2 months of administration. These effects of atorvastatin were also significant when compared with effects of placebo treatment (p = 0.008) (Fig. 2).
Figure 2. Percent Change in HbA1C and Insulin.
The SEM is identified by the bars. ANOVA = analysis of variance; A10 = atorvastatin 10 mg; A20 = atorvastatin 20 mg; A40 = atorvastatin 40 mg; A80 = atorvastatin 80 mg; HbA1C = glycated hemoglobin A1C; Pl = placebo.
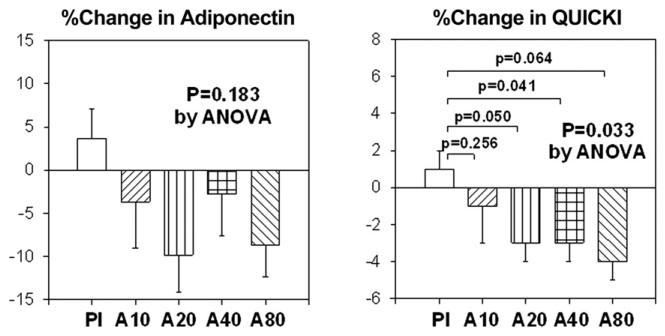
Placebo treatment did not significantly change fasting insulin or glucose levels from baseline. Atorvastatin 10, 20, 40, and 80 mg did not significantly change glucose levels after 2 months of administration when compared with baseline. Atorvastatin 10, 20, 40, and 80 mg substantially increased fasting insulin levels (mean changes: 25%, 42%, 31%, and 45%, respectively) after 2 months of therapy when compared with baseline (p = 0.222, p = 0.01, p < 0.001, and p = 0.005, respectively). These effects of atorvastatin to raise fasting insulin levels were significant when compared with placebo treatment (p = 0.009) (Fig. 2). Placebo treatment did not significantly change plasma adiponectin levels or insulin sensitivity relative to baseline measurements. However, atorvastatin 10, 20, 40, and 80 mg all decreased plasma adiponectin levels (mean changes: 4%, 10%, 3%, and 9%, respectively) after 2 months of therapy when compared with baseline (p = 0.124, p = 0.004, p = 0.084, and p = 0.040, respectively). However, when compared with placebo treatment, these effects of atorvastatin to reduce adiponectin levels were not significant (p = 0.183). Atorvastatin 10, 20, 40, and 80 mg decreased insulin sensitivity (mean changes: 1%, 3%, 3%, and 4%, respectively) after 2 months of therapy when compared with baseline (p = 0.312, p = 0.008, p < 0.001, and p = 0.008, respectively). Moreover, when compared with placebo treatment, the effect of atorvastatin to reduce insulin sensitivity was significant (p = 0.033) (Fig. 3). The magnitude of percent changes in HbA1C and adiponectin were not significantly different among the 4 different doses of atorvastatin tested. We investigated whether changes in hsCRP, HbA1C, insulin, adiponectin, or insulin resistance were related to changes in lipoprotein levels. There were no significant correlations.
Figure 3. Percent Change in Adiponectin and QUICKI.
The SEM is identified by the bars. QUICKI = Quantitative Insulin-Sensitivity Check Index; other abbreviations as in Figure 2.
Patients with metabolic syndrome/type 2 diabetes
We performed a subgroup analysis of our data in subjects with metabolic syndrome or type 2 diabetes (Table 3). The effects of atorvastatin versus placebo in the group of patients without metabolic syndrome/type 2 diabetes were not significantly different from those of the group of patients with metabolic syndrome/type 2 diabetes.
Table 3.
Effects of Placebo or Atorvastatin on Lipids and Endocrine Parameters in Hypercholesterolemic Patients With Metabolic Syndrome/Type 2 Diabetes
| Placebo (n = 19) |
Atorvastatin 10 mg (n = 18) |
Atorvastatin 20 mg (n = 18) |
Atorvastatin 40 mg (n = 20) |
Atorvastatin 80 mg (n = 18) |
Global ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | ||
| Age (yrs) | 57 ± 8 | 57 ± 6 | 61 ± 10 | 60 ± 9 | 57 ± 10 | 0.588 | |||||
| Sex (M:F) | 10:9 | 10:8 | 10:8 | 9:11 | 8:10 | ||||||
| BMI (kg/m2) | 25.8 ± 2.3 | 25.7 ± 2.2 | 26.2 ± 4.1 | 26.0 ± 3.5 | 26.6 ± 3.9 | 26.6 ± 3.8 | 26.7 ± 3.2 | 26.6 ± 3.1 | 27.4 ± 2.8 | 27.2 ± 2.8 | 0.890 |
| Body weight (kg) | 68.2 ± 8.6 | 67.8 ± 8.5 | 64.0 ± 8.8 | 63.8 ± 8.3 | 65.5 ± 9.6 | 65.3 ± 9.1 | 64.9 ± 7.4 | 64.7 ± 7.4 | 66.1 ± 4.9 | 65.9 ± 5.0 | 0.890 |
| Lipids (mg/dl) | |||||||||||
| Total cholesterol | 236 ± 39 | 225 ± 30 | 237 ± 24 | 169 ± 21‡§ | 251 ± 32 | 168 ± 39‡§ | 249 ± 33 | 150 ± 33‡§ | 252 ± 39 | 146 ± 38‡§ | <0.001 |
| Triglycerides | 209 ± 101 | 203 ± 92 | 164 ± 68 | 140 ± 51 | 171 ± 76 | 141 ± 62 | 190 ± 111 | 135 ± 61† | 160 ± 70 | 114 ± 39† | 0.308 |
| LDL cholesterol | 149 ± 33 | 140 ± 35 | 155 ± 18 | 94 ± 15‡§ | 165 ± 23 | 89 ± 35‡§ | 163 ± 30 | 78 ± 22‡§ | 171 ± 29 | 79 ± 25‡§ | <0.001 |
| Apo B | 117 ± 30 | 114 ± 19 | 116 ± 17 | 80 ± 13‡§ | 126 ± 23 | 80 ± 26‡§ | 124 ± 22 | 68 ± 17‡§ | 127 ± 25 | 65 ± 20‡§ | <0.001 |
| HDL cholesterol | 44 ± 11 | 44 ± 10 | 49 ± 13 | 48 ± 13 | 52 ± 13 | 51 ± 10 | 48 ± 12 | 46 ± 12 | 49 ± 10 | 44 ± 11* | 0.316 |
| Apo A-I | 131 ± 19 | 131 ± 26 | 134 ± 20 | 143 ± 23* | 136 ± 18 | 140 ± 15 | 129 ± 21 | 131 ± 19 | 132 ± 20 | 135 ± 27 | 0.369 |
| Inflammation hsCRP (mg/l) | 1.70 (0.73–2.68) | 1.00 (0.63–2.35) | 1.70 (0.50–3.50) | 0.80 (0.60–1.20)* | 1.40 (0.70–2.40) | 0.75 (0.40–1.20)† | 1.65 (0.95–3.55) | 0.75 (0.50–1.60)* | 1.75 (1.00–3.60) | 1.35 (0.40–2.80) | 0.436 |
| HbA1C (%) | 5.9 ± 0.6 | 5.8 ± 0.7 | 6.0 ± 0.7 | 6.3 ± 0.8‡§ | 6.1 ± 1.0 | 6.5 ± 1.1*§ | 6.5 ± 0.9 | 6.9 ± 1.0*§ | 6.4 ± 0.7 | 6.9 ± 1.3*§ | 0.004 |
| Insulin resistance | |||||||||||
| ADP (μg/ml) | 2.3 ± 0.9 | 2.3 ± 1.0 | 2.9 ± 2.4 | 2.8 ± 2.4 | 2.8 ± 1.9 | 2.1 ± 1.5*§ | 3.1 ± 1.7 | 3.0 ± 1.8 | 3.2 ± 1.7 | 2.9 ± 2.0§ | 0.147 |
| Insulin (μU/ml) | 7.99 ± 2.94 | 7.69 ± 2.60 | 9.03 ± 6.07 | 10.79 ± 9.03 | 7.81 ± 4.75 | 11.30 ± 7.88†§ | 9.71 ± 6.06 | 11.82 ± 6.04* | 7.96 ± 5.34 | 11.69 ± 9.13†§ | 0.011 |
| Glucose (mg/dl) | 110 ± 24 | 102 ± 23 | 116 ± 19 | 116 ± 22 | 118 ± 22 | 127 ± 25 | 127 ± 30 | 130 ± 30 | 118 ± 28 | 119 ± 32 | 0.157 |
| QUICKI | 0.35 ± 0.02 | 0.35 ± 0.02 | 0.35 ± 0.04 | 0.34 ± 0.04 | 0.35 ± 0.03 | 0.33 ± 0.03*§ | 0.34 ± 0.03 | 0.32 ± 0.03†§ | 0.35 ± 0.04 | 0.33 ± 0.02†§ | 0.006 |
Data are expressed as mean ± SD or median. There were no significant differences among each baseline values. Global analysis of variance (ANOVA) indicates group differences. Quantitative Insulin-Sensitivity Check Index (QUICKI) = 1/[log (insulin) + log (glucose)] (15).
p < 0.05,
p < 0.01,
p < 0.001 for comparison with each baseline value;
p < 0.05 for comparison with the value after therapy with placebo.
Abbreviations as in Table 2.
Discussion
In the present study, our primary outcome of HbA1C levels was significantly increased in patients treated with atorvastatin. This was accompanied by increased fasting insulin levels, reduced insulin sensitivity, and lower adiponectin levels. Because HbA1C levels are a sensitive indicator of ambient glycemia, our results strongly suggest that atorvastatin causes glucose intolerance that is due, in part, to decreased insulin sensitivity. These off-target detrimental metabolic effects of atorvastatin occur despite beneficial effects to improve lipid profile, flow-mediated dilation, and circulating pro-inflammatory markers. Furthermore, there were no significant correlations between lipoprotein changes and endothelial dysfunction and metabolic parameters. We previously observed that simvastatin reduces adiponectin levels and insulin sensitivity (12) and only pravastatin improved insulin sensitivity, even though both statins caused comparable improvements in lipid profiles and endothelium-dependent vasodilation in hypercholesterolemic patients (13). Thus, different statins have differential metabolic effects that might depend on their lipophilic properties.
Statin therapy might directly alter adiponectin levels independent of adiposity. In 3T3-L1 adipocytes, pravastatin increases expression of adiponectin messenger ribonucleic acid and enhances adiponectin secretion into conditioned media. This corresponds to increased plasma levels of adiponectin and enhanced insulin sensitivity in C57BL/6J mice without changes in body weight (16). Simvastatin inhibits the glucose-stimulated elevations of free calcium in beta cells, leading to suppressed insulin secretion (4). Atorvastatin reduces sensitivity to insulin in rats (5). Atorvastatin but not pravastatin attenuates expression of the glucose transporter GLUT-4 in adipocytes, impairing glucose tolerance (6).
It is not clear why atorvastatin has beneficial metabolic actions in some studies but not in others.
The effects of atorvastatin might be different between patients with and without metabolic syndrome and diabetes. However, when we compared effects of atorvastatin on metabolic parameters in patients with and without metabolic syndrome and diabetes, there were no significant differences.
In the current study, the effects of atorvastatin on fasting glucose levels were not significant; however, the effects of atorvastatin on fasting insulin levels and HbA1C levels were significant when compared with placebo. The surrogate measure of insulin sensitivity we employed, QUICKI, is the most extensively validated and accurate surrogate index of insulin sensitivity currently available in humans; QUICKI measures primarily hepatic insulin resistance (15,17). Under most conditions, peripheral and hepatic insulin sensitivity runs in parallel. Glycated hemoglobin A1C represents prevailing glycemia over long periods of time. Elevated HbA1C is a reflection of glucose intolerance. Glucose intolerance results from impaired insulin sensitivity and/or insulin secretion and/or non-insulin–mediated glucose disposal.
Clinical studies have demonstrated that lipophilic statins, atorvastatin, simvastatin, and rosuvastatin might increase the onset of new diabetes (7-9). A nested case-control study reported that an adjusted odds ratio for simvastatin use alone compared with nonexposed odds ratio of 1.0 and for pravastatin use alone compared with nonexposed odds ratio of 0.7 (18). Indeed, pravastatin reduces the rate of onset of new diabetes by 30% (19), although it does not in another study (20). Meta-analysis of randomized controlled trials suggests potential differences between statins (10). Thus, it is possible that different statins might have differential effects on the rate of new onset diabetes, but to be certain, head-to-head comparative studies are required.
In patients with type 2 diabetes the benefits of lowering glucose levels by any means is unclear. In several recently published clinical trials, improving glycemic control did not reduce cardiovascular events (21). This is a complicated issue. In patients with early reversible cardiovascular and metabolic pathophysiology benefits from lower glycemia might diminish cardiovascular risk (22). However, in advanced patients with irreversible atherosclerotic disease, it might be unfavorable, due to hypoglycemia, weight gain, and other adverse effects (21).
We reported that statin lowers CRP levels in hyperlipidemic coronary patients (23). In the current study, we observed that atorvastatin lowers CRP levels relative to baseline levels in hyperlipidemic patients. However, these results did not achieve statistical significance when compared with placebo. This might be due, in part, to very low baseline CRP levels in our study subjects.
Acknowledgment
The authors thank Myron A. Waclawiw, PhD (Office of Biostatistics Research, National Heart, Lung, and Blood Institute, NIH), for his comments regarding statistical comments.
This study was supported by grants from established investigator award (2005-1, 2006-1) (Dr. Koh), Gachon University Gil Hospital, and the Intramural Research Program, National Center for Complementary and Alternative Medicine, National Institutes of Health (NIH) (Dr. Quon).
Abbreviations and Acronyms
- ANOVA
analysis of variance
- CI
confidence interval
- HbA1C
glycated hemoglobin A1C
- hsCRP
high-sensitivity C-reactive protein
- LDL
low-density lipoprotein
- QUICKI
Quantitative Insulin-Sensitivity Check Index
Footnotes
Presented at the European Society of Cardiology 2009, Barcelona, Spain, August 29 to September 2, 2009, and American Heart Association Scientific Sessions 2009, Orlando, Florida, November 14 to 18, 2009.
REFERENCES
- 1.Kim J, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 2.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–91. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 3.Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648–57. doi: 10.1016/s0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 4.Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol. 1999;126:1205–13. doi: 10.1038/sj.bjp.0702397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanda M, Satoh K, Ichihara K. Effects of atorvastatin and pravastatin on glucose tolerance in diabetic rats mildly induced by streptozotocin. Biol Pharm Bull. 2003;26:1681–4. doi: 10.1248/bpb.26.1681. [DOI] [PubMed] [Google Scholar]
- 6.Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycemic control. Diabetologia. 2006;49:1881–92. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 7.Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 8.Sever PS, Dahlof B, Poulter NR, et al. ASCOT investigators Prevention of coronary and stroke events with atorvastatin in hyper-tensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial– Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Danielson E, Fonseca FA, et al. for the JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 10.Coleman CI, Reinhart K, Kluger J, White CM. The effect of statins on the development of new-onset type 2 diabetes: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2008;24:1359–62. doi: 10.1185/030079908x292029. [DOI] [PubMed] [Google Scholar]
- 11.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 12.Koh KK, Quon MJ, Han SH, et al. Simvastatin improves flow-mediated dilation, but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Diabetes Care. 2008;31:776–82. doi: 10.2337/dc07-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh KK, Quon MJ, Han SH, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis. 2009;204:483–90. doi: 10.1016/j.atherosclerosis.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of patients with combined hyperlipidemia. J Am Coll Cardiol. 2005;45:1649–53. doi: 10.1016/j.jacc.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 16.Takagi T, Matsuda M, Abe M, et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis. 2008;196:114–21. doi: 10.1016/j.atherosclerosis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 18.Jick SS, Bradbury BD. Statins and newly diagnosed diabetes. Br J Clin Pharmacol. 2004;58:303–9. doi: 10.1111/j.1365-2125.2004.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–62. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]
- 20.Keech A, Colquhoun D, Best J, et al. for the LIPID Study Group Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care. 2003;26:2713–21. doi: 10.2337/diacare.26.10.2713. [DOI] [PubMed] [Google Scholar]
- 21.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298–304. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Reaven PD, Moritz TE, Schwenke DC, et al. for the Veterans Affairs Diabetes Trial Intensive glucose lowering therapy reduces cardiovascular disease events in VADT participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58:2642–8. doi: 10.2337/db09-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh KK, Son JW, Ahn JY, et al. Comparative effects of diet and statin on NO bioactivity and matrix metalloproteinases in hypercholesterolemic patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:e19–23. doi: 10.1161/01.atv.0000030997.02059.bb. [DOI] [PubMed] [Google Scholar]