Abstract
CD74, a transmembrane glycoprotein that associates with MHC II, is an important chaperone that regulates antigen presentation for immune response. In addition, CD74 is the receptor for macrophage migration-inhibitory factor which, when bound to CD74, initiates survival pathways and cell proliferation. Formalin fixed, paraffin embedded clinical specimens were evaluated by immunohistochemical procedures for expression of CD74. Overall, expression of CD74 within gastrointestinal carcinomas showed a statistically greater expression than in the normal tissue counterparts (P<0.001 or better). CD74 expression was observed in 95% of pancreatic carcinomas with the majority of cases presenting a mostly intense, diffuse labeling pattern. The results suggested a trend towards greater expression within the higher grade carcinomas (P=0.06). Colorectal and gastric carcinomas gave similar results with 60% and 86%, respectively, positive for CD74 with an intense, diffuse staining pattern. We hypothesized that precursor lesions would express levels of CD74 as high, or higher, than their respective carcinomas, since activation of survival pathways would be of particular importance at the early stages of neoplastic development. For PanIN lesions there was greater expression of CD74 within higher grade, PanIN-3 lesions, whereas the colonic adenomas showed no such trend, but overall, a higher frequency and intensity of CD74 labeling than was observed within the colon carcinomas. These findings are supportive of a role for CD74 in the development and maintenance of gastrointestinal neo-plasia, and provide a rationale for development of therapeutic agents that are able to block CD74 function, specifically within the tumor cell.
Keywords: CD74, invariant chain, pancreatic carcinoma, colon carcinoma, gastric carcinoma
Introduction
CD74 (invariant chain, Ii) is a type-II transmembrane glycoprotein that associates with the MHC II a and b chains and directs the transport of the abIi complexes to intracellular endosomes and lysosomes, thus initiating antigen presentation for immune response [1-3]. Within normal tissues, CD74 is expressed at high levels by antigen-presenting cells (APC), including B cells, monocytes, macrophages, dendritic cells, and Langerhans cells [4]. Although cell surface expression of CD74 is low in many cell types, rapid internalization with concomitant re-expression at the cell surface provides a steady-state level of CD74-MHC II complex at the cell surface that is sufficient for biological function [5,6].
In addition to its role in antigen presentation, the binding of the proinflammatory cytokine, macrophage migration-inhibitory factor (MIF), to a cell surface CD74 initiates signaling cascades resulting in cell survival [7]. Although MIF is able to bind to CD74 itself, it is the CD74-CD44 complex that generates intracellular signals that activate cell proliferation and survival pathways [8,9]. MIF binding to the CD74 receptor can also up-regulate expression of CD74 at the cell surface [10].
It is perhaps relevant that with respect to these biological functions, CD74 is also expressed on a variety of malignant cells. Its expression has been observed in ∼90% of B-cell malignancies, as well as the majority of cell lines derived from these cancers [11,12]. CD74 expression has also been described in non-hematologic cancers, including gastrointestinal [13, 14,15], renal [16], non-small cell lung [17] and, recently, glioblastoma cell lines [18]. CD74 expression in many of these cancers has been suggested to be a prognostic factor with higher relative expression of CD74 behaving as a marker of tumor progression or poor clinical outcome [19,20]. The biological functions of CD74, combined with its expression on malignant cells and limited expression on normal tissues, suggest CD74 as a potential therapeutic target. In the present report, we describe the morphological distribution of CD74 within invasive carcinomas of the gastrointestinal system, their respective precursor lesions and inflammation, as a basis for evaluating the role of this protein in the development and/or maintenance of the neoplastic state.
Materials and methods
Materials
Tissue microarrays were purchased from ISU-ABXIS through Accurate Chemical & Scientific Corp (Westbury, NY) (pancreatic cancer A207-IV and -V, colon cancer A203-IV and -VI, and stomach cancer A209-II) and US BioMax (Rockville, MD) (pancreatic cancer PA961, colon cancer BC051110, and stomach cancer ST811). In addition, standard whole sections of formalin-fixed, paraffin-embedded carcinomas were also evaluated. A microarray containing core tissues of PanIN lesions was kindly provided by Dr. Ralph Hruban at Johns Hopkins Medical Institutes (Baltimore, MD). A colorectal carcinoma progression microarray that included adenoma-tous lesions was obtained from the Cooperative Human Tissue Network's Mid-Atlantic Division at the University of Virginia Health System (Charlottesville, VA). Murine and humanized versions of the LL1 antibody (mLL1 and hLL1 [milatuzumab], respectively) reactive with the CD74 protein [21] were obtained from Immu-nomedics, Inc. (Morris Plains, NJ). A non-binding isotype-matched control antibody, murine Ag8, was purified in our laboratory from the P3X63-Ag8 murine myeloma. Additional control MAbs, hMN14 (anti-CEACAM5; labetuzumab) and hA20 (anti-CD20; veltuzumab) for evaluation of human cell lines by flow cytometry and immuno-histochemistry of xenotransplants grown in athymic nude mice, were also obtained from Immunomedics, Inc.
Immunohistochemistry
Immunohistochemistry was performed essentially as described previously [22]. Unstained sections were deparaffinized by routine methods. The sections were then heated to 95°C for 20 min in a pH-6.0 citrate buffer Target Retrieval Solution (Dako, Carpinteria, CA), allowed to cool to room temperature, and then quenched with 3% H2O2 in methanol for 15 minutes at room temperature. Primary antibodies, murine for human tissues and humanized for xenotransplants, were then employed at a concentration of 5 μg/ml followed by use of the appropriate species-reactive ABC Vectastain kit (Vector Laboratories, Burlingame, CA) for labeling the tissues. CD74 expression was scored for intensity (scale of +1 to +4) and pattern of distribution: negative, <1% of tumor cells were labeled; focal - between 1% and 25% of tumor cells were labeled; diffuse – greater than 25% of tumor cells were labeled. Only the carcinoma cells were considered for assessment of CD74 expression.
Flow cytometry
To evaluate antigen expression levels in human tumor cell-lines of gastrointestinal origin, flow cytometry was performed with direct-labeled, ALEXA-488-conjugated, hMAbs. The ALEXA-488 conjugates were prepared by the manufacturer's instructions (Invitrogen Corp., Carlsbad, CA). For certain studies, cells were permeabilized by use of a BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit(Becton Dickinson, San Jose, CA), prior to labeling with fluorescent MAbs. All flow cytometry studies were performed and analyzed using a FACSCalibur (Becton Dickinson, San Jose, CA).
Statistical analyses
Data are expressed as the mean ± standard deviation. Statistical differences between groups were evaluated by comparison of antigen content as defined by the above scoring paradigms. Comparison of any two groups was performed by use of Student's t-test by use of the Med-Calc statistical software package (version 7.5; Med-Calc, Mariakerke, Belgium).
Results
CD74 is expressed by several cell types normally present within organs of the gastrointestinal system (and other organs as well). Thus, stromal cells, including vascular endothelium and immune and inflammatory cells were clearly positive to varying levels of intensity and distribution within all normal and neoplastic tissues examined. CD74 expression within fibroblasts was never observed. Tissue sections of Raji and/or Daudi B-cell lymphoma cell lines grown as xenografts in athymic mice were included with all immunostaining studies as positive controls for CD74 expression.
Pancreatic tissues
The frequency of CD74 expression was highest within pancreatic carcinomas (Table 1). Positive antigen expression was observed in 53 of 56 specimens (95%) with the overwhelming majority of CD74-positive specimens (91%) displaying a diffuse, heterogeneous pattern of marker expression (Figures 1A and 1C). The pattern of distribution (focal vs diffuse) did not correlate with tumor grade or stage of disease. However, the data suggested a trend that approached statistical significance (P=0.06; nonparametric Spearman correlation) between intensity of stain and grade of tumor differentiation; well-differentiated tumors exhibited lower levels of stain intensity (2.50 + 0.84, mean ± SD; N=6) than moderately-well differentiated (2.83 + 1.14; N=29) and poorly differentiated tumors (3.1 + 0.94; N=21). For the most part, the CD74 protein was observed in the apical cytoplasm of the cell with intensification at the cell surface (Figure 1B). Of the 3 CD74-negative specimens, 2 were poorly differentiated and 1 was moderately-well differentiated.
Table 1.
Immunohistochemical staining results for expression of CD74
| Mean Intensity | Labeling Patterna | ||||
|---|---|---|---|---|---|
| Diagnosis | N | Scale: 1-4 | Focal | Diffuse | Total Positiveb |
| Pancreatic CA | 56 | 2.89 ± 1.04 | 5 | 48 | 53 (95%) |
| PanIN precursor | 22 | 1.14 ± 1.21c | 5 | 7 | 12 (55%) |
| Pancreatitis | 19 | 2.42 ± 1.07d | 4 | 13 | 17 (89%) |
| Normal pancreas | 11 | 1.44 ± 0.53c | 7 | 2 | 9 (82%) |
| Colon CA | 98 | 1.54 ± 1.44 | 14 | 45 | (60%) |
| Colonic Adenoma | 13 | 2.69 ± 1.38e | 3 | 8 | 11 (85%) |
| Colitis | 11 | 1.5 | 0 | 2 | 2 (18%) |
| Normal Colon | 19 | 0.58 ± 1.38c | 3 | 3 | 6 (32%) |
| Gastric CA | 56 | 3.25 ± 1.40 | 0 | 48 | 48 (86%) |
| Gastritis | 47 | 1.13 ±1.01c | 0 | 29 | 29 (62%) |
| Normal Stomach | 8 | 2.00 ± 0.82c | 1 | 6 | 7 (88%) |
| Total CA | 210 | 19 | 141 | 160 (76%) | |
Number of specimens with focal labeling (1%-25% of carcinoma cells positive) or diffuse labeling (>25% of carcinoma cells positive)
Total number of specimens with positive labeling of carcinoma cells; numbers in parenthesis represent the percentage of specimens labeled as positive with respect to the total number of specimens examined.
Statistically significant difference from CA group (P <0.001).
No significant difference from CA group.
Statistically significant difference from CA group (P<0.01).
Figure 1.
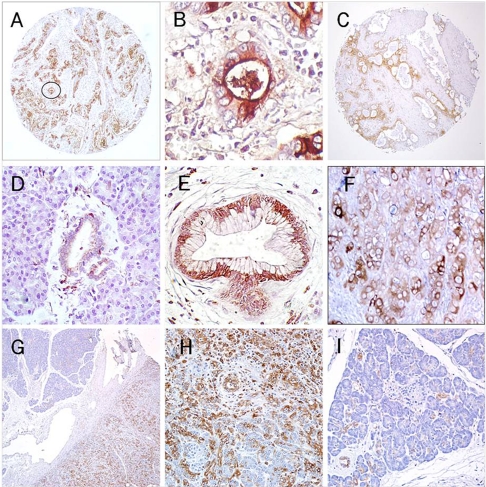
Expression of CD74 in pancreatic tissues. (A) and (C) (50×) – an intense diffuse reaction for CD74 expression is noted within specimens of invasive pancreatic carcinoma with labeling of the apical cytoplasm and cell surface evident in (B) (circled field from A; 400×). (D) normal pancreatic acinar cells are mostly negative with weak, limited labeling of normal ductal tissue (200×). (E) PanIN-3 lesion shows evidence of a mostly cytoplasmic/perinuclear staining (200×). (F) Focal, CD74 expression within the CaPan1 human pancreatic carcinoma cell line grown as a xenograft in athymic nude mice (200×). (G) A specimen demonstrating chronic pancreatitis with fields of both involved and noninvolved tissue (50×); (H) the involved fields demonstrate CD74 staining of acinar and ductal cells (100×), whereas in I – only focal staining of ductal tissue is evident within the noninvolved field (100×).
Within normal pancreas (N=11), CD74 was not observed in acinar and islet cells; however, ducts and ductules exhibited a weak, yet positive labeling (intensity = 1.44 ± 0.53) of the cytoplasm in 82% of specimens (Figure 1D). For the most part, only a few ductules within these specimens showed evidence of CD74 staining. Cell surface expression of CD74 was not apparent in these normal ductal cells.
Considering that CD74 has a specific role in activation of survival pathways [23] and that activation of these pathways might be involved as early procarcinogenic events in the development of a cancer [24], we next examined a group of PanIN lesions for expression of CD74. These lesions are considered to be the direct precursors towards development of invasive pancreatic carcinoma [25]. Overall, CD74 expression was observed in 55% of such lesions (N=22), which was significantly lower than that observed for the group of invasive carcinomas (P <0.002) (Figure 1E). However, all of the PanIN-3 lesions (N=6) were positive for CD74 with staining pattern and intensity statistically similar to the invasive pancreatic carcinomas (P=0.162). On the other hand, only 38% of the low grade, PanIN-1 and -2 lesions (together N=16) were positive.
We were also able to evaluate CD74 expression in surgical specimens obtained from patients diagnosed with chronic pancreatitis, a condition that provides significantly increased risk for development of pancreatic cancer. Of 19 specimens, 17 (89%) were CD74-positive with a mean intensity of 2.42 ± 1.07. In the majority of cases, a diffuse pattern of staining was observed with both acinar and ductal cells expressing the protein within the cytoplasm and at the cell surface. CD74 expression was specifically noted within inflamed, atrophic lobules, whereas in neighboring noninflamed lobules, CD74 was observed in a more focal pattern within apparently normal ductules, blood vessel endothelium and infiltrating inflammatory cells (Figures 1G, 1H, and 1I).
Colonic tissues
CD74 expression was also observed within colon carcinomas, but, at substantially lower frequency (60%) than observed for pancreatic carcinomas. The majority of positive specimens (45 of 59; 76%) displayed a diffuse pattern of antigen expression (Figures 2A and 2B), with an average staining intensity of only 1.54 ± 1.44 (Table 1), again, considerably lower than that observed for pancreatic carcinomas (P<0.001). Labeling of the protein was observed within the cytoplasm, with notable intensification of stain in the apical and perinuclear regions (Figures 2C and 2D), but with little to no staining observed at the cell surface. Expression of CD74 did not correlate with stage of disease or staining pattern.
Figure 2.
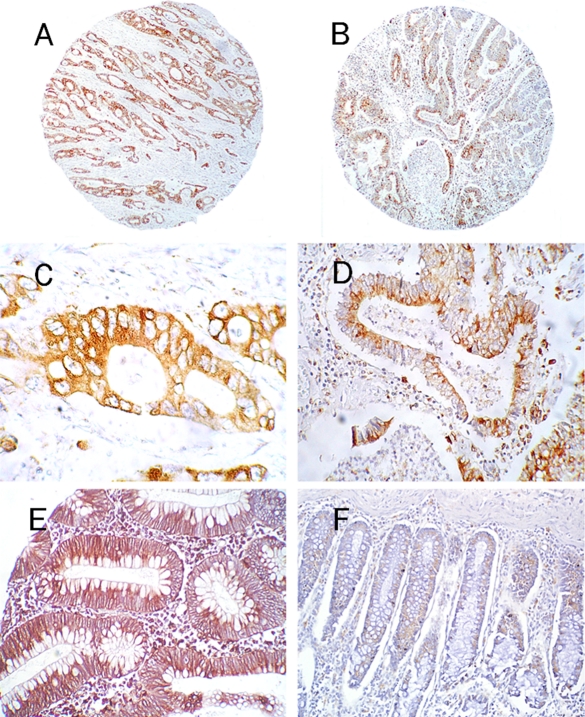
Expression of CD74 in colon tissues. A and B (50×) – invasive colon carcinomas display a mostly intense, diffuse labeling of CD74, with intensification in the perinuclear region of the cytoplasm, as noted in C and D (400× and 200×, respectively). In contrast to the pancreatic carcinomas, significant labeling at the cell surface was not observed. E – a low-grade adenoma shows intense, diffuse labeling of the cytoplasm beneath the mucin goblets (200×). F – a weak, diffuse labeling of normal colonic goblet cells was observed in most of the cases examined (100×).
Interestingly, colorectal adenoma had a statistically significant and considerably greater expression of CD74 as compared to the group of carcinomas (average staining intensity of 2.69 ± 1.38; P <0.01) (Figure 2E). Unfortunately, there were insufficient numbers of these precursor lesions (N=13) to evaluate possible differences in expression of CD74 between low and high grade lesions. Specimens of ulcerative colitis had very low expression of CD74; only 2 of 18 (18%) were positive with an average intensity of 1.5. In normal colon (N=20), staining was most obvious within the cytoplasm surrounding the mucin goblets, and was concentrated within the perinuclear region in many cells (Figures 2F). CD74 was expressed similarly at all levels of the crypts.
Gastric tissues
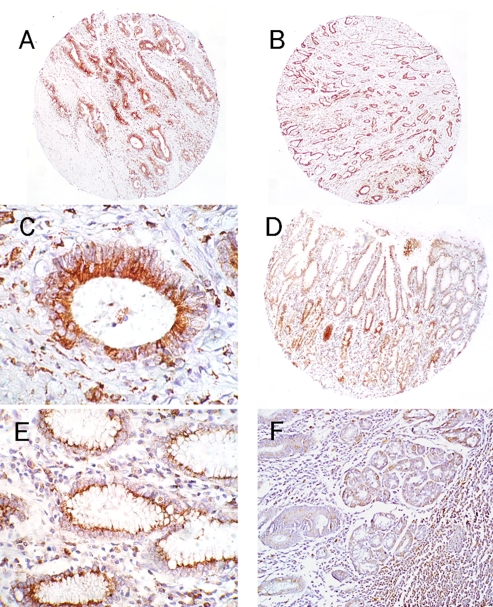
Similar to pancreatic carcinoma, the overwhelming majority of gastric carcinomas were CD74-positive (48 of 56; 86%) (Figures 3A and 3B), with localization of the marker to the apical and perinuclear region of the cytoplasm (Figure 3C). Within normal gastric mucosa (N=8), it appeared that mucin-containing glandular cells expressed the CD74 protein at high levels (Figure 3D). While cell-surface expression was occasionally observed, it was unremarkable compared to the cytoplasmic localization of the marker (Figure 3E). CD74 expression in specimens of gastritis was moderate in frequency (N=47, 62% positive); however, the average intensity of stain was quite low (1.13 ± 1.01) (Figure 3F).
Figure 3.
Expression of CD74 in gastric tissues. Gastric carcinomas usually presented with an intense, diffuse labeling of CD74 (A and B; 50×), and as with colorectal carcinomas, the major fraction of staining was localized to the cytoplasm (C; 400×). Moderate to sometimes intense labeling of CD74 is observed within normal stomach (D; 50×), mostly within the mucous secreting cells, with the major fraction of staining located within the perinuclear region of the cell (E; 200×). (F) a specimen of gastritis demonstrating weak staining of the gastric glands with significant CD74 positive inflammatory cells evident in the surrounding tissue (100×).
CD74 expression by gastrointestinal cancer cell-lines
To expand upon these studies, we examined a number of gastrointestinal carcinoma cell lines grown both in vitro and in vivo as xenotransplants in athymic nude mice, in order to identify a model that could be used for evaluation of the activity and function of CD74 in solid tumors. By flow cytometry employing MAbs conjugated with the fluorescent ALEXA-488 dye, we noted that CD74 expression at the cell surface of in vitro cultivated cells was unremarkable except for the CaPan1 pancreatic carcinoma and positive control Daudi lymphoma cells (Table 2). We also examined the levels of intracellular CD74 by permeabilization of the cells prior to labeling with fluorescent MAbs. As noted in the table, most of the cell lines showed a slight increase in absolute value of mean fluorescent intensity (MFI), but again expression of the CD74 biomarker was unremarkable. However, for the CaPan1 cell line, an approximate 10-fold increase in the MFI was observed with greater than 90% of the cells present in the positive gate. The positive control anti-CEACAM5 MAb, and negative control anti-CD20 MAb, gave the expected results for each cell line. We also examined specimens of the respective tumor xenotransplants derived from these same cell lines with a humanized anti-CD74 MAb, hLL1 (milatuzumab). The results confirmed that CD74 expression was highest in the CaPan1 tumor model, with approximately 25% of the cells reactive for CD74 distributed in a focal manner within the tissue section (Figure 1G). Cytoplasmic staining was the major feature observed, with cell-surface staining detected occasionally. Although CD74 expression was observed in the overwhelming number of individual cells within the HT29 colorectal carcinoma xenotransplants, the expression of this protein was very weak.
Table 2.
Flow cytometric and immunohistochemical phenotyping of cell lines and xenotransplants for the expression of CD74
| Cell line | CD74 | CEACAM5 | CD20 | Immunohistology (CD74) | |
|---|---|---|---|---|---|
| Panc CA | ASPC-1 | 3.8a | 76.2 | 3.8 | Focal (5%) +1-3b |
| ASPC-1 Perm | 7.1c | 87.7 | 5.2 | ||
| BxPC3 | 4.2 | 89.0 | 4.1 | Focal (1%) +1 | |
| BxPC3 Perm | 5.1 | 43.3 | 4.5 | ||
| Capan-1 | 22.0 | 61.3 | 12.3 | Focal (25%) +2-3 | |
| Capan-1 Perm | 248.0 | 70.8 | 8.2 | ||
| Panc-1 | 7.7 | 8.2 | 7.8 | Negative | |
| Panc-1 Perm | 16.1 | 14.0 | 29.7 | ||
| Colon CA | LS174T | 6.8 | 54.4 | 9.4 | Negative |
| LS174T Perm | 19.7 | 112.0 | 11.1 | ||
| Moser | 6.3 | 145.8 | 5.7 | Negative | |
| Moser Perm | 4.7 | 62.5 | 4.1 | ||
| HT29 | 5.2 | 174.0 | 5.3 | Diffuse (>90%), weak | |
| HT29 Perm | 30.0 | 567.0 | 32.5 | blush (<+1) | |
| Lovo | 4.6 | 140.0 | 4.3 | Not Done | |
| Lovo Perm | 36.0 | 281.0 | 37.7 | ||
| GW-39 | Does not grow as a cell-line | Focal (5%) +1-2 | |||
| Lymphoma | Daudi | 34.0 | 14.7 | 344.6 | Diffuse (>90%) +2-4 |
| Daudi Perm | 561.0 | 5.7 | 28.6 | ||
Mean fluorescent intensity of positive gated cells after direct labeling with ALEXA-488-MAb constructs. hLL1 anti-CD74 was used to detect the biomarker under evaluation; hMN-14 anti-CEACAM5 was employed as the positive control; hA20 anti-CD20 was employed as the negative control. Note that the controls are the reverse for the Daudi lymphoma cell line, itself a positive control for expression of CD74.
Immunohistochemical staining was interpreted in the same manner as the human tissues with description of distribution (Focal <25% of tissue stained, Diffuse >25% of tissue stained) and approximate percentage of cells within the xenograftthat express CD74, as well as an indication of staining intensity (+1 to +4).
Cells were permeabilized using the BD cytofix/cytoperm kit priorto evaluation for reactivity with the ALEXA-488-MAbs.
Discussion
A major function of CD74 (invariant chain) is as a chaperone to transport MHC II to the appropriate intracellular compartments for antigen presentation and initiation of an immune response. CD74-trimers, formed within the ER, bind to MHC II directing its transport to the endosomes. Importantly, CD74, through direct interaction with the MHC II molecule, prevents inappropriate binding of endogenous self-antigens to the peptide-binding cleft within MHC II [2,3,26]. However, within late-stage endosomes and/or lysosomes, CD74 undergoes proteolytic digestion to enable MHC II to bind exogenously-derived immunogenic peptides. The peptide-MHC II complex is then transported to the cell surface for presentation of immunogen to CD4+ T cells. As such, CD74 is detectable at the cell surface of antigen-presenting cells, as well as malignant cells derived from these tissues, particularly tumors of B-cell origin. By use of immunohistochemistry, the CD74 marker is clearly observed as a component within the membrane, where it exists as part of a functional receptor complex [11].
Recent evidence has suggested another important role for the CD74 molecule in the activation of cell survival pathways. CD74 is a cell receptor for the proinflammatory cytokine, MIF [7]. Although CD74 itself is able to bind MIF, when bound to surface-expressed CD44, the CD74-CD44 complex is able to initiate several survival pathways, including the ERK-1/2 MAP kinase signaling cascade, and to stimulate cell proliferation by enhanced expression of cyclins and other regulatory factors [7,8]. Furthermore, inhibition of MIF or the CD74 receptor by siRNA [27,28], antibody [12,29], or small molecule antagonists [30], has been shown to provide a growth inhibitory effect upon malignant cells. Our group has demonstrated the specific growth-inhibitory effect of the hLL1, anti-CD74 antibody (milatuzumab) upon B-cell malignancies carried as xenografts in athymic nude mice, and is actively pursuing the clinical use of this antibody for the therapy of multiple myeloma [31,32]. In view of this, we began an investigation into the expression and morphological distribution of CD74 in non-hematological tumors.
While there are several reports on the enhanced expression of CD74 in solid tumors [16-18], including those of the gastrointestinal tract [10,14,15,20], to the best of our knowledge this is the first detailed morphologic description of CD74 distribution within clinical specimens of invasive carcinomas, precursor lesions, and inflamed tissues of the gastrointestinal system. There is a significantly enhanced expression of the CD74 marker within carcinomas of the pancreas, colon, and stomach, as compared to their respective normal epithelia. Although overall CD74 expression was highest in the pancreatic carcinomas, in fact almost universal, considerable numbers of colonic and gastric carcinomas also expressed high levels of CD74. A trend towards higher expression of CD74 within poorly-differentiated as compared to well- and moderately-differentiated carcinomas of pancreatic origin suggests, at the very least, an association between CD74 expression and tumor behavior. This has been recognized by others, and may correlate with prognosis [13,20]. A similar trend for CD74 expression with grade of tumor has been noted for colorectal tumors [33]; however, we did not observe this association.
We hypothesized that CD74 expression would be as high, or higher, in the precursor lesions, PanINs and adenomas, than in pancreatic and colorectal carcinomas, respectively, since activation of survival pathways and a potential for decreased presentation of early, tumor-associated peptides for immune response would be of particular importance during the early phase of neoplastic growth. Although colorectal adenomas, in general, did show a higher expression of CD74 than colorectal carcinoma and normal colonic mucosa, both in frequency and intensity of staining, the same correlation was not true for the PanIN lesions and pancreatic carcinoma. Rather, it appeared that with higher grade dysplasia, the expression of CD74 increased to levels similar to that for invasive carcinomas. It is of interest to speculate whether downregualtion of CD74 within precursors could prevent development of invasive carcinoma.
In general, CD74 was localized to the cytoplasm in an apical and/or perinuclear region, with cell-surface expression observed mostly within the pancreatic carcinomas and not the colorectal and gastric tumors. Flow cytometry and immunohistochemistry studies with several human cell lines of pancreatic and colonic carcinoma origin confirmed the finding that, in contrast to hematological tumors, there was little cell-surface expression of CD74 in these tumors. However, one caveat is that our current studies evaluated a static presentation of antigen rather than a dynamic, time-dependent, profile. It is possible that CD74 is present within the membrane of these tumors, both primary tumors and cell lines, at concentrations below the detection limits of the technologies employed here. Prior studies by our group employing radiolabeled antibodies that bound to cell-surface CD74 demonstrated that HT-29 human colon carcinoma cells expressed little to no CD74 at the surface. However, when grown in the presence of IFN-γ, CD74 was detectable at the surface of these cells, but still only at low levels; approximately 104 molecules/cell. By use of antibody labeled with a residualizing radioisotope, a continuous, rapid turnover of surface CD74 was observed with internalization of approximately 106 molecules/cell/24h [5,6]. Thus, the absence of observable surface CD74 noted by immunohistochemistry in the current study may be less important than the fact that the overwhelming majority of primary gastrointestinal carcinomas had considerably higher total levels of CD74 than normal tissues. It may be that this large intracellular pool of CD74 provides greater opportunity for maintenance of a low amount of surface-expressed CD74 receptor.
Several reports [23,24, and review 34], together with data presented here, have suggested potential roles that enhanced CD74 expression may have in the development and maintenance of neoplasia. Chronic inflammation is a predisposing factor in the progression to invasive neoplasia.
Within the gastrointestinal system, chronic pancreatitis, inflammatory bowel disease, and H. pylori infection are all well known as high-risk factors for development of the corresponding carcinoma. In the latter case, H. pylori has been demonstrated to bind directly to CD74 within the normal gastric mucosa, up-regulating surface expression of CD74 and proinflammatory cytokines [35,36]. CD74 and MIF act in concert, controllingtheir own production in a positive feedback loop that provides increased expression of each during inflammation, and activates cell EGFR-mediated proliferation [36] and NF-κB survival cascades [9,37]. However, persistent, uncontrolled proliferation of epithelial cells provides greater opportunity for genetic mutations to occur and the eventual development of neoplasia. Continued activation of the survival pathways may then provide an advantage for the cancer cells to become established within the tissue.
Furthermore, MHC II presentation of endogenous, potentially immunogenic peptides (tumor antigens) may be blocked by the increased expression of CD74, with a consequent decrease of tumor immunogenicity. High intracellular concentrations of CD74 may provide a more efficient blocking of the MHC II peptide-binding cleft, thus preventing uptake of endogenous tumor-related peptides. The very aggressive mouse sarcoma model SaI (MHC II−/CD74−), when transfected with syngeneic MHC II (MHC II+/CD74−), became immunogenic and was routinely rejected [38]. Upon further transfection with CD74 (MHC II+/CD74+), the aggressive, non-immunogenic profile of the tumor was restored. Similarly, studies have demonstrated that suppression of CD74 can convert tumor cells into potent vaccines [27,28,39]. In the absence of the CD74 chaperone, MHC II molecules are able to bind and present endogenous peptides as immunogenic tumor antigens.
Our findings, together with other reports in the literature, suggest that overexpression of CD74 is a common and important phenomenon for development and maintenance of invasive carcinomas of the gastrointestinal organs. Thus, when considering the functions attributed to this protein, induction of survival and cell proliferation pathways, and its role in regulation of immune response, we speculate that CD74 may prove useful as a target for therapeutic intervention, perhaps by use of targeted-siRNA and/or antagonism of cell-surface expressed CD74 receptor. Towards this purpose, we have defined the CaPan1 human pancreatic carcinoma cell line as a model for evaluation of anti-CD74 reagents.
References
- 1.Claesson L, Larhammer D, Rask L, Peterson PA. cDNA clone for the human invariant gamma chain of class II histocompatibility antigens and its implications for the protein structure. Proc Natl Acad Sci USA. 1983;80:7395–9. doi: 10.1073/pnas.80.24.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roche PA, Cresswell P. Intracellular transport of class II MHC molecules directed by invarient chain. Nature. 1990;345:615–18. [Google Scholar]
- 3.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA. 1993;90:8581–5. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moller P, Henne C, Moldenhauer G. CD74 Workshop Panel Report. In: Schlossman SF, Boumsell L, Gilks W, et al., editors. Leukocyte Typing V, White cell differentiation Antigens, Vol 1. New York: Oxford University Press; 1995. pp. 568–571. [Google Scholar]
- 5.Hansen HJ, Ong GL, Diril H, Valdez A, Roche PA, Griffiths GL, Goldenberg DM, Mattes MJ. Internalization and catabolism of radiolabeled antibodies to the MHC class-II invariant chain by B-cell lymphomas. Biochem J. 1996;320:293–300. doi: 10.1042/bj3200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong GL, Goldenberg DM, Hansen HJ, Mattes MJ. Cell surface expression and metabolism of major histocompatibility complex class II invariant chain (CD74) by diverse cell lines. Immunology. 1999;98:296–302. doi: 10.1046/j.1365-2567.1999.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I. Cell surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–16. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 10.Beswick EJ, Das S, Pinchuk IV, Adegboyega P, Suarez G, Yamaoka Y, Reyes VE. Helicobactor pylori-induced IL-8 production by gastric epithelial cells up-regulates CD74 expression. J Immunol. 2005;175:171–6. doi: 10.4049/jimmunol.175.1.171. [DOI] [PubMed] [Google Scholar]
- 11.Burton JD, Ely S, Reddy PK, Stein R, Gold DV, Cardillo TM, Goldenberg DM. CD74 is expressed by multiple myeloma cells and is a promising target for therapy. Clin Cancer Res. 2004;10:6606–11. doi: 10.1158/1078-0432.CCR-04-0182. [DOI] [PubMed] [Google Scholar]
- 12.Stein R, Qu Z, Cardillo TM, Chen S, Rosario A, Horak ID, Hansen HJ, Goldenberg DM. Anti-proliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies. Blood. 2004;104:3705–11. doi: 10.1182/blood-2004-03-0890. [DOI] [PubMed] [Google Scholar]
- 13.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Iwashige H, Aridome K, Hokita S, Aikou T. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87–91. doi: 10.1016/s0304-3835(01)00503-1. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbert RJ, Wilson JM, Scott N, Coletta PL, Hull MA. Differential CD74 (major histocompatiability complex Class II invariant chain) expression in mouse and human intestinal adenomas. Eur J Cancer. 2009;145:318–32. doi: 10.1016/j.ejca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Hustinx SR, Cao D, Maitra A, Sato N, Martin ST, Sudhir D, Iacobuzio-Donahue C, Cameron JL, Yeo CJ, Kern SE, Goggins M, Mollenhauer J, Pandey A, Hruban RH. Differentially expressed genes in pancreatic ductal adenocarcinomas identified through serial analysis of gene expression. Cancer Biol Therapy. 2004;3:1254–61. doi: 10.4161/cbt.3.12.1238. [DOI] [PubMed] [Google Scholar]
- 16.Young AN, Amin MB, Moreno CS, Lim SD, Cohen C, Petros JA, Marshall FF, Neish AS. Expression profiling of renal epithelial neoplasms: a method for tumor classification and discovery of diagnostic molecular markers. Am J Pathol. 2002;158:1639–51. doi: 10.1016/S0002-9440(10)64120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioachim HL, Pambuccian SE, Hekimgil M, Giancotti FR, Dorsett BH. Lymphoid monoclonal antibodies reactive with lung tumors. Diagnostic applications. Am J Surg Pathol. 1996;20:64–71. doi: 10.1097/00000478-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Kitange GJ, Carlson BL, Schroeder MA, Decker PA, Morlan BW, Wu W, Ballman KV, Giannini C, Sarkaria JN. Expression of CD74 in high grade gliomas: a potential role in temozolomide resistance. J Neurooncol. 2010 doi: 10.1007/s11060-010-0186-9. May 5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamuleau ME, Souwer Y, Van Ham SM, Zevenbergen A, Westers TM, Berkhof J, Meijer CJ, van de Loosdrecht AA, Ossenkoppele GJ. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res. 2004;64:5546–50. doi: 10.1158/0008-5472.CAN-04-1350. [DOI] [PubMed] [Google Scholar]
- 20.Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, Aiura K, Shimazu M, Hirohashi S, Nimura Y, Sakamoto M. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12:2419–26. doi: 10.1158/1078-0432.CCR-05-1852. [DOI] [PubMed] [Google Scholar]
- 21.Pawlak-Byczkowska EJ, Hansen HJ, Dion AS, Goldenberg DM. Two new monoclonal antibodies, EPB-1 and EPB-2, reactive with human lymphoma. Cancer Res. 1989;49:4568–77. [PubMed] [Google Scholar]
- 22.Gold DV, Karanjawala Z, Modrak DE, Goldenberg DM, Hruban RH. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7380–7. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 23.Maharshak N, Cohen S, Lantner F, Hart G, Leng L, Bucala R, Shachar I. CD74 is a survival receptor on colon epithelial cells. World J Gastroenterol. 2010;16:3258–66. doi: 10.3748/wjg.v16.i26.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beswick EJ, Pinchuk IV, Suarez G, Sierra JC, Reyes VE. Helicobacter pylori CagA-dependent macrophage migration inhibitory factor produced by gastric epithelial cells binds to CD74 and stimulates procarcinogenic events. J Immunol. 2006;176:6794–801. doi: 10.4049/jimmunol.176.11.6794. [DOI] [PubMed] [Google Scholar]
- 25.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306–16. [PMC free article] [PubMed] [Google Scholar]
- 26.Morris P, Shaman J, Attaya M, Amaya M, Goodman S, Bergman C, Monaco JJ, Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 27.Hillman GG, Kallinteris NL, Li J, Wang Y, Lu X, Li Y, Wu S, Wright JL, Slos P, Gulfo JV, Humphreys RE, Xu M. Generating MHC Class II+/Ii- phenotype after adenoviral delivery of both an expressible gene for MHC Class II inducer and an antisense Ii-RNA construct in tumor cells. Gene Ther. 2003;10:1512–8. doi: 10.1038/sj.gt.3302027. [DOI] [PubMed] [Google Scholar]
- 28.Lu X, Kallinteris NL, Li J, Wu S, Li Y, Jiang Z, Hillman GG, Gulfo JV, Humphreys RE, Xu M. Tumor immunotherapy by converting tumor cells to MHC class II-positive, Ii protein-negative phenotype. Cancer Immunol Immunother. 2003;52:592–8. doi: 10.1007/s00262-003-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, Govindan S, Goldenberg DM. CD74: A new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13 doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 30.Xu M, Li J, Gulfo JV, Von Hofe E, Humphreys RE. MHC class II allosteric site drugs: new immuno-therapeutics for malignant, infectious and autoimmune diseases. Scand J Immunol. 2001;54:39–44. doi: 10.1046/j.1365-3083.2001.00964.x. [DOI] [PubMed] [Google Scholar]
- 31.Stein R, Smith MR, Chen S, Zalath M, Goldenberg DM. Combining milatuzumab with bortezomib, doxorubicin, or dexamethasone improves responses in multiple myeloma. Clin Cancer Res. 2007;15:2808–17. doi: 10.1158/1078-0432.CCR-08-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman JL, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H, Teoh N, Wegener WA, Goldenberg DM. Dose-escalation trial of milatuzumab (humanized anti-CD74 monoclonal antibody) in multiple myeloma [abstract] J Clin Oncol. 2009;27:15s. doi: 10.1111/bjh.12565. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z, Xu M, Savas L, LeClair P, Banner BF. Invariant chain expression in colon neoplasms. Virchows Arch. 1999;435:32–6. doi: 10.1007/s004280050391. [DOI] [PubMed] [Google Scholar]
- 34.Beswick EJ, Reyes VE. CD74 in antigen presentation, inflammation, and cancers of the gastrointestinal tract. World J Gastroenterol. 2009;15:2855–61. doi: 10.3748/wjg.15.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrera CA, Beswick EJ, Sierra JC, Bland D, Espejo R, Mifflin R, Adegboyega P, Crowe SE, Ernst PB, Reyes VE. Polarized expression of CD74 by gastric epithelial cells. J Histochem Cytochem. 2005;53:1481–9. doi: 10.1369/jhc.4A6552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beswick EJ, Reyes VE. Macrophage migration inhibitory factor and interleukin-8 produced by gastric epithelial cells during Helicobacter pylori exposure induce expression and activation of epidermal growth factor receptor. Infect Immun. 2008;76:3233–40. doi: 10.1128/IAI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, Bucala R, Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–92. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- 38.Clements VK, Baskar S, Armstrong TD, Ostrand-Rosenberg S. Invariant chain alters the malignant phenotype of MHC class II+ tumor cells. J Immunol. 1992;149:2391–96. [PubMed] [Google Scholar]
- 39.Armstrong TD, Clements VK, Martin BK, Ting JP, Ostrand-Rosenberg S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA. 1997;94:6886–91. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]