Abstract
The World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissue, 2008 edition, states that anaplastic large cell lymphoma (ALCL) is “consistently negative for Epstein-Barr virus (EBV)". The statement made by the WHO has led to the widespread belief that EBV can have no pathogenic role in ALCL. Herein we report a case of an immunocompetent 35-year-old male who presented with hemophagocytic syndrome secondary to lymphoma for which diagnostic material consisted solely of a bone marrow biopsy. The biopsy demonstrated large anaplastic cells which were uniformly positive for surface CD3, CD30 (strong membranous and Golgi expression), CD45, TIA-1 and Granzyme B but negative for ALK-1. In-situ hybridization was strongly positive for EBER in the large neoplastic cells. The uniformity of CD30 expression and positivity for cytotoxic markers on the anaplastic tumor cells raised the diagnostic possibility of an EBV-associated ALCL, ALK-. Discussion of this case as well as a retrospective review of 64 cases of reported of EBV+ ALCL are presented.
Keywords: Epstein-Barr virus, anaplastic large cell lymphoma, ALK
Introduction
The World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissue, 2008 edition, states that anaplastic large cell lymphoma (ALCL) is “consistently negative for Epstein-Barr virus (EBV)” [1]. However, numerous reports suggest that the lack of EBV in ALCL is less absolute. We describe herein a patient with a T-cell lymphoma and hemophagocytic syndrome, where morphology and immunohistochemical staining (IHC) led to the diagnostic consideration of ALCL, ALK-, in addition to PTCL, NOS. However, by in-situ hybridization (ISH), the neoplastic large cells had strong EBV positivity. Further review of the literature suggested that, while EBV is certainly not required in the pathogenesis of ALCL, nor is it even highly associated with ALCL, at least 64 cases of systemic lymphoma have been identified as ALK- ALCL of either T- or Null-cell pheno-type which all demonstrated strong CD30 expression as well as evidence of EBV positivity.
These cases all suggest that the presence of EBV does not categorically exclude the diagnosis of ALCL.
Case presentation
The patient was a 35-year-old Hispanic male who for one month experienced intermittent low grade fever and soaking night sweats. Two days prior to presentation, he developed nausea and vomiting, decreased appetite and early satiety. At that time, he also reported dark-colored urine. Prior CT scan of the abdomen showed splenomegaly (20 cm), extensive retroperitoneal lymphadenopathy and multiple hypodense lesions in the liver and spleen. On presentation, he complained of fatigue and vomiting. He had no significant past medical or surgical history. The patient had no rash or other cutaneous lesions. CBC obtained showed pancytopenia (WBC 1.6 thousand/dL, hemoglobin 7.2 g/dL, hematocrit 21.6%, and platelets 52 thousand/ dL). He was then admitted to the hospital for further workup.
A bone marrow biopsy showed a markedly hypercellular marrow (90% cellularity) with virtually no normal residual hematopoiesis (Figure 1A). There were scattered large cells with large pleomorphic (occasionally multinucleated or horseshoe-shaped) nuclei with open or speckled chromatin, prominent inclusion-like eosinophilic nuclei and moderate to abundant eosinophilic cytoplasm. The scattered distribution of the large cells was due to a background of numerous activated macrophages with ingested cells with associated scattered small lymphocytes. Due to the focality of the disease, the bone marrow aspirate smears demonstrated evidence of active hemophagocytosis, but otherwise contained trilineage hematopoiesis with no definitive morphologic evidence of lymphomatous involvement (Figure 1B). Flow cytometry, therefore, reflected normal maturing hematopoietic elements.
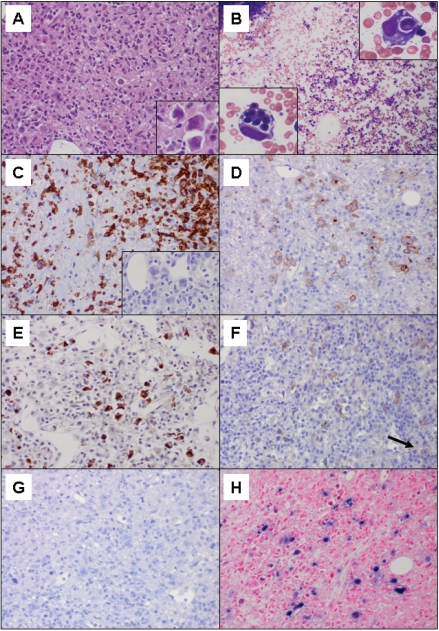
Figure 1.
A. Hematoxylin and eosin (H&E) of bone marrow biopsy with marrow space virtually entirely replaced by the neopiastic process and hemophagocytosis (400×); insert: neopiastic large cells (1000×). B. Wright stained aspirate smear with trilineage hematopoiesis and no neopiastic large cells (100×); insert: hemophagocytic macrophages (1000×). C. CD3 stain demonstrating surface positivity on the neopiastic large cells as well as numerous background small T-cells (400×); insert: PAX-5 stain negative on the large cells (400×). D. CD30 stain demonstrating membrane and Golgi uniform positivity on the neopiastic large cells (400×). E. Granzyme B stain demonstrating positivity on the neopiastic large cells (400×). F. CD56 stain demonstrating positivity on scattered small background lymphocytes as well as faint staining on rare lymphoma cells (arrow) (400×). G. ALK-1 stain negative on lymphoma cells (400×). H. EBER in situ hybridization demonstrating strong uniform positivity on the neopiastic large cells (400×).
Immunohistochemical studies performed on the bone marrow biopsy revealed that the scattered large cells were uniformly positive for surface CD3 (Figure 1C), CD30 (Figure 1D), CD43, CD45, ZAP-70, TIA-1, and Granzyme B (Figure 1E). The CD30 showed a uniformly strong membranous and Golgi staining pattern on all neo-plastic cells. There was apparent weak subset positivity for EMA and CD56 on the neoplastic large cells (Figure 1F), with scattered positivity of CD56 on background small lymphocytes. The following stains were negative: CDla, CD2, CD4, CD5, CD8, CD15, CD20, CD57, CD138, CD163, clusterin, ALK-1 (Figure 1G), PAX-5, S-100, Melan-A and CAM 5.2. In-situ hybridization stains on the bone marrow biopsy demonstrated that the neoplastic large atypical cells were strongly positive for EBER (Figure 1H). Unfortunately, additional molecular tests, such as T-cell gene rearrangement studies, were not feasible since the neoplastic cells were only identified in the decalcified bone marrow biopsy specimen.
Additional relevant laboratory values were as follows: LDH 1812 U/L, haptoglobin <10 mg/ dl_, Direct Coombs test negative and ferritin 55,349 ng/mL with normal iron studies. A full immunological history of the patient was obtained and there was no evidence of any history of immunoincompetence. An HIV immunoassay was negative.
The patient was subsequently started on a hyper-CVAD (fractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone) regimen with an alternating dose of methotrexate and cytarabine every 3 to 4 weeks for 8 cycles [2]. After a course complicated by respiratory failure due to an excessive cytokine response and circulatory overload, the patient recovered from his first round of chemotherapy and hemo-phagocytic syndrome. Sadly, the patient subsequently expired from infection during the final course of hyper-CVAD.
Discussion
In 1985, Stein et al. introduced the notion of a Ki-1 (CD30) lymphoma composed of anaplastic large cells which often involved the sinuses of lymph nodes [3]. Of the 45 Ki-1 positive cases of anaplastic large cell lymphomas described in the paper, 26 were of T-cell origin (58%), 3 of Null-cell origin (7%), 9 of mixed cell origin (20%) and 7 of B-cell origin (16%). The authors concluded that Ki-1 lymphomas are a heterogeneous group with both T-cell and B-cell lineages represented. In the ensuing years (and multiple lymphoma classification schemes), the name has officially become anaplastic large cell lymphoma with the exclusion of those lymphomas of B-cell origin in the World Health Organization (WHO) monographs published in 2001 and 2008 [1, 4].
However, to this day, anaplastic large cell lymphoma has continued to elicit debate regarding its definition. During a session of the 2005 Society of Hematopathology/European Association for Haematopathology Workshop focused on anaplastic large cell lymphoma, no clear consensus for the histologic and immunohistochemical categorization of ALK- ALCL could be agreed upon [5]. It was suggested that any anaplastic T- or Null-cell neoplasm that strongly and uniformly expressed CD30 and had some of the histological features of ALK+ ALCL but is ALK- should be defined as an ALK- ALCL. Other participants thought that an ALK- ALCL should be indistinguishable from an ALK+ ALCL but lack ALK expression. Another predominant opinion was that ALK- ALCL lies within the spectrum of peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS).
Included in this series of cases examined by a panel of hematopathologists was a case that was positive for EBV-encoded small nuclear RNAs (EBER) for which the expert panel diagnosis was ALK- ALCL [5]. This case was one of only four cases for which the panel rendered a definitive ALK- ALCL diagnosis out of twenty total cases (including twelve ALK+ ALCL cases, three peripheral T-cell lymphomas, and one undetermined diagnosis). This diagnosis of an EBV+ ALK- ALCL was rendered despite the statement in the current WHO classification system [1], which states, “ALCL, ALK-, are consistently negative for Epstein-Barr virus (EBV)."
The categorical dissociation of EBV infection and ALCL expressed by the WHO is supported by several studies. Benharroch et al. investigated 64 cases of ALK+ ALCL by ISH and LMP-1 IHC [6]. All 64 cases were negative for EBV by in-situ hybridization and/or latent membrane protein 1 (LMP-1) IHC. Although this study did not rigorously exclude anaplastic tumors of B-cell origin, no EBV positivity was found in any of the cases, including those that met WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, 2008 edition, ALCL classification criteria [1]. Similarly, Herling et al. found no evidence of EBV by ISH and IHC methods in 64 cases of ALCL, classified according to WHO 2001 criteria [4,7]. These cases consisted of twenty-seven ALK+ and 37 ALK- cases of ALCL. In the study, all of the cases were negative for B-cell antigens, including PAX-5, CD20 and CD79a. Their conclusion was that EBV infectivity had no tu-morigenic role in ALCL arising in Western patients. They also theorized that published reports of EBV+ ALCL cases in Western patients may be the result of inclusion of cases that were thought to be ALCL, but no longer fit the current criteria for ALCL classification, such as CD30 positive anaplastic tumors of B-cell origin.
As mentioned above, many hematopathologists believe that ALK- ALCL should be diagnosed as PTCL, NOS, rather than forming a separate diagnostic category [5]. The main reason for this belief is that ALK- ALCL has no defining morphologic, immunophenotypic or genetic profile. An initial epidemiologic study comparing clinico-pathological data of ALK+ (n = 28) and ALK- (n = 46) ALCL; PTCL, NOS (n = 47); and angioim-munoblastic T-cell lymphoma (AILT) (n = 12) identified no statistically significant differences between age, disease stage and overall or progression-free survival of these different subcate-gories of T-cell lymphomas, suggesting that perhaps they should not be split into distinct entities [8].
However, based upon a large multi-institutional review of clinical, immunohistochemical and genetic characteristics of 1314 patients, Savage et al. reports that, contrary to prior studies, the 5-year failure-free survival was significantly better for ALK- ALCL patients than for PTCL, NOS, patients (36% vs 20%, respectively; P = 0.012) as was 5-year overall survival (49% vs 32%, respectively; P = 0.032) [9]. ALK- ALCL is more often CD30 (strong membranous and Golgi-pattern positivity), EMA, TIA-1 and Gran-zyme-B positive than PTCL, NOS [9]. Furthermore, in a immunohistochemical study by Went et a/., only 3 out of 145 cases of PCTL, NOS, stained positive for CD30 (6%) and only a minority of cases (27%) were positive for TIA-1 [10]. Lastly, losses of 5q (26%) and 9p (31%) found in PTCL, NOS, from a previous study were not found in 31 cases of ALK- ALCL, supporting the continued separation of these two diseases as distinct clinicopathologic entities [11].
However, when reviewing the earlier literature, the uncertainty of whether ALK- ALCL is a separate entity from PTCL, NOS, makes the association of EBV with ALK- ALCL even more difficult to assess. Several reports have shown EBV infectivity in a subset of PTCL, NOS. One review cited the presence of EBV in a variable proportion of neopiastic cells in a limited number of cases of PTCL, NOS [12]. Another study found integration of EBV into 5% of neoplastic T-cells [13], while Dupuis et al. found EBV in 5-50% of neoplastic cells in ten of 45 cases of PTCL, NOS, and rarely saw EBER marking >50% of cells (two cases) [14]. While PTCL, NOS, may be EBV+ in a variable number of neoplastic cells, likewise, CD30 expression can be seen in a subset of the cells with variable intensity. However, uniformly strongly positive expression of CD30 should favor ALK- ALCL [1,5]. Thus, PTCL, NOS, has been characterized as lacking both uniform EBV and CD30 positivity.
When faced with an EBV+ T-cell lymphoma, extra nodal NK/T-cell lymphoma, nasal type, is also a diagnostic consideration. Extranodal NK/T-cell lymphoma, nasal type, is strongly associated with EBV and may have similar morphologic and immunophenotypic characteristics of ALCL [1,25,16]. These extranodal NK/T-cell lympho-mas, nasal type, are characterized by their expression of CD2 and CD56 with a clear association with EBV, present in a clonal episomal form [1]. Like PTCL, NOS, extranodal NK/T-cell lymphoma, nasal type, may occasionally be positive for CD30.
Thus, one could make the argument that all EBV+ T-cell neoplasms with systemic involvement (non-cutaneous) should be classified as PTCL, NOS, or extranodal NK/T-cell lymphoma, nasal type. However, there are reports that have linked EBV infectivity to cases which fulfill all other diagnostic criteria for systemic ALCL, such as pleomorphic morphology and uniform strong membrane and Golgi expression of CD30. After extensive literature review, these reports have been summarized in Table 1. Of note, the case numbers were carefully examined based upon the available data to exclude all cases with a B-cell lineage or mixed B- and T-cell lineage, as well as cases of primary cutaneous ALCL. Although some authors in the past have suggested that the alleged cases of EBV+ ALCL must have derived from B-cell neoplasms which, in the earlier Updated Kiel and the Revised European-American Lymphoma (REAL) classification systems, were included in the ALCL category, we have eliminated all those cases from our analysis. In addition, where notation was available, only cases in which the EBV positivity was noted on the neoplastic cells by ISH or IHC are included. Of course, using PCR methods, non-neoplastic tumor-infiltrating lymphocytes may contribute to the PCR detection of EBV, leading to falsely elevated numbers of EBV+ ALCL. Wherever possible, the immune status of the patients in the study is noted.
Table 1.
Meta-analysis of studies demonstrating the presence of EBV in ALCL
| Date of Study | Total Number of Cases | Country of origin of the patients | Method of EBV Assessment | Number of EBV+ Cases* | ALCL Classification Scheme | Immunocompetency Status | Comments | Reference |
|---|---|---|---|---|---|---|---|---|
| 1991 | 47 | Germany | PCR | 5/19 | Updated Kiel | Competent | Positive cases: T-cell phenotype (18 T-cell, 1 Null-cell) All LMP1+ cases were PCR and EBER positive as well. | [33] |
| LMP1-IHC | 2/19 | |||||||
| EBNA2-IHC | 0/19 | |||||||
| EBER1/2- ISH | 2/2 | |||||||
| 1992 | 13 | Netherlands | PCR | 6/13 | Updated Kiel | Nl | All “ALCL” cases characterized as >75% CD30+ cells (4/11 T-cell phenotype EBV+ by PCR, 2/2 Null-cell phenotype EBV+ by PCR) | [34] |
| EBER1/2- ISH | 2/5 | |||||||
| LMP1-IHC | 1/13 | |||||||
| EBNA2-IHC | 0/13 | |||||||
| 1992 | 8 | Germany | Southern Blot | 1/3 | Updated Kiel | NI | 4 cases with immunophenotyping available are T-cell in origin | [35] |
| Dot Blot | 1/4 | |||||||
| BamH1W-ISH | 2/4 | |||||||
| 1992 | 8 | USA | PCR (2 rounds) | 8/8 | NI | NI | PCR positivity may be from tumor infiltrating non-neoplastic lymphocytes | [36] |
| Southern Blot | 0/4 | |||||||
| BamH1W-ISH | 0/4 | |||||||
| 1992 | 26 | Denmark | LMP1-IHC | 1/26 | Updated Kiel | Competent | Positive case: “Null-cell” phenotype (19 T-cell, 7 Null-cell) | [37] |
| EBNA2-IHC | 0/26 | |||||||
| 1994 | 4 | Netherlands, Hungary, France | EBER1/2-ISH | 1/2 | Updated Kiel | NI | Cutaneous cases excluded | [38] |
| PCR | 2/4 | |||||||
| LMP1-IHC | 1/4 | |||||||
| 1995 | 16 | Germany and Austria | PCR | 5/16 | Updated Kiel | Competent | 11 T-cell, 3 Null-cell (the 2 B-cell phenotype cases cannot be subtracted from available data) | [39] |
| EBER-ISH | 1/5 | |||||||
| 1995 | 13 | USA and Hong Kong | EBER1-ISH | 3/13 | NI | Competent | T-cell lineage determined by CD43 and CD45RO (15 T-cell, 3 Null-cell) | [40] |
| LMP1-IHC | 1/13 | |||||||
| 1996 | 40 | Italy | LMP1-IHC | 10/40 | Updated Kiel, REAL | 3 pts HIV+ | Phenotype of LMP+ cases not specified (18 T-cell, 22 Null-cell) | [41] |
| 1996 | 14 | Denmark | EBER1/2- ISH | 1/14 | NI | NI | [42] | |
| 1997 | 67 | Japan | EBERV2-ISH | 4/54 | Stein et al. and | NI | All EBER+ cases: ALK-; All | [44] |
| LMP1-IHC | 7/31 | Suchi et al. [3,43] | LMP+ cases: ALK+ (cases of cutaneous ALCL and HD-like ALCL were excluded in this analysis) | |||||
| 1997 | 6 | Japan | EBER (Dia-latron) | 1/55 | NI | Competent | (4 T-cell, 2 Null-Cell) | [45] |
| EBER(DAKO) | 2/5 | |||||||
| BamH1W-ISH | 6/6 | |||||||
| LMP1-IHC | 1/1 | |||||||
| PCR | 5/5 | |||||||
| 1999 | 66 | Japan | EBER1/2- ISH | 4/42 | Updated Kiel, REAL | NI | All positive cases: ALK-(0/32 EBV+ ALK+ cases) | [46] |
| 1999 | 13 | S. Korea | EBER-ISH | 3/13 | REAL | NI | EBER type not specified | [47] |
| 2000 | 48 | Netherlands and Austria | EBER1/2-ISH | 2/48 | REAL | Competent | [48] | |
| LMP1-IHC | 1/2 | |||||||
| 2000 | 143 | Japan | EBER1/2- ISH | 12/58 | Stein et al. and Suchi et al. [3,43] | Competent | All positive cases: ALK1-(0/80 EBV+ ALK+ cases) | [28] |
| 2002 | 46 | India | EBER1-ISH | 9/46 | NI | Competent | All LMP1+ cases also EBER+, all PCR+ cases also EBER+ | [49] |
| LMP1-IHC | 5/46 | |||||||
| PCR | 6/6 | |||||||
| 2002 | 74 | Netherlands | EBER1/2-ISH | 6/74 | WHO 2001 | Competent | All positive cases: ALK- | [8] |
| 2004 | 37 | Pakistan | EBER1-ISH | 2/12 | REAL, WHO 2001 | NI | [50] | |
| PCR | 7/28 | |||||||
| 2006 | 42 | S. Korea | EBER1-ISH | 2/3 | EORTC | Competent | Only 3 systemic cases cited | [51] |
| LMP1-IHC | 2/3 | |||||||
| 2007 | 16 | NI | EBER-ISH | 1/16 | WHO 2001 and 2005 SHP/EAHP consensus panel | NI | Positive case: ALK- T-cell phenotype (0/12 EBV+ ALK+ cases)+ | [5] |
| 2008 | 63 | USA | EBER-ISH | 3/45 | WHO 2001 | NI | Positive Cases: 1 ALK+, 2 ALK- | [17] |
NI: Not indicated; IHC: immunohistochemical stain; ISH : In situ hybridization; LMP1: Latent membrane protein-1; EBER: EBV-encoded RNA-1; EBNA2: EBV nuclear protein-2; BamHIW: BamH1-W internal repetitive fragment of the EBV genome.
EBV positivity is restricted to cases of T-cell or Null-cell phenotype (with EBV evident on the neoplastic cells in IHC and ISH studies), excluding whenever possible the B-cell phenotypes included in ALCL in the earlier papers and cases of primary cutaneous ALCL.
#Cases which could not be determined to NOT be of B-cell origin were excluded from the analysis. Probable ALCL cases determined by uniform CD30 expression and some indication of either T- or null-cell phenotype (CD2, CD3, CD5, CD43, EMA) with no expression of CD15.
Only cases which the panel firmly decided were ALK- ALCL are included.
Several conclusions from this retrospective analysis are evident. A total of 64 cases of lymphomas were identified which were morphologically compatible with the diagnosis of ALCL and showed ISH evidence of EBV in the neoplastic cells of T- or Null-cell phenotype (shown in bold font). Of the twenty-two studies from which these cases were gleaned, nine definitively commented upon the immunocompetent status of the patients. Numerous studies in the last thirteen years have come out of Asia or Southeast Asia. Perhaps in consideration of this considerable body of work from Asia, Herling et al. stated in their report that “there is no role for EBV in ALCL arising in Western patients [7].” However, a number of the cases in the earlier studies came largely out of Europe, and the most recent paper by Tan et al. is from the United States of America [17]. None of the studies specifically examined Hispanic populations, the population from which our patient originated.
Interestingly, where EBER ISH and LMP-1 IHC were performed on the same cases, the two EBV studies are not always equivalents positive. In latency pattern type II, which is seen in cases of extranodal NK/T-cell lymphoma and Hodgkin's lymphoma, EBNA-1, and LMP-1 and 2 are expressed. Latency pattern type III, seen in post-transplant lymphoproliterative disorders, has expression of all nine latency-associated proteins, including six EBNA's and three LMP's [18,19]. In the majority of cases from the retrospective analysis, there were more EBER+ cases than LMP-1+ cases. Only Nakagawa et al. demonstrated greater LMP-1 expression than EBER positivity but the LMP-1+ cases were in ALK+ cases while EBER+ cases were ALK- [20]. Thus, the EBV that can be seen in ALCL is not consistently associated with either latency patterns type II or III, both of which should be both EBER+ and LMP-1+. In fact, the mix of EBER and LMP-1 patterns may suggest a corresponding mixture of latency patterns and thereby suggest heterogeneous pathogenic processes.
In addition, of the EBER+ cases identified in studies since the routine use of ALK immunohistochemical staining, only one of the thirty cases for which ALK status was specified was ALK+ [17]. The remaining cases were all ALK-. Thus, the association with EBV in ALCL, when present, appears to be predominantly with cases of ALK-ALCL.
The question of EBV infectivity specifically of T-cells is significant because of the causal role EBV can play in tumorigenesis. EBV is a human herpes virus that has a well-established association with several lymphoproliferative disorders, including Burkitt's lymphoma, post-transplant lymphoproliferative disorders, extranodal NK/T-cell lymphoma, nasal type, and Hodgkin lymphoma. In the B-cell processes, the EBV envelope glycoprotein gp350/220 binds to the complement receptor, CD21, that is present on naive B-cells and enters a latent phase in memory B-cells until reactivation. Once the B-cell is infected, LMP-1 may at that point play a critical role in EBV-associated B-cell lymphomas in in-ducingthe phenotypic alterations found in these lymphomas, such as expression of CD23, and activation of TNF pathways [21]. EBV nuclear antigens 1 and 3C as well as LMP-1 have also been found to promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints [22]. Although EBV infectivity is well-established in B-cells, there are studies that have shown EBV infectivity in cell types besides B-cells, including T-cells [23] and epithelial cells [24]. However, the role of EBV in T-cells differs from its role in B -cells. For example, while LMP-1 in B-cells resides in the cell membrane or cytoplasm, in T-cells, LMP-1 localizes to the nucleus [18]. In addition, there is a suggestion that LMP-2 expression may be driven through different promoters in B-cell neoplasms, such as Hodgkin's lymphoma, than in T-cell neoplasms such as extra nodal NK/T-cell lymphoma [25]. So, although EBV can certainly play a pathogenic role in T-cell neoplasms, the mechanisms are less well understood than for B-cell lymphomas and are likely different.
EBV infectivity has been seen in ALCL cases where the patients are immunocompromised or immunosuppressed. For example, ALCL may arise in a background of immunosuppression from transplantation as a post-transplant lymphoproliferative disease (PTLD) [1]. In this context, it is well established that EBV infectivity of neoplastic T-cells can be seen. In the Society for Hematopathology/European Association for Haematopathology Workshop on NK/T-cell malignancies in 2007, they reported EBV infectivity in over one-third of cases of NK/T-cell PTLD [26]. While the most common types of NK/T-cell PTLD were PTCL, NOS, and hepatosplenic T-cell lymphoma, EBV was found in almost 50% of PTCL, NOS, and, not surprisingly, all cases of extranodal NK/T-cell lymphomas. However, most of the cases listed in Table 1 were immunocompetent as was our patient. Thus, the presence of EBV in these cases cannot be accounted for by immunosuppression.
We have reported a case of a T-cell lymphoma for which the diagnosis of ALK- ALCL was considered. Unfortunately, for clinical reasons, the only available diagnostic material was a bone marrow biopsy. Thus, we were unable to assess any lymph node histologic patterns or perform any molecular studies on the decalcified material. However, cytologically the malignant cells were highly pleomorphic and anaplastic. The neoplasm was positive for surface CD3, CD30, CD43, EBER, TIA-1 and Granzyme B, and Zap-70 and weak subset positive for EM A and CD56. All B-cell markers were negative, as was clusterin. EBER and CD30 were strongly positive in all of the large neoplastic cells. This case was CD4-/CD8-.
Differential diagnoses also included PTCL, NOS, and possibly an extranodal NK/T-cell lymphoma, nasal type, or an EBV-associated lymphoproliferative disorder. There was extremely weak CD56 positivity on only a very small subset of neoplastic cells. Having subset CD56 positivity is not uncommon in ALCL [17,28]. CD2 was also negative. Together, the lack of uniform CD56 expression and the complete absence of CD2 expression disfavored an extranodal NK/T-cell lymphoma, nasal type, despite the suggestive Hispanic ethnicity of the patient. Importantly, our patient had no evidence of immuno-compromise (HIV-, unremarkable past medical history), disfavoring an EBV-associated lymphoproliferative disorder. Although there is certainly a mixed lymphohistiocytic background without significant clustering of neoplastic cells, this can be seen in ALCL and may also be a histologic consequence of the profound background hemophagocytosis. The anaplastic morphology and uniformly strong expression of CD30, with the classic membrane and Golgi staining pattern, and coexpression of cytotoxic markers disfavored a PTCL, NOS. Although the majority of ALCL cases are CD4+/CD8-, 20-25% of cases of ALCL may be CD4-/CD8-, and the double negative phenotype is slightly more common in ALCL than in PTCL [9,29,30] . It was felt that the CD43 and Zap-70 were not discriminatory between these entities, since CD43 and ZAP-70 expression can be found in a significant subset of ALK- ALCL cases, albeit modestly more common in PTCL [9,31]. Clusterin is found in only 71% of ALK- ALCLs, so the absence of this marker alone does not preclude the diagnosis of ALCL [32]. However, because CD43 and ZAP-70 were positive (favoring PTCL, NOS), and the clusterin was negative (disfavoring ALK-ALCL), PTCL, NOS, remains high on the differential. The combined morphology, uniform CD30, EMA subset positivity and cytotoxic markers seen in this case raised the diagnostic possibility of an ALK- ALCL, despite the clear EBV positivity of this neoplasm. Examination of the literature suggests that this entity might not be as uncommon as previously accepted.
In conclusion, all diagnoses are an attempt to place individual cases into rigid classification categories. Yet, those cases are as varied as the patients are themselves, reflecting the difficulties inherent in classification schemes. Thus, although this specific case might be classified as a PTCL, NOS or an extranodal NK/T-cell lymphoma, nasal type, by some, certain features strongly suggest that, despite the EBV positivity, the case might be classified as an ALCL. Given the strong EBER positivity in the neoplastic cells, the presence of EBV in this case is likely not to be incidental, but critical to the pathogenesis of the lymphoma, despite the apparent immunocompetence of the individual. Thus, EBV involvement may not preclude the diagnosis of ALK- ALCL. We recommend that EBV studies be conducted more routinely in the standard work-up of any T-cell lymphoma, including those which fit the morphologic and immunophenotypic profile of ALK- ALCL.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein J, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2008. [Google Scholar]
- 2.Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, Ha CS, Keating MJ, Murphy S, Freireich EJ. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 3.Stein H, Mason DY, Gerdes J, O'Conner N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, Schwarting R, Lennert K. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 4.Jaffe ES, Harris NL, Stein H, Vardiman JW. WHO Classification of Tumours. Pathology and Genetics of Tumours of Hematopoietic and Lymphoid Tissues. 3rd ed. World Health Organization; 2001. [Google Scholar]
- 5.Medeiros U, Elenitoba-Johnson KSJ. Anaplastic large cell lymphoma. Am J Clin Pathol. 2007;127:707–722. doi: 10.1309/r2q9ccuvtlrycf3h. [DOI] [PubMed] [Google Scholar]
- 6.Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugieres L, Terrier-Lacombe MJ, Haralambieva E, Pulford K, Pileri S, Morris SW, Mason DY, Delsol G. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998;91:2076–2084. [PubMed] [Google Scholar]
- 7.Herling M, Rassidakis GZ, Jones D, Schmitt-Graeff A, Sarris AH, Medeiros U. Absence of Epstein-Barr virus in anaplastic large cell lymphoma: a study of 64 cases classified according to World Health Organization criteria. Hum Pathol. 2004;35:455–459. doi: 10.1016/j.humpath.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 8.ten Berge RL, de Bruin PC, Oudejans JJ, Ossenkoppele GJ, van der Valk P, Meijer CJLM. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology. 2003;43:462–469. doi: 10.1046/j.1365-2559.2003.01726.x. [DOI] [PubMed] [Google Scholar]
- 9.Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD. ALK anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK + ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 10.Went P, Agostinelli C, Gallamini A, Piccaluga PP, Ascani S, Sabattini E, Bacci F, Falini B, Motta T, Paulli M, Artusi T, Piccioli M, Zinzani PL, Pileri SA. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24:2472–2479. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]
- 11.Salaverria I, Bea S, Lopez-Guillermo A, Lespinet V, Pinyol M, Burkhardt B, Lamant L, Zettl A, Horsman D, Gascoyne R, Ott G, Siebert R, Delsol G, Campo E. Genomic profiling reveals different genetic aberrations in systemic ALK-positive and ALK-negative anaplastic large cell lymphomas. Br J Haematol. 2008;140:516–526. doi: 10.1111/j.1365-2141.2007.06924.x. [DOI] [PubMed] [Google Scholar]
- 12.de Leval L, Gaulard P. Pathobiology and molecular profiling of peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2008:272–279. doi: 10.1182/asheducation-2008.1.272. [DOI] [PubMed] [Google Scholar]
- 13.Agostinelli C, Picculuga PP, Went P, Rossi M, Gazzola A, Righi S, Sista T, Campidelli C, Zinzani PL, Falini B, Pileri SA. Peripheral T-cell lymphoma, not otherwise specified: the stuff of genes, dreams and therapies. J Clin Pathol. 2008;61:1160–1167. doi: 10.1136/jcp.2008.055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuis J, Emile JF, Mounier N, Gisselbrecht C, Martin-Garcia N, Petrella T, Bouabdallah R, Berger F, Delmer A, Coiffier B, Reyes F, Gaulard P. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: a Groupe d'Etude des Lymphomes de I'Adulte (GELA) study. Blood. 2006;108:4163–4169. doi: 10.1182/blood-2006-04-017632. [DOI] [PubMed] [Google Scholar]
- 15.Ferenczi K, Summers P, Aubert P, Cooper B, Meyerson H, Cooper KD, Honda K. A case of CD30+ nasal natural killer/T-Cell lymphoma. Am J Dermatopathol. 2008;30:567–571. doi: 10.1097/DAD.0b013e318184bc3f. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz EJ, Molina-Kirsch H, Zhao S, Marinelli RJ, Warnke RA, Natkunam Y. Immunohistochemical characterization of nasal-type extranodal NK/T-Cell lymphoma using a tissue microarray: an analysis of 84 cases. Am J Clin Pathol. 2008;130:343–351. doi: 10.1309/V561QTM6854W4WAV. [DOI] [PubMed] [Google Scholar]
- 17.Tan BT, Seo K, Warnke RA, Arber DA. The frequency of immunoglobulin heavy chain gene and T-cell receptor D-chain gene rearrangements and Epstein-Barr virus in ALK+ and ALK-anaplastic large cell lymphoma and other peripheral T-cell lymphomas. J Mol Diagn. 2008;10:502–512. doi: 10.2353/jmoldx.2008.080054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu ZG, Iwatsuki K, Oyama N, Ohtsuka M, Satoh M, Kikuchi S, Akiba H, Kaneko F. The latency pattern of Epstein-Barr infection and viral IL-10 expression in cutaneous natural killer/T-cell lymphomas. Br J Cancer. 2001;84:920–925. doi: 10.1054/bjoc.2000.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa A, Nakamura S, Ito M, Shiota M, Mori S, Suchi T. CD3O-positive anaplastic large cell lymphoma in childhood: expression of p80npm/alk and absence of Epstein-Barr virus. Mod Pathol. 1997;10:210–215. [PubMed] [Google Scholar]
- 21.Xu J, Ahmad A, Menezes J. Preferential localization of the Epstein-Barr virus (EBV) oncoprotein LMP-1 to nuclei in human T-cells: implications for its role in the development of EBV genome-positive T-cell lymphomas. J Virol. 2002;76:4080–4086. doi: 10.1128/JVI.76.8.4080-4086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruhne B, Sompallae R, Massuci MG. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene. 2009;28:3997–4008. doi: 10.1038/onc.2009.258. [DOI] [PubMed] [Google Scholar]
- 23.Calattini S, Sereti I, Scheinberg P, Kimura H, Childs RW, Cohen JI. Detection of EBV genomes in plasmablasts/plasma cells and non-B cells in the blood of most patients with EBV lymphoproliferative disorders using Immuno-FISH. Blood. 2010 doi: 10.1182/blood-2010-05-285452. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgos JS, Vera-Sempere FJ. Immunohistochemical absence of CD21 membrane receptor in nasopharyngeal carcinoma cells infected by Epstein-Barr virus in Spanish patients. Laryngoscope. 2000;110:2081–2084. doi: 10.1097/00005537-200012000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Fox CP, Haigh TA, Taylor GS, Long HM, Lee SP, Shannon-Lowe C, O'Connors S, Bollard CM, Iqbal J, Chan WC, Rickinson AB, Bell Al, Rowe M. A novel latent membrane 2 transcript expressed in Epstein-Barr virus-positive NK and T-cell lymphoproliferative disease encodes a target for cellular immunotherapy. Blood. 2010 doi: 10.1182/blood-2010-06-292268. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow SH. T-Cell and NK-cell posttransplan-tation lymphoproliferative disorders. Am J Clin Pathol. 2007;127:887–895. doi: 10.1309/LYXN3RGF7D7KPYG0. [DOI] [PubMed] [Google Scholar]
- 27.Dunphy CH, DeMello DE, Gale GB. Pediatric CD56+ anaplastic large cell lymphoma: a review of the literature. Arch Pathol Lab Med. 2006;130:1859–1864. doi: 10.5858/2006-130-1859-PCALCL. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki R, Kagami Y, Takeuchi K, Kami M, Okamoto M, Ichinohasama R, Mori N, Kojima M, Yoshino T, Yamabe H, Shiota M, Mori S, Ogura M, Hamajima N, Seto M, Suchi T, Morishima Y, Nakamura S. Prognostic significance of CD56 expression for ALK-positive and ALK-negative anaplastic large-cell lymphoma of T/null cell phenotype. Blood. 2000;96:2993–3000. [PubMed] [Google Scholar]
- 29.Kesler MV, Paranjape GS, Asplund SL, McKenna RW, Jamal S, Kroft SH. Anaplastic large cell lymphoma: a flow cytometric analysis of 29 cases. Am J Clin Pathol. 2007;128:314–322. doi: 10.1309/GUHKGAJEJ72CEAL7. [DOI] [PubMed] [Google Scholar]
- 30.Juco J, Holden JT, Mann KP, Kelley LG, Li S. Immunophenotypic analysis of anaplastic large cell lymphoma by flow cytometry. Am J Clin Pathol. 2003;119:205–212. doi: 10.1309/HEFL-7KC4-35KF-WEX8. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Young L, Win W, Taylor CR. Distribution and ZAP-70 expression of WHO lymphoma categories in Shanxi, China. Appl Immunohistochem Mol Morphol. 2005;13:323–332. doi: 10.1097/01.pai.0000176161.38402.b2. [DOI] [PubMed] [Google Scholar]
- 32.Saffer H, Wahed A, Rassidakis GZ, Medeiros U. Clusterin expression in malignant lymphomas: a survey of 266 cases. Mod Pathol. 2002;15:1221–1226. doi: 10.1097/01.MP.0000036386.87517.AA. [DOI] [PubMed] [Google Scholar]
- 33.Herbst H, Dallenbach F, Hummel M, Niedobitek G, Finn T, Young LS, Rowe M, Muller-Lantzsch N, Stein H. Epstein-Barr virus DNA and latent gene products in Ki-1 (CD 3O)-positive anaplastic large cell lymphomas. Blood. 1991;78:2666–2673. [PubMed] [Google Scholar]
- 34.Kanavaros P, Jiwa NM, de Bruin PC, van der Valk P, Noorduyn LA, van Heerde P, Gordijn R, Horstman A, Mullink R, Willemze R, Walboomers JMM, Meijer CJLM. High incidence of EBV genome in CD3O-positive non-Hodgkin's lymphomas. J Pathol. 1992;168:307–315. doi: 10.1002/path.1711680311. [DOI] [PubMed] [Google Scholar]
- 35.Ott G, Ott MM, Feller AC, Seidl S, Muller-Hermelink HK. Prevalence of Epstein-Barr virus DNA in different T-cell lymphoma entities in a European population. Int J Cancer. 1992;51:562–567. doi: 10.1002/ijc.2910510410. [DOI] [PubMed] [Google Scholar]
- 36.Ross CW, Schlegelmilch JA, Grogan TM, Weiss LM, Schnitzer B, Hanson CA. Detection of Epstein-Barr virus genome in Ki-1 (CD3O)-positive, large-cell anaplastic lymphomas using the polymerase chain reaction. Am J Pathol. 1992;141:457–465. [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton-Dutoit SJ, Pallesen G. A survey of Ep stein-Barr virus gene expression in sporadic non-Hodgkin's lymphomas: detection of Epstein-Barr virus in a subset of peripheral T-cell lymphomas. Am J Pathol. 1992;140:1315–1325. [PMC free article] [PubMed] [Google Scholar]
- 38.de Bruin PC, Jiwa M, Oudejans JJ, van der Valk P, van Heerde P, Sabourin JC, Csanaky G, Gaulard P, Noorduyn AL, Willemze R, Meijer CJLM. Presence of Epstein-Barr virus in extranodal T-cell lymphomas: differences in relation to site. Blood. 1994;83:1612–1618. [PubMed] [Google Scholar]
- 39.Peris K, Niedermeyer H, Chimenti S, Radaskiewicz T, Kerl H, Hoefler H. Detection of Epstein-Barr virus in cutaneous and lymph nodal anaplastic large cell lymphomas (Ki-1+) Br J Dermatol. 1995;133:542–546. doi: 10.1111/j.1365-2133.1995.tb02701.x. [DOI] [PubMed] [Google Scholar]
- 40.Lopategui JR, Gaffey MJ, Chan JKC, Frierson HF, Sun LH, Bellafiore FJ, Chang KL, Weiss LM. Infrequent association of Epstein-Barr virus with CD3O-positive anaplastic large cell lymphomas from American and Asian patients. Am J Surg Pathol. 1995;19:42–49. doi: 10.1097/00000478-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Clavio M, Rossi E, Truini M, Carrara P, Ravetti JL, Spriano M, Vimercati AR, Santini G, Canepa L, Pierri I, Celesti L, Miglino M, Castellaneta A, Damasio E, Gobbi M. Anaplastic large cell lymphoma: a clinicopathologic study of 53 patients. Leuk Lymphoma. 1996;22:319–327. doi: 10.3109/10428199609051763. [DOI] [PubMed] [Google Scholar]
- 42.d'Amore F, Johansen P, Houmand A, Weisenburger DD, Mortensen LS. Epstein-Barr virus genome in non-Hodgkin's lymphomas occurring in immunocompetent patients: highest prevalence in nonlymphoblastic T-cell lymphoma and correlation with a poor prognosis. Blood. 1996;87:1045–1055. [PubMed] [Google Scholar]
- 43.Suchi T, Lennert K, Tu LY, Kikuchi M, Sato E, Stansfeld AG, Feller AC. Histopathology and immunohistochemistry of peripheral T cell lymphomas: a proposal for their classification. J Clin Pathol. 1987;40:995–1015. doi: 10.1136/jcp.40.9.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura S, Shiota M, Nakagawa A, Yatabe Y, Kojima M, Motoori T, Suzuki R, Kagami Y, Ogura M, Morishima Y, Mizoguchi Y, Okamoto M, Seto M, Koshikawa T, Mori S, Suchi T. Anaplastic large cell lymphoma: a distinct molecular pathologic entity: a reappraisal with special reference to p80NPM/ALK expression. Am J Surg Pathol. 1997;21:1420–1432. doi: 10.1097/00000478-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Shimakage M, Nakamine H, Tamura S, Takenaka T, Yutsudo M, Hakura A. Detection of Epstein-Barr virus transcripts in anaplastic large-cell lymphomas by mRNA in situ hybridization. Hum Pathol. 1997;28:1415–1419. doi: 10.1016/s0046-8177(97)90232-x. [DOI] [PubMed] [Google Scholar]
- 46.Kagami Y, Suzuki R, Taji H, Yatabe Y, Takeuchi T, Maeda S, Kondo E, Kojima M, Motoori T, Mizoguchi Y, Okamoto M, Ohnishi K, Yamabe H, Seto M, Ogura M, Koshikawa T, Takahashi T, Kurita S, Morishima Y, Suchi T, Nakamura S. Nodal cytotoxic lymphoma spectrum: a clinicopathologic study of 66 patients. Am J Surg Pathol. 1999;23:1184–1200. doi: 10.1097/00000478-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Huh J, Cho K, Heo DS, Kim JE, Kim CW. Detection of Epstein-Barr virus in Korean peripheral T-cell lymphoma. Am J Hematol. 1999;60:205–214. doi: 10.1002/(sici)1096-8652(199903)60:3<205::aid-ajh7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 48.Brink AATP, ten Berge RL, van den Brule AJC, Willemze R, Chott A, Meijer CJLM. Epstein-Barr virus is present in neoplastic cytotoxic T cells in extranodal, and predominantly in B cells in nodal T non-Hodgkin lymphomas. J Pathol. 2000;191:400–406. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal S, Ramanathan U, Naresh KN. Epstein-Barr virus association and ALK gene expression in anaplastic large-cell lymphoma. Hum Pathol. 2002;33:146–152. doi: 10.1053/hupa.2002.31925. [DOI] [PubMed] [Google Scholar]
- 50.Noorali S, Pervez S, Yaqoob N, Moatter T, Nasir MI, Haroon S, Hodges E, Smith JL. Prevalence and characterization of anaplastic large cell lymphoma and its association with Epstein-Barr virus in Pakistani patients. Pathol Res Pract. 2004;200:669–679. doi: 10.1016/j.prp.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Kim YC, Yang WI, Lee MG, Kim SN, Cho KH, Lee SJ, Lee MW, Koh JK. Epstein-Barr virus in CD30+ anaplastic large cell lymphoma involving the skin and lymphomatoid papulosis in South Korea. Int J Dermatol. 2006;45:1312–1316. doi: 10.1111/j.1365-4632.2006.02951.x. [DOI] [PubMed] [Google Scholar]