Abstract
Filamentous fungi are widely known for their industrial applications, namely, the production of food-processing enzymes and metabolites such as antibiotics and organic acids. In the past decade, the full genome sequencing of filamentous fungi increased the potential to predict encoded proteins enormously, namely, hydrolytic enzymes or proteins involved in the biosynthesis of metabolites of interest. The integration of genome sequence information with possible phenotypes requires, however, the knowledge of all the proteins in the cell in a system-wise manner, given by proteomics. This review summarises the progress of proteomics and its importance for the study of biotechnological processes in filamentous fungi. A major step forward in proteomics was to couple protein separation with high-resolution mass spectrometry, allowing accurate protein quantification. Despite the fact that most fungal proteomic studies have been focused on proteins from mycelial extracts, many proteins are related to processes which are compartmentalised in the fungal cell, e.g. β-lactam antibiotic production in the microbody. For the study of such processes, a targeted approach is required, e.g. by organelle proteomics. Typical workflows for sample preparation in fungal organelle proteomics are discussed, including homogenisation and sub-cellular fractionation. Finally, examples are presented of fungal organelle proteomic studies, which have enlarged the knowledge on areas of interest to biotechnology, such as protein secretion, energy production or antibiotic biosynthesis.
Keywords: Aspergillus niger, Trichoderma, Cell-organelle proteomics, Protein secretion, Metabolites, Antibiotics
Introduction
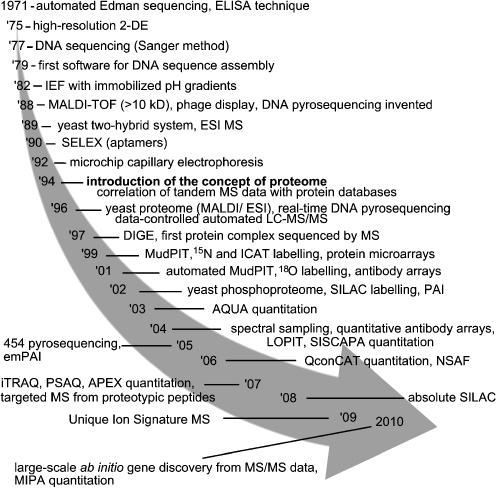
In 1994, Wilkins defined the proteome as the ‘protein complement to a genome’ (Wilkins 1994). Proteomics, i.e. the study of the proteome, was only introduced as a concept in the mid 1990s but has deep roots in the early 1970s, as a result of important technical advances in biochemistry and genetics (Fig. 1). Similar to genomics and transcriptomics, proteomics has evolved to incorporate high-throughput processes, which have allowed faster analyses of larger numbers of proteins (Lueking et al. 1999; Wolters et al. 2001). Proteomics is therefore a core part of functional genomics, as the latter relies on high-throughput approaches for determining the relation between a given genome and the corresponding phenotypes.
Fig. 1.
Timeline of major events important for the development of proteomics
High-throughput procedures depend on increased speed, accuracy and automation to feasibly manage the increasingly larger sizes of samples and data. In the context of proteome studies, an example of a high-throughput technique is the protein array, for quantification of individual proteins in mixtures of proteins. Like oligonucleotide arrays, protein arrays are based on analyte detection through spotted probes. These spotted probes however can be various types of molecules, ranging from antibodies, and sample proteins themselves to specifically engineered aptamers. Thus far, the most standardised protein arrays make use of spotted antibodies (Borrebaeck and Wingren 2007). Despite of the high-throughput nature of protein arrays, these also present some important limitations. Similarly to oligonucleotide arrays whose results need to be validated by quantitative techniques e.g. quantitative PCR (QPCR), protein arrays also require validation by quantitative techniques such as ELISA (Zichi et al. 2008). Another important limitation is the cost and availability of the antibody reagents for this technique. In the case of filamentous fungi there are virtually no such antibodies specifically developed for protein array detection. Last but not least, until now it has been virtually impossible to distinguish protein isoforms, mutated proteins or post-translational modifications using this system.
In addition to protein arrays, other techniques exist for protein quantification, such as mass spectrometry (MS). Depending on the instrumentation used, mass spectrometers may achieve an astonishing mass accuracy below the part per million (Olsen et al. 2005). MS has many applications in proteomics, such as (1) the analysis of protein complexes (Link et al. 1999), (2) the detection and quantification of post-translational modifications (Jensen 2006), (3) protein identification in complex mixtures (Eng et al. 1994); (Wolters et al. 2001), (4) protein quantification (Bantscheff et al. 2007) and (5) proteome profiling, just to mention a few. Proteome profiling is most often carried out to compare the proteome of mutants (Dong et al. 2007), a phenotype-specific proteome (Mallick et al. 2007), and the proteome of cell organelles (Yan et al. 2009).
Quantitative MS-based proteomics
The first systematic approaches for protein quantification in protein mixtures were based on high-resolution two-dimensional electrophoresis (2-DE). Quantification by 2-DE still presents advantages, not only due to the overall sensitivity of this technique but also because of its high resolution power as it is able to discriminate protein isoforms and proteins with post-translational modifications. Difference gel electrophoresis (DIGE) further increased the potential of 2-DE as different samples could be labelled with specific dyes and analysed on the same gel. However, quantification through 2-DE also suffers from major disadvantages. The technique is still laborious, of limited reproducibility and semi-quantitative. Moreover, 2-DE shows poor quantification performance for proteins with particular features such as extreme sizes (large or small), large hydrophobicity, low abundance or extreme isoelectric point. In contrast, MS has been adapted in the past two decades to enable truly quantitative proteomics. The most standardised methods for quantitative MS-based proteomics are based on selective labelling of proteins or peptides with stable isotopes, e.g. 2 H, 13 C, 15 N or 18O. Nowadays, there are several choices for MS-based quantification in proteomics, and for this reason, only a few examples will be given in this review. Depending on the scope of the experiment, absolute or relative quantification can be used (Table 1).
Table 1.
Label-based quantification of proteins
| Absolute quantification | |
| PSAQ | + Allows extrapolation |
| − Expensive special software | |
| A: Detection of protein isoforms/variants; quantification of biomarkers | |
| SISCAPA | + Allows extrapolation; allows multiplexing |
| − Dependent on antibodies (expensive, difficult to implement for general use) | |
| A: Peptidomics; quantification of peptide biomarkers | |
| QconCAT | + One standard yields multiple proteins, can be obtained by expression vector |
| − Underestimation; digestion not reproducible; expensive; special software | |
| A: Quantification of protein subunits or proteins from a small pathway | |
| AQUA | + Very precise, rigorous peptide mixture quantification, detects PTMs |
| − Underestimation; expensive | |
| A: Peptidomics and phosphoproteomics | |
| Relative quantification | |
| In vivo or metabolic | |
| 15N | + High signal for protein labelling; separation by 2-DE possible, detects PTMs |
| − Only pair-wise; special software; tissue samples not possible | |
| A: Cell culture of microorganisms, also PTMs | |
| SILAC | + Specific accurate quantification, allows multiplexing, detects PTMs |
| − Labelled AA essential and not converted to other AA; growth media of controlled composition; requires special software | |
| A: Cell culture; phosphoproteomics; quantifies other PTMs | |
| In vitro | |
| ICAT | + Sample size reduction; upstream of tryptic digestion; detects PTMs |
| − Cys-lacking proteins missed; unwanted side-reactions | |
| A: Cys-containing proteins in complex samples | |
| iTRAQ, TMT | + Multiplex up to 8 samples; precise co-migration in LC (iTRAQ); detects PTMs |
| − MS interference; unwanted side-reactions; underestimation | |
| A: Peptidomics and phosphoproteomics, medium-to-low complexity samples | |
| ICPL | + Allows extrapolation; compatible with 2-DE; PTMs |
| − Trypsin does not cleave modified lysine; unwanted side-reactions | |
| A: Virtually any biological sample | |
| 18O | + Simple setting, chemical properties not affected, no side-reactions; also PTMs |
| − 18O-16O back-exchange; requires high resolution MS (4 Da); | |
| A: Virtually any biological sample | |
+ major features, − major limits, A major applications, AA amino acids, AQUA absolute quantification method, ICAT isotope-coded affinity tags, ICPL isotope-coded protein labelling, iTRAQ isobaric tag for absolute and relative quantification, PSAQ protein standard absolute quantification, PTM post-translational modification, QconCAT quantitative concatenated standard, SILAC stable isotope labelling by amino acids in cell culture, TMT tandem mass tags
Absolute quantification has a 2-log dynamic range and typically involves spiking of labelled protein or peptide standards into a sample protein or peptide mixture. Most often, absolute quantification only allows the quantification of one or few proteins from multiple biological samples. One of the largest applications of absolute quantification of proteins is the quantification of protein biomarkers, e.g. related to cells from a healthy or diseased state. The labelled molecule can be introduced at different stages of the experimental workflow, viz. before pre-fractionation, before tryptic digestion or after digestion. The step in which protein or peptide standards are spiked into samples is determinant for protein quantification in biological samples, since later spiking results in underestimation of protein amounts in the original unprocessed sample. In this respect, two methods give good estimations of protein amount in biological samples. These methods are Protein Standard Absolute Quantification, or PSAQ (Brun et al. 2007), and Stable Isotope Standards and Capture by Anti-Peptide Antibodies, or SISCAPA (Anderson et al. 2004). In PSAQ, labelled protein standards are directly mixed with the sample, whereas, in SISCAPA, labelled peptide standards are mixed with the sample and anti-peptide antibodies are used for screening and quantification of peptides. A different approach can be used for the quantification of protein complexes, e.g. quantification using concatenated proteotypic peptides or QconCAT (Pratt et al. 2006). Proteotypic peptides are signature peptides that are unique to a protein and yield good-quality MS peaks. For this approach, an artificial gene is constructed coding for a protein of concatenated proteotypic peptides. The gene is then expressed and the isolated standard protein is labelled. The standard protein is pooled with a sample mixture, and after tryptic digestion, each standard protein yields different labelled peptides, each probing for a specific protein. Finally, in a method described as absolute quantification (AQUA) of proteins (Gerber et al. 2003), the tryptic peptides from sample digestion are mixed with labelled peptide standards. This method is very well suited for the quantification of peptides and proteins with post-translational modifications, most notably in the field of phosphoproteomics. Absolute quantification can be applied to the quantification of fungal proteins, such as: (a) the enzymes involved in the production of mycotoxins, antibiotic or other target secondary metabolites, (b) extracellular enzymes used in biotechnological processes, (c) potential allergens or (d) subunits of protein complexes of interest to fungal metabolism and physiology.
As mentioned before, label-based absolute quantification makes use of an external standard to quantify a small subset of target proteins regardless of the number of biological samples analysed. Label-based relative quantification, on the other hand, labels all the proteins within a single biological sample and compares these to all the unlabelled proteins of another sample. Exceptionally, up to eight samples can be compared in multiplexed systems; however, this complicates the corresponding MS/MS analysis. The dynamic range of relative quantification is usually in the order of 1–2 logs, thus slightly lower than the 2-log dynamic range of absolute quantification. According to its nature, the labelling procedure in relative quantification can be performed in one of two ways: in vivo, also known as metabolic labelling, or in vitro. In the in vivo system, an organism is grown in strictly controlled minimal medium supplemented with isotope-enriched nutrients. For this, two major methods exist: 15N labelling (Oda et al. 1999; Krijgsveld et al. 2003) and stable isotope labelling by amino acids in cell culture or SILAC (Ong et al. 2002). The target organism is grown on 15N-medium (15N labelling) or on medium containing one or two labelled amino acids (SILAC). The control organism is grown on normal medium. After protein extraction, the light and heavy proteins are pooled. The mixed light and heavy proteins can undergo tryptic digestion and LC-MS/MS. For these two methods of in vivo labelling, MS analysis usually requires special software, capable of detecting most isotope-labelled peptides. In the in vitro system, all proteins or tryptic peptides in one of the samples are chemically or enzymatically labelled. Most often, chemical labelling in the in vitro system targets the free amino groups (including lysine side chain), cysteine residues or free carboxyl groups (including aspartate and glutamate side chains). One common problem with chemical labelling is the occurrence of unwanted side reactions. Enzymatic labelling also called proteolytic labelling or 18O labelling is based on tryptic digestion of protein in water containing heavy oxygen H182O, resulting in the labelling of all tryptic peptides. A frequent problem with this approach was the occurrence of back-exchange of isotopes, which nowadays is prevented by incubating the tryptic peptides in H182O. Another common problem is that high-resolution MS is required to resolve 4-Da mass differences between the two isotopic forms. Label-based relative quantification can also find applications in fungal research for the investigation of the effects on the proteome of two contrasting conditions, such as high or low temperature, oxygen, pH or specific salts. Label-based relative quantification presents a smaller dynamic range compared to absolute quantification; however, despite the lower dynamic ranges of measured protein concentration, relative quantification is able to generate proteome-wide quantification of proteins.
Nowadays, with the increasing resolution achieved by one- or multidimensional LC-MS instruments and the development of new algorithms, another group of quantification methods is becoming increasingly more standard for proteomic quantification. This group is collectively called label-free MS-based quantification of proteins. This type of quantification contrasts much with label-based quantification, in that it presents a lower accuracy (>30% uncertainty) compared to label-based methods. However, label-free quantification has a larger dynamic in the order of 2–3 logs of protein concentration and, in general, it is much cheaper and simpler compared to label-based quantification. Currently, the methods for label-free quantification of proteins are mainly based on two parameters: total ion current from chromatogram (TIC) measured prior to MS and total spectral counts (SpC) for the tryptic peptides identified during MS/MS. TIC-based quantification is overall more accurate, whereas SpC-based quantification is more sensitive and has a larger dynamic range (Zybailov et al. 2005). Another difference between the two is that TIC-based quantification requires all tryptic peptides corresponding to a protein to be detected for quantification, whereas with SpC all tryptic peptides detected can be used for protein quantification (Old et al. 2005). SpC-based quantification has recently been refined by the incorporation of other parameters, such as the theoretical number of tryptic peptides per protein or the protein length in amino acids (Ishihama et al. 2005; Zybailov et al. 2006).
MS-based proteomics applied to filamentous fungi
Mass spectrometry-based proteomics has benefitted significantly from the many genomes sequenced in the past decade. With regard to the kingdom of fungi, genomes have become available for many species. The ascomycetes, whose genomes were recently sequenced, belong to all subphyla of Ascomycota, viz., Pezizomycotina or true ascomycetes (Machida et al. 2005; Pel et al. 2007; Espagne et al. 2008; Martínez et al. 2008; Van den Berg et al. 2008; DiGuistini et al. 2009), Saccharomycotina or true yeasts (Jones et al. 2004; Wei et al. 2007), and Taphrinomycotina, i.e. dimorphic ascomycetes (Wood et al. 2002). In addition to the large group represented by the ascomycetes, basidiomycetous genomes have also been sequenced, namely from a white-rot fungus (Martínez et al. 2004), an ectomycorrhisal fungus (Martin et al. 2008) and an encapsulated yeast (Loftus et al. 2005). Full-genome DNA sequencing of these fungal species has many implications as it becomes possible to, e.g. (1) predict novel open reading frames (ORFs), (2) determine genome organisation and its relation with fungal evolution, (3) contribute to a better gene annotation and gene function prediction, e.g. if genomes of different organisms are compared, (4) the unravelling of DNA regulatory sequences and corresponding putative regulators and (5) a near-complete understanding of fungal strain differences, including not only naturally occurring strains but also mutant strains used, for instance, in biotechnological processes.
Genome sequence information and systematic ORF prediction are vital to bottom-up proteomics, i.e. identification of full proteins from the total corresponding tryptic peptides. For shotgun proteomics, this is even more the case, as these analyses typically result in the identification of hundreds to thousands of individual proteins. Therefore, only a complete overview of all the ORFs from the genome will allow the identification of such large numbers of proteins. Notwithstanding and parallel to this dependence on genome sequence, shotgun proteomics can also validate gene models and, most importantly, identify protein coding regions ab initio (Bitton et al. 2010).
Sample preparation in fungal organelle proteomics
To date, most MS-based proteomic analyses in filamentous fungi have had three targets: the whole cell (mycelial extract), the cytosolic proteins and the secretome. Many important biological processes, however, occur within specific cell compartments. Most often, the production and accumulation of industrially relevant metabolites within the cell are time- and location-dependent processes. Thus, organelle proteome mapping and quantification of specific proteins across time in different cell compartments may better reveal various aspects of fungal metabolite production, e.g. penicillin production in the microbodies of Penicillium chrysogenum (Kiel et al. 2009).
The analysis of fungal cell organelles by proteomics is an expanding field and is expected to yield significant biotechnological advances as exemplified above for the case of penicillin production. A typical experimental setup for the proteome analysis of organelles comprises steps, such as organelle enrichment, protein separation, mass spectrometry and bioinformatics of MS data. Each of these steps can be approached differently, and it is still a challenging task to combine different approaches in such a way that the final protein lists obtained are indeed unbiased representations of the organelle proteomes.
A crucial step in organelle proteomics is organelle enrichment, as downstream analysis is dependent on good-quality organelle preparations. A typical workflow for organelle enrichment comprises three major steps, from cell disruption, to crude organelle separation and subsequent enrichment by additional separation techniques (Fig. 2). In this respect, filamentous fungi are difficult subjects for a number of reasons. First, filamentous fungi secrete proteases, which enhance the common problem of protein degradation common to other eukaryotes. Second, these microorganisms display polarised growth supported by microtubules. These structures increase the clustering of different organelles, which makes organelle separation more difficult. Last but not least, depending on the species, filamentous fungi may possess very thick, compact cell walls. This feature brings difficulties to the cell disruption process, as the latter must be gentle enough to preserve organelle integrity as much as possible.
Fig. 2.
Proposed workflow for organelle proteomics in filamentous fungi
Special attention must be given to the different steps of organelle proteomics in filamentous fungi. Homogenisation buffers must be isotonic, near neutral to slightly basic and invariably contain protease inhibitors. Ideally, the cell disruption procedures should be standardised by automation, to maintain reproducibility throughout the homogenisation procedures. For this, the use of instruments such as the French pressure cell or automatic mortar grinders is recommended. It is also preferable to use less harsh conditions by using the highest biomass-to-homogenisation buffer ratio possible for feasible operation. Bead beating (bead milling) is not always recommended, as it may be too harsh for large organelles such as the nucleus or vacuoles and may require extensive optimisation. Still, for the analysis of proteins from cell extracts, bead beating can be a preferred method to the use of lysing buffers under extreme conditions, such as boiling or the use of detergents (Nandakumar and Marten 2002). Enzymatic degradation of the cell wall or protoplast formation followed by gentle lysis is one of the best methods to obtain intact organelles. This method is in fact still preferred for the enrichment of very large organelles, such as large vacuoles. Yet, there are two arguments against the general use of protoplasts in fungal organelle proteomics. First and most important, protoplast formation typically extends for a minimum period of 2 to 3 h, during which fungal physiology is severely disturbed and, consequently, this would introduce an extra variable in the experiment. Second, different batches of enzyme cocktails may have very different protoplast-forming efficiencies, thereby decreasing the overall reproducibility of this method. Another popular method with decreased reproducibility is cell disruption by manual grinding in mortar with sterile sand. Though simple and cost-effective, this is a low-throughput method that is largely dependent on a number of factors, such as mortar and buffer temperature, amount and type of sand, operator skill, time and amount of biomass used.
Following cell disruption, unbroken mycelium is removed from the homogenate suspension, for instance, by suction-filtration through multiple layers of nylon gauze or Miracloth, and sub-fractionation methods are used to obtain crude or enriched organelle fractions. Most often, the first step in sub-cellular fractionation is low-speed centrifugation to allow cell debris and nuclei to be separated from less heavy organelles. Alternative, but less used, techniques are differential detergent fractionation (Ramsby and Makowski 1999) and centrifugal elutriation (Lin et al. 1985). After this step, different strategies may be applied, namely, ultracentrifugation on density gradients, immunomagnetic separation and free-flow electrophoresis. In density gradient centrifugation, linear or step density gradients can be used. Linear density gradients do not require extensive optimisation and allow organelles to migrate in such a way that, after ultracentrifugation, there will be a continuum of organelle distribution between different fractions, allowing for more flexibility in the choice of different fractions and, therefore, different organelles or organelle combinations. The use of step density gradients in general only allows the enrichment of one or few organelles. The main advantage of step gradients compared to linear gradients is that, once protocols are optimised, high concentrations of the target organelle are achieved. Moreover, step preparation dispenses the need for special equipment commonly used to create linear gradients. Immunomagnetic separation (IMS) is based on the binding to organelle-antigens of antibodies that coat super-paramagnetic beads. Common bottlenecks are the often insufficient antibody affinity or specificity, as well as the large amount of antibodies necessary to coat the magnetic beads. IMS can be used for the enrichment of many organelles, viz. mitochondria (Hornig-Do et al. 2009), vacuoles (Urwyler et al. 2009), microbodies (Luers et al. 1998), endosomes (Vergés et al. 1999), secretory organelles (Kawajiri et al. 1977) and vesicles (Abe et al. 2009). Finally, one method increasingly used to enrich organelles is free-flow electrophoresis (FFE). In FFE, the organelle mixture moves along carrier medium between two slanted plates. Simultaneous to this, a perpendicular electrical field deflects the organelles that concentrate in specific spots at the bottom of the slanted plates. Recently, this system has been miniaturised to microchip format also known as microfluidic FFE (μ-FFE), making it attractive for the separation of minute amounts of sample coupled to very sensitive downstream MS techniques (Kohlheyer et al. 2008).
Proteomics in fungal biotechnology
In the past decade, several mass spectrometry-based proteomic studies were carried out in filamentous fungi. Most often, these microorganisms have been studied for a number of practical reasons relative to either a positive or a negative impact on humans and economy. Positive impact of filamentous fungi ranges from being components of foodstuffs and participating in fermentation processes to producing molecules of interest to biotechnological industries, namely enzymes, antibiotics, organic acids and other metabolites. Depending on the species, however, filamentous fungi may have a negative impact, varying from damage of buildings to more serious issues such as food spoilage, crop, animal or specifically human diseases and mycotoxin production.
High-resolution 2-DE has been used in several studies to generate fungal protein maps and, in some cases, even to quantify proteins by DIGE. A pioneer proteomic study in Hypocrea jecorina (Trichoderma reesei) used high-resolution 2-DE and was able to identify all the subunits of the 26S proteasome, a large multi-subunit complex involved in intracellular proteolysis (Grinyer et al. 2007). Two years later, the same team processed the isolated complex by tandem anion exchange/size exclusion chromatography prior to 2-DE separation, resulting in increased operation speed and overall spot resolution (Kautto et al. 2009).
Oda and co-workers (2006) cultivated Aspergillus oryzae under solid-state and under submerged culture conditions. From the analysis of the secretome, they concluded that some enzymes were specifically secreted either on solid-state or on submerged culture conditions, regardless of medium composition. Enzymes such as glucoamylase A and B showed different expression behaviour, e.g. a xylanase gene was expressed under both conditions, while glucoamylase A was secreted only under the submerged condition, whereas the glucoamylase B gene was highly expressed under the solid-state condition. This knowledge is of particular relevance to industry, as some biotechnological processes that employ fungal hosts can only be performed under submerged or solid-state conditions. Proteome differences were found between the in vitro and in planta secretomes with many proteins being expressed in only one of the two conditions as, e.g. in Fusarium graminearum (Paper et al. 2007).
Two-dimensional electrophoresis was also applied in the study of proteins involved in osmoadaptation in Aspergillus nidulans (Kim et al. 2007b). Osmoadapted A. nidulans showed increased expression of glyceraldehyde-3-phosphate dehydrogenase and aldehyde dehydrogenase, and decreased expression of enolase, which suggests an increased glycerol biosynthesis. Moreover, TCA cycle enzymes were less abundant. Altogether, these results suggest that osmoadapted A. nidulans shifts from energy metabolism to the biosynthesis of glycerol, most likely important for control of cell turgor. Also, a hypothetical protein containing a domain of unknown function DUF1349 was also found to be more abundant on osmoadapted cells, suggesting a possible role of this protein domain in osmoadaptation.
More recently, a 2-DE system has been set up to identify the major proteome differences under hypoxia in A. nidulans (Shimizu et al. 2009). For this, the proteome was compared for cells growing under hypoxia and for cells under normal aeration. Important differences were observed, such as differences in metabolic enzymes and enzymes for energy production. Furthermore, given the higher abundance of proteins involved in nucleotide salvage in the hypoxic condition, it was concluded that activation of nucleotide salvage is a fungal mechanism of adaptation to oxygen deprival.
The major intracellular proteins of A. fumigatus have been identified by 2-DE proteomics (Carberry et al. 2006). Apart from identifying the main intracellular proteins, this group also identified the main glutathione transferase protein, by purification on glutathione-bound sepharose. Kniemeyer and co-workers (2006) established another 2-DE protein map of A. fumigatus. By comparing 2-DE maps from A. fumigatus grown on either glucose or ethanol as sole carbon sources, the main enzymes involved in alcohol metabolism were identified. Apart from alcohol dehydrogenase, a particular aldehyde dehydrogenase, out of several putatively expressed from the genome, was very abundant upon growth on ethanol. Thus, this protein most likely functions as acetaldehyde dehydrogenase in A. fumigatus for the production of acetyl-CoA. In another study of A. fumigatus, 2-DE based proteomics was used to investigate the proteins differentially present in fungus cultivated with the antifungal drug amphotericin B (Gautam et al. 2008). Two of the most abundant proteins under amphotericin B cultivation were Rho-GDP dissociation inhibitor (GDI) and secretory GDI. Interestingly, the corresponding genes were also more expressed under cultivation on medium containing amphotericin B. Such genes are potential targets for the development of new antifungal drugs. In another study A. nidulans was grown in the presence of the macrolide antibiotic concanamycin A (Melin et al. 2002). No over-expressed proteins were found compared to the proteome from the fungus grown in the absence of the anibiotic. However, besides glyceraldehydes-3-phosphate dehydrogenase, three proteins were found down-regulated under these conditions. These three proteins were, respectively, homologous to a cadmium-induced protein, to LovC involved in the biosynthesis of the secondary metabolite lovastatin and to a protein of unknown function. Down-regulation of expression of these proteins could in part explain the growth inhibition and compact morphology of A. nidulans when grown in solid-medium with macrolide antibiotics.
Following these major advances in 2-DE-based proteomics, another group reported the use of high-resolution 2-DE to analyse protein changes during penicillin biosynthesis in three strains of Penicillium chrysogenum (Jami et al. 2010). The strains used were the wild type, a strain with a small improvement for penicillin biosynthesis and a strain with a large improvement for penicillin biosynthesis. As a result of the experimental conditions used, the corresponding protein maps generated showed an amazing number of up to a thousand distinct spots per gel, of which 950 proteins could be readily identified. The large number of proteins identified allowed for estimating the main proteome changes between the high producer strains and the wild-type strain. In the high producer strains, a number of pathways were more prominent, like cysteine biosynthesis, enzymes of the pentose phosphate pathway and stress response proteins together, whereas proteins for biosynthesis of other secondary metabolites different from penicillin (pigments and isoflavonoids) were reduced. For studies of metabolic processes by proteomics, it is of interest to combine proteomic analysis with metabolic measurements. The process of degradation of aromatic compounds in Phanerochaete chrysosporium was followed by metabolite analysis and by high-resolution 2-DE coupled to LC-MS/MS (Matsuzaki et al. 2008). Conclusions could be drawn for the process of lignin degradation based on enzyme expression values as assessed by proteomics and the amount of chemical species related to these enzymes, assessed by metabolomics. A second example of an approach combining proteomics with metabolomics comes from the analysis of the effect of lactate and starch on fumonisin B2 biosynthesis in A. niger (Sørensen et al. 2009). By means of an approach similar to the one adopted by Matsuzaki and co-workers, Sørensen and colleagues were able to show a specific relation between the increase in fumonisin B2 and the enzymes affecting the intracellular levels of acetyl-CoA. From this observation Sørensen and colleagues were able to conclude that fumonisin B2 production in A. niger is most likely regulated by acetyl-CoA.
In addition to the study of metabolism, the study of secreted enzymes is an important field in research on filamentous fungi. Many filamentous fungi have evolved to secrete high amounts of specialised enzymes responsible, e.g. for plant cell-wall degradation. For this reason these fungi are applied in various biotechnological processes. In an attempt to disclose the cellulose-degrading system in the model fungus Neurospora crassa, Tian and co-workers combined gene expression data with proteomic data from the secretome of the fungus (Tian et al. 2009). By applying microarray and shotgun proteomics analysis on strains grown on different media, they were able to identify strong candidate genes involved in cellulose degradation. In addition, some of these candidate genes were further validated by the observation that the corresponding deletion strains grew poorly on cellulose. Another possible approach to identify a given secreted enzyme is to separate all secreted proteins, screen for a certain enzyme activity and identify the isolated protein. Following this approach in A. fumigatus, the secretome was separated by 2-DE and, after fluorescence assay for β-glucosidase activity, positively identified spots were analysed and the proteins identified by MS/MS (Kim et al. 2007a). If the purpose of the analysis is to have an overview of the largest number possible of secreted enzymes, then several media must be tested containing different carbon substrates. Two such experimental approaches were carried out in aspergilli grown under varying conditions. Medina and colleagues (Medina et al. 2004) were able to identify proteins specifically secreted on medium containing rutin in Aspergillus flavus. For this, the genome sequence information of seven different species of Aspergilli was used for protein identification to increase the number of identified proteins. In this way, proteins may still be identified even if the corresponding gene model from A. flavus would be mis-annoted, e.g. by incorrect intron-exon boundaries or mis-annotated C- or N-termini.
Oda and co-workers on the other hand cultivated Aspergillus oryzae under solid-state or under submerged culture conditions. Following this procedure, they concluded that some enzymes were specifically secreted either on solid-state or on submerged culture conditions, regardless of medium composition. Also, proteome differences may be found between the in vitro and in planta secretomes with many proteins being expressed in only one of the two conditions as e.g. in Fusarium graminearum (Paper et al. 2007).
The A. niger secretome was subject of several studies the last 2 years. Tsang and co-workers (Tsang et al. 2009) used defined and complex media to increase the spectrum of enzymes secreted by A. niger. In this study the analysis of secreted proteins was combined with genome-wide predictions of signal-peptide containing proteins. In this way, secreted proteins could be validated as secreted proteins and not as contaminants resulting from cell lysis. In another study (Jacobs et al. 2009), gene expression data were crossed with 2-DE-based proteomic data from three strains of A. niger that are overproducers of lipase, protease and hydrolase. In this work, automated sample processing was used for 2-DE spots, allowing the identification of 898 individual proteins. Protein samples from 2-DE gels were normalised for spot volume and relative expression was estimated. The overproducing strains showed up-regulation of proteins involved in carbon and nitrogen metabolism, protein folding and protein degradation. Furthermore, using the information of up-regulated protein folding and protein degradation in overproducing strains, this group was able to increase the secretion of glucuronidase from a GUS reporter, by overexpressing the sttC gene predicted to be involved in protein glycosylation and by simultaneously knocking-out the doaA gene involved in protein degradation.
Proteomics of the secretome is also used to search for new biopolymer degrading enzymes like, e.g. polysaccharidases and proteases. Very recently, the proteome and secretome, related to the utilisation of two sugars d-xylose and d-maltose by A. niger, were compared by high-resolution 2-DE (Lu et al. 2010). Also part of the intracellular proteome was quantified by DIGE. The utilisation of the sugars strongly influenced the composition of secretome, but had only a minor effect on the intracellular proteome. From the changes in the proteome found for the different conditions, it was concluded that the changes due to variation in the shake flask cultures. However, the culture conditions like pH control or oxygen control had a large effect on composition of the intracellular proteome, reflecting the importance of these parameters for the metabolic processes. In a study of Adav et al. (2010) 102 proteins were identified from the culture broth of A.niger after 144 h of fermentation. Although of the 102 proteins many are hydrolytic enzymes like polysaccharidases and proteases, one should realise these are starvation conditions that do not represent lignocellulose induced conditions. Braaksma et al. (2010) studied the secretome of A. niger cultures grown on d-galacturonic acid, under which condition the pectinases were the more dominant enzymes and d-sorbitol for which the carbohydrases in particular alpha and beta-glucosidases were the most dominant enzymes. Under conditions of starvation, due to carbon source depletion proteases were dominantly found in the secretome, similar to the results described by Adav et al. (2010).
Organelle proteomics in fungal biotechnology
All the methods downstream of organelle enrichment procedures are basically identical to the ones pointed earlier for general MS-based proteomic procedures. These include separation of isolated proteins by 2-DE or multidimensional LC followed by MS/MS analysis. The workflow of organelle proteomics is much dependent on the experimental aim and nature of the organelles to analyse.
Whenever the experimental aim is to study protein secretion, besides the secretome itself it is desirable to study the proteomes of secretory organelles and traffic vesicles. In a previous study, microsomal proteins from biological duplicates of A. niger were separated by SDS-PAGE and gel slices were processed for LC-MS/MS (Ferreira de Oliveira et al. 2010). It was subsequently shown that specific microsomal proteome changes occurred after the addition of D-xylose to the culture medium. Quantification was performed based on normalised spectral abundance factors, calculated from MS spectra. Among other processes the differentially expressed proteins were related to protein secretion and thus pointed to novel mechanisms of regulation.
Other aspects of fungal biotechnology such as energy production, organic acid metabolism or cell apoptosis are best studied from examination of the mitochondrial proteome. The first study to use enriched mitochondrial preparations in filamentous fungi was in Trichoderma harzianum (Grinyer et al. 2004). A crude mitochondrial pellet was obtained by differential centrifugation and a 2-DE protein map was created. Despite the low number of proteins identified (n = 25), this work presented the first fungal mitochondrial proteome map. Another similar study reports the characterisation of the mitochondrial proteome of Aspergillus fumigatus (Vödisch et al. 2009). In contrast to the previous work, crude mitochondria were further separated on a three-step density gradient for enrichment and confirmed by electron microscopy. Moreover, up to six 2-DE gels were used to construct the protein reference maps for the mycelium and the mitochondria, with a superior overall resolution.
One fungal organelle of particular industrial importance is the microbody. Microbodies in filamentous fungi can be divided into three categories: peroxisomes, responsible for β-oxidation of long-chain fatty acids; glyoxysomes, which additionally participate in the glyoxylate cycle; and the Woronin body, which functions as a septal plug in case of cell injury. Microbodies have been implicated in the production of β-lactam antibiotics, most notably of penicillin. These organelles were recently analysed in Penicillium chrysogenum, the major penicillin producer (Kiel et al. 2009). Microbody enrichment was carried out by differential centrifugation and confirmed by electron microscopy. After SDS-PAGE separation of proteins and processing for LC-MS/MS, 89 proteins were identified of which 79 possessed a putative microbody targeting signal, including the penicillin biosynthesis protein isopenicillin N:acyl CoA acyltransferase.
Noteworthy, due to technical advances in MS-based proteomics, the preparation of highly enriched organelle fractions though desirable is no longer mandatory in many situations. In the past decade, a number of studies have shown that protein profiles can be created from direct LC-MS/MS analyses of complex proteins mixtures originating from separate fractions of linear gradients (Andersen et al. 2003; Dunkley et al. 2004; Dunkley et al. 2006). These profiles can be used to predict protein localisation, as assessed by labelled in vitro peptide standards. More recently, label-free protein profiling was achieved, increasing the power of such analyses for establishing organelle proteomes (Foster et al. 2006; Gilchrist et al. 2006; Kislinger et al. 2006; Takamori et al. 2006). Notwithstanding the simplicity of these label-free comprehensive studies, identical studies have not been yet applied to organelle proteomics of filamentous fungi.
Concluding remarks
This review summarises the major aspects of proteomics applied to filamentous fungi, including protein quantification methods and sample preparation for organelle proteomics. In filamentous fungi proteomics still developing and in particular in organelle proteomics is suffering from many constraints. First, in many conditions the experimental setup is not adapted to more rigorous protein quantification or to the identification of a maximal number of proteins representative of a given sample or condition. Second, reproducibility and performance of different steps, including cell disruption and sub-cellular fractionation are often neglected. Third, the potential of bioinformatics and statistics are often ignored or not efficiently exploited. For each of these common problems, adapted suggestions were presented. In the first place, depending on the goal, protein quantification methods exist that are targeted to small subsets of proteins from various samples (absolute quantification) or to most proteins of two contrasting samples (relative quantification); alternatively, less expensive label-free techniques can be used as these support increased dynamic ranges and rely solely on bioinformatics of LC-MS/MS parameters. In the second place, reproducibility and performance in proteomics can be much improved by increased automation and sample processing speed, namely during the steps of homogenisation and sub-fractionation. Third, bioinformatics is essential to proteomics, not only to identify proteins and predict their functions, but also to predict the presence of targeting sequences in proteins. Statistics also plays a major role in organelle proteomics since the analysis of complex protein samples e.g. from sub-cellular fractions requires statistical validation of protein ratios. Moreover, depending on the experimental scope protein correlation profiling can be used to confidently identify protein candidates associated with organelles or functional complexes.
Fungal proteomics is still in its infancy, but is developing rapidly. Significant advances are expected for the improved production of enzymes and metabolites that can contribute to existing and novel biotechnological processes. The secretome studies show the wealth of enzymes secreted by filamentous fungi. This will provide a major contribution in the understanding of the mechanisms of metabolic processes that are of importance in biotechnology. Organelle proteomics can contribute in this, since compartmentalisation is an important aspect in the underlying mechanisms of, e.g. antibiotic, organic acid and protein production.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abe N, Almenar-Queralt A, Lillo C, Shen Z, Lozach J, Briggs SP, Williams DS, Goldstein LS, Cavalli V. Sunday driver interacts with two distinct classes of axonal organelles. J Biol Chem. 2009;284:34628–34639. doi: 10.1074/jbc.M109.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adav SS, Li AA, Manavalan A, Punt P, Sze SK. Quantitative iTRAQ secretome analysis of Aspergillus niger reveals novel hydrolytic enzymes. J Proteome Res. 2010;9:3932–3940. doi: 10.1021/pr100148j. [DOI] [PubMed] [Google Scholar]
- Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW. Mass spectrometric quantitation of peptides and proteins using stable isotope standards and capture by anti-peptide antibodies (SISCAPA) J Proteome Res. 2004;3:235–244. doi: 10.1021/pr034086h. [DOI] [PubMed] [Google Scholar]
- Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem. 2007;389:1017–1031. doi: 10.1007/s00216-007-1486-6. [DOI] [PubMed] [Google Scholar]
- Bitton DA, Smith DL, Connolly Y, Scutt PJ, Miller CJ. An integrated mass-spectrometry pipeline identifies novel protein coding-regions in the human genome. PLoS ONE. 2010;5:e8949. doi: 10.1371/journal.pone.0008949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrebaeck CA, Wingren C. High-throughput proteomics using antibody microarrays: an update. Expert Rev Mol Diagn. 2007;7:673–686. doi: 10.1586/14737159.7.5.673. [DOI] [PubMed] [Google Scholar]
- Braaksma M, Martens-Uzunova EM, Punt PJ, Schaap PJ (2010) An inventory of the Aspergillus niger secretome by combining in silico predictions with shotgun proteomics data. BMC Genom (in press) [DOI] [PMC free article] [PubMed]
- Brun V, Dupuis A, Adrait A, Marcellin M, Thomas D, Court M, Vandenesch F, Garin J. Isotope-labeled protein standards: toward absolute quantitative proteomics. Mol Cell Proteomics. 2007;6:2139–2149. doi: 10.1074/mcp.M700163-MCP200. [DOI] [PubMed] [Google Scholar]
- Carberry S, Neville CM, Kavanagh KA, Doyle S. Analysis of major intracellular proteins of Aspergillus fumigatus by MALDI mass spectrometry: identification and characterisation of an elongation factor 1B protein with glutathione transferase activity. Biochem Biophys Res Commun. 2006;341:1096–1104. doi: 10.1016/j.bbrc.2006.01.078. [DOI] [PubMed] [Google Scholar]
- DiGuistini S, Liao NY, Platt D, Robertson G, Seidel M, Chan SK, Docking TR, Birol I, Holt RA, Hirst M, Mardis E, Marra MA, Hamelin RC, Bohlmann J, Breuil C, Jones SJ. De novo genome sequence assembly of a filamentous fungus using Sanger, 454 and Illumina sequence data. Genome Biol. 2009;10:R94. doi: 10.1186/gb-2009-10-9-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong MQ, Venable JD, Au N, Xu T, Park SK, Cociorva D, Johnson JR, Dillin A, Yates JR., 3rd Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 2007;317:660–663. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- Dunkley TP, Watson R, Griffin JL, Dupree P, Lilley KS. Localization of organelle proteins by isotope tagging (LOPIT) Mol Cell Proteomics. 2004;3:1128–1134. doi: 10.1074/mcp.T400009-MCP200. [DOI] [PubMed] [Google Scholar]
- Dunkley TP, Hester S, Shadforth IP, Runions J, Weimar T, Hanton SL, Griffin JL, Bessant C, Brandizzi F, Hawes C, Watson RB, Dupree P, Lilley KS. Mapping the Arabidopsis organelle proteome. Proc Natl Acad Sci USA. 2006;103:6518–6523. doi: 10.1073/pnas.0506958103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J, McCormack AL, Yates JR., 3rd An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Espagne E, Lespinet O, Malagnac F, Da Silva C, Jaillon O, Porcel BM, Couloux A, Aury JM, Ségurens B, Poulain J, Anthouard V, Grossetete S, Khalili H, Coppin E, Déquard-Chablat M, Picard M, Contamine V, Arnaise S, Bourdais A, Berteaux-Lecellier V, Gautheret D, de Vries RP, Battaglia E, Coutinho PM, Danchin EG, Henrissat B, Khoury RE, Sainsard-Chanet A, Boivin A, Pinan-Lucarré B, Sellem CH, Debuchy R, Wincker P, Weissenbach J, Silar P. The genome sequence of the model ascomycete fungus Podospora anserina. Genome Biol. 2008;9:R77. doi: 10.1186/gb-2008-9-5-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira de Oliveira JMP, van Passel MW, Schaap PJ, de Graaff LH. Shotgun proteomics of Aspergillus niger microsomes upon D-xylose induction. Appl Environ Microbiol. 2010;76:4421–4429. doi: 10.1128/AEM.00482-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LJ, de Hoog CL, Zhang Y, Zhang Y, Xie X, Mootha VK, Mann M. A mammalian organelle map by protein correlation profiling. Cell. 2006;125:187–199. doi: 10.1016/j.cell.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Gautam P, Shankar J, Madan T, Sirdeshmukh R, Sundaran CS, Gade WN, Basir SF, Sarma PU. Proteomic and transcriptomic analysis of Aspergillus fumigatus on exposure to Amphotericin B. Antimicrob Agents Chemoter. 2008;52:4220–4227. doi: 10.1128/AAC.01431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci USA. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernández-Rodríguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–1281. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- Grinyer J, McKay M, Herbert B, Nevalainen H. Fungal proteomics: mapping the mitochondrial proteins of a Trichoderma harzianum strain applied for biological control. Curr Genet. 2004;45:170–175. doi: 10.1007/s00294-003-0475-3. [DOI] [PubMed] [Google Scholar]
- Grinyer J, Kautto L, Traini M, Willows RD, Te’o J, Bergquist P, Nevalainen H. Proteome mapping of the Trichoderma reesei 20S proteasome. Curr Genet. 2007;51:79–88. doi: 10.1007/s00294-006-0108-8. [DOI] [PubMed] [Google Scholar]
- Hornig-Do HT, Günther G, Bust M, Lehnartz P, Bosio A, Wiesner RJ. Isolation of functional pure mitochondria by superparamagnetic microbeads. Anal Biochem. 2009;389:1–5. doi: 10.1016/j.ab.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- Jacobs DI, Olsthoorn MMA, Maillet I, Akeroyd M, Breestraat S, Donkers S, van der Hoeven RAM, van den Hondel CAMJJ, Kooistra R, Lapointe T, Menke H, Meulenberg R, Misset R, Müller WH, van Peij NNME, RamA RS, Roelofs MS, Roubos JA, van Tilborg MWEM, Verkleij AJ, Pel HJ, Stam H, Sagt CMJ. Effective lead selection for improved protein production in Aspergillus niger based on integrated genomics. Fungal Genet Biol. 2009;46:S141–S152. doi: 10.1016/j.fgb.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Jami MS, Barreiro C, García-Estrada C, Martín JF. Proteome analysis of the penicillin producer. Penicillium chrysogenum: characterization of protein changes during the industrial strain improvement. Mol Cell Proteomics. 2010;9:1182–1198. doi: 10.1074/mcp.M900327-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ON. Interpreting the protein language using proteomics. Nat Rev Mol Cell Biol. 2006;7:391–403. doi: 10.1038/nrm1939. [DOI] [PubMed] [Google Scholar]
- Jones T, Federspiel NA, Chibana H, Dungan J, Kalman S, Magee BB, Newport G, Thorstenson YR, Agabian N, Magee PT, Davis RW, Scherer S. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci USA. 2004;101:7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautto L, Grinyer J, Birch D, Kapur A, Baker M, Traini M, Bergquist P, Nevalainen H. Rapid purification method for the 26S proteasome from the filamentous fungus Trichoderma reesei. Protein Expr Purif. 2009;67:156–163. doi: 10.1016/j.pep.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Kawajiri K, Ito A, Omura T. Subfractionation of rat liver microsomes by immunoprecipitation and immunoadsorption methods. J Biochem. 1977;81:779–789. doi: 10.1093/oxfordjournals.jbchem.a131516. [DOI] [PubMed] [Google Scholar]
- Kiel JA, van den Berg MA, Fusetti F, Poolman B, Bovenberg RA, Veenhuis M, van der Klei IJ. Matching the proteome to the genome: the microbody of penicillin-producing Penicillium chrysogenum cells. Funct Integr Genomics. 2009;9:167–184. doi: 10.1007/s10142-009-0110-6. [DOI] [PubMed] [Google Scholar]
- Kim KH, Brown KM, Harris PV, Langston JA, Cherry JR. A proteomics strategy to discover β-glucosidases from Aspergillus fumigatus with two-dimensional page in-gel activity assay and tandem mass spectrometry. J Proteome Res. 2007;6:4749–4757. doi: 10.1021/pr070355i. [DOI] [PubMed] [Google Scholar]
- Kim Y, Nandakumar MP, Marten MR. Proteome map of Aspergillus nidulans during osmoadaptation. Fungal Genet Biol. 2007;44:886–895. doi: 10.1016/j.fgb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Cox B, Kannan A, Chung C, Hu P, Ignatchenko A, Scott MS, Gramolini AO, Morris Q, Hallett MT, Rossant J, Hughes TR, Frey B, Emili A. Global survey of organ and organelle protein expression in mouse: combined proteomic and transcriptomic profiling. Cell. 2006;125:173–186. doi: 10.1016/j.cell.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Kniemeyer O, Lessing F, Scheibner O, Hertweck C, Brakhage AA. Optimisation of a 2-D gel electrophoresis protocol for the human pathogenic fungus Aspergillus fumigatus. Curr Genet. 2006;49:178–189. doi: 10.1007/s00294-005-0047-9. [DOI] [PubMed] [Google Scholar]
- Kohlheyer D, Eijkel JC, van den Berg A, Schasfoort RB. Miniaturizing free-flow electrophoresis—a critical review. Electrophoresis. 2008;29:977–993. doi: 10.1002/elps.200700725. [DOI] [PubMed] [Google Scholar]
- Krijgsveld J, Ketting RF, Mahmoudi T, Johansen J, Artal-Sanz M, Verrijzer CP, Plasterk RH, Heck AJ. Metabolic labeling of C. elegans and D. melanogaster for quantitative proteomics. Nat Biotechnol. 2003;21:927–931. doi: 10.1038/nbt848. [DOI] [PubMed] [Google Scholar]
- Lin JT, Griffith OM, Corse JW. Subcellular fractionation of wheat leaf protoplasts by centrifugal elutriation. Anal Biochem. 1985;148:10–14. doi: 10.1016/0003-2697(85)90621-9. [DOI] [PubMed] [Google Scholar]
- Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., 3rd Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, Vamathevan J, Miranda M, Anderson IJ, Fraser JA, Allen JE, Bosdet IE, Brent MR, Chiu R, Doering TL, Donlin MJ, D’Souza CA, Fox DS, Grinberg V, Fu J, Fukushima M, Haas BJ, Huang JC, Janbon G, Jones SJ, Koo HL, Krzywinski MI, Kwon-Chung JK, Lengeler KB, Maiti R, Marra MA, Marra RE, Mathewson CA, Mitchell TG, Pertea M, Riggs FR, Salzberg SL, Schein JE, Shvartsbeyn A, Shin H, Shumway M, Specht CA, Suh BB, Tenney A, Utterback TR, Wickes BL, Wortman JR, Wye NH, Kronstad JW, Lodge JK, Heitman J, Davis RW, Fraser CM, Hyman RW. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–1324. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Sun J, Nimtz M, Wissing J, Zeng AP, Rinas U. The intra- and extracellular proteome of Aspergillus niger growing on defined medium with xylose or maltose as carbon substrate. Microb Cell Fact. 2010;9:23. doi: 10.1186/1475-2859-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueking A, Horn M, Eickhoff H, Büssow K, Lehrach H, Walter G. Protein microarrays for gene expression and antibody screening. Anal Biochem. 1999;270:103–111. doi: 10.1006/abio.1999.4063. [DOI] [PubMed] [Google Scholar]
- Luers GH, Hartig R, Mohr H, Hausmann M, Fahimi HD, Cremer C, Volkl A. Immuno-isolation of highly purified peroxisomes using magnetic beads and continuous immunomagnetic sorting. Electrophoresis. 1998;19:1205–1210. doi: 10.1002/elps.1150190722. [DOI] [PubMed] [Google Scholar]
- Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto K, Arima T, Akita O, Kashiwagi Y, Abe K, Gomi K, Horiuchi H, Kitamoto K, Kobayashi T, Takeuchi M, Denning DW, Galagan JE, Nierman WC, Yu J, Archer DB, Bennett JW, Bhatnagar D, Cleveland TE, Fedorova ND, Gotoh O, Horikawa H, Hosoyama A, Ichinomiya M, Igarashi R, Iwashita K, Juvvadi PR, Kato M, Kato Y, Kin T, Kokubun A, Maeda H, Maeyama N, Maruyama J, Nagasaki H, Nakajima T, Oda K, Okada K, Paulsen I, Sakamoto K, Sawano T, Takahashi M, Takase K, Terabayashi Y, Wortman JR, Yamada O, Yamagata Y, Anazawa H, Hata Y, Koide Y, Komori T, Koyama Y, Minetoki T, Suharnan S, Tanaka A, Isono K, Kuhara S, Ogasawara N, Kikuchi H. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- Mallick P, Schirle M, Chen SS, Flory MR, Lee H, Martin D, Ranish J, Raught B, Schmitt R, Werner T, Kuster B, Aebersold R. Computational prediction of proteotypic peptides for quantitative proteomics. Nat Biotechnol. 2007;25:125–131. doi: 10.1038/nbt1275. [DOI] [PubMed] [Google Scholar]
- Martin F, Aerts A, Ahrén D, Brun A, Danchin EG, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V, Salamov A, Shapiro HJ, Wuyts J, Blaudez D, Buée M, Brokstein P, Canbäck B, Cohen D, Courty PE, Coutinho PM, Delaruelle C, Detter JC, Deveau A, DiFazio S, Duplessis S, Fraissinet-Tachet L, Lucic E, Frey-Klett P, Fourrey C, Feussner I, Gay G, Grimwood J, Hoegger PJ, Jain P, Kilaru S, Labbé J, Lin YC, Legué V, Le Tacon F, Marmeisse R, Melayah D, Montanini B, Muratet M, Nehls U, Niculita-Hirzel H, Oudot-Le Secq MP, Peter M, Quesneville H, Rajashekar B, Reich M, Rouhier N, Schmutz J, Yin T, Chalot M, Henrissat B, Kües U, Lucas S, Van de Peer Y, Podila GK, Polle A, Pukkila PJ, Richardson PM, Rouzé P, Sanders IR, Stajich JE, Tunlid A, Tuskan G, Grigoriev IV. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature. 2008;452:88–92. doi: 10.1038/nature06556. [DOI] [PubMed] [Google Scholar]
- Martínez D, Larrondo LF, Putnam N, Gelpke MD, Huang K, Chapman J, Helfenbein KG, Ramaiya P, Detter JC, Larimer F, Coutinho PM, Henrissat B, Berka R, Cullen D, Rokhsar D. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol. 2004;22:695–700. doi: 10.1038/nbt967. [DOI] [PubMed] [Google Scholar]
- Martínez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, Danchin EG, Grigoriev IV, Harris P, Jackson M, Kubíček CP, Han CS, Ho I, Larrondo LF, de León AL, Magnuson JK, Merino S, Misra M, Nelson B, Putnam N, Robbertse B, Salamov AA, Schmoll M, Terry A, Thayer N, Westerholm-Parvinen A, Schoch CL, Yao J, Barabote R, Nelson MA, Detter C, Bruce D, Kuske CR, Xie G, Richardson P, Rokhsar DS, Lucas SM, Rubin EM, Dunn-Coleman N, Ward M, Brettin TS. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina) Nat Biotechnol. 2008;26:553–560. doi: 10.1038/nbt1403. [DOI] [PubMed] [Google Scholar]
- Matsuzaki F, Shimizu M, Wariishi H. Proteomic and metabolomic analyses of the white-rot fungus Phanerochaete chrysosporium exposed to exogenous benzoic acid. J Proteome Res. 2008;7:2342–2350. doi: 10.1021/pr700617s. [DOI] [PubMed] [Google Scholar]
- Medina ML, Kiernan UA, Francisco WA. Proteomic analysis of rutin-induced secreted proteins from Aspergillus flavus. Fungal Genet Biol. 2004;41:327–335. doi: 10.1016/j.fgb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Melin P, Schnürer J, Wagner EGH. Proteome analysis of Aspergillus nidulans reveals proteins associated with the response to the antibiotic concanamycin A, produced by Streptomyces species. Mol Genet Genomics. 2002;267:695–702. doi: 10.1007/s00438-002-0695-0. [DOI] [PubMed] [Google Scholar]
- Nandakumar MP, Marten MR. Comparison of lysis methods and preparation protocols for one- and two-dimensional electrophoresis of Aspergillus oryzae intracellular proteins. Electrophoresis. 2002;23:2216–2222. doi: 10.1002/1522-2683(200207)23:14<2216::AID-ELPS2216>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Oda K, Kakizono D, Yamada O, Iefuji H, Akita O, Iwashita K. Proteomic analysis of extracellular proteins from Aspergillus oryzae grown under submerged and solid-state culture conditions. Appl Environ Microbiol. 2006;72:3448–3457. doi: 10.1128/AEM.72.5.3448-3457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Huang K, Cross FR, Cowburn D, Chait BT. Accurate quantitation of protein expression and site-specific phosphorylation. Proc Natl Acad Sci USA. 1999;96:6591–6596. doi: 10.1073/pnas.96.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- Olsen JV, de Godoy LM, Li G, Macek B, Mortensen P, Pesch R, Makarov A, Lange O, Horning S, Mann M. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics. 2005;4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.M200025-MCP200. [DOI] [PubMed] [Google Scholar]
- Paper JM, Scott-Craig JS, Adhikari ND, Cuomo CA, Walton JD. Comparative proteomics of extracellular proteins in vitro and in planta from the pathogenic fungus Fusarium graminearum. Proteomics. 2007;7:3171–3183. doi: 10.1002/pmic.200700184. [DOI] [PubMed] [Google Scholar]
- Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K, Andersen MR, Bendtsen JD, Benen JAE, van den Berg M, Breestraat S, Caddick MX, Contreras R, Cornell M, Coutinho PM, Danchin EGJ, Debets AJM, Dekker P, van Dijck PWM, van Dijk A, Dijkhuizen L, Driessen AJM, d’Enfert C, Geysens S, Goosen C, Groot GSP, de Groot PWJ, Guillemette T, Henrissat B, Herweijer M, van den Hombergh JPTW, van den Hondel CAMJJ, van der Heijden RTJM, van der Kaaij RM, Klis FM, Kools HJ, Kubíček CP, van Kuyk PA, Lauber J, Lu X, van der Maarel MJEC, Meulenberg R, Menke H, Mortimer MA, Nielsen J, Oliver SG, Olsthoorn M, Pal K, van Peij NNME, Ram AFJ, Rinas U, Roubos JA, Sagt CMJ, Schmoll M, Sun JB, Ussery D, Varga J, Vervecken W, de Vondervoort PJJV, Wedler H, Wösten HAB, Zeng AP, van Ooyen AJJ, Visser J, Stam H. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol. 2007;25:221–231. doi: 10.1038/nbt1282. [DOI] [PubMed] [Google Scholar]
- Pratt JM, Simpson DM, Doherty MK, Rivers J, Gaskell SJ, Beynon RJ. Multiplexed absolute quantification for proteomics using concatenated signature peptides encoded by QconCAT genes. Nat Protoc. 2006;1:1029–1043. doi: 10.1038/nprot.2006.129. [DOI] [PubMed] [Google Scholar]
- Ramsby ML, Makowski GS. Differential detergent fractionation of eukaryotic cells. Analysis by two-dimensional gel electrophoresis. Methods Mol Biol. 1999;112:53–66. doi: 10.1385/1-59259-584-7:53. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Fujii T, Masuo S, Fujita K, Takaya N. Proteomic analysis of Aspergillus nidulans cultured under hypoxic conditions. Proteomics. 2009;9:7–19. doi: 10.1002/pmic.200701163. [DOI] [PubMed] [Google Scholar]
- Sørensen LM, Lametsch R, Andersen MR, Nielsen PV, Frisvad JC. Proteome analysis of Aspergillus niger: lactate added in starch-containing medium can increase production of the mycotoxin fumonisin B2 by modifying acetyl-CoA metabolism. BMC Microbiol. 2009;9:255. doi: 10.1186/1471-2180-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grübmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tian C, Beeson WT, Iavarone AT, Sun J, Marletta MA, Cate JH, Glass NL. Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proc Natl Acad Sci USA. 2009;106:22157–22162. doi: 10.1073/pnas.0906810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A, Butler G, Powlowski J, Panisko EA, Baker SE. Analytical and computational approaches to define the Aspergillus niger secretome. Fungal Genet Biol. 2009;46(Suppl 1):S153–S160. doi: 10.1016/j.fgb.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Urwyler S, Nyfeler Y, Ragaz C, Lee H, Müller LN, Aebersold R, Hilbi H. Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic. 2009;10:76–87. doi: 10.1111/j.1600-0854.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- van den Berg MA, Albang R, Albermann K, Badger JH, Daran JM, Driessen AJ, García-Estrada C, Fedorova ND, Harris DM, Heijne WH, Joardar V, Kiel JA, Kovalchuk A, Martín JF, Nierman WC, Nijland JG, Pronk JT, Roubos JA, van der Klei IJ, van Peij NN, Veenhuis M, von Döhren H, Wagner C, Wortman J, Bovenberg RA. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat Biotechnol. 2008;26:1161–1168. doi: 10.1038/nbt.1498. [DOI] [PubMed] [Google Scholar]
- Vergés M, Havel RJ, Mostov KE. A tubular endosomal fraction from rat liver: biochemical evidence of receptor sorting by default. Proc Natl Acad Sci USA. 1999;96:10146–10151. doi: 10.1073/pnas.96.18.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vödisch M, Albrecht D, Leßing F, Schmidt AD, Winkler R, Guthke R, Brakhage AA, Kniemeyer O. Two-dimensional proteome reference maps for the human pathogenic filamentous fungus Aspergillus fumigatus. Proteomics. 2009;9:1407–1415. doi: 10.1002/pmic.200800394. [DOI] [PubMed] [Google Scholar]
- Wei W, McCusker JH, Hyman RW, Jones T, Ning Y, Cao Z, Gu Z, Bruno D, Miranda M, Nguyen M, Wilhelmy J, Komp C, Tamse R, Wang X, Jia P, Luedi P, Öfner PJ, David L, Dietrich FS, Li Y, Davis RW, Steinmetz LM. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc Natl Acad Sci USA. 2007;104:12825–12830. doi: 10.1073/pnas.0701291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MR. 2D electrophoresis: from protein maps to Genomes. Proceedings of the International Meeting. Siena, Italy, September 5–7, 1994. Electrophoresis. 1994;16:1077–1322. [PubMed] [Google Scholar]
- Wolters DA, Washburn MP, Yates JR., 3rd An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem. 2001;73:5683–5690. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, Basham D, Bowman S, Brooks K, Brown D, Brown S, Chillingworth T, Churcher C, Collins M, Connor R, Cronin A, Davis P, Feltwell T, Fraser A, Gentles S, Goble A, Hamlin N, Harris D, Hidalgo J, Hodgson G, Holroyd S, Hornsby T, Howarth S, Huckle EJ, Hunt S, Jagels K, James K, Jones L, Jones M, Leather S, McDonald S, McLean J, Mooney P, Moule S, Mungall K, Murphy L, Niblett D, Odell C, Oliver K, O’Neil S, Pearson D, Quail MA, Rabbinowitsch E, Rutherford K, Rutter S, Saunders D, Seeger K, Sharp S, Skelton J, Simmonds M, Squares R, Squares S, Stevens K, Taylor K, Taylor RG, Tivey A, Walsh S, Warren T, Whitehead S, Woodward J, Volckaert G, Aert R, Robben J, Grymonprez B, Weltjens I, Vanstreels E, Rieger M, Schäfer M, Müller-Auer S, Gabel C, Fuchs M, Dusterhoft A, Fritzc C, Holzer E, Moestl D, Hilbert H, Borzym K, Langer I, Beck A, Lehrach H, Reinhardt R, Pohl TM, Eger P, Zimmermann W, Wedler H, Wambutt R, Purnelle B, Goffeau A, Cadieu E, Dréano S, Gloux S, Lelaure V, Mottier S, Galibert F, Aves SJ, Xiang Z, Hunt C, Moore K, Hurst SM, Lucas M, Rochet M, Gaillardin C, Tallada VA, Garzon A, Thode G, Daga RR, Cruzado L, Jiménez J, Sánchez M, del Rey F, Benito J, Domínguez A, Revuelta JL, Moreno S, Armstrong J, Forsburg SL, Cerutti L, Lowe T, McCombie WR, Paulsen I, Potashkin J, Shpakovski GV, Ussery D, Barrell BG, Nurse P. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- Yan W, Aebersold R, Raines EW. Evolution of organelle-associated protein profiling. J Proteomics. 2009;72:4–11. doi: 10.1016/j.jprot.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zichi D, Eaton B, Singer B, Gold L. Proteomics and diagnostics: let’s get specific, again. Curr Opin Chem Biol. 2008;12:78–85. doi: 10.1016/j.cbpa.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res. 2006;5:2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]