Abstract
Endometrial serous carcinoma (ESC) is the most aggressive subtype of endometrial cancer. Its aggressive behavior and poor clinical outcome may be partially attributed to lack of early diagnostic markers and unclear patho-genesis. The transcription factor Erythroid–E2-related factor 2 (Nrf2) is a recently identified protein marker, which plays a role in carcinogenesis as well as responsible for poor prognosis of many human cancers. The aim of this study is to determine the Nrf2 expression in benign endometrium (n=28), endometrial cancers (n=122) as well as their precursor lesions (n=81) trying to see whether Nrf2 has any diagnostic usage and is potentially involved in endometrial carcinogenesis. The level of Nrf2 was evaluated by immunohistochemical (IHC) and verified by using Western blots. Among the malignant cases, Nrf2 was positive in 28 (68%) of 50 ESCs, which was significantly more than in 3 (6%) of 50 endometrioid carcinomas (p < 0.001) and 2 (13%) of 15 clear cell carcinomas (p = 0.001) and other histologic types of endometrial cancers. Among endometrial precursor lesions, both serous endometrial glandular dysplasia (EmGD, 40%) and serous endometrial intraepithelial carcinoma (EIC, 44%) showed a significantly higher Nrf2 expression than that in atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (0%), clear cell EmGD (10%), and clear cell EIC (25%), respectively. We conclude that Nrf2 overexpression is closely associated with endometrial neoplasms with serous differentiation. Alteration of Nrf2 expression may represent one of the early molecular events in ESC carcinogenesis and overexpression of Nrf2 may used as a diagnostic marker in surgical pathology.
Keywords: Nrf2, endometrial cancer, precancer, endometrial serous carcinoma, endometrial glandular dysplasia
Introduction
Endometrial cancer, the most common gynecologic malignancy [1, 2], has been divided into two major categories: type I and type II [3, 4]. Endometrial endometrioid adenocarcinoma (EEC), the prototype of the Type I carcinoma has a fairly good prognosis, while endometrial serous carcinoma (ESC) or uterine papillary serous carcinoma, a prototype of Type II, bears a significantly worse prognosis. The dismal outcome of ESC was mainly attributed to lack of diagnostic markers as well as inadequately defined carcinogenesis.
Erythroid–E2-related factor 2 (Nrf2), is a significant nuclear transcription factor maintaining intracellular redox homeostasis through regulating the transcription of a series of target genes [5-7]. The main biologic function of Nrf2 is antioxidant insult [8-10]. It is known that activation of Nrf2 confers protection against many chronic diseases including cardiovascular diseases, lung inflammation, and diabetic nephropathy [5, 6, 11-18]. Mechanistic studies of Nrf2 showed that it is generally maintained in a low basal level and constantly regulated at the protein level by a negative regulator named Keap1 through ubiquitination and proteasomal machinery [19]. Chemopreventive compounds are able to stabilize Nrf2 through inhibition of Nrf2 degradation, thus enhancing the protein level of Nrf2 and activating the Nrf2-dependant antioxidant response [20, 21]. However, the dark side of Nrf2 has recently been revealed [22, 23]. Mutations or change of Nrf2 expression levels have been identified in many cancers [24-27] including endometrial cancers [28]. Deregulation of Nrf2 by its regulatory genes results in overexpression of its downstream genes that intensify the Nrf2-dependent protective mechanism and provide cancer cells with a growth advantage [24-27] and chemoresistance to many chemotherapeutic drugs in common human cancers [23, 29-31]. Addionally, Nrf2 may play a role in initiation of cancer development. It was demonstrated that increased Nrf2 and GSTP1 were closely associated with the early hepatocarcinogenesis [32] and arsenic induced skin cancer [33]. Based on above understanding, we suspected that, among the perplexing role in many human cancers, constitutive Nrf2 activation or overexpression may be involved in the process of endometrial serous carcinogenesis.
In this study, we investigated the level of Nrf2 protein expression in a large number of pathological endometrial samples including benign endometrial, precancerous lesions, and different type of human endometrial cancers to determine whether Nrf2 is differentially expressed in the endometrial tissues and whether expression differences among the different types of endometrial samples including those precancers could be exploited to facilitate our understanding of type II endometrial carcinogenesis and to serve as an additional diagnostic marker.
Materials and methods
Pathologic features of the cases studied
All endometrial samples were retrospectively retrieved from the Department of Pathology at the University of Arizona from 1996 to 2009 under the approval of the Institutional Review Board for human tissue usage. Among all the studied cases, no malignancies with a history of prior radiation or chemotherapy were included; while benign cases with personal cancer history or transplantation therapy were excluded. Case review was accomplished under the light microscopy using corresponding hematoxylin and eosin-stained slides to reconfirm the endometrial histogical types (Olympus BX41). Carcinomas with more than 10% mixed histological component were ruled out. A total of 231 specimens were studied which included benign endo-metrium (n=28), atypical endometrial hyperpla-sia or endometrial intraepithelial neoplasia (n=18), serous endometrial glandular dysplasia (EmGD) (n=20), serous endometrial intraepithelial carcinoma (EIC) (n=25), clear cell EmGD (n=10), clear cell EIC (n=8), endometrioid carcinoma (n=50), serous carcinoma (n=41), clear cell carcinoma (n=15), mucinous carcinoma (n=8), and other malignancies (n=8). Cancers grouped in the other category included those undifferentiated carcinoma (n=3), carcinosarcoma (n=3), squamous cell carcinoma (n=2).
Cases matched both atypical hyperplasia or EIN were included into the hyperplasia/EIN category [34-37]. EmGD was diagnosed as defined by Zheng et al [38, 39] and the diagnostic criteria for serous EIC were based on Ambros et al [40]. Clear cell EmGD and EIC were diagnosed according to previously described morphologic criteria [41]. All malignant cases were diagnosed, graded and staged using criteria of the International Federation of Gynecology Oncology (FIGO)[42, 43].
Tissue handling
Tissue sections were all derived from hysterectomy specimens. All uteri obtained were processed for 10% formalin fixation and paraffin embedding. Paraffin sections were cut at 5-um thickness and placed on Super Plus slides (Fisher Scientific, Pittsburgh, PA). For every case, one section was stained with H&E and examined microscopically to confirm the pathologic diagnosis.
Nrf2 immunohistochemical (IHC) analysis
A rabbit monoclonal antibody (EP1808Y) specifically reacted with human Nrf2 (IgG2) was purchased from Abcam, Inc. (Cambridge, MA). The antibody and IHC has been successfully used in previous studies [28]. Sections of ESC (known to express Nrf2) served as positive controls [28]. The staining reaction was evaluated only in epithelial cells. For those cases containing multiple lesions (such as EmGD or EIC in cases of ESC), each of the foci was separately evaluated. All IHC slides were reviewed independently by two investigators (N.C. and W.Z.). The percentage and intensity were arbitrarily divided into 5 and 4 tiers, respectively (see Table 1). For the purpose of better description and statistical analysis, we created positive score index which represented a sum of total Nrf2 expression in each category. Majority Nrf2 expression was observed in cytoplasm, while only small percentage of cases showed nuclear staining. Therefore, no data were analyzed for nuclear staining.
Table 1.
Nrf2 cytoplasmic immunoreactivity in endometrial tissue
| Case/Foci | Extent of Tissue Staining (Cytoplasmic) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessed | 80-100% | 50-79% | 25-49% | <25% | 0% | Index | |||
| Scores | 4 (%) | 3 (%) | 2 (%) | 1 (%) | 0 (%) | ||||
| Benign | 28 | ||||||||
| Atrophic | 10 | 0(0) | 0(0) | 1(10) | 1(10) | 8(80) | 30 | ||
| Proliferative | 10 | 0(0) | 0(0) | 0(0) | 2(20) | 8(80) | 20 | ||
| Secretory | 8 | 0(0) | 0(0) | 0(0) | 1(13) | 7(87) | 13 | ||
| Precursor lesion | 81 | ||||||||
| AEH or EIN | 18 | 0(0) | 0(0) | 0(0) | 6(33) | 12 (67) | 33 | ||
| Serous EmGD | 20 | 6(30) | 2(10) | 3(15) | 2(10) | 7(35) | 190 | ||
| Serous EIC | 25 | 8(32) | 3(12) | 2(88) | 6(24) | 6(24) | 204 | ||
| CC EmGD | 10 | 1(10) | 1(10) | 1(10) | 1(10) | 6(60) | 100 | ||
| CCEIC | 8 | 0(0) | 2(25) | 1(13) | 1(12) | 4(50) | 114 | ||
| Invasive Camcer | 122 | ||||||||
| Endometrioid | 50 | 1(2) | 2(4) | 7(14) | 7(14) | 33 (66) | 62 | ||
| Serous | 41 | 15 (37) | 13 (32) | 7(16) | 2(5) | 4(10) | 279 | ||
| Clear Cell | 15 | 1(7) | 1(7) | 2(13) | 3(20) | 8(53) | 95 | ||
| Mucinous | 8 | 0(0) | 0(0) | 0(0) | 1(13) | 7(87) | 13 | ||
| Others | 8 | 0(0) | 2(25) | 1(13) | 1(12) | 4(50) | 113 | ||
| Intensity of the Immunereactivity (cytoplasmic) | |||||||||
| Assessed | Strong | Moderate | Weak | Absent | Index | ||||
| Scores | 3 (%) | 2 (%) | 1 (%) | 0 (%) | |||||
| Benign | 28 | ||||||||
| Atrophic | 10 | 0(0) | 0(0) | 2(20) | 8(80) | 20 | |||
| Proliferative | 10 | 0(0) | 0(0) | 2(20) | 8(80) | 20 | |||
| Secretory | 8 | 0(0) | 0(0) | 1(13) | 7(87) | 13 | |||
| Precursor lesion | 81 | ||||||||
| AEH or EIN | 18 | 0(0) | 3(17) | 3(16) | 12 (67) | 50 | |||
| Serous EmGD | 20 | 3(15) | 6(30) | 4(20) | 7(35) | 125 | |||
| Serous EIC | 25 | 4(16) | 10 (40) | 5(20) | 6(24) | 164 | |||
| CC EmGD | 10 | 0(0) | 2(20) | 2(20) | 6(60) | 60 | |||
| CCEIC | 8 | 1(13) | 2(25) | 3(37) | 2(25) | 126 | |||
| Invasive Cancer | 122 | ||||||||
| Endometrioid | 50 | 0(0) | 5(10) | 12 (24) | 33 (66) | 44 | |||
| Serous | 41 | 8(19) | 25 (61) | 4(10) | 4(10) | 189 | |||
| Clear Cell | 15 | 1(7) | 3(20) | 3(20) | 8(53) | 81 | |||
| Mucinous | 8 | 0(0) | 0(0) | 1(13) | 7(87) | 13 | |||
| Others | 8 | 2(25) | 2(25) | 0(0) | 4(50) | 125 | |||
Note: Serous EmGD is equivalent to serous type endometrial dysplasia grade 1 and 2; Serous EIC is considered as grade 3 dysplastic glands; Clear cell (CC) EmGD covers grade 1 and grade 2 CC carcinoma precursor lesions; while CC EIC is the grade 3 precursor lesion of CC carcinoma. The positive score index represents the sum of either staining extent or intensity times the number of positive cases in each category.
Western blotting analysis of Nrf2 expression
Twenty frozen endometrial tissues and endometrial cancer cell lines (Ishkawa and SPEC-2) were homogenized and the samples were loaded in SDS gels and subjected to im-munoblot analysis as previously reported [44]. Intensity of the signal was measured by densi-tometry software (NIH Image 1.61). Relative expression of Nrf2 was normalized by the amount of the β-actin in every lane (calculated as the ratio of Nrf2 to β-actin).
Statistical analysis
Comparison of IHC Nrf2 expression in different status of the endometrium was assessed by Chi-squares and Fisher's exact test when an expected cell value was 5 or less. Nrf2 immunoreactivity index between type I and type II endometrial cancers including their putative precursor lesions were calculated by paired-comparisons t test by PROC MEANS in the SAS system.
Results
Nrf2 cytoplasmic expression in benign endometrial tissue
Most of the benign endometria showed a non-detective level of Nrf2 expression. Only a few showed weak positive staining of Nrf2 in a small percentage of the cells. Of the 28 tested benign cases, 1 (4%) atrophic, 2 (7%) proliferative, and 1 (4%) secretory endometria showed weak Nrf2 staining in less than 25% of the cells. The Nrf2 staining index overall was 30 or less for the staining extents and 20 or less for the intensity in the benign endometrial glandular cells. Detailed data are summarized in Table 1.
Nrf2 cytoplasmic expression in endometrial cancer precursors
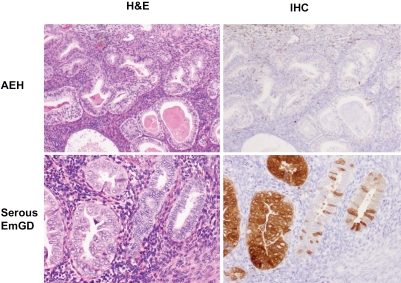
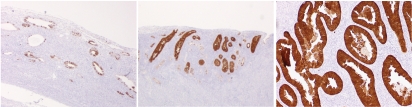
A total of 81 precursor lesions studied, the extent of Nrf2 (score index) was the highest in serous EIC (204), followed by serous EmGD (190), clear cell EIC (114), clear cell EmGD (100), and AEH/EIN (33). The score index for the staining intensity among the above lesions showed a similar order with the highest in serous EIC (164), followed by serous EmGD (125), clear cell EIC (100), clear cell EmGD (60), and AEH/EIN (50). Detailed data are summarized in Table 1. Representative pictures displaying Nrf2 expression in endometrial precursor lesions are presented in Figures 1 and 2.
Figure 1.
Nrf2 immunostaining in endometrial precancerous lesions. Negative Nrf2 immunostaining was seen in a case of atypical endometrial hyperplasia (right upper panel). In contrast, intense diffuse Nrf2 immunoreactivity was observed in serous EmGD glands (left side of the right lower panel). Stromal cells were negative. (original magnifications: 200×).
Figure 2.
Nrf2 staining in endometrial serous carcinoma and its precursor lesions. Nrf2 was strongly and focally positive in cytoplasm of serous EmGD (left). Strong and diffuse positive staining was seen in both serous EIC (mid) and endometrial serous carcinoma (right). (original magnifications: 200×).
Nrf2 cytoplasmic expression in endometrial cancers
Among all 122 studied endometrial cancers, Nrf2 expression was the highest in cases of ESC with the extent and intensity score index of 279 and 189, respectively. This was followed by other carcinoma category (113 and 125), clear cell carcinoma (96 and 81), endometrioid carcinoma (62 and 44), and mucinous carcinoma (13 and 13).
In cases of ESC, 37 (90%) of 41 had Nrf2 expression with 37% showing staining in more than 80% of the tumor cells and at least moderate intensity in more than 80% of the cases. This was dramatically different from endometrioid carcinomas, which only had 1 (2%) of 50 with more than 80% cells and 5 (10%) cases with moderate intensity of Nrf2 expression. Overall there were 17 (44%) of 50 endometrioid carcinomas showed various levels of Nrf2 expression in cytoplasm. Based on FIGO grade, the distribution of FIGO grades in the 17 endometrioid carcinomas with Nrf2 staining was as follows: grade 3 (n=7), grade 2 (n=9), and grade 1 (n=1). The mucinous carcinoma showing Nrf2 staining was a FIGO grade 2 tumor. Since there were not enough cases of type I endometrial cancer that showed any Nrf2 expression, the relationship between Nrf2 staining and tumor grade and the depth of myometrial invasion were notevaluable.
The level of Nrf2 expression was intermediate in other carcinoma category. This was probably due to the mixture of cancers including undifferentiated carcinomas and squamous cancers in this category. The level of Nrf2 expression in clear cell carcinomas was similar to those cancers in the other category, while the lowest expression was seen in mucinous carcinomas.
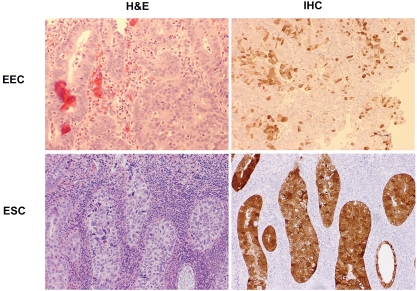
Nrf2 immunoreactivity was negative in stromal cells, myometrium and vascular endothelia among all cases studied. The summarized data are presented in Table 1. Representative images displaying Nrf2 expression patterns in endometrial cancers are presented in Figure 3.
Figure 3.
Nrf2 staining in endometrial cancers. Nrf2 was focally positive in an endometrioid carcinoma (upper panel), while diffuse cytoplasmic and focal nuclear staining was seen in an endometrial serous carcinoma (lower panel), (original magnifications: 200×).
Comparison of Nrf2 staining patterns in endometrial cancers and their precursor lesions
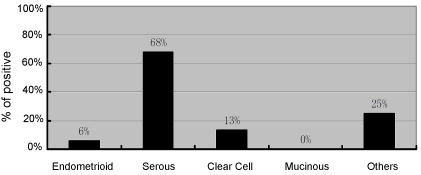
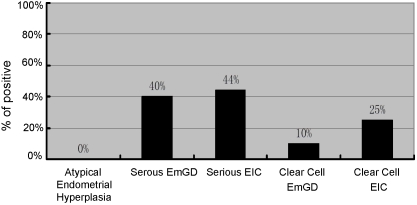
Nrf2 expression was mainly in cytoplasm and the level of expression was heterogeneous. If positivity for Nrf2 is defined as ≥50% staining, irrespective of intensity of staining, the results can be summarized as follows: Of all endometrial cancer precursors, Nrf2 staining was positive in none of AEH or EIN cases, 40% serous EmGDs, 44% serous EICs, 20% clear cell EmGDs, and 25% clear cell EICs. Among all endometrial cancers, we observed that Nrf2 was positive in 6% EECs, 68% ESCs, 14% CCCs, none of mucinous carcinomas, and 25% of other endometrial cancers. The data are summarized in Figures 4 and 5 and corresponding statistical comparison results are listed in Table 2
Figure 4.
Nrf2 expression in endometrial cancers. The graph was generated when ≥50% positively stained cells irrespective of intensity of staining was defined as positive.
Figure 5.
Nrf2 expression in endometrial intraepithelial neoplastic lesions. The graph was generated when >50% positively stained cells irrespective of intensity of staining was defined as positive.
Table 2.
Nrf2 nuclear immunoreactivity in neoplastic endometrium
| Case/Foci Assessed | Positive | |
|---|---|---|
| Precursor Lesion | 81 | |
| AEH/EIN | 18 | 0 (0%) |
| Serous EmGD | 20 | 3(15%) |
| Serous EIC | 25 | 6 (24%) |
| Clear cell EmGD | 10 | 2 (20%) |
| Clear cell EIC | 8 | 1 (13%) |
| Invasive Carcinoma | 122 | |
| Endometrioid | 50 | 3 (6%) |
| Serous | 41 | 9 (22%) |
| Clear cell | 15 | 1 (7%) |
| Mucinous | 8 | 0 (0%) |
| Others | 8 | 1 (13%) |
Note: Serous EmGD is equivalent to serous type endometrial dysplasia grade 1 and 2; Serous EIC is considered as grade 3 dysplastic glands; Clear cell EmGD covers grade 1 and grade 2 clear cell carcinoma precursor lesions; while clear cell EIC is the grade 3 precursor lesion of clear cell carcinoma.
Overall, ESCs including their precursor lesions showed a significant different staining pattern for Nrf2 compared with type I cancers and their precursor lesions (p < 0.001). Both ESC and CCC are classified into type II cancers, however, ESC showed a significantly higher frequency of Nrf2 expression (68%) than CCC (13%) (p = 0.001). Although CCCs showed a higher Nrf2 friequency (13%) than EECs (6%), the difference was not statistically different (p = 0.325). When comparison was carried out inside those serous subgroups, Nrf2 expression was significantly higher in ESCs than that in lesions of serous EmGD (p = 0.035) and serous EIC (p = 0.052). However, there were no significant differences for serous EIC when compared with serous EmGD. For all clear cell lesions, no statistical differences were found among EmGD, EIC and CCC. Among all EIC lesions, the difference of Nrf2 expression approached a statistical significance between serous and clear cell EICs (p = 0.05). Within the category of EmGD, there were no significant differences of Nrf2 expression between serous EmGD and clear cell EmGDs (p = 0.119). Compared with both serous and clear cell EIC, AEH or EIN showed less Nrf2 positive cases (p < 0.001) than the former but no difference than the later. The similar findings were observed in the corresponding type II precancerous lesions. The above data are summarized in Table 3.
Table 3.
Statistical comparison of endometrial neoplastic lesions
| Parameter a vs. Perameter b | Positive rates (Parameter a) | Positive rates (Parameter b) | P (X2-test) |
|---|---|---|---|
| Endometrioid Ca vs. ESC | 3/50 (6%) | 28/41 (68.3%) | <0.001 |
| ESC vs. CCC | 28/41 (68.3%) | 2/15 (13.3%) | 0.001 |
| Endometrioid Ca vs. CCC | 3/50 (6%) | 2/15 (13.3%) | 0.325 |
| Serous EmGD vs. ESC | 8/20 (40%) | 28/41 (68.3%) | 0.035 |
| Serous EIC vs. ESC | 11/25 (44%) | 28/41 (68.3%) | 0.052 |
| Serous EmGD vs. serous EIC | 8/20 (40%) | 11/25 (44%) | 0.787 |
| Serous EIC vs. clear cell EIC | 11/25 (44%) | 2/8 (25%) | 0.05 |
| serous EmGD vs. clear cell EmGD | 8/20 (40%) | 2/20 (10%) | 0.119 |
| AEH vs. selous EIC | 0/18 (0%) | 11/25 (44%) | < 0.001 |
| AEH vs. clear cell EIC | 0/18 (0%) | 2/8 (25%) | 0.278 |
| AEH vs. serous EmGD | 0/18 (0%) | 8/20 (40%) | 0.003 |
| AEH vs. clear cell EmGD | 0/18 (0%) | 2/20 (10%) | 0.419 |
AEH: atypical endometrial hyperplasia; EmGD: endometrial glandular dysplasia; Endometrioid Ca: endometrioid carcinoma; ESC: endometrial serous carcinoma; CCC: clear cell carcinoma; EIC: endometrial intraepithelial carcinoma.
Western blot detecting Nrf2 protein expression in neoplastic endometrial tissues/cells
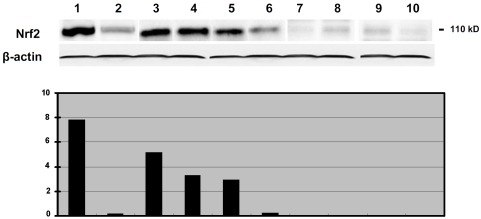
We also used western blots to measure the reactivity of Nrf2 antibody with Nrf2 protein produced by endometrial cancer cell lines and neoplastic endometrial tissues. Nrf2 antibodies reacted with a protein at about 110 kD, the expected molecular weight for Nrf2 (Figure 6). Nrf2 was highly expressed in SPEC-2 cells, an endometrial carcinoma cell line derived from tumor tissue of ESC. In contrast, marginal expression of Nrf2 was detected in Ishikawa cells, which was derived from a well differentiated EEC. Among the endometrial tissues tested, the highest level of Nrf2 was seen in cases of ESC and serous EIC. The level of Nrf2 expression in a case of EmGD was comparable to a case of endometrioid carcinoma. No Nrf2 was detected in cases of mucinous carcinoma or AEH/EIN or benign proliferative endometrium. The cases with highly expressed Nrf2 detected by western blot also showed high level of expression in IHC slides, while the lower or none expression cases were negative on IHC slides. Therefore, the results of IHC and western blot were correlated to each other.
Figure 6.
Western blotting of Nrf2 expression in endometrial specimens. Nrf2 protein was shown as a 110 kD band on western blot. Actin used as a load control. Bar graph (low panel) represented a ratio of Nrf2 and actin. Lane 1, SPEC 2 cells (derived from an endometrial serous carcinoma and known to express a high level Nrf2) served as a positive control; Lane 2, Ishikawa cells (derived from a well differentiated endometrioid carcinoma); Lanes 3 and 4: endometrial serous carcinomas; Lane 5, serous EIC; Lane 6, serous EmGD; Lane 7, endometrioid carcinoma; Lane 8, mucinous carcinoma; Lane 9, Atypical endometrial hyperplasia; and Lane 10, benign proliferative endometrium.
Discussion
In the last 2 decades, there have been great progress in the study of carcinogenesis of type I endometrial cancer [45]. However, studies on type II carcinogenesis have been remarkably limited until recently. Type II endometrial cancer represents only about 15% of the endometrial cancers but accounts for approximately 80% of endometrial cancer-related deaths [46]. In contrast, type I cancers constitute up to 85% of all endometrial cancers which are typically with a good prognosis. Therefore, the development of molecular markers specific for the different histologic types would provide useful adjuncts to the morphologic assessment of endometrial lesions and thus facilitate patient care. Meanwhile, it is clinically important if the molecular markers actually play a role in carcinogenesis so that an effective therapeutic intervention may be administered.
In this report, we examined the expression of Nrf2 in a large number of endometrial tissue samples. We found that Nrf2 is an excellent cytoplasmic marker by immunohistochemistry to differentiate ESC from other endometrial cancers. Nrf2 was expressed in a significantly higher proportion of ESC (68%), as compared with endometrioid carcinomas (6%) (p < 0.001), clear cell carcinoma (13%) (p = 0.001), and other endometrial cancers (Tables 1 and 2). Mucinous carcinoma showed negative staining in 7 of the 8 cases tested. Non-neoplastic endometrial tissues were mostly negative. Therefore, assimilation of entire data indicates that Nrf2 expression pattern appears to be completely different between ESC and other endometrial cancers. Nrf2 seems to be a highly sensitive and specific cytoplasmic marker for the serous type of endometrial cancers. Positive staining, particularly in more than 50% of the neoplastic cells with at least moderate staining intensity, may lead us toward the diagnosis of an endometrial serous neoplasm, which has a serious impact in clinic [47, 48].
Furthermore, Nrf2 protein expression was dramatically different among the endometrial precursor lesions. EmGD including both serous and clear cell types are recognized precancers of ESC and CCC, respectively [38, 39, 49, 50]. Serous EIC, although develops earlier than ESC, is considered an early form of ESC due to its high association of extra uterine disease [47, 51, 52]. AEH and/or EIN are well recognized type I pre-cancer [50]. Nrf2 was negative in such lesions (Table 2), whereas it was positive in a substantial number of precursor lesions of type II cancer, which illustrated an interesting contrast. The expression rates of Nrf2 were dramatically increased in both serous EmGD (40%) and EIC (44%) as compared with lesions of AEH/EIN (0%) or corresponding clear cell precursors (10% and 25%, respectively) (Table 2). In our opinion, the congruence in the expression rates between serous EmGD and serous EIC suggests that endometrial neoplasia with serous differentiation is not only different from those with endometrioid as previously known [45] but also different from those with clear cell differentiation. Moreover, the high level of Nrf2 expression in serous EmGD is a solid line of evidence linking this precancer to the full-blown ESC.
More evidence has indicated a positive role of Nrf2 in carcinogenesis and chemoresistance. The first evidence came from the finding that Nrf2 was elevated during hepatocarcinogenesis [32]. Its elevation was then found in many cancers including lung, ovary, and breast when the Nrf2 negative regulator Keap1 lost function [23-27, 30, 31]. Using genetic manipulation, Zhang's group and others have demonstrated a strong positive correlation between Nrf2 levels and resistance to chemotherapeutic drugs such as cisplatin in both endometrial and ovarian cancer cells [23, 31]. Furthermore, we have recently shown that reduced levels of Nrf2 (either directly or via manipulation of its negative regulator) significantly sensitized SPEC-2 cells and its xenografts to chemotherapeutic drugs [28]. Collectively, these findings may explain why Nrf2 is associated with tumor progression and the aggressiveness of ESC. Further studies are required to investigate how the level of Nrf2 is regulated in ESC and its precancers.
It is interesting to note that Nrf2 expression in CCC and its precursors was closer to that in EEC, rather than that in ESC. There is, thus far, no molecular study on endometrial cancer that included a sufficient number of clear cell carcinoma has been published. In various studies, a small number of CCCs were analyzed, mostly together with ESC. Thus, it has been speculated that CCC is similar to ESC. However, evidence supporting that they have different molecular pathogenesis pathways does exist. Compared to ESC, alterations of p53, PTEN, K-Ras, and EGFR were significantly different in CCC [53, 54]. Therefore, it is not clear if pathogenesis of CCC is similar to ESC. Significantly different Nrf2 expression between CCC and ESC and their precursors is supportive of a different pathogenetic mechanism in these type II endometrial cancers.
In summary, our results in this study indicate that expression of Nrf2 is closely associated with endometrial serous cancer, while it seems not related to type I endometrial cancer. Importantly, strong and diffuse Nrf2 cytoplasmic expression is highly sensitive for ESC including its early and pre-cancers. As a result, Nrf2 may serve as a useful diagnostic marker in the assessment of endometrial cancers and their precursor lesions, particularly when the amount of available tissue material is limited and morphology is ambiguous. Alteration of Nrf2 expression may represent one of the early molecular events in the neoplastic transformation of endometrial serous cancers. Furthermore, overexpression of Nrf2 may be partially responsible to the aggressive biological behavior and dismal clinical outcome of ESC due to its known effect of increased resistance to chemotherapeutic drugs. The findings may also provide an opportunity for therapeutic intervention against chemoresistancevia applications of either Nrf2 inhibitors or gene knockdown approaches for ESC.
Acknowledgments
The project was supported in part by Better Than Ever Fund, P30 CA23074 from Arizona Cancer Center and Department of Pathology, University of Arizona Startup fund to WZ. Dr. Ning Chen is a Ph.D. candidate of Shandong University, Jinan, China. Partial funding of her training was supported by Department of Obstetrics and Gynecology, Qilu Hospital, Shandong University.
Glossary
Abbreviations
- Nrf2
Erythroid–E2-related factor 2
- ESC
endometrial serous carcinoma
- EEC
endometrial endometrioid carcinoma
- CCC
clear cell carcinoma
- EIC
endoemtrial intraepithelial carcinoma
- EmGD
endometrial glandular dysplasia
- AEH
atypical endometrial hyperplasia
- EIN
endometrial intraepithelial carcinoma
- FIGO
International Federation of Obstetrics and Gynecology
References
- 1.Gurpide E. Endometrial cancer: biochemical and clinical correlates. J Natl Cancer Inst. 1991;83:405–416. doi: 10.1093/jnci/83.6.405. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000;13:295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- 4.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 5.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 6.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 7.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 12.Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-Imidazolide. Eur J Pharmacol. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in STZ-induced diabetic nephropathy. Diabetes. 2010 doi: 10.2337/db09-1342. Diabetes (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho HY, Kleeberger SR. Genetic mechanisms of susceptibility to oxidative lung injury in mice. Free Radic Biol Med. 2007;42:433–445. doi: 10.1016/j.freeradbiomed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 27.Nioi P, Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun. 2007;362:816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 28.Jiang T, Chen N, Zhao F, Wang XJ, Kong B, Zheng W, Zhang DD. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 70:5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, Gabrielson E, Feinstein E, Biswal S. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JM, Manandhar S, Lee HR, Park HM, Kwak MK. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatimplication to cancer cell resistance. Cancer Lett. 2008;260:96–108. doi: 10.1016/j.canlet.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda H, Nishi S, Sakai M. Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem J. 2004;380:515–521. doi: 10.1042/BJ20031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pi J, Diwan BA, Sun Y, Liu J, Qu W, He Y, Styblo M, Waalkes MP. Arsenic-induced malignant transformation of human keratinocytes: involvement of Nrf2. Free Radic Biol Med. 2008;45:651–658. doi: 10.1016/j.freeradbiomed.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutter GL. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol. 2000;76:287–290. doi: 10.1006/gyno.1999.5580. [DOI] [PubMed] [Google Scholar]
- 35.Mutter GL. Histopathology of genetically defined endometrial precancers. Int J Gynecol Pathol. 2000;19:301–309. doi: 10.1097/00004347-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Mutter GL. Diagnosis of premalignant endometrial disease. J Clin Pathol. 2002;55:326–331. doi: 10.1136/jcp.55.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mutter GL, Baak JP, Crum CP, Richart RM, Ferenczy A, Faquin WC. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol. 2000;190:462–469. doi: 10.1002/(SICI)1096-9896(200003)190:4<462::AID-PATH590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Liang SX, Chambers SK, Cheng L, Zhang S, Zhou Y, Zheng W. Endometrial glandular dysplasia: a putative precursor lesion of uterine papillary serous carcinoma. Part II: molecular features. Int J Surg Pathol. 2004;12:319–331. doi: 10.1177/106689690401200405. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W, Liang SX, Yu H, Rutherford T, Chambers SK, Schwartz PE. Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma. Part I: morphologic features. Int J Surg Pathol. 2004;12:207–223. doi: 10.1177/106689690401200302. [DOI] [PubMed] [Google Scholar]
- 40.Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: A distinctive lesion specifically associated with tumors displaying serous differentiation. Human Pathol. 1995;26:1260–1267. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 41.Fadare O, Liang SX, Ulukus EC, Chambers SK, Zheng W. Precursors of Endometrial Clear Cell Carcinoma. Am J Surg Pathol. 2006;30:1519–1530. doi: 10.1097/01.pas.0000213296.88778.db. [DOI] [PubMed] [Google Scholar]
- 42.Announcements FIGO stages–1988 revision. Gynecol Oncol. 1989;35:125–127. [Google Scholar]
- 43.Kurman RJ, Zaino RJ, Norris HJ. Endometrial carcinoma. In: Kurman RJ, editor. Blaustein's pathology of the Female Genital Tract. New York: Springer-Verlag; 1994. pp. 439–486. [Google Scholar]
- 44.Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD. Direct interaction between Nrf2 in endometrial serous carcinoma Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 46.Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, Husain A, Teng NN, Berek JS, Chan JK. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198:218. doi: 10.1016/j.ajog.2007.08.075. e211-216. [DOI] [PubMed] [Google Scholar]
- 47.Zheng W, Schwartz PE. Serous EIC as an early form of uterine papillary serous carcinoma: recent progress in understanding its pathogenesis and current opinions regarding pathologic and clinical management. Gynecol Oncol. 2005;96:579–582. doi: 10.1016/j.ygyno.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 48.Zheng W, Liang SX, Yi X, Ulukus EC, Davis JR, Chambers SK. Occurrence of endometrial glandular dysplasia precedes uterine papillary serous carcinoma. Int J Gynecol Pathol. 2007;26:38–52. doi: 10.1097/01.pgp.0000228138.56222.4e. [DOI] [PubMed] [Google Scholar]
- 49.Fadare O, Liang SX, Ulukus EC, Chambers SK, Zheng W. Precursors of endometrial clear cell carcinoma. Am J Surg Pathol. 2006;30:1519–1530. doi: 10.1097/01.pas.0000213296.88778.db. [DOI] [PubMed] [Google Scholar]
- 50.Yi X, Zheng W. Endometrial glandular dysplasia and endometrial intraepithelial neoplasia. Curr Opin Obstet Gynecol. 2008;20:20–25. doi: 10.1097/GCO.0b013e3282f2fd50. [DOI] [PubMed] [Google Scholar]
- 51.Zheng W, Khurana R, Farahmand S, Wang Y, Zhang ZF, Felix JC. p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am J Surg Pathol. 1998;22:1463–1473. doi: 10.1097/00000478-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Wheeler DT, Bell KA, Kurman RJ, Sherman ME. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Pathol. 2000;24:797–806. doi: 10.1097/00000478-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004;444:213–223. doi: 10.1007/s00428-003-0947-3. [DOI] [PubMed] [Google Scholar]
- 54.Lax SF. Molecular genetic changes in epithelial, stromal and mixed neoplasms of the endometrium. Pathology. 2007;39:46–54. doi: 10.1080/00313020601146822. [DOI] [PubMed] [Google Scholar]