Abstract
Urachal adencarcinoma is rare and its metastasis to the ovary is extremely rare. I report here on a case of urachal adenocarcinoma that metastasized to bilateral ovaries in a 72-year-old female. She presented with vaginal spotting. Abdominal CT revealed a huge multiloculated cystic mass in the recto-uterine pouch and a solid mass with dot calcification in the anterior pelvic cavity. The resected ovaries were equal-sized at about 10cm at the greatest diameter. The sectioned surfaces were predominantly multicystic with a solid nodule. Microscopically, both ovaries were mainly composed of cystic glands lined by mucin-containing epithelium with atypical nuclei. The solid nodule consisted of irregularly infiltrating glands and single tumor cells. Two years later, the patient was admitted with hema-turia. The kidney CT revealed a solid mass with calcification in the bladder dome, which suggested urachal carcinoma. The partial cystectomy specimen revealed an ill-defined ulcerative tumor. Histologically, the tumor corresponded to mucinous adenocarcinoma and centered at the bladder wall with predominant invasion of the muscularis. The immunohistochemical profiles of the ovarian and urachal tumors were exactly the same. The tumor cells were diffusely positive for CK20, CDX-2, MUC2 and MUC5AC, focally positive for 34(3E12 and negative for CK7.
Keywords: Urachus, ovarian neoplasm, metastasis, mucinous, adenocarcinoma
Introduction
Urachal adencarcinoma is rare and its metastasis to the ovary is extremely rare with only a few such case reports available in the English medical literature [1-5]. When urachal adenocarcinoma metastasizes to the ovary, it may mimics primary ovarian mucinous carcinoma and this can lead to misdiagnosis. I present here a case of urachal adenocarcinoma that metastasized to the bilateral ovaries and mimicked primary ovarian mucinous adenocarcinoma, and I discuss the differential diagnostic points.
Case report
A 72-year-old female was referred to my hospital with a pelvic mass that was found on pelvic ul-trasonography. She had complaints of vaginal spotting for the previous a couple of days. Abdominal computed tomography (CT) revealed a huge multiloculated cystic mass in the recto-uterine pouch that extended to the right pelvic cavity, suggesting mucinous or serous cystade-nocarcinoma of an ovary origin. A 3.9×2.1cm sized enhancing solid mass with dot calcification was also noted in the anterior pelvic cavity at just superior aspect to the bladder, which suggested a seeded mass on the pelvic peritoneum. Bilateral salpingooophorectomy, total hysterectomy, omentectomy and excisional biopsy of the peritoneum were performed. At that time, the ovarian tumors were diagnosed as mucinous cystadenocarcinoma of the bilateral ovaries. The patient underwent chemotherapy with a combination of docetaxel and carboplatin.
Two years postoperatively, the patient was admitted with hematuria. The kidney CT revealed a 3.9 × 2.1 cm sized exo- and endophytically growing heterogenous solid mass with calcification in the bladder dome suggesting urachal carcinoma. Partial cystectomy with umbilectomy was performed. At this time, the bladder tumor was diagnosed as urachal adenocarcinoma and the previous ovarian tumors were revised as metastatic mucinous adenocarcinoma from urachus.
Immediately after partial cystectomy, the patient progressively suffered with abdominal pain that suggested mechanical ileus. Seven months after this, she underwent explo-laparotomy and the peritoneal biopsy demonstrated peritoneal carcinomatosis. She was managed with conservative therapy for 3 months and she is alive currently with disease for 36 months after the initial operation.
Pathologic findings
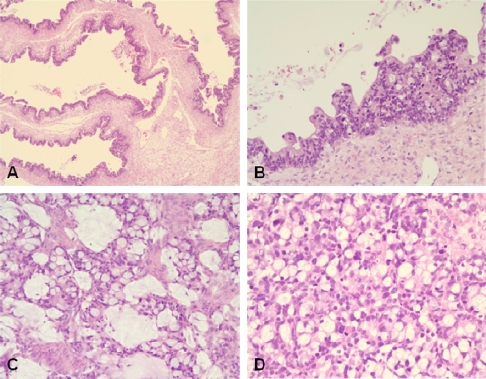
Both the resected ovaries were markedly enlarged and nearly equal in size at about 10cm at the greatest diameter. The external surfaces of both ovaries were smooth. The sectioned surface of the right ovary was totally multicystic (Figure 1A), but the left ovary was predominantly multicystic along with a 4 cm-sized circumscribed solid nodule (Figure 1B). The content of cysts was mucinous. The uterus and omentum were unremarkable grossly. Microscopically, both ovaries revealed basically similar histology and they were mainly composed of dilated and markedly cystic glands (Figure 2A) that were lined by columnar, mucin-producing, single layered or stratified epithelium (Figure 2B). The stratified epithelium as well as single layered epithelium revealed a considerable degree of nuclear atypia and frequent mitosis. The solid nodule found grossly corresponded to a definitely infiitrative histology that consisted of irregularly infiltrating glands and single tumor cells with desmoplastic stroma. The tumor contained many goblet cells (Figure 2C) and signet ring cells (Figure 2D). Some tumor cells were surrounded by corpora albicantia. There were focal surface implants.
Figure 1.
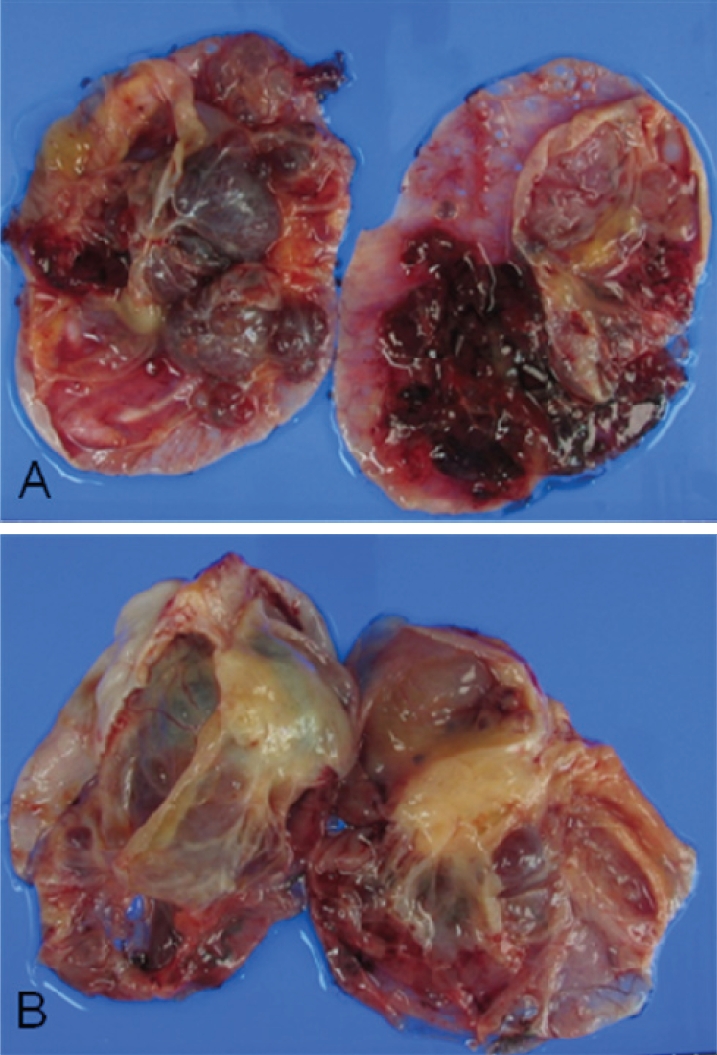
Macroscopic photograph of the bilateral ovarian tumors. The sectioned surfaces of the right (A) and left (B) ovaries are predominantly multicystic. The left ovary has a solid nodule.
Figure 2.
Microscopic photograph of the metastatic ovarian tumor. A. Tumor consisting of cystically dilated glands that simulate primary mucinous ovarian tumor (H&E, X100). B. Cyst-lining stratified epithelium with nuclear atypia (H&E, X200). C. Irregular glands having many goblet cells and mucin (H&E, X200). D. Single tumor cells admixed with many signet ring cells (H&E, X200).
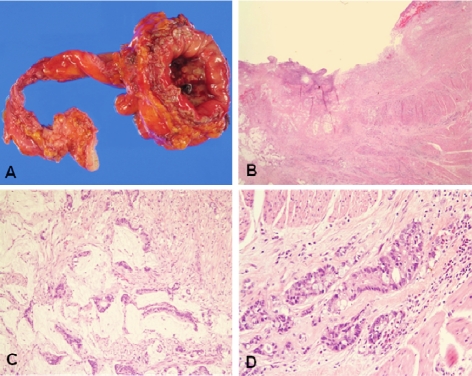
The partial cystectomy specimen revealed an ill defined ulcerative tumor that measured 2.5 cm × 1.5cm on the cross section (Figure 3A). Microscopically, the tumor centered at the muscular wall of the bladder with predominat invasion into the muscle layer and was associated with overlying mucosal ulceration (Figure 3B). The tumor corresponded to mucinous carcinoma showing atypical glands with abundant pools of extracellular mucin (Figure 3C). Signet ring cells and goblet cells were also admixed (Figure 3D). No urachal remnant was observed.
Figure 3.
Primary urachal carcinoma. A. Gross findings of the partial cystectomy with the urachal tract. B. On low power view, the tumor centers at the muscularis with overlying surface ulceration. Abundant mucin pools are present on left side. (H&E, X12.5). C. Tumor cell nests floating on the extracellular mucin pools (H&E, X200). D. Neoplastic glands with abundant goblet cells (H&E, X200).
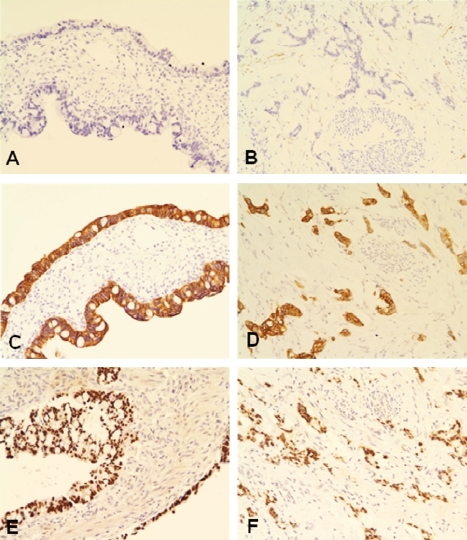
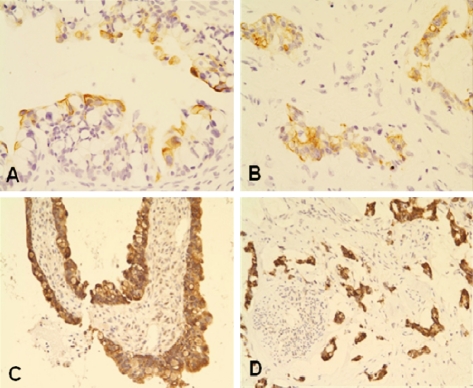
Comprehensive immunohistochemical studies were done. The primary antibodies used were listed in Table 1 and the immunohistochemical results were summarized in Table 2. The immunohistochemical profiles of both the ovarian and urachal tumors were exactly the same. The tumor cells were diffusely immunorective for cytokeratin (CK)20, CDX2 and CD15, but absolutely not reactive for CK7 (Figure 4A-4F). The tumor cells were associated with the focal expression of 34PE12 (Figure 5A and 5B) and a membranous expression of p-catenin without a nuclear expression. The tumor cells diffusely expressed MUC2 (Figure 5C and 5D) and MUC5AC, and they focally expressed MUC1, but they didn't express MUC6.
Table 1.
Primary antibodies used for immunohistochemistry
| Primary Antibody | Clone | Manufacture | Dilution | Antigen retrieval* |
|---|---|---|---|---|
| CK7 | OV-TL 12/30 | Dako, Carpinteria, Denmark | 1:100 | yes |
| CK20 | Ks20.8 | Dako, Carpinteria, Denmark | 1:50 | yes |
| CDX2 | AMT28 | Novocastra, Newcastle, UK | 1:50 | yes |
| CD15 | MMA | Thermo, Fremont, CA, USA | 1:50 | yes |
| 34βE12 | 34βE12 | Dako, Carpinteria, Denmark | 1:50 | yes |
| β-catenin | CAT-5H10 | Zymed, San Francisco, CA, USA | 1:50 | yes |
| MUC1 | Ma695 | Novocastra, Newcastle, UK | 1:100 | yes |
| MUC2 | M53 | Thermo, Fremont, CA, USA | 1:400 | yes |
| MUC5AC | 45M1 | Thermo, Fremont, CA, USA | 1:100 | yes |
| MUC6 | CLH5 | Thermo, Fremont, CA, USA | 1:50 | yes |
Microwave in lOmM sodium citrate buffer
Table 2.
Immunohistochemical findings of ovarian and urachal tumors
| Primary Antibody | Ovarian tumor | Urachal tumor |
|---|---|---|
| CK7 | Negative | Negative |
| CK20 | Diffusely positive | Diffusely positive |
| CDX2 | Diffusely positive | Diffusely positive |
| CD15 | Diffusely positive | Diffusely positive |
| 34βE12 | Focally positive | Focally positive |
| β-catenin | Membranous expression | Membranous expression |
| MUC1 | Focally positive | Focally positive |
| MUC2 | Diffusely positive | Diffusely positive |
| MUC5AC | Diffusely positive | Diffusely positive |
| MUC6 | Negative | Negative |
Figure 4.
Immunohistochemistry of the ovarian (left side) and urachal carcinomas (right side). A and B. No expression of CK7. C and D. Diffuse cytoplasmic expression of CK20. E and F. Diffuse nuclear expression of CDX2.
Figure 5.
Immunohistochemistry of the ovarian (left side) and urachal carcinomas (right side). A and B. Focal cytoplasmic expression of 34βE12. C and D. Diffuse cytoplasmic expression of MUC2.
Discussion
Urachal carcinoma is a rare tumor which account for less than 1% of all bladder cancers [6]. It usually is adenocarcinoma and account for approximately 30% of vesical adenocarcinomas [6]. The subtypes of adenocarcinoma cover the enteric subtype, including mucinous adenocarcinoma, and not otherwise specified (NOS) subtype [6-8]. The criteria for making a diagnosis of urachal carcinoma includes tumor in the dome of the bladder, the absence of cystitis cystica and cystitis glandularis, predominant invasion of the muscularis or deeper tissues with a sharp demarcation between the tumor and the surface bladder epithelium, the presence of urachal remnants within the tumor, extension of tumor into the bladder wall with involvement of the space of Rezius, the anterior abdominal wall or the umbilicus, and no evidence of primary neoplasm elsewhere [6,9]. Although the present case was not accompanied by urachal remnants within the bladder tumor, it fulfilled the rest of above diagnostic criteria. Benign and adenomatous urachal remnants are helpful if present, but their absence does not preclude a urachal origin [9].
The most common sites of metastasis of urachal carcinoma are the regional lymph node and lungs [9]. The uncommon sites of metastasis are the peritoneum, the anterior abdominal wall, bone, soft tissue and ovary [9]. To the best of my knowledge, only 5 cases of urachal carcinoma that have metastasized to the ovary have been previously reported [1-5]. Among them, 2 cases were identified simultaneously with the primary urachal carcinoma and 3 cases were detected postoperatively. In four of the 5 reported cases, the histology of the metastatic ovarian cancers was mucinous adencarcinoma.
Macroscopically as well as microscopically, metastatic ovarian mucinous carcinoma is very similar to primary ovarian mucinous carcinoma [10-11]. So, it is sometimes very problematic to differentiate metastatic carcinoma from primary ovarian mucinous carcinoma. According to the literature [10], the frequent findings of metastatic carcinoma are bilaterality, microscopic surface involvement by epithelial cells and an infiltrative pattern of stromal invasion. The findings that are less frequent but present exclusively or almost exclusively in metastatic carcinoma are a nodular invasive pattern, ovarian hilar involvement, single cell invasion, signet-ring cells, vascular invasion and microscopic surface mucin. In the present case, the findings of bilaterality, microscopic surface implants, infiltrative stromal invasion and many signet-ring cells supported metastasis. Even the cystlining single-layered epithelium revealed a degree of nuclear atypia that was similar to that of the stratified epithelium, and any benign-appearing areas lined by bland looking epithelium were not observed in the present case. This finding could be incompatible with primary ovarian mucinous tumor and it may be an important factor favoring metastatic tumor.
A bilateral presentation of primary ovarian mucinous carcinoma is uncommon. When bilateral ovarian mucinous carcinoma is considered in the diagnosis, it is essential to completely exclude the possibility of metastatic carcinoma. The most common primary sites for the metastatic ovarian mucinous carcinoma are colon, pancreas, gallbladder, stomach, appendix, uterine cervix and etc [12-14]. The bladder adenocarcinoma, including urachal carcinoma, can be less common candidate of the primary tumor on these occasions.
Although many immunohistochemical studies have tried to differentiate between primary and metastatic ovarian mucinous tumors, there is a considerable overlap in the immunohistochemical staining patterns of both these tumors. The CK7/CK20 coordinate expression profile is useful to distinguish primary ovarian mucinous tumors from metastatic mucinous carcinomas of the lower gastrointestinal tract [14,15]. A CK7-/CK20+ immunoprofile is the most common profile in lower intestinal tract tumors and was uncommon in upper gastrointestinal tract tumors, rarely seen in primary ovarian tumors, and not seen in endocervical tumors [14]. A CK7+/ CK20- profile is observed in some primary ovarian, upper gastrointestinal tract and endocervical tumors but not in lower intestinal tract tumors [14]. The staining distribution of CK7 and CK20 is also informative, that is, the staining distribution of CK7 is often focal when present in the colorectal and appendiceal carcinomas but not in the low-grade appendiceal tumors, and the staining distribution of CK20 and CDX2 is often focal when present in the primary ovarian tumors and the nonlower intestinal tract tumors, whereas it is almost always diffuse in the lower intestinal tract tumors [14,15].
There are only limited studies on the immunohistochemical profiles of urachal carcinomas and all the studies had a small sample size. According to the literature [9], all the urachal carcinomas are diffusely and strongly positive for CK20 and CDX-2, and about half of the urachal carcinomas are positive for CK7. Unfortunately, their staining distribution was not described. Pantuck et al [16] reported that adencarcinoma of the urachus and bladder expressed a unique colonic epithelial epitope immunohistochemically. The immunohistochemical profiles of urachal carcinomas are similar to that of colorectal or appendiceal cancers [9,17]. So, the coordinate expression of CK7 and CK20 or CDX2 might be helpful to discriminate metastatic urachal carcinoma from primary ovarian mucinous tumor. According to the reported cases, the immunohistochemical mucin pheno-type of urachal carcinoma is also similar to colorectal or appendiceal cancers, that is, there is unique positivity for MUC2 [9,17]. In the present case, the negativity for CK7 and the diffuse positivity for CK20, CDX2 and MUC2 opposed a diagnosis of primary ovarian mucinous carcinoma and it supported the diagnosis of metastatic carcinoma from the colorectum, appendix, urachus, etc.
Immunostains do not unequivocally discriminate between a urachal malignancy and a colonic malignancy. Generally, 34βE12 is not expressed in neither primary ovarian mucinous tumor nor in lower gastrointestinal tract tumors [9]. The expression of 34βE12 might be useful to differentiate urachal origin from ovarian or lower gastrointestinal tract origin. The majority of urachal carcinomas rarely show nuclear reactivity for β-catenin but a considerable proportion of colorectal cancers show nuclear reactivity for β-catenin [10]. In the present case, the positivity for 34βE12 and the absence of β-catenin nuclear staining are more likely compatible with urachal origin rather than an origin from the colorectum or appendix.
If the mucinous carcinoma is encountered in the ovary, and especially bilateral ovaries, metastasis, including metastasis from urachal adenocarcinoma should be seriously considered in the differential diagnosis. The immunohistochemical study, particulary using a panel of CK7, CK20, CDX2, MUC2, 34βE12 and β-catenin, might be helpful to discriminate the metastasis of urachal adenocarcinoma from primary ovarian mucinous carcinoma and metastatic carcinoma from other organs.
Acknowledgments
This research was supported by the research fund of Dankook University in 2008.
References
- 1.El-Ghobashy A, Ohadike C, Wilkinson N, Lane G, Campbell JD. Recurrent urachal mucinous adenocarcinoma presenting as bilateral ovarian tumors on cesarean delivery. Int J Gynecol Cancer. 2009;19:1539–1541. doi: 10.1111/IGC.0b013e3181a84177. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami S, Kageyama Y, Yonese J, Fukui I, Kitahara S, Arai G, Hyouchi N, Suzuki M, Masuda H, Hayashi T, Okuno T, Kihara K. Successful treatment of metastatic adenocarcinoma of the urachus: report of 2 cases with more than 10-year survival. Urology. 2001;58:462. doi: 10.1016/s0090-4295(01)01259-6. [DOI] [PubMed] [Google Scholar]
- 3.Ohira S, Shiohara S, Itoh K, Ashida T, Fukushima M, Konishi I. Urachal adenocarcinoma metastatic to the ovaries: case report and literature review. Int J Gynecol Pathol. 2003;22:189–193. doi: 10.1097/00004347-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Yanagisawa S, Fujinaga Y, Kadoya M. Urachal mucinous cystadenocarcinoma with a cystic ovarian metastasis. AJR Am J Roentgenol. 2003;180:1183–1184. doi: 10.2214/ajr.180.4.1801183. [DOI] [PubMed] [Google Scholar]
- 5.Young RH. Urachal adenocarcinoma metastatic to the ovary simulating primary mucinous cystadenocarcinoma of the ovary: report of a case. Virchows Arch. 1995;426:529–532. doi: 10.1007/BF00193178. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol. 1984;131:1–8. doi: 10.1016/s0022-5347(17)50167-6. [DOI] [PubMed] [Google Scholar]
- 7.Molina JR, Quevedo JF, Furth AF, Richardson RL, Zincke H, Burch PA. Predictors of survival from urachal cancer: a Mayo Clinic study of 49 cases. Cancer. 2007;110:2434–2440. doi: 10.1002/cncr.23070. [DOI] [PubMed] [Google Scholar]
- 8.Paras FA, Jr, Maclennan GT. Urachal adenocarcinoma. J Urol. 2008;180:720. doi: 10.1016/j.juro.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Gopalan A, Sharp DS, Fine SW, Tickoo SK, Herr HW, Reuter VE, Olgac S. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol. 2009;33:659–668. doi: 10.1097/PAS.0b013e31819aa4ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003;27:281–292. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hart W. mucinous tumors of the ovary: A review. Int J Gynecol Pathol. 2004;24:4–25. [PubMed] [Google Scholar]
- 12.Khunamornpong S, Lerwill MF, Siriaunkgul S, Suprasert P, Pojchamarnwiputh S, Chiangmai WN, et al. Carcinoma of extrahepatic bile ducts and gallbladder metastatic to the ovary: a report of 16 cases. Int J Gynecol Pathol. 2008;27:366–379. doi: 10.1097/PGP.0b013e31815d6903. [DOI] [PubMed] [Google Scholar]
- 13.Khunamornpong S, Siriaunkgul S, Suprasert P, Pojchamarnwiputh S, Na Chiangmai W, Young RH. Intrahepatic cholangiocarcinoma metastatic to the ovary: a report of 16 cases of an underem-phasized form of secondary tumor in the ovary that may mimic primary neoplasia. Am J Surg Pathol. 2007;31:1788–1799. doi: 10.1097/PAS.0b013e3180674ded. [DOI] [PubMed] [Google Scholar]
- 14.Vang R, Gown AM, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, Ronnett BM. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006;30:1130–1139. doi: 10.1097/01.pas.0000213281.43036.bb. [DOI] [PubMed] [Google Scholar]
- 15.Vang R, Gown AM, Wu LS, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, Ronnett BM. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod Pathol. 2006;19:1421–1428. doi: 10.1038/modpathol.3800698. [DOI] [PubMed] [Google Scholar]
- 16.Pantuck AJ, Bancila E, Das KM, Amenta PS, Cummings KB, Marks M, Weiss RE. Adenocarcinoma of the urachus and bladder expresses a unique colonic epithelial epitope: an immunohistochemical study. J Urol. 1997;158:1722–1727. doi: 10.1016/s0022-5347(01)64109-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim T, Joo M, Kim M, Kim H, Chi J, Ro J. Urachal adenocarcinoma with a concomitant urachal remnant-a case report- Kor J Pathol. 2004;38:280–283. [Google Scholar]