Abstract
The effect of exogenous Gal-1 on cellular response and adhesion molecule expression was investigated in a classical model of acute inflammation induced by zymosan. C57BL6 mice, treated or not with human recombinant (hr) Gal-1, received i.p. injection of zymosan and peritoneal exudate, blood and mesentery were processed for cellular, biochemical, light and electron microscopic analysis after 4 and 24 h. Zymosan peritonitis provoked the expected signs of inflammation at 4 h, including a significant increase in extravasated PMNs in the mesentery and peritoneal exudate, mirrored by blood neutrophilia. These changes subsided after 24 h. Ultrastructural immunocytochemical analysis of PMNs showed significant Gal-1 expression and co-localization with L-selectin and β2-integrin in the plasma membrane and cytoplasm. Pharmacological treatment with hrGal-1 at 4 h produced an inhibition of PMN migration, associated with diminished expression of adhesion molecules, particularly β2-integrin, and TNF-α and IL-1β release by peritoneal cells. At 24 h, Gal-1 induced an increase in mononuclear phagocytic cell recruitment. In conclusion, our data propose an important mechanism of anti-inflammatory action of Gal-1, initially by modulation of pro-inflammatory cytokine release and PMN migration through an imbalance between adhesion molecule expression and, later, by promoting monocyte-macrophage recruitment.
Keywords: CD11b, CD62L, monocyte, neutrophil, zymosan peritonitis, immunocytochemistry
Introduction
One of the important steps of inflammation is polymorphonuclear leukocyte (PMN) recruitment from the bloodstream to inflammatory sites through interactions with postcapillary venule endothelial cells [1]. The mechanism of cellular migration is triggered by a series of pro-inflammatory mediators that are produced by mast cells, macrophages, activated endothelial cells, and leukocytes that have transmigrated to the inflamed tissue [2]. In addition, the inflammatory response is controlled by the action of anti-inflammatory mediators, which act to maintain homeostasis of the immune response and prevent tissue damage. Among these mediators, highlight the galectin-1 (Gal-1), a 14.5-kDa protein which acts as an endogenous modulator of inflammation [3].
Gal-1 belongs to a family of proteins involved in inflammatory processes, characterized by an affinity for galactosides-β and a conserved sequence of 130 amino acids in the carbohydrate recognition domain (CRD) [4, 5]. The expression of Gal-1 was observed in various cell types associated with the inflammatory response, especially neutrophils and mast cells [3, 6], macrophages [7], T and B lymphocytes [8, 9], and endothelial cells [3, 6, 10], suggesting an important role in the generation and maintenance of immune tolerance.
In acute inflammation, the anti-inflammatory action of Gal-1 was observed in an experimental model of paw edema in rats [11]. Exogenous administration of this protein, 30 minutes before or together with phospholipase A2 from bee venom, was able to inhibit PMN extravasation, mast cell degranulation, and arachidonic acid and prostaglandin E2 release by lipopolysaccha-ride (LPS)-stimulated macrophages.
The anti-inflammatory role of Gal-1 in PMN recruitment was also shown in in vitro and in vivo experimental models [3, 6, 10, 12]. Incubation of human PMN with human recombinant (hr) Gal-1 significantly decreased the extent of capture, rolling, and adhesion on activated endothelial monolayers [10, 12]. Furthermore, Gal-1 inhibited the platelet-activating factor-induced increase in β2-integrin expression in PMNs in a concentration-dependent manner, as assessed by flow cytometry [12]. Investigations using in vivo models showed that pre-administration of Gal-1 promoted an inhibition of PMN extravasation to the peritoneal cavity after 4 hours of car-rageenan application in rats [3] and IL-1β in mice [10]. Similarly, the exogenous Gal-1 reduced the capture process of PMNs by endothelial cells in mesenteric circulation [10], and the lack of endogenous Gal-1 caused a significant increase in PMN adhesion and emigration in cremasteric circulation in Gal-1-null mice [12]. These studies suggest a role of this protein in the modulation of adhesion molecules associated with interaction of PMNs and the endothe-lium, particularly L-selectin and β2-integrin.
Given the anti-inflammatory role of Gal-1, the purpose of this study was to investigate the effect of pharmacological pretreatment with hrGal-1 in experimental peritonitis induced by zymosan in mice, focusing on the recruitment of inflammatory cells, release of proinflammatory cytokines, and modulation of L-selectin and β2-integrin adhesion molecules. The co-locatization of Gal-1 and adhesion molecules during PMN interaction with endothelial cells was assessed by ultrastructural immunocytochemical studies.
Materials and methods
Animals
Male C57BL/6 mice (20–25 g of body weight), maintained on a standard chow pellet diet with tap water ad libitum, were used for all experiments. Animals were housed at a density of 6 animals per cage in a room with controlled lighting (lights on from 8:00 a.m. to 8:00 p.m.), in which the temperature was maintained at 21–23°C. Animal work was performed according to the Committee on Care and Use of Laboratory Animal Resources of the School of Medicine Protocol n° 3015/06), São Jose do Rio Preto, SP.
Zymosan peritonitis
Experimental peritonitis was induced by i.p. injection of 1 mg of boiled zymosan A (Sigma-Aldrich) in 0.5 ml of sterile saline [13], whereas control animals were injected with an equal volume of saline. At different time points (0, 4, and 24 hours), animals (n=6 per group) were anesthetized with ketamine and xylazine (100 and 20 mg/Kg, i.p.) for collection of blood aliquots (maximum 1 ml) before sacrifice and washing of the peritoneal cavity with 3 ml of PBS supplemented with 3 mM EDTA. Then, fragments of the mesentery were collected and processed as described below.
In another set of experiments, mice were treated with an i.p. injection of 0.3 μg of hrGal-1 (Peprotech, EC Ltd, London, UK) in 100 μl of sterile saline 15 minutes before the zymosan injection. This dose was scaled up from a previous study of an inflammation model in mouse [10]. PMN recruitment into the peritoneal cavity, blood, and mesentery was assessed at the 4- and 24-h time-points.
Cellular analyses
Cell Quantification. Aliquots of blood (20 μl) or peritoneal lavage fluid (100 μl) were diluted 1/10 in Turk's solution (0.1% crystal violet in 3% acetic acid); total and differential counting was obtained with a Neubauer chamber using a 40× objective and a light microscope (Zeiss). Peritoneal cells and blood were distinguished in PMNs and monocytes/macrophages (mono-M φ). Data were reported as mean ± SEM of the average number of cells × 105 / mL from blood samples and the number of cells × 105 per animal from the peritoneal exudate.
Blood Flow Cytometry. In order to quantify L-selectin (CD62L) or β2-integrin (CD11b) expression, leukocytes were isolated from abdominal aorta blood collected in EDTA (100 mg/mL). Erythrocyte lysis was performed using an ammonium chloride solution (0.13 M), and leukocytes were recovered after washing the preparation with PBS. The cells (1.0 × 106) were incubated for 30 min at 4°C in the dark with 10 μL of monoclonal antibodies against L-selectin conjugated to PE (anti-mouse CD62L) and β2-integrin conjugated to FITC (anti-mouse CD11b) (BD Pharmigen, San Diego, USA). Immediately after incubation, the cells were analyzed using a FAC-SCalibur flow cytometer (Becton & Dickinson, San Jose, CA, USA). Data were obtained from 10,000 cells and only morphologically viable neutrophils were considered for analysis. Leukocytes were separated based on size and granularity. Fluorescence was determined and the results are expressed as the mean fluorescence of two assays performed in duplicate.
Biochemical analysis
Cytokine levels. Aliquots of peritoneal lavage fluid were centrifuged at 400 × g for 10 min and tested for TNF-α, IL-1β, and IL-6 according to the manufacturer's protocol (R&D Systems).
Histological analysis
Mesentery flat mount. Fragments of mesentery were stretched with pins and fixed in formalin 10% for 30 minutes. After, they were washed with distilled water, stained by toluidine blue solution (30 min), separated from the small intestine and distended on the slides for histological quantification of the inflammatory cells. Analysis of cells in the mesenteric connective tissue was performed at 0, 4, and 24 hours post-zymosan administration with or without prior drug treatment (hrGal-1) with a high-power objective (40×), counting mast cells and PMNs in 1 mm2 area (analyzing at least 10 sections of tissues per group). Values are reported as mean ± SEM of the number of cells per mm2.
Ultrastructural immunocytochemical analysis
Blood aliquots and mesentery fragments at 4h post-zymosan administration were fixed in 4% paraformaldehyde and 0.5% glutaraldehyde, 0.1% sodium cacodylate buffer (pH 7.4) for 24 h at 4°C. They were then washed in sodium cacodylate, dehydrated through a graded series of methanol, and embedded in LR Gold (London Resin Co., Reading, Berkshire, UK). Sections (0.5 μm) were stained with 1% toluidine blue in 1% Borax solution (TAAB Laboratories, Aldermaston, Berkshire, UK). Sections (70 nm) were cut on an ultramicrotome (Reichert Ultracut; Leica, Austria) and placed on nickel grids for immunogold labeling.
To detect the expression of Gal-1 and adhesion molecules (CD11b and CD62L) on PMNs of blood and mesenteries (intravascular and transmigrated cells), a post-embedding immunogold double labeling reaction was performed. Ultrathin sections were incubated with: (1) phosphate-buffered solution (PBS) containing 1% egg albumin (PBEA); (2) PBS containing 5% egg albumin (PBEA) for 30 min; (3) polyclonal rabbit anti-Gal-1 (1:100) (Peprotech, London, UK) and polyclonal goat anti-CD11b (1:200) or monoclonal rat anti-CD62L (1:50) (Santa Cruz Biotechnology, California, USA) for 2 h; (4) normal rabbit serum and normal goat or rat serum, respectively, were used as control; (5) after washes in PBEA, in order to detect Gal-1, goat anti-rabbit IgG antibody (1:100 in PBEA) conjugated to 15 nm colloidal gold (British Biocell, Cardiff, UK) was added, and to detect CD11b or CD62L, rabbit anti-goat or goat anti-rat IgG antibody (1:100 in PBEA) conjugated to 10 nm colloidal gold (British Biocell) was added; and (6) after 1 h, the sections were washed in PBEA and then in distilled water.
Ultrathin sections were stained with uranyl acetate and lead citrate before examination on a ZEISS LEO 906 electron microscope (Electron Microscope Centre, IBILCE-UNESP). Randomly photographed sections of intravascular and transmigrated PMN to the tissue were used for the immunocytochemical analysis. The area of the cell compartment was determined with Axio-vision software. The density of immunogold (number of gold particles per μm2) was calculated and expressed for each cell compartment. Values are reported as mean ± SEM of n electron micrographs analyzed.
Statistical analysis
Statistical differences between means were determined by one-way analysis of variance (ANOVA), followed, if significant, by Bonferroni test. When 2 variables were analyzed, Student's t-test was used. A probability value less than 0.05 was taken as significant.
Results
Effect of exogenous Gal-1 on migration of inflammatory cells
Initially, we analyzed the effect of i.p. administration of zymosan in mice at different times (0, 4, and 24 hours). After 4 h of peritonitis induction, an intense inflammatory response was characterized by an increase in the number of PMNs in blood (16 ± 3 × 105 cells/ml) and peritoneal exudate (89 ± 18 × 105 cells/animal), compared with the control group (8 ± 3 × 105 cells/ml and 3 ± 2 × 105 cells/animal, respectively). In the latter experimental time, 24 h, the levels of PMN decreased in the blood (5 ± 1 × 105 cells/ml) and peritoneal wash (7 ± 2 × 105 cells / animal), as observed in the control group.
Mononuclear phagocytic cells (mono-Mφ) increased significantly at 4 h in the peritoneal wash (51 ± 6 × 105 cells/animal) compared to the control group (14 ± 5 × 105 cells/animal), followed by a decrease after 24 hours (28 ± 9 × 105 cells/animal). In the blood, no significant change was demonstrated in the number of monocytes at differential times.
The effect of pharmacological pretreatment with human recombinant (hr) Gal-1 on the migration of inflammatory cells was evaluated in this model of zymosan peritonitis. After 4 h, a significant decrease in the number of blood and peritoneal PMNs was demonstrated in the pretreated group compared to untreated animals (Figure 1A and C). No effect of exogenous Gal-1 was noted on PMNs at 24 h (Figure 1A and C). Similarly, the quantification of monocytes revealed no significant differences in the blood samples of animals pretreated or not with hrGal-1 in the experimentally investigated times (Figure 1B). However, the peritoneal exudate of pretreated animals showed a significant increase of these cells compared to the untreated group after 24 h (Figure 1D).
Figure 1.
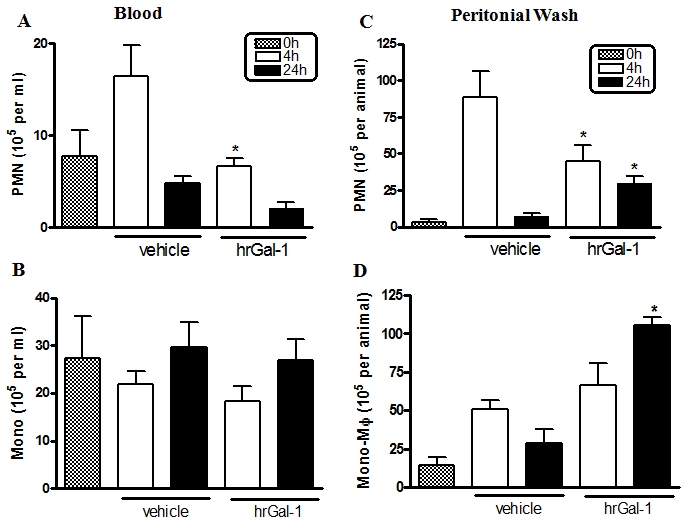
Effect of hrGal-1 on leukocyte migration during acute peritonitis model. Density of neutrophils (PMNs) and mononuclear phagocytic cells (mono-Mφ) from blood (A-B) and peritoneal wash (C-D) after 4h and 24h of zymosan administration in mice pretreated intraperitoneally with vehicle (PBS) or hrGal-1 (0.3 μg). Data are mean ± SEM from two separate experiments with 4 mice each. * P < 0.05 versus corresponding vehicle group value.
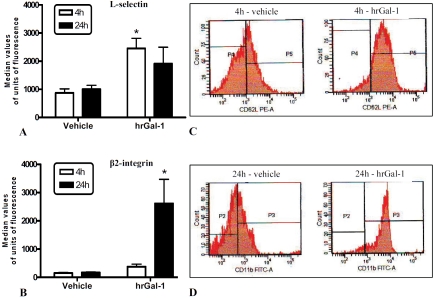
Besides alteration in blood cell counts, PMN of mice pretreated with hrGal-1 displayed a significantly higher degree of activation at 4h, as assessed by CD62L expression (Figure 2A and C). At 24h, the effect of pharmacological treatment was associated with high significant levels of CD 11b on PMN compared to cells of untreated mice (Figure 2B and D).
Figure 2.
L-selectin and β2-integrin expressions on circulating leukocytes. Mice pretreated intraperitoneally with vehicle (PBS) or hrGal-1 (0.3 μg) received 1mg of zymosan i.p. at time 0. At different time points after zymosan, blood was collected and L-selectin (A) and β2-integrin (B) expressions on leukocytes quantified by flow cytometry. Representative flow histograms of L-selectin (C) and β2-integrin (D) after 4 and 24h respectively showing differences between vehicle and hrGal-1 treated groups. Data are mean ± SEM from two experiments performed with three mice each. *P < 0.05 versus correspondent vehicle group value.
Cytokine detection
Similar to cell trafficking, the profiles of cytokine levels were markedly altered by pharmacological treatment with hrGal-1 at 4 h. Table 1 illustrates the values obtained for the cytokine content from peritoneal lavage fluid. In untreated mice, zymosan provoked the expected transient increase in TNF-α, followed by changes in inter-leukin (IL)-1β and IL-6. In mice pretreated with hrGal-1, the TNF-α and IL-1β response was diminished significantly at this time point. In the presence of exogenous hrGal-1, the IL-6 response was not changed. At 24h, cytokine levels were not detected in any experimental group.
Table 1.
Effect of hrGal-1 on soluble mediators release
| Group/Treatment | TNF-α(pg/ml) | IL-1β(pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|
| Control | ND | ND | ND |
| 4h/vehicle | 91.93 ± 13.5 | 374.4 ±44.38 | 668 ± 46.94 |
| 4h/ hrGal-1 | 30.07 ± 10.93* | 139.8 ±35.57* | 724.6 ± 20.13 |
| 24h/vehicle | ND | ND | ND |
| 24h/hrGal-1 | ND | ND | ND |
Mice pretreated intraperitoneally with vehicle (PBS) or hrGal-1 (0.3 μg) received 1mg of zymosan i.p. at time 0. Lavage fluid was collected at 0, 4 and 24h later, and serum concentrations of TNF-α, IL-1β, and IL-6 were determined. Results are from 6 mice per group. ND, not detected.
P < 0.05 versus corresponding vehicle group values.
Histological studies
Similar to cytokine release and cell trafficking, the profiles of the histological analysis were markedly altered on pharmacological treatment with hrGal-1. Morphological analysis of the mesentery of mice, 4 h post-zymosan, showed intense migration of PMNs (44 ± 10 cells/mm2) from postcapillary venules to the connective tissue compared to control animals (1 ± 0.5 cells/mm2). At this time, pharmacological treatment with hrGal-1 caused a reduction in the number of transmigrated PMN (2 ± 1 cells/ mm2) in relation to untreated animals. Although a high mast cell density (14 ± 2 cells/mm2) and activation (7 ± 1 degranulated cells/mm2) were noted in the 4-h-inflamed mesentery compared to control (5 ± 2 cells/mm2; 3 ± 1 degranulated cells/mm2), no significant effect of administration of hrGal-1 was detected in these cells (11 ± 2 cells/mm2; 9 ± 2 degranulated cells/mm2).
After 24 h, a significant reduction in PMN infiltration and mast cell number (55%) was observed in the mesentery of untreated animals. Treatment with hrGal-1 did not produce different changes in the response of PMNs (4 ± 2 cells/mm2) or mast cells (9 ± 2 cells/mm2) compared to untreated animals at 24 h (4 ± 2 PMN/mm2; 5 ± 1 mast cells/mm2).
Immunogold analysis of Gal-1 and adhesion molecules in the neutrophils
The expression of Gal-1 and the adhesion molecules L-selectin (CD62L) and β2-integrin (CD11b) was detected by postembedding immunogold labelingand analyzed to define their sub-cellular co-localization in the neutrophils of blood and those that were undergoing recruitment at 4 h in mesentery.
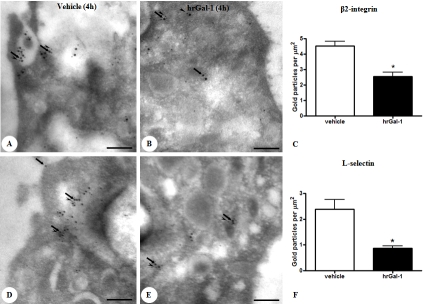
In this acute phase of inflammation, blood neutrophils of untreated animals presented co-localization between Gal-1 and adhesion molecules (CD62L and CD11b) that was detected throughout the cytosol, with a significant proportion also being observed in the plasma membrane (Figure 3A and D). Pretreatment of mice with hrGal-1 greatly diminished the CD11b (Figure 3B) and CD62L expression (Figure 3E), confirmed by the density of immunogold particles associated with them (Figure 3C and F).
Figure 3.
Analysis of Gal-1 and adhesion molecule (L-selectin and β2-integrin) co-localization in peripheral blood neu-trophils. At 4 h of zymosan administration, several points of co-localization of Gal-1 (arrows; 15 nm colloidal gold) and β2-integrin and L-selectin (A and D, respectively; arrowheads; 10 nm colloidal gold) were localized in the plasma membrane and cytoplasm of neutrophils. Pretreatment with hrGal-1 induced diminished β2-integrin (B) and L-selectin (E) expression. Density of β2-integrin (C) and L-selectin (F) immunogold particles in blood neutrophils at 4h post-zymosan administration in mice pretreated or not (vehicle) with hrGal-1. Data are mean ± SEM of 10 distinct cells for each group analyzed in the peripheral blood samples of 4 mice. *P < 0.001 versus correspondent vehicle group value.
Similarly, mesenteric neutrophils (intravascular and transmigrated) of untreated animals showed points of co-localization between Gal-1 and CD11b expression in the plasma membrane and cytosol (Figure 4A), which was poorly observed for CD62L (Figure 4B). The exogenous administration of hrGal-1 produced in the neutrophils a significant decrease of CD11b immu-noreactivity in their subcellular compartments (plasma membrane and cytosol) (Figure 4C), while CD62L expression was greatly increased in these cells (Figure 4D). No immunogold labeling was detected in sections incubated with control nonimmune sera (Figure 4E). The ultrastruc-tural observations were confirmed by quantification of gold particles associated with adhesion molecules, shown in Figure 4, F and G.
Figure 4.
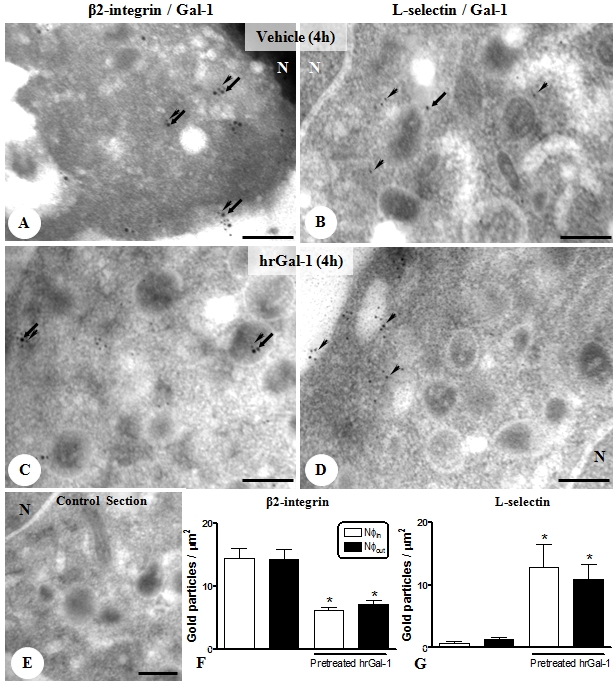
Expression of Gal-1 and adhesion molecule (β2-integrin and L-selectin) in transmigrated neutrophils (Nφs) of mesenteric tissue. Nφs showed a significant proportion of Gal-1 (arrows; 15 nm colloidal gold) and β2-integrin (arrowheads; 10 nm colloidal gold) co-localization in the plasma membrane and cytoplasm (A) that was poorly observed for L-selectin (B; arrowheads; 10 nm colloidal gold) at4h of peritonitis. Nucleus (N). Pretreatment with hrGal-1 induced diminished β2-integrin (C) and augmented L-selectin expression (D). (E) Absence of gold labeling in control section of reaction. Scale bars, 0.5μm. Density of β2-integrin (F) and L-selectin (G) immunogold particles in intravascular (Nφin) and transmigrated Nφs (Nφout) at 4 h post-zymosan administration in mice pretreated or not with hrGal-1. Data are mean ± SEM of 10 distinct cells for each group analyzed in the mesenteric tissue samples of 4 mice. *P < 0.01 versus correspondent Nφin and Nφout values.
Discussion
In spite of significant advances in elucidating the role of galectin-1 (Gal-1) within models of autoimmune conditions, focusing on T- and B-cells [14-16], the effects of this protein toward the cells of innate immunity have not been studied in such detail. We began the present study by validating this effect using a classical model of acute inflammation induced by zymosan in mice [17, 18]. Furthermore, the pharmacological treatment with human recombinant (hr) Gal-1 was investigated in innate immune cell recruitment, particularly polymorphonuclear leukocytes (PMNs) and mononuclear phagocytic cells, and in the expression of the adhesion molecules β2-integrin (CD11b) and L-selectin (CD62L) during the process of PMN transmigration in the mouse mesentery.
Administration of exogenous hrGal-1 before induction of zymosan peritonitis produced an inhibition of PMN recruitment to the peritoneal cavity (∼70%), blood (50%), and mesentery (∼80%), after 4 h, the peak of the inflammatory process. This anti-migratory effect of the protein was associated with inhibition of the release of the proinflammatory cytokines TNF-α and IL-1β but not IL-6. Similar results were described in vitro assays for Gal-1 that abrogated the production of the pro-inflammatory cytokines TNF-α and interferon (IFN)-g by activated macrophages and T cells and in plasma from mice with concanavalin A-induced hepatitis [19, 20]. On the other hand, the Gal-1 anti-migratory effect in peritonitis induced by IL-1β in wild-type and knockout mice [10, 12], was not associated with chemokine KC (CXCL1) or prostaglandin E2 (PGE2) reduction [10]. These data indicate that Gal-1 regulates the acute phase of the inflammatory response by controlling specific proinflammatory cytokine release, particularly those associated with macrophage activation.
Mesentery inflammation induced by zymosan was characterized by a significant proportion of mast cell degranulation (∼70%) at 4 h, along extensive PMN infiltration, followed by a decrease at 24 h (∼30%). The importance of mast cells in PMN migration in this peritonitis model was demonstrated by selective depletion of these cells, which provoked a lower influx of PMNs to the peritoneal cavity [18], as well as in the arthritis model in mice treated with a mast cell stabilizer, nedocromil [21]. Although our results showed diminished PMN migration associated with hrGal-1 pharmacological treatment, no differences were seen in the number or pattern of mast cell degranulation compared to the untreated group. On the other hand, a study using PLA2-induced paw edema in rats described a diminished number of degranulated mast cells associated with exogenous Gal-1 administration at different doses, but no effect was noted in histamine-induced edema, showing a selective role of Gal-1 for PLA2 at the level of arachidonic acid release, particularly by macrophages [11]. In this sense, our findings suggest that the anti-inflammatory role of Gal-1 in the recruitment of PMNs in the initial phase of inflammation occurs through a mechanism independent of mast cell modulation.
In light of our previous studies in which endogenous Gal-1 in mononuclear phagocytic cells could be modulated under different inflammatory stimuli [3], we also addressed the exogenous administration of Gal-1 to these cells. The main changes provoked by pretreatment with hrGal-1 in mononuclear phagocytic cells were observed in mouse peritoneal exudate at 24 h, with a significant increase in the number of these cells compared to the untreated group, revealing a role of this protein in monocyte chemotaxis. This chemotactic effect of Gal-1 is associated with its carbohydrate binding function (reduced by 65% in the presence of lactose) and the MAP kinase pathways, as demonstrated by in vitro assays [22]. Additionally, administration of exogenous Gal-1 appears to play a role in the activation of these cells, particularly dendritic cells, increasing the secretion of proinflammatory cytokines [23].
The effect of CD11b and CD62L expression in PMNs from animals treated (or not) with hrGal-1 was tested in this study. In early acute inflammation, blood and mesenteric neutrophils of untreated animals presented a significant proportion of co-localization between Gal-1 and adhesion molecules (CD11b and CD62L) in the plasma membrane and cytosol, observed particularly in the blood cells. In fact, integrins act as ligands of Gal-1 protein for cell adhesion to extracellular matrix, as demonstrated by molecular investigations carried out with macrophages [24] and muscle cells [25-27]. Our data indicate that pharmacological treatment with hrGal-1 greatly augmented expression of the CD62L on blood PMN plasma membranes, as detected by flow citometry, while endogenous CD62L and CD11b levels were significantly diminished in these cells compared to untreated animals, showing an important mechanism of this protein to inhibit cell migration. Curiously, once in contact with the mesentery, PMNs continue to express low levels of CD11b associated with hrGal-1 administration, but, in contrast, CD62L levels augment significantly. This latter effect could explain the high number of PMNs at 24 h in the hrGal-1-treated animals compared to the vehicle group. However, no Gal-1 effect was found on CD62L expression in the studies conducted by Cooper et al. [12], which demonstrated an inhibitory role of hrGal-1 administration in the steps of rolling and firm adhesion of platelet-activating factor (PAF)-stimulated PMNs to endothelial cells by significantly decreasing CD11b expression. The data showed clearly that exogenous Gal-1 downregulates CD11b expression, but its effect on CD62L warrants further investigation.
Several studies have demonstrated the importance of the CD11b and CD62L adhesion molecules in the transmigration of PMNs at sites of injury, representing critical points of the immune response of host defense [28-30]. Concurrent with the increased levels of CD11b during PMN activation by inflammatory stimuli, the adhesion molecule CD62L is rapidly cleaved in the cytoplasmic membrane of this cell type, a process necessary to stop the rolling of these cells in postcapillary venules [31] and the subsequent firm adhesion to the endothelium [32]. From these sets of experiments, we can propose that the effects of hrGal-1 produce a break in the balance between adhesion molecule expression on PMNs, significantly diminishing CD11b and CD62L, with a consequent interference in the cellular transmigration at 4 h in this experimental model of zymosan peritonitis. In contrast, at 24h high significant levels of CD11b expression was detected by flow citometry analysis on blood PMN from pretreated animals, but this effect was not associated with an increase in cell transmigration on mesentery. Studies should be conducted to further elucidate the correlation between exogenous Gal-1 and CD11b expression at later phases of inflammatory process.
In conclusion, we have revealed an exogenous anti-inflammatory effect of Gal-1, associated with the modulation of innate immune cells and cytokine release involved in the acute inflammation response. In the initial phase of inflammation, Gal-1 was effective in regulating pro-inflammatory IL-1β and TNF-α release and PMN migration to the mesentery through an imbalance between CD11b and CD62L expression in these cells. In the late phase, the main effect of Gal-1 was an increase in the recruitment of peritoneal mononuclear phagocytic cells, important for process resolution. Thus, Gal-1 protein should be considered a potential target for the development of new therapeutic strategies for inflammatory diseases.
Acknowledgments
We are grateful to Domingos Zanchetta Netto for technical assistance. This work was supported by Fundação de Amparo a Pesquisa - FAPESP [grant 2007/14055-0], Brazil. CDG was supported by the School of Medicine - FAMERP [grant 4349/2007], CEG by FAPESP [grant 2008/00557-7], and SMO by Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq [grant 306074/2007-9], Brazil.
References
- 1.Weber C. Novel mechanistic concepts for the control of leukocyte transmigration: specialization of integrins, chemokines, and junctional molecules. J Mol Med. 2003;81:4–19. doi: 10.1007/s00109-002-0391-x. [DOI] [PubMed] [Google Scholar]
- 2.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–78. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 3.Gil CD, Cooper D, Rosignoli G, Perretti M, Oliani SM. Inflammation-induced modulation of cellular galectin-1 and -3 expression in a model of rat peritonitis. Inflamm Res. 2006;55:99–107. doi: 10.1007/s00011-005-0059-4. [DOI] [PubMed] [Google Scholar]
- 4.Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport EM, Kurmyshkina OV, Bovin NV. Mammalian galectins: structure, carbohydrate specificity, and functions. Biochemistry (Mosc) 2008;73:393–405. doi: 10.1134/s0006297908040032. [DOI] [PubMed] [Google Scholar]
- 6.Gil CD, La M, Perretti M, Oliani SM. Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell Biol Internat. 2006;30:338–344. doi: 10.1016/j.cellbi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Iglesias MM, Modesti NM, Castagna LF, Wolfenstein-Todel C, Riera CM, Sotomayor CE. Activated rat macrophages produce a galectin-1-like protein that induces apoptosis of T cells: biochemical and functional characterization. J Immunol. 1998;160:4831–4840. [PubMed] [Google Scholar]
- 8.Blaser C, Kaufmann M, Muller C, Zimmermann C, Wells V, Mallucci L, Pircher H. Beta-galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur J Immunol. 1998;28:2311–2319. doi: 10.1002/(SICI)1521-4141(199808)28:08<2311::AID-IMMU2311>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 9.Zuniga E, Rabinovich GA, Iglesias MM, Gruppi A. Regulated expression of galectin-1 during B-cell activation and implications for T-cell apoptosis. J Leukoc Biol. 2001;70:73–79. [PubMed] [Google Scholar]
- 10.La M, Cao TV, Cerchiaro G, Chilton K, Hirabayashi J, Kasai K, Oliani SM, Chernajovsky Y, Perretti M. A novel biological activity for galectin-1: inhibition of leukocyte-endothelial cell interactions in experimental inflammation. Am J Pathol. 2003;163:1505–1515. doi: 10.1016/s0002-9440(10)63507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000;30:1331–1339. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Cooper D, Norling LV, Perretti M. Novel insights into the inhibitory effects of Galectin-1 on neutrophil recruitment under flow. J Leukoc Biol. 2008;83:1459–1466. doi: 10.1189/jlb.1207831. [DOI] [PubMed] [Google Scholar]
- 13.Perretti M, Solito E, Parente L. Evidence that endogenous interleukin-1 is involved in leukocyte migration in acute experimental inflammation in rats and mice. Agents Actions. 1992;35:71–78. doi: 10.1007/BF01990954. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recom-binant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–398. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum LG, Blackall DP, Arias-Magallano S, Nanigian D, Uh SY, Browne JM, Hoffmann D, Emmanouilides CE, Territo MC, Baldwin GC. Amelioration of graft versus host disease by galectin-1. Clin Immunol. 2003;109:295–307. doi: 10.1016/j.clim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–182. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- 17.Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajuebor MN, Das AM, Virág L, Flower RJ, Szabó C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: Evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;1767:1685–1691. [PubMed] [Google Scholar]
- 19.Santucci L, Fiorucci S, Cammilleri F, Servillo G, Federici B, Morelli A. Galectin-1 exerts im-munomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:399–406. doi: 10.1002/hep.510310220. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovich GA, Ilarregui JM. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev. 2009;230:144–159. doi: 10.1111/j.1600-065X.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 21.Pimentel TA, Sampaio AL, D'Acquisto F, Perretti M, Oliani SM. An essential role for mast cells as modulators of neutrophils influx in collagen-induced arthritis in the mouse. Lab Invest. 2010 doi: 10.1038/labinvest.2010.140. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik RK, Ghurye RR, Lawrence-Watt DJ, Stewart HJ. Galectin-1 stimulates monocyte chemotaxis via the p44/42 MAP kinase pathway and a pertussis toxin-sensitive pathway. Glycobiology. 2009;19:1402–1407. doi: 10.1093/glycob/cwp077. [DOI] [PubMed] [Google Scholar]
- 23.Fulcher JA, Hashimi ST, Levroney EL, Pang M, Gurney KB, Baum LG, Lee B. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J Immunol. 2006;177:216–226. doi: 10.4049/jimmunol.177.1.216. [DOI] [PubMed] [Google Scholar]
- 24.Avni O, Pur Z, Yefenof E, Baniyash M. Complement receptor 3 of macrophages is associated with galectin-1-like protein. J Immunol. 1998;160:6151–6158. [PubMed] [Google Scholar]
- 25.Gu M, Wang W, Song WK, Cooper DN, Kaufman SJ. Selective modulation of the interaction of alpha 7 beta 1 integrin with fibronectin and laminin by L-14 lectin during skeletal muscle differentiation. J Cell Sci. 1994;107:175–181. doi: 10.1242/jcs.107.1.175. [DOI] [PubMed] [Google Scholar]
- 26.Moiseeva EP, Spring EL, Baron JH, De Bono DP. Galectin 1 modulates attachment, spreading and migration of cultured vascular smooth muscle cells via interactions with cellular receptors and components of extracellular matrix. J Vasc Res. 1999;36:47–58. doi: 10.1159/000025625. [DOI] [PubMed] [Google Scholar]
- 27.Moiseeva EP, Williams B, Goodall AH, Samani NJ. Galectin-1 interacts with beta-1 subunit of integrin. Biochem Biophys Res Commun. 2003;310:1010–1016. doi: 10.1016/j.bbrc.2003.09.112. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee BE, Yona S, Rosignoli G, Young RE, Nourshargh S, Flower RJ, Perretti M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J Leukoc Biol. 2005;78:639–646. doi: 10.1189/jlb.0405206. [DOI] [PubMed] [Google Scholar]
- 29.Damazo AS, Yona S, D'Acquisto F, Flower RJ, Oliani SM, Perretti M. Critical protective role for annexin 1 gene expression in the endotoxemic murine microcirculation. Am J Pathol. 2005;166:1607–1617. doi: 10.1016/S0002-9440(10)62471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damazo AS, Yona S, Flower RJ, Perretti M, Oliani SM. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176:4410–4418. doi: 10.4049/jimmunol.176.7.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogowski O, Sasson Y, Kassirer M, Zeltser D, Maharshak N, Arber N, Halperin P, Serrov J, Sorkin P, Eldor A, Berliner S. Downregulation of the CD62L antigen as a possible mechanism for neutrophilia during inflammation. Br J Haematol. 1998;101:666–669. doi: 10.1046/j.1365-2141.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- 32.Diez-Fraile A, Meyer E, Duchateau L, Burvenich C. L-selectin and β2-integrin expression on circulating bovine polymorphonuclear leukocytes during endotoxin mastitis. J Dairy Sci. 2003;86:2334–2342. doi: 10.3168/jds.S0022-0302(03)73826-0. [DOI] [PubMed] [Google Scholar]