Abstract
We have developed a universally applicable system for conditional gene expression in embryonic stem (ES) cells that relies on tamoxifen-dependent Cre recombinase-loxP site-mediated recombination and bicistronic gene-trap expression vectors that allow transgene expression from endogenous cellular promoters. Two vectors were introduced into the genome of recipient ES cells, successively: (i) a bicistronic gene-trap vector encoding the β-galactosidase/neoR fusion protein and the Cre-ERT2 (Cre recombinase fused to a mutated ligand-binding domain of the human estrogen receptor) and (ii) a bicistronic gene-trap vector encoding the hygroR protein and the human alkaline phosphatase (hAP), the expression of which is prevented by tandemly repeated stop-of-transcription sequences flanked by loxP sites. In selected clones, hAP expression was shown to be regulated accurately by 4′hydroxy-tamoxifen. Strict hormone-dependent expression of hAP was achieved (i) in vitro in undifferentiated ES cells and embryoid bodies, (ii) in vivo in virtually all the tissues of the 10-day-old chimeric fetus (after injection of 4′hydroxy-tamoxifen to foster mothers), and (iii) ex vivo in primary embryonic fibroblasts isolated from chimeric fetuses. Therefore, this approach can be applied to drive conditional expression of virtually any transgene in a large variety of cell types, both in vitro and in vivo.
Embryonic stem (ES) cells are pluripotential cells derived from the inner cell mass of the blastocyst. They can proliferate indefinitely in vitro while retaining pluripotency. After reintroduction into the blastocyst, they are able to adopt all of the cell fates within the developing embryo. The molecular mechanisms underlying the control of self-renewal and differentiation of mouse ES cells is still understood poorly. A major obstacle toward this goal is the lack of an inducible expression system that would allow any gene whose constitutive expression is detrimental to self-renewal to be turned on in undifferentiated ES cells as well as in their differentiated derivatives both in vitro and in vivo.
ES cells are capable of differentiating into a very large variety of cell types in vitro, but commitment into different lineages occurs simultaneously. Procedures for coaxing differentiation into specific cell types with growth factors (1) or for sorting out the desired cells with selectable genes (2) have been developed. Another approach is based on the ectopic expression of lineage-specific transcription factors that coax differentiation into lineages of interest. Constitutive expression of myoD, HoxB4, and Hox11 has been shown to promote differentiation of mouse ES cells into muscle (3) and hematopoietic cells (4, 5). However, constitutive expression of differentiation-promoting genes in ES cells is likely to be detrimental to self-renewal and therefore promotes uncontrolled differentiation during the engineering procedure. Here too inducible expression would be a valuable asset.
Conditional expression vectors have already been reported for ES cells. Whyatt et al. (6) developed an interferon-based inducible expression system to drive conditional expression of the chloramphenicol acetyltransferase (CAT) gene. Expression of the reporter gene was shown to be controlled tightly. However, only undifferentiated ES cells, and none of their in vitro- or in vivo-differentiated derivatives, were tested for interferon-mediated CAT expression. In addition, continuous stimulation with α- and β-interferons to keep the responsive promoter “on” is likely to interfere with cellular and developmental processes (7). Zhang et al. (8) reported on tamoxifen-regulated Cre recombinase and its use to induce Cre-mediated recombination, thereby activating a stably integrated lacZ reporter gene in ES cells. However, the dose of tamoxifen required to induce lacZ expression far exceeded the threshold above which tamoxifen becomes detrimental to cell growth and pregnancy in mice (9), and strong background expression was observed consistently. Further, only undifferentiated ES cells were tested for tamoxifen-dependent expression of lacZ. Recently, Niwa et al. (10) reported on a tetracycline-regulated transactivator tTA to drive conditional expression of the Oct-3/4 transgene. Expression of the transgene was shown to be controlled tightly in response to low doses of tetracycline in undifferentiated ES cells, but induction of Oct-3/4 expression in their differentiated derivatives was not reported.
Here, we report on the generation of an inducible expression system that overcomes the shortcomings of earlier approaches successfully. It relies on (i) a Cre/lox-based strategy, making use of a Cre recombinase fused to a mutated estrogen receptor that is highly sensitive to induction by 4′OH-tamoxifen (4′OHT) (11, 12) and (ii) the use of bicistronic gene-trap expression vectors that allow expression of the ligand-dependent Cre and of the transgene from endogenous promoters. This system allows tightly controlled expression of human alkaline phosphatase (hAP), both in undifferentiated ES cells and in their differentiated derivatives in vitro and in vivo.
Materials and Methods
Plasmid Construction.
To engineer pGTEV-Cre-ERT2, the Cre-ERT2 coding sequence from pCre-ERT2 (11) was subcloned first between the SmaI and XbaI sites of plasmid pIRES-neo (CLONTECH) to generate pIRES-Cre-ERT2. In parallel, the SAβgeo sequence was amplified from ROSAβgeo (13) by using the following oligonucleotides: 5′-AGAACCAATGCATGCTGATCACGCAGGTTTA-3′ and 5′-AAGGAAAAAAGGGGCGCCTATGGCTCGTACTCTATAG-3′. Then the PCR product (3.7 kb) was subcloned between the SpeI and NsiI sites of plasmid pIRES-Cre-ERT2 to generate pGTEV-Cre-ERT2.
To engineer PGK-lox-STOP-lox-EGFP, a 1.1-kb NheI–MluI fragment from pEGFP-C1 (CLONTECH) containing the enhanced green fluorescent protein (EGFP) coding sequence was subcloned between the HindIII and XbaI sites in pYS3 (14).
To engineer pCAG-lox-STOP-lox-ADh, a 2.5-kb SalI–XhoI fragment encompassing the CAG promoter in pHPCAG (15) was subcloned into the SalI site in pBSK to generate pCAG. In parallel, the 2.0-kb NdeI insert containing loxP-pgk-neor-pA-pA-loxP (see above) was subcloned into the EcoRI site in pCAG to generate pCAG-lox-STOP-lox. A 1.0-kb XhoI–EcoRI fragment encompassing the alcohol dehydrogenase (ADh) coding sequence in pRC-CMVTM-ADH-DRO-SV40-Neo plasmid (16) was subcloned into the XbaI site in pCAG-lox-STOP-lox to generate pCAG-lox-STOP-lox-ADh.
To engineer pIGTE2, a 0.3-kb SpeI–HindIII fragment encompassing the splice acceptor (SA) in ROSAβgeo was subcloned between the SpeI and HindIII sites in pIRES-Cre-ERT2 to generate pSA. In parallel, the plasmid pT102 (a generous gift from T. Lamonerie, Ecole Normale Supérieure, Lyon, France) was digested with EcoRI and BstEII and religated to eliminate a sequence encoding tk driven by the pgk promoter. The resulting plasmid was digested with NdeI, and the 2.0-kb NdeI insert containing loxP-pgk-neor-pA-pA-loxP was subcloned between the EcoRI and XhoI sites in pSA to generate the pSA-lox-STOP-lox plasmid. A 2.0-kb BamHI fragment containing the full coding sequence of the hygror–EGFP fusion protein from pHyg-EGFP (CLONTECH) was subcloned between the ApaI and NcoI sites in pSA-lox-STOP-lox to generate pIGTE2. To engineer pIGTE2-hAP, a 2.7-kb SalI–SpeI fragment encompassing the IRES-hAP coding sequence in pHW3 (17) was subcloned into the XbaI site in pIRES-hyg (CLONTECH). The resulting plasmid (pIRES-hyg-IRES-hAP) was digested with BglII and XhoI, and the 2.7-kb insert containing IRES-hAP-pA was subcloned into the XbaI site in pIGTE2 to generate pIGTE2-hAP.
ES Cell Culture and Manipulation.
ES cells (ENS cell line; ref. 18) were maintained routinely on feeder cells (γ-irradiated hygro and neo primary fibroblasts) in medium supplemented with human leukemia inhibitory factor. For isolation of genetically engineered cells, 5 × 106 cells were electroporated with 30 μg of linearized plasmid at 0.26 kV and 960 μF in a 0.4-cm cuvette and then selected in the presence of G418 (200 μg/ml) or hygromycine B (80 μg/ml) for 8 days. Drug-resistant colonies were transferred individually into 96-well microtiter plates, expanded, and frozen. To detect Cre activity in transient-expression assays, ES cells were transfected by using calcium phosphate precipitation (19). Detection of Cre activity was performed after 48 h as described in Gene Expression and Histological Analyses. ES cells were induced to differentiate either by incubation with 10−6 M retinoic acid for 2 days or culture onto nonadherent Petri dishes (105 cells in 50 ml of leukemia inhibitory factor-deprived culture medium) for 7–14 days to allow the formation of embryoid bodies. 4′OHT (Calbiochem) was dissolved in ethanol (100 mM) and added to culture medium for the time and concentration indicated.
Chimera Generation, Tamoxifen Injection, and Primary Culture.
Chimeras were produced by ES-cell injection into C57bl/6 blastocysts. 4′OHT was dissolved in ethanol (100 mg/ml) and mixed with sunflower oil by sonication (final concentration 10 mg/ml) before i.p. injection. Primary fibroblasts were prepared from individual chimeras as described (20). Preparation of primary fibroblasts was cultured in 200 μg/ml G418 and 80 μg/ml hygromycine B for 7 days to kill host-derived fibroblasts.
Gene Expression and Histological Analyses.
Histochemical staining for β-galactosidase activity in cultured cells, tissue sections, and visceral yolk sac was carried out with 5-bromo-4-chloro-3-indolyl β-D-galactoside as described (21). Histochemical staining for ADh was carried out as described (16). Histochemical staining for hAP activity in cultured cells, tissue sections, and whole-mount embryos was carried out as described (22). Quantification of hAP activity in protein lysates was carried out by using the AP substrate kit (Bio-Rad). Quantification of EGFP-expressing cells was performed by fluorescence-activated cell sorting (FACS).
Results
Design of Tamoxifen-Controlled Inducible Expression System in ES Cells.
The system that we have established makes use of a Cre recombinase fused to the ligand-binding domain (LBD) of the human estrogen receptor (called Cre-ERT2). The recombinase is activated by 4′OHT but not by endogenous estrogens because of specific point mutations in the LBD (11, 12). A DNA fragment containing transcription termination signals flanked by loxP sites is inserted between the promoter and the transgene, thereby preventing its transcription. Activated Cre induces deletion of this DNA fragment and allows transgene expression. This system has been used successfully to drive 4′OHT-dependent expression of several genes in transgenic mice (12, 23–26). The system that we designed specifically for ES cells has two important features: (i) it makes use of the ligand-dependent recombinase Cre-ERT2, which is approximately 10-fold more sensitive to 4′OHT induction than the original Cre-ERT (11, 12), and (ii) it makes use of bicistronic gene-trap expression vectors that allow expression of the ligand-dependent Cre and the reporter gene, both driven off of cellular promoters. By using this strategy, we aim to achieve robust and stable expression of Cre-ERT2 and the reporter gene in ES cells. A double-step engineering procedure was implemented to generate this system: (i) engineering ES cell lines to stably express Cre-ERT2 (GTEV-ES cells) and (ii) engineering GTEV ES cell lines to express a reporter transgene conditionally in response to 4′OHT (GTEV-IGTE ES cells).
Generation of ES Cell Lines Stably Expressing Cre-ERT2 and lacZ (GTEV-ES cells).
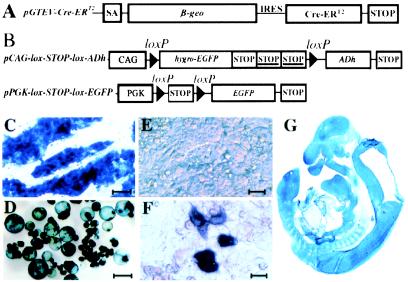
The construct used to drive stable expression of Cre-ERT2, pGTEV-Cre-ERT2 (GTEV, gene-trap expression vector), is shown in Fig. 1A. It carries, downstream of the LacZ-neomycin-phosphotransferase fusion gene (β-geo), the Cre-ERT2 coding sequence fused to the IRES of the encephalomyocarditis virus. After integration downstream of an endogenous promoter, β-geo and Cre-ERT2 are likely to be coexpressed from a single transcript. ENS ES cells were electroporated with the pGTEV-Cre-ERT2 gene-trap vector. Individual G418R clones (110) were screened for three criteria: (i) stable and robust expression of β-galactosidase in undifferentiated ES cells and in 14-day-old embryoid bodies, (ii) widespread distribution of β-galactosidase-positive cells in the 8-day-old embryo after aggregation with morulaes, and (iii) 4′OHT-dependent expression of EGFP after transient transfection of ES cells with the pPGK-lox-STOP-lox-EGFP reporter plasmid (transient expression of EGFP depends on Cre recombinase-mediated recombination in transfected cells; Fig. 1B). Six clones were selected by using this triple assay (Table 1). All six clones display few EGFP-positive cells before treatment with 4′OHT (0.04–0.22%), and their number increases by a factor of 3–9 (0.20–0.91%) after treatment with hormone. 4′OHT-dependent Cre activity in undifferentiated ES cells was demonstrated conclusively by transfecting the six GTEV clones with the pCAG-lox-STOP-lox-ADh reporter plasmid (Fig. 1B). All display a marked increase in the ADh-positive/ADh-negative cells ratio in the presence of 4′OHT (Table 1). Control experiment with pCAG-ADh, a plasmid that expresses ADh constitutively, returns similar ratios of ADh-positive/ADh-negative cells, indicating that Cre activity is not rate-limiting in GTEV clones (data not shown). GTEV clones that do not express β-galactosidase do not display any Cre activity in the transient-expression assay (data not shown), indicating that β-galactosidase expression reflects 4′OHT-dependent Cre activity in GTEV cells.
Figure 1.
Generation of GTEV ES cells stably expressing pGTEV-Cre-ERT2. (A) Structure of the bicistronic gene-trap expression vector pGTEV-Cre-ERT2. SA, adenovirus major late-transcription splice acceptor; β-geo, LacZ–neomycin phosphotransferase fusion gene conferring resistance to G418 and expression of β-galactosidase; Cre-ERT2, gene encoding the ligand-dependent Cre recombinase Cre-ERT2; IRES, internal ribosomal entry site of the encephalomyocarditis virus; STOP, transcription termination from simian virus 40. (B) Structure of the pPGK-lox-STOP-lox-EGFP and pCAG-lox-STOP-lox-ADh reporter plasmids. CAG, cytomegalovirus/chicken β-actin fusion promoter; PGK, promoter of the mouse pgk-1 gene; STOP, transcription termination from simian virus 40; STOP, transcription termination from the mouse pgk-1 gene. (C and D) Histochemical analysis of β-galactosidase expression in undifferentiated ES cells from clone GTEV49 (C) and in 14-day-old embryoid bodies made from GTEV49 ES cells (D). (E and F) Histochemical analysis of Cre activity in undifferentiated ES cells from clone GTEV49. To detect Cre activity, GTEV49 ES cells were transfected with the pCAG-lox-STOP-lox-ADh reporter plasmid in the presence of 1 μM 4′OHT (F) or in vehicle alone (E). Cells were harvested after 48 h and processed for detection of ADh activity. (G) Histochemical analysis of β-galactosidase expression on a sagittal section of transgenic fetus tgGTEV49 at 13 days of gestation. [Scale bars = 10 μM (E and F) and 100 μM (C and D).]
Table 1.
Characterization of G418R ES cell clones generated by electroporation with pGTEV-Cre-ERT2 for lacZ expression and Cre-ERT2 activity
| GTEV clone | LacZ expression in ES cells (+++, strong; ++, moderate) | LacZ expression in embryoid bodies (14 days) (+++, strong; ++, moderate) | Distribution of LacZ-expressing cells in aggregation chimeras (at E8) | ES cells expressing EGFP
after transfection with
pCAG-lox-STOP-lox-EGFP*
|
ES cells
expressing ADh after transfection with
pCAG-loxSTOPlox-ADh†
|
Pattern
of LacZ expression in tgGTEV transgenic lines
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −4′OHT, % | +4′OHT,‡ % | % +4′OHT % −4′OHT | −4′OHT | +4′OHT‡ | E8 | E10 | E13 | ||||
| GTEV09 | +++ | +++ | Ub. | 0.07 | 0.20 | 2.8 | − | +++ | — | — | — |
| GTEV39 | +++ | mosaic | Ub. | 0.06 | 0.23 | 3.8 | + | +++ | — | — | — |
| GTEV49 | +++§ | +++¶ | Ub. | 0.04 | 0.34 | 8.5 | −‖ | +++** | Ub. | Ub. | Ub.‡‡ |
| GTEV55 | ++ | ++ | Ub. | 0.04 | 0.35 | 8.7 | − | +++ | Ub. | Ub. | Ub. |
| GTEV70 | +++ | mosaic | Ub. | 0.08 | 0.47 | 5.9 | + | +++ | Ub. | mosaic | mosaic |
| GTEV85 | +++ | mosaic | Ub. | 0.22 | 0.91 | 4.1 | + | +++ | Ub. | mosaic | none |
The percentage of EGFP-expressing cells is measured by FACS.
ADh-expressing cells are identified by in situ histochemistry.
1 μM, 48 h.
Shown in Fig. 2C;
Shown in Fig. 2D.
Shown in Fig. 2E.
Shown in Fig. 2F.
Shown in Fig. 2G. Ub, ubiquitous; E8, E10, and E13, 8th, 10th, and 13th day of gestation, respectively.
Four clones (GTEV-49, -55, -70, and -85) that display the strongest expression of β-galactosidase and the highest induction of Cre activity were selected for further studies. Data obtained with clone GTEV49 are shown in Fig. 1 C–F. Chimeric mice were generated from each one of these four clones. The stability of the βgeo-CreERT2 expression in the major differentiated cell types was assessed by means of β-galactosidase-expression analysis in the developing F1 progeny (between 8th and 13th day of gestation). Of the four transgenic lines analyzed, two (tgGTEV49 and tgGTEV55, derived from GTEV49 and GTEV55 ES cells) display ubiquitous expression of β-galactosidase throughout the developmental period analyzed (Table 1 and Fig. 1G). In contrast, tgGTEV70 and tgGTEV85 embryos (derived from GTEV70 and GTEV85 ES cells) display mosaic patterns of β-galactosidase expression from 10 days of gestation onward (Table 1).
Two Cre-ERT2-expressing GTEV cell lines were selected for the subsequent generation of stable ES cell lines conditionally expressing a reporter transgene: (i) GTEV49, which is characterized by ubiquitous albeit moderate expression of lacZ-Cre-ERT2, and (ii) GTEV85, which is characterized by a stronger expression of lacZ-Cre-ERT2, although this expression appears to be down-regulated in the midgestation embryo.
Generation of ES Cell Lines Conditionally Expressing the Reporter hAP Gene (GTEV/IGTE-ES cells).
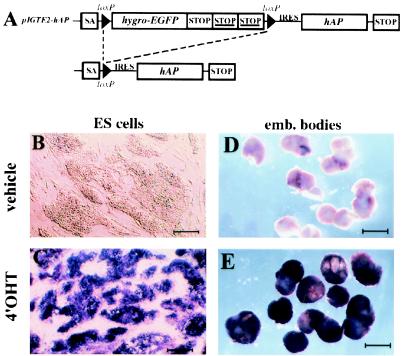
To determine whether the GTEV ES cell lines are able to regulate the expression of a transgene in response to 4′OHT, GTEV49 and GTEV85 ES cells were electroporated with the gene-trap vector pIGTE2-hAP (Fig. 2A). pIGTE2-hAP (IGTE, inducible gene trap expression) carries the hAP coding sequence fused to the IRES of the Moloney murine leukemia virus (17). After integration downstream of an active promoter, transcription of hAP is prevented by a loxP-flanked intervening cassette containing the hygror–EGFP fusion gene followed by three tandemly repeated transcriptional termination sequences. Individual hygroR clones (190) were assayed for expression of hAP after treatment with 4′OHT (1 μM, 48 h). Of the 190 clones, 172 (90%) display no expression of hAP after induction by 4′OHT (not shown); 15 clones display expression of hAP that does not depend on, or is activated weakly by, 4′OHT (Table 2). Five clones (≈3%) display strong 4′OHT-dependent expression of hAP with levels of induction ranging from 7-fold to ∞. Among them, clones 49–146 and 85–149 display no background expression of hAP and strong activation of expression after treatment with 4′OHT. Although a hygror–EGFP fusion gene was cloned between the two loxP sites, EGFP expression was not detected in any clone before treatment with 4′OHT. Each of the five 4′OHT-responsive clones was tested for hormone-dependent expression of hAP after differentiation into embryoid bodies. All clones but one (49–146) display strong activation of hAP expression in response to 1 μM 4′OHT for 48 h (Table 2 and Fig. 2 D and E). In situ staining of hAP activity in undifferentiated 49–110 ES cells (hereafter referred to as GTEV49-IGTE110) show that hAP expression is activated in virtually all cells after treatment with 1 μM 4′OHT for 48 h (Fig. 2C). No hAP-positive cells can be detected in the untreated cell population, indicating that hAP expression is repressed fully in the absence of hormone (Fig. 2B).
Figure 2.
Generation of GTEV/IGTE ES cells accurately regulating expression of the hAP reporter gene. (A) Structure of the bicistronic gene-trap expression vector pIGTE2-hAP. SA, adenovirus major late-transcription splice acceptor; hygror-EGFP, fusion gene encoding resistance to hygromycine B and expression of EGFP; hAP, gene encoding hAP; IRES, internal ribosomal entry site of the Moloney murine leukemia virus; STOP, transcription termination from simian virus 40; STOP, transcription termination from the mouse pgk-1 gene. (B–E) Histochemical analysis of hAP activity in undifferentiated ES cells (B and C) and 8-day-old embryoid bodies (D and E) cultured with 4′OHT (1 μM, 48 h; C and E) or with vehicle alone (B and D). (Scale bars = 100 μM.)
Table 2.
Characterization of GTEV/IGTE clones generated by electroporation of GTEV49 and GTEV85 ES cells with pIGTE2-hAP
| GTEV/IGTE clone | Dosage of hAP in cell lysates
|
Expression of hAP‖ in 8-day-old embryoid bodies (in situ histochemistry) | Expression of hAP** in chimeras at E10 (in situ histochemistry) | ||
|---|---|---|---|---|---|
| −OHT (OD) | +OHT‖ (OD) | Induction +OHT−OHT | |||
| 49–30 | 1539 | 769 | — | ND | ND |
| 49–33* | 15 | 96 | 7 | +++ | ND |
| 49–110* | 4† | 157‡ | 39 | +++§ | +++¶ |
| 49–113 | 62 | 177 | 3 | ND | ND |
| 49–126 | 1280 | 1099 | — | ND | ND |
| 49–134 | 104 | 57 | — | ND | ND |
| 49–143 | 910 | 999 | 1.1 | ND | ND |
| 49–146* | 0 | 150 | ∞ | — | ND |
| 49–160 | 0 | 21 | ∞ | ND | ND |
| 49–162 | 24 | 31 | 1.3 | ND | ND |
| 49–181 | 29 | 15 | — | ND | ND |
| 49–184* | 15 | 271 | 18 | +++ | ND |
| 49–185 | 0 | 32 | ∞ | ND | ND |
| 85–1 | 402 | 263 | — | ND | ND |
| 85–12 | 24 | 93 | 3.9 | ND | ND |
| 85–39 | 63 | 87 | 1.4 | ND | ND |
| 85–47 | 108 | 113 | 1 | ND | ND |
| 85–101 | 5 | 26 | 5.2 | ND | ND |
| 85–149* | 0 | 332 | ∞ | +++ | ++++ |
| 85–150 | 21 | 44 | 2.1 | ND | ND |
+++/++++, strong/very strong induction of hAP expression; −, no induction of hAP expression; ND, not determined; OD, optic density at 420 nM.
Indicates clones selected for strong induction of hAP expression in response to 4′OHT treatment.
Shown in Fig. 3B.
Shown in Fig. 3C.
Shown in Fig. 3E.
Shown in Fig. 5D.
+4′OHT (1 μM, 48 hours).
+4′OHT (1 mg i.p.).
Optimization of 4′OHT-Induced hAP Expression.
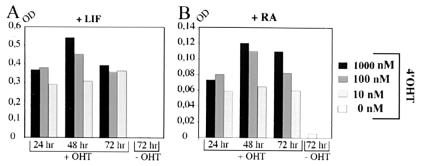
In the previous assay, induction of hAP expression was tested by using 1 μM 4′OHT for 48 h, a concentration near the threshold above which 4′OHT becomes detrimental to ES cell growth. Time- and dose-response assays were carried out therefore to determine the optimal conditions required for induction of hAP expression in undifferentiated GTEV49-IGTE110 ES cells (Fig. 3A). A strong (25-fold) activation of hAP expression at 72 h is observed after treatment with 10 nM 4′OHT for 24 h. Longer treatment with 10 nM 4′OHT does not increase the level of hAP expression significantly. The use of higher concentrations of 4′OHT increases the level of hAP expression up to 32-fold (100 nM 4′OHT) or up to 36-fold (1 μM 4′OHT), depending on the duration of hormone treatment. Time- and dose-response assays were carried out also on ES cells that were induced to differentiate with 10−6 M retinoic acid (RA) for 2 days before hormone induction. Very similar results are obtained regarding both the optimal concentration of 4′OHT and the optimal duration of treatment (Fig. 3B). To conclude, abrupt activation of hAP expression can be achieved after treatment with 10 nM 4′OHT for as little as 24 h, both in undifferentiated ES cells and in their RA-induced derivatives.
Figure 3.
Dose- and time-dependent activation of hAP expression in GTEV49-IGTE110 ES cells. Undifferentiated (A) or differentiating (10−6 M retinoic acid for 2 days before addition of 4′OHT) (B) ES cells were treated with 10, 100, or 1,000 nM 4′OHT (+OHT) for 24, 48, and 72 h or with vehicle alone (−OHT) for 72 h. hAP activities in protein lysates were analyzed at 72 h in every experimental condition. OD, optic density at 420 nM; +LIF (leukemia inhibitory factor), undifferentiated ES cells; +RA, retinoic acid-induced cells.
hAP Gene Is Switched on by 4′OHT in Midgestation Chimeras in Utero.
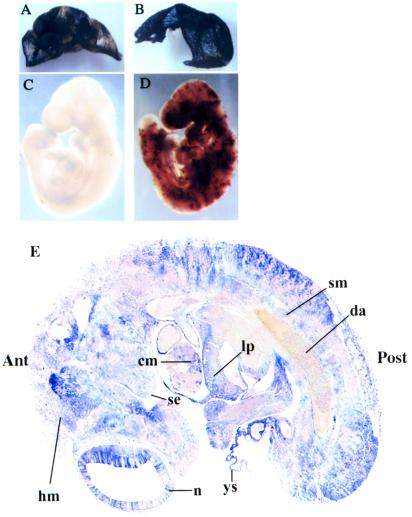
To determine whether hAP expression can be activated in the developing chimeras in utero, GTEV49-IGTE110 cells were injected into blastocysts. At the 7th day of gestation, foster mothers were injected i.p. with 1 mg 4′OHT (16). At day 10, chimeric embryos were identified by β-galactosidase expression in the visceral yolk sac (Fig 4 A and B), and hAP expression was examined in the embryo proper (Fig. 4 C and D). Strong hAP activity is evidenced in whole-mount embryos (Fig. 4D). Differences in staining intensity merely reflect variations in the overall level of chimerism (data not shown). hAP-positive cells are undetectable in chimeras dissected from pregnant females injected with vehicle (Fig. 4C). To determine more thoroughly the spectrum of differentiated cells that are able to activate hAP expression in response to a single i.p. injection of 4′OHT, a 12-day-old chimeric fetus that had received a single injection of 4′OHT on the 8th day of gestation was processed for detection of hAP activity and sectioned serially (Fig. 4E). hAP-positive cells are present in a large panel of tissues, including neuroepithelium, surface ectoderm, head mesenchyme, somitic mesoderm, cardiac mesoderm, liver primordium, and yolk sac mesoderm. Only the red blood cells within the dorsal aorta display little or no hAP-staining. This result shows that hAP is expressed in virtually all tissues in the 12-day-old chimera after activation by 4′OHT.
Figure 4.
Regulation of hAP expression by 4′OHT in the developing chimeras generated from GTEV49-IGTE110 ES cells. Foster mothers were injected with 4′OHT (1 mg i.p.) on the 7th day of gestation. Embryos were recovered the 10th day (A–D) or the 12th day of gestation (E) and processed for detection of lacZ (visceral yolk sac) and hAP activities (embryo proper). (A and B) Histochemical analysis of β-galactosidase expression in visceral yolk sac to identify chimeric embryos from 4′OHT-injected (B) or mock-injected (A) females. (C and D) Histochemical analysis of hAP activity in 10-day-old whole-mount embryos dissected from 4′OHT-injected (D) or mock-injected (C) females. (E) Parasaggital section of a 12-day-old hAP-positive chimera. n, neuroepithelium; se, surface ectoderm; hm, head mesenchyme; sm, somitic mesoderm; cm, cardiac mesoderm; lp, liver primordium; ys, yolk sac mesoderm; da, dorsal aorta; Ant, anterior; Post, posterior.
hAP Gene Is Switched on by 4′OHT in Primary Embryonic Fibroblasts Prepared from Chimeric Fetuses.
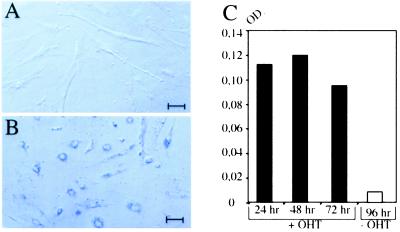
We next wanted to determine whether expression of hAP could be activated by 4′OHT in primary embryonic fibroblasts isolated from chimeras made from GTEV49-IGTE110 ES cells. Chimeric embryos at the 15th day of gestation are identified on the basis of β-galactosidase expression in the visceral yolk sac (not shown). Primary embryonic fibroblasts are isolated, selected in G418 and hygromycine B to kill host-derived cells, and treated with 1 μM 4′OHT for 48 h. All the embryonic fibroblasts are shown to activate hAP expression in response to hormone (Fig. 5B). No hAP-positive cells are evidenced in the untreated cell population (Fig. 5A). Quantification of hAP activity in protein lysates shows that expression of hAP increases by a factor of 12 in response to treatment with 1 μM 4′OHT for 24 h (Fig. 5C). Longer treatment with hormone does not increase hAP expression significantly. These data provide evidence that hAP expression is up-regulated accurately by 4′OHT in differentiated derivatives such as embryonic fibroblasts.
Figure 5.
Regulation of hAP expression by 4′OHT in primary embryonic fibroblasts isolated from 15-day-old chimeric embryos generated from GTEV49-IGTE110 ES cells. (A and B) Histochemical analysis of hAP activity in fibroblasts cultured with 4′OHT (1 μM, 48 h) (B) or with vehicle alone (A). (C) Quantification of hAP activity in protein lysates prepared from embryonic fibroblasts treated with 4′OHT for 24, 48, and 72 h with 1 μM 4′OHT (+OHT) or for 96 h with vehicle alone (−OHT). OD, optic density at 420 nm. (Scale bars = 100 μM.)
Discussion
We have established a system that allows gene expression in ES cells and their differentiated derivatives to be controlled tightly in response to a nondetrimental dose of the synthetic hormone 4′OHT. Our system is characterized by very low background, indicating that transcription of the reporter gene is repressed efficiently in the absence of ligand. First, this repression is likely to rely on the insertion of the selectable gene (hygror) between the two loxP site in pIGTE2-hAP. Cells that excise the loxP-hygror-loxP spontaneously because of background activity of Cre-ERT2 are killed by antibiotic selection. Second, the insertion of three tandemly repeated sites for transcription termination is likely to prevent leakiness of hAP expression before 4′OHT treatment.
Activation of hAP expression is shown to be highly sensitive to hormone treatment because abrupt activation of hAP is observed following 24-h treatment with 4′OHT at concentrations as low as 10 nM (Fig. 4). This result is in sharp contrast with a previous work in which tamoxifen-regulated expression of LacZ in undifferentiated ES cells was shown to require 100- to 1,000-fold higher concentrations of 4′OHT for 3 days to reach optimum activation of LacZ expression in only 50–90% of the cell population (8). Strong background also was observed consistently in this latter case. Here, virtually 100% of GTEV49-IGTE110 cells were shown to turn hAP expression on in response to 4′OHT. The sensitivity of our system is likely to rely crucially on three factors. First, we used Cre-ERT2 recombinase, which is approximately 10-fold more sensitive to 4′OHT induction than the original Cre-ERT (11, 12). By optimizing the sensitivity of ligand-dependent Cre to ligand binding, we minimized the dose of ligand required to obtain 100% activated transgenes as well as the time required to achieve maximum transgene expression. Second, we used the gene-trap vectors pGTEV-Cre-ERT2 and pIGTE2-hAP. Gene-trap strategy has been used successfully to establish the ROSA26 cell line, an ES cell line stably expressing lacZ in all tissues of the developing fetus at high levels (13, 27, 28). By using this strategy, we aimed to achieve robust and stable expression of both Cre-ERT2 and hAP in ES cells and their differentiated derivatives. Third, GTEV clones were selected carefully on the basis of stable and robust expression of β-galactosidase, thereby indirectly on a high level of Cre-ERT2. Coexpression of Cre-ERT2 and β-galactosidase was achieved by using a bicistronic expression vector and the IRES of encephalomyocarditis virus. This IRES sequence is used commonly to drive ectopic expression of foreign genes in transgenic mice (29, 30).
As opposed to existing systems (6, 8, 10) that are designed and tested for inducible expression restricted to undifferentiated ES cells only, we show that inducible expression with GTEV49 ES cells can be achieved both in undifferentiated ES cells and their ectodermal, mesodermal, and endodermal differentiated derivatives in midgestation embryos. Because these embryos are chimeric, we could not determine precisely the fraction of ES-derived cells that actually activates the expression of hAP after injection of 4′OHT to foster mothers. However, this fraction is likely to be close to 100%, because the proportion of hAP-expressing cells (induced cells) and the proportion of β-galactosidase-expressing cells (all ES-derived cells) in 10- and 13-day-old embryos are identical (data not shown).
The GTEV49 ES cell line will be highly valuable for inducing ectopic expression of genes in a large variety of ES-derived progenitor cells in vitro. Conditions to promote differentiation of mouse ES cells specifically into neural precursors have been developed (31, 32). GTEV49 ES cells will allow conditional expression of genes in those precursors to coax their differentiation into specific neuron subtypes (32). GTEV49 cells also will allow the isolation, from the developing chimeras, of genetically engineered primary embryonic cells in which a gene of interest is expressed ectopically in response to a brief treatment with 4′OHT. We have demonstrated the feasibility of this experimental approach with primary embryonic fibroblasts. In principle, it could be applied to any other primary cell types to overcome the shortcomings of immortalized cell lines.
Acknowledgments
We thank J.-L. Darlix for the gift of pHW3, T. Jaffredo for the gift of pRC-CMVTM-ADH-DRO-SV40-Neo, Yvan Lallemand for the gift of pYS3, T. Lamonerie for the gift of pT102, and Clarence Deffaux for helpful discussions regarding the use of IRES sequences. This work was supported by the Hôpital Universitaire de Strasbourg, the Fondation pour la Recherche Médicale, the Association pour la Recherche contre le Cancer, the Conseil Régional de la Région Rhône-Alpes (Neurosciences/Cognisciences Program), the Human Frontier Science Program, and the 5th Framework Program (European Union).
Abbreviations
- ES
embryonic stem
- 4′OHT
4′hydroxy-tamoxifen
- hAP
human alkaline phosphatase
- EGFP
enhanced green fluorescent protein
- ADh
alcohol dehydrogenase
- IRES
internal ribosomal entry site
References
- 1.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton D A, Benvenisty N. Proc Natl Acad Sci USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M, Pevny L, Lovell-Badge R, Smith A. Curr Biol. 1998;8:971–974. doi: 10.1016/s0960-9822(98)70399-9. [DOI] [PubMed] [Google Scholar]
- 3.Dekel I, Magal Y, Pearson-White S, Emerson C P, Shani M. New Biol. 1992;4:217–224. [PubMed] [Google Scholar]
- 4.Helgason C D, Sauvageau G, Lawrence H J, Largman C, Humphries R K. Blood. 1996;87:2740–2749. [PubMed] [Google Scholar]
- 5.Keller G, Wall C, Fong A Z, Hawley T S, Hawley R G. Blood. 1998;92:877–887. [PubMed] [Google Scholar]
- 6.Whyatt L M, Duwel A, Smith A G, Rathjen P D. Mol Cell Biol. 1993;13:7971–7976. doi: 10.1128/mcb.13.12.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangfelt O, Erickson S, Grander D. Front Biosci. 2000;5:D479–D487. doi: 10.2741/sangfelt. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Riesterer C, Ayrall A M, Sablitzky F, Littlewood T D, Reth M. Nucleic Acids Res. 1996;15:543–548. doi: 10.1093/nar/24.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danielian P S, Muccino D, Rowitch D H, Michael S K, McMahon A P. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 10.Niwa H, Miyazaki J, Smith A G. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 11.Feil R, Wagner J, Metzger D, Chambon P. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 12.Indra A K, Warot X, Brocard J, Bornert J M, Xiao J H, Chambon P, Metzger D. Nucleic Acids Res. 1999;15:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich G, Soriano P. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 14.Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- 15.Niwa H, Burdon T, Chambers I, Smith A. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautier R, Drocourt D, Jaffredo T. Exp Cell Res. 1996;224:291–301. doi: 10.1006/excr.1996.0139. [DOI] [PubMed] [Google Scholar]
- 17.Torrent C, Berlioz C, Darlix J-L. Hum Gene Ther. 1996;7:603–612. doi: 10.1089/hum.1996.7.5-603. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier K, Chassande O, Plateroti M, Roux J P, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. EMBO J. 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson, E. J. (1987) ed., Robertson, E. J. (IRL, Oxford), pp. 71–112.
- 21.Savatier P, Morgenstern J, Beddington R S P. Development (Cambridge, UK) 1990;109:655–665. doi: 10.1242/dev.109.3.655. [DOI] [PubMed] [Google Scholar]
- 22.Fekete D M, Cepko C L. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brocard J, Wendling O, Messaddeq N, Vonesch J L, Chambon P, Metzger D. Proc Natl Acad Sci USA. 1997;94:14559–14563. doi: 10.1073/pnas.94.26.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwenk F K R, Angrand P O, Rajewsky K, Stewart A F. Nucleic Acids Res. 1998;26:1427–1432. doi: 10.1093/nar/26.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasioukhin V D L, Wise B, Fuchs E. Proc Natl Acad Sci USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmel R A, Blanquet V, Wurst W, Loomis C A, Joyner A L. Genes Dev. 2000;14:377–389. [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrowicz B P, Imamoto A, Fiering S, Herzenberg L A, Kerr W G, Soriano P. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soriano P. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 29.Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mountford P S, Smith A G. Trends Genet. 1995;11:179–184. doi: 10.1016/S0168-9525(00)89040-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okabe S, Forsberg-Nilsson K, Spiro A C, Segal M, McKay R D. Mech Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee S H, Lumelsky N, Studer L, Auerbach J M, McKay R D. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]