Abstract
Maternal cocaine addiction is a significant public health issue particularly affecting children, with high rates of reported abuse, neglect and foster care placement. This review examines both preclinical and clinical evidence for how cocaine abuse may impact maternal care and infant development, exploring brain, behavioral and neuroendocrine mechanisms. There is evidence that cocaine may affect infant development both directly, via in utero exposure, and indirectly via alterations in maternal care. Two neural systems known to play an important role in both maternal care and cocaine addiction are the oxytocin and dopamine systems, mediating social and reward-related behaviors and stress reactivity. These same neural mechanisms may also be involved in the infant’s development of vulnerability to addiction. Understanding the neuroendocrine pathways involved in maternal behavior and addiction may help facilitate earlier, more effective interventions to help substance abusing mothers provide adequate care for their infant, and perhaps prevent the intergenerational transmission of risk.
Keywords: addiction, cocaine, maternal, dopamine, oxytocin, mother-child bonding, stress
For most mothers, interacting and engaging with one’s own infant is a rewarding and pleasurable experience that promotes mother-infant attachment, ensures optimal care for the developing infant, and motivates maternal behavior even in the face of extreme fatigue and competing needs for attention.1,2 However, animal and human research suggests that mothers who are addicted to substances, particularly cocaine, even when not actively using the drug, may be less able to respond appropriately to their infant’s cues, finding these interactions less intrinsically rewarding or more stress-invoking.3,4 This may put the infant or child at risk for neglect or abuse.
Cocaine abuse continues to be a significant public health issue in the United States, with one million first time users estimated during 2004. Most significantly, almost 90% of drug-abusing women are of reproductive age, with an estimated 4.6 million women users of cocaine in the United States, and 750 000 drug-exposed births annually.5 This has long-lasting implications for families, their children—and society, which bears much of the cost for future educational and therapeutic services.
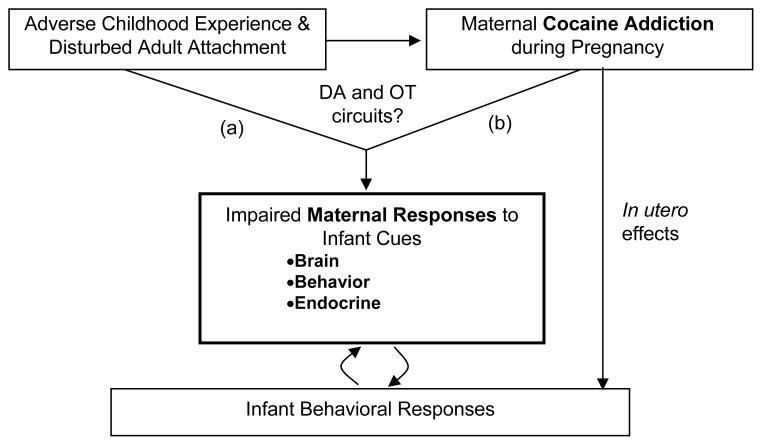
The purpose of this review article is to examine the potential impact of cocaine addiction on maternal care and infant development, exploring brain, behavioral and neuroendocrine mechanisms. (Fig 1). We focus especially on women with active cocaine dependence because (1) cocaine addiction co-opts neural circuitry that has recently been shown to be key to early parenting, that is, dopaminergically regulated mesocorticolimbic and nigrostriatal brain pathways;6,7 (2) cocaine abuse is highly correlated with maternal neglect of offspring and poorer maternal-infant interactions in both human and animal models;3,8–10 and (3) parallel studies of cocaine-exposure in the parturient rodent model suggests that cocaine may inhibit both central and peripheral oxytocin production11–13 altering maternal sensitivity to offspring’s cries, olfactory cues, tactile stimulation, and activity patterns such that mothers are more neglectful of their offspring and more reactive to stress. In particular, these preclinical studies suggest basic mechanisms by which cocaine abuse may disrupt the earliest and most fundamental aspects of parenting behavior.
Figure 1.
Proposed factors influencing maternal responses and infant development. DA, dopamine; OT, oxytocin.
In addition, we aim to explore how the mothers’ own adverse childhood experience may predispose to addiction behaviors, and interact with cocaine exposure to affect maternal responses to infant cues (Fig 1). Studies have shown a strong graded relationship between stressful or traumatic childhood experiences and subsequent illicit drug use,14 as well as impaired parenting practices.15 The lack of responsive, nurturant caregiving in early childhood — which is associated with disturbed patterns of adult attachment — may influence the development of both dopaminergic and oxytocinergic brain systems via epigenetic mechanisms,16–18 thus making an individual more vulnerable to addiction and adverse parenting responses toward their own infants.
There are also findings from both human and preclinical models that suggest prenatal exposure to cocaine may disrupt offspring behavior, as expressed in poor state lability, decreased social behavior, abnormal activity patterns and irritability/aggression.19–23 These behavioral disruptions may, in turn, impact the parenting behaviors of both cocaine-using and non-cocaine-using mothers. Together these observations suggest that, in pregnant women, cocaine abuse may impair parenting both by impairing maternal sensitivity to salient infant cues and by distorting infant cueing behavior.
While information about the neural circuitry of parenting in humans is still sparse, the few studies available to date suggest concordance with a growing body of work in preclinical rodent models. For example, recent functional neuroimaging studies with a normative sample of first-time mothers responding to visual and auditory infant cues from their own and another baby, suggest convergence with preclinical data regarding limbic-hypothalamic-midbrain brain circuits including the amygdala, insula, hypothalamus, ventral tegmental area, striatum and medial prefrontal cortex24,25 Whether or not these circuits are activated in response to infant cues when parents have abused drugs that impact similar circuitry has never before been studied in humans.
The hope is that understanding the interaction between these factors in cocaine exposed mothers will inform us on how to most effectively intervene, at primary through tertiary prevention levels. For example, cocaine exposed mothers with differing adult attachment patterns may respond differently to their infants’ cues, activating different neural systems, which may require different forms of behavioral or pharmacological intervention. Understanding how maternal responses to infant cues are altered in particular groups may help us to formulate more effective parent-child interaction therapies or home visitation programs, assisting new mothers to recognize and appropriately respond to their infants’ cues.26,27 It may also ultimately lead to improved pharmacotherapy for cocaine addicted mothers, targeting specific brain regions or neuroendocrine systems important in addiction—such as oxytocin and the amygdala, the ventral striatum or insula.28,29
Understanding the neuroendocrine mechanisms that are critical to parental investment in infants will facilitate more refined and presumably earlier interventions to help substance abusing parents invest in and provide sufficient and necessary care for their infant despite the earlier compromises they may bring to their parenting role. Earlier and more sustained parental care of infants may also diminish the intergenerational impact of substance abuse and neglect on later risk for addiction and related psychopathology among adolescents and young adults, especially those at risk for initiation of drug use and addiction.
Cocaine Addiction, Maternal Care and Infant Development
Accumulating evidence from both preclinical and clinical laboratories indicate that early failures in parental care have a compromising and enduring impact on the stress regulatory capacities of offspring and on the parenting abilities of those offspring as adults, such that offspring are more vulnerable to stress and hence to a range of psychopathologies including substance abuse. Further, recent work in both preclinical and clinical settings is delineating a specific neural circuitry that is key to early parenting and attachment to offspring. Substance abuse in general, and cocaine abuse specifically, has long been recognized to be associated with many factors that may compromise an adults’ ability to parent, including higher rates of serious psychopathology such as depression and disorders of attachment, related in part to abuse and neglect experiences in childhood. The mechanism of action for cocaine involves similar dopaminergically regulated reward systems in the brain that overlap with those identified as key to the initiation of parenting behaviors in adults (e.g., amygdala, hippocampus, striatum). These same systems are key to reward and stress/fear regulatory systems, the balance between which is considered critical in models of drug abuse and addiction.30–32 Thus, it may be that among the many factors impacting a substance abusing adult’s ability to parent, one may be the disruption or co-optation of basic neural circuitry key to infant attachment by drugs of abuse. While there has been considerable work on the impact of exposure to cocaine during fetal development on long-term child outcome,23,33–35 there has been far less systematic work on the impact of cocaine on adults’ parenting ability. Preclinical studies in models of cocaine abuse suggest that cocaine disrupts basic parenting behaviors and thus, at least a part of the impact of cocaine abuse on child outcome is mediated through disrupted parental care. In the next sections we review work on the impact of parenting behavior on infant development, the impact of substance abuse on parenting behaviors, from both the preclinical and clinical/human perspective, and the emerging work on the neural circuitry of parenting and attachment.
Impact of maternal care on infant development
There has been considerable work characterizing key aspects of maternal behavior in animal models and linking individual variation in these behaviors to offspring development. In general, these findings suggest that maternal behavior in the days and weeks following birth serves to “program” the subsequent maternal behavior of the adult offspring as well as establishing the pups’ level of hypothalamic-pituitary-adrenal responsiveness to stress.36–38 This complex programming also appears to influence aspects of learning and memory. Further, many of the brain regions implicated in these experimental interventions are the same as those identified in the knockout gene and lesioning studies (see below).
Repeated handling of pups in conjunction with brief maternal separations induces more licking and grooming by the rat dams. As adults, the offspring of mothers that exhibited more licking and grooming of pups during the first 10 days of life showed reduced plasma adrenocorticotropic hormone (ACTH) and corticosterone responses to acute restraint stress, as well as increased hippocampal glucocorticoid receptor mRNA expression, and decreased levels of hypothalamic corticotropin-releasing factor (CRF) mRNA.39 Subsequent studies by the same group of investigators have shown that the offspring of these high licking and grooming mothers also show reduced acoustic startle responses, and enhanced spatial learning and memory.40,41 In contrast, repeated handling of pups in conjunction with prolonged maternal separations induces deranged maternal behavior including a reduction in licking and grooming by the rat dams and reduced maternal aggression.40 Similarly, the adult offspring show increased neuroendocrine responses to acute restraint stress and airpuff startle, including elevated levels of paraventricular nucleus (PVN) CRF mRNA and elevated plasma levels of ACTH and corticosterone40,42. These animals also show an increased acoustic startle response, and enhanced anxiety or fearfulness to novel environments.40
Early adoption (3–6 hours after birth) has been found to be associated with increased maternal licking behavior43 and to prevent the prolonged stress-induced secretion of corticosterone evident in early separated offspring that were returned to the nest with their biological mother. Similarly, as adults the early-adopted pups demonstrated lower novelty-induced locomotion and improved recognition performance in a Y-maze compared to the early separated offspring. However, later adoption at either 5 or 10 days resulted in a prolonged stress-induced corticosterone secretion, increased the locomotor response to novelty, and disrupted cognitive performance in the adult offspring. This has been further supported by work on maternal separation of mice which suggests a role for nerve growth factor to mediate the effects of external manipulations on the developing brain.44 It also has been shown that the amount of licking and grooming that a female pup receives in infancy is associated with how much licking and grooming she provides to her offspring as a new mother.37 Low licking and grooming dams could be transformed into high licking and grooming dams by handling. These changes are also passed on to the next generation – that is that the female offspring of the low licking and grooming dams became high licking and grooming mothers if they were either cross-fostered by high licking and grooming dams or if they were handled. The converse was also true, namely that the female offspring of the high licking and grooming dams became low licking and grooming mothers if they were cross-fostered by low licking and grooming dams. These naturally occurring variations in licking, grooming, and arched back nursing have also been associated with the development of individual differences in behavioral responses to novelty in adult offspring. Adult offspring of the low licking, grooming, and arched back nursing mothers’ show increased startle responses, decreased open-field exploration, and longer latencies to eat food provided in a novel environment.37
Furthermore, Francis and coworkers demonstrated that the influence of maternal care on the development of stress reactivity was mediated by changes in gene expression in regions of the brain that regulate stress responses. For example, adult offspring of high licking, grooming, and arched back nursing dams showed increased hippocampal glucocorticoid receptor mRNA expression as well as increased expression of NMDA receptor subunit and brain-derived neurotrophic factor mRNA, and increased cholinergic innervation of the hippocampus.37 In the amygdala there are increased central benzodiazepine receptor levels in the central and basolateral nuclei. In the PVN there is decreased CRF mRNA. These adult pups also show a number of changes in receptor density in the locus ceruleus including: increased alpha2 adrenoreceptors, reduced GABA A receptors, and decreased CRF receptors.45,46 In another recent study, oxytocin receptor binding levels were examined in brain sections from high and low licking, grooming, and arched back nursing animals sacrificed either as non-lactating virgins or during lactation.47 Examination of the medial preoptic area (MPOA) and the intermediate and ventral regions of the lateral septum disclosed that oxytocin receptor levels were significantly higher in lactating females compared with non-lactating females. Lactation-induced increases in oxytocin receptor binding were greater in high compared with low licking, grooming, and arched back nursing females in the BNST and ventral region of the septum. Francis and colleagues suggest, therefore, that variations in maternal behavior in the rat may be reflected in, and influenced by differences in oxytocin receptor levels in the brain.
In sum, the nature of early caregiving experiences can have enduring consequences on individual differences in subsequent maternal behavior, anxiety regulation and patterns of stress response through specific neuropharmacological mechanisms.16 Data from animal studies indicate that the interval surrounding birth is a critical period in the life of the offspring that likely has enduring neurobiological and behavioral consequences.
Impact of cocaine addiction on maternal care and infant development—preclinical models
Reports generally agree that all varieties of cocaine exposure (acute, intermittent, and chronic) disrupt some aspects of maternal behavior, the extent dependent on dose and time of testing and exposure.9,10,48–50 Cocaine has been proposed as mediating maternal behavior/aggression in the early postpartum period in the rat through its effects on the oxytocin (OT) system.51 Only one human study involving mothers who used cocaine during gestation has been published, showing reduced plasma oxytocin levels in cocaine abusing mothers.51 The presence of OT seems critical to initiating the onset of maternal behavior in several mammalian species including the rat and sheep.52–57 Recently a study by Johns and colleagues8 examined maternal behavior following treatment with chronic or intermittent cocaine treatment using cross fostering of prenatally exposed and non-drug exposed offspring. While both drug treatment regimens disrupted maternal behavior towards all offspring, chronic gestational treatment had the greatest impact on early maternal behavior in the dams regardless of the pups’ prenatal condition. Interestingly, however, all dams, both cocaine treated and controls including untreated dams, displayed differential maternal behavior to the chronic cocaine exposed pups. While the strength of this effect varied depending on the behavior examined, differences were clearly apparent. Differences suggest that rat dams regardless of treatment condition, detected and responded to cocaine exposed pups differently. Abnormal behavioral or physical attributes of cocaine exposed offspring may thus make them more vulnerable to neglect or perhaps unable to elicit normal caretaking, and drug-exposure may compromise the adult’s capacity to care for the pup, especially one with abnormal behavioral or physical characteristics.
Impact of cocaine addiction on maternal care and infant development—human models
Maternal substance abuse, more than most other psychiatric or social problem (with the exception of poverty) is the most common factor involved when children are referred to the child welfare system because of suspected parental abuse or neglect.58,59 Observations of mother-child interactions involving mothers with histories of abuse and/or dependence on illicit drugs (e.g., heroin and cocaine) have indicated poor sensitivity, unresponsiveness to children’s emotional cues, and heightened physical provocation and intrusiveness.60,61 Studies reporting drug abusing mothers’ views about parenting have indicated a lack of understanding about basic child development issues and ambivalent feelings about having and keeping children.4,62 As a group, drug-dependent mothers fare worse than non-drug-dependent mothers on a wide range of parenting indices and more frequently lose their children to foster care than non-drug-dependent mothers.63,64 Self-reported behaviors among drug-dependent mothers have also revealed harsh, threatening, overly-involved, authoritarian parenting styles juxtaposed with permissiveness, neglect, poor involvement, low tolerance of child demands and misbehavior, and parent-child role reversals.4,65,66
To date, fewer than 20 studies have examined the interactions between cocaine-using mothers and their infants and young children. The findings are varied in part because of variations in sample size (from 5 to 364 mother-child pairs across the studies), use of a comparison or control group, inclusion of the average amount of maternal cocaine use in the analyses and taking into account postnatal changes in amount of use, place of the assessment and age of the child, and the interactive behaviors assessed. With these caveats in mind, findings across the available studies point to a general disengagement, lack of pleasure in the interaction or attention to the infant, and poor attention to the infant’s cues. Cocaine-using mothers, whose children were six months of age or less, were found to spend more time passively looking at their infant but were more disengaged from the infant in terms of responding to the infant’s cues.67 They were lacking in social initiative and resourcefulness,60 showed less flexibility, engagement, and higher non-contingency in interaction,68 had shorter feeding episodes and were found less attentive to interaction. Non-attentiveness and tendency to interrupt the interaction also increased towards the six month point.3 With young infants, mothers who relapsed back to cocaine use after the birth of the child had more negative interaction behavior as a whole than those who remained drug free following their prenatal use.69 Infants less than six months of age of cocaine-using mothers showed longer periods drowsy, asleep or distressed as newborns,67 showed less enjoyment during play, continued to show negative expressions and slow recovery after short interruption of interaction,70 higher stress to novelty68 and less readiness to interact with the mother at six months compared to three months of age.3 In their dyadic interactions, there was a notable lack of enthusiasm and mutual enjoyment,60 less engagement in dyadic interactions,3 higher dyadic conflict68 and less mutual arousal within the dyad.60
Cocaine-using mothers of children six months to three years of age continued to show less enjoyment and pleasure in interaction,60 less emotional engagement,71 were more intrusive and hostile, showed lower self confidence, and tended to give commands or instructions not appropriate for the child’s developmental age.22 Maternal interaction was found to be most impaired with mothers continuing cocaine use during three-year postnatal follow-up than with mothers who were drug free.22 The children of these mothers more often ignored their mothers’ departures,19 cried less during separation-reunion and showed more avoidance in reunion,20 showed either lower71 or higher negative affect in response to stress,23 less emotional engagement in follow-up play after short interruption71 and diminished ability to persist in task.22
A few studies have explored issues of attachment between cocaine-using mothers and their children. Sensitive maternal behavior in interaction has been considered the crucial component leading to secure attachment in the child, although adult attachment patterns have not been specifically measured. Insecure attachment is a potent risk factor for a child’s later socio-affective and behavioral maladaptation.72,73 Disorganized attachment pattern is considered most worrisome, because it appears to be associated with higher stress, aggression, externalizing problem behavior and psychiatric symptomatology in later childhood.74–76 Findings relating maternal cocaine-use status and child attachment organization have varied. Three studies report insecure and especially disorganized attachment pattern as clearly more prevalent among cocaine-exposed children compared to normative samples, measured at 15 to 18 months of child’s age.77,78
Thus, while not entirely consistent, what seems clear from these few studies is that the combination of maternal depression, early abuse and neglect, unstable early and current attachments, and continued substance use come together to markedly impair an adult’s ability to care for her infant. In turn, the infant and young children may have a biologically conveyed vulnerability to becoming easily overaroused, behaviorally disorganized or withdrawn, more impulsive, and less attentive34,79,80 —a behavioral profile that would be challenging for the most competent of caregivers and surely stressful for a mother who is herself more fragile and easily disorganized.
Neural circuitry of maternal behavior—oxytocin and dopamine systems
Although the central nervous system events that accompany parental care in humans are largely unknown, it is likely that there is a substantial degree of conservation across mammalian species.81 Classical lesion studies done in rodent model systems (rats, mice, and voles) have implicated the medial preoptic area (MPOA) of the hypothalamus, the ventral part of the bed nucleus of the stria terminalis (BNST), and the lateral septum (LS) as regions pivotal for regulation of pup-directed maternal behavior.82–84 Estrogen, prolactin, and oxytocin can act on the MPOA to promote maternal behavior.85–87 Oxytocin is primarily synthesized in the magnocellular secretory neurons of two hypothalamic nuclei, the PVN and the supraoptic nucleus (SON). The PVN and SON project to the posterior pituitary gland. Pituitary release of oxytocin into the bloodstream results in milk ejection during nursing and uterine contraction during labor. It has also been shown that oxytocin fibers, which arise from parvocellular neurons in the PVN, project to areas of the limbic system including the amygdala, BNST, and LS.88
There are several reports that oxytocin facilitates maternal behavior (sensitization) in estrogen-primed nulliparous female rats. Intracerebroventricular (ICV) administration of OT in virgin female rats induces full maternal behavior within minutes.89 Conversely, central injection of an OT antagonist, or a lesion of OT-producing cells in the PVN, suppresses the onset of maternal behavior in postpartum female rats.55,56 However, these manipulations have no effect on maternal behavior in animals permitted several days of postpartum mothering. This result suggests that oxytocin plays an important role in facilitating the onset, rather than the maintenance, of maternal attachment to pups.90 Brain areas that may inhibit maternal behavior in rats have been also identified.91 For example, the vomeronasal and primary olfactory systems have been identified as brain regions that mediate avoidance behavior in virgin female rats exposed to the odor cues of pups.92
Ascending dopaminergic and noradrengeric systems associated with reward pathways also appear to play a crucial role in facilitating maternal behavior.93 For example, rat dams given microinfusions of the neurotoxin 6-hydroxydopamine (6-OHDA) in the ventral tegmental area (VTA) to destroy catecholaminergic neurons during lactation showed a persistent deficit in pup retrieval but were not impaired with respect to nursing, nest building, or maternal aggression.94 There also appears to be an important interaction between dopaminergic neurons and oxytocin pathways.95 Specifically, pup retrieval and assuming a nursing posture over pups were blocked in parturient dams by infusions of an oxytocin antagonist into either the VTA or MPOA.96
In summary, the initiation and maintenance of maternal behavior involves a specific neural circuit based in reward, affiliation and stress response systems (e.g., striatum, amygdala). With pregnancy or with repeated exposure to pups, structural and molecular changes occur, most of which are not yet completely understood, in specific limbic, hypothalamic, and midbrain regions that reflect, in part, an adaptation to the various homeostatic demands associated with maternal care. Many of the same cell groups implicated in the control of maternal behavior have been implicated in the control of ingestive (eating and drinking) behavior, thermoregulatory (energy homeostasis), social (defensive and sexual) behaviors, as well as general exploratory or foraging behaviors (with locomotor and orienting components) that are required for obtaining any particular goal object. Many of these same structures are also intimately involved in stress response. Swanson has conceptualized this set of limbic, hypothalamic, and midbrain nuclei as being the “behavioral control column” that is voluntarily regulated by cerebral projections.97 Consistent with this formulation, it is readily apparent that motherhood presents a major homeostatic challenge within each of these behavioral domains.
Neural circuitry of maternal behavior—human studies using functional brain imaging
Functional brain imaging studies examining brain responses to emotionally charged infant stimuli in healthy parents are just emerging to provide data regarding the neural substrates of normal parenting.98 Lorberbaum and colleagues99 provided the first work in this area using baby cries as stimuli and presenting these stimuli to mothers in a functional imaging paradigm. Building on the thalamocingulate theory of maternal behavior in animals developed by MacLean (1990), Lorberbaum predicted that baby cries would selectively activate cingulate, thalamus, medial, and orbitofrontal prefrontal cortex. Mothers who were less than 3.5 months postpartum and were exposed to 30 seconds of a standard baby cry versus white noise stimuli99 showed increased activity in anterior cingulate and right medial prefrontal cortex. In the follow-up study of brain activity in breastfeeding first-time mothers 4–8 weeks postpartum listening to standard baby cry compared with intensity and pattern matched white noise,99 all of the regions activated were those known to be important for rodent maternal behavior including midbrain, hypothalamus, striatum and septal regions.82,100 Other groups are now finding similar patterns of activation in thalamo-cortical-basal ganglia based circuits in mothers responding to infant cries.101–103
In addition to baby cry stimuli, several groups, including our own, are using baby visual stimuli.2,102–111 We demonstrated that dopaminergic reward pathways are activated when mothers view pictures of their own baby’s happy—but not sad—faces.112 Hypothesizing that reward and oxytocin circuits, which are important for aspects of romantic love, might also be involved in maternal love and using photographs of familiar and unfamiliar infants, Bartels and colleagues107 reported activations in anterior cingulate, insula, basal ganglia (striatum) and midbrain (periaqueductal gray), regions potentially mediating the emotionally rewarding aspects of maternal behavior, and a decrease in activity in areas important for negative emotions, avoidance behavior and social assessment. This may suggest a push-pull mechanism for maternal behavior in which child stimuli activate reward and shut down avoidance circuits.107 A similar study comparing parents and non-parents responding to infant pictures reported bilateral orbitofrontal cortex activations that were correlated with ratings of pleasant mood.109 Another study, using video of infant images, reported activations in amygdala, temporal pole, & occipital regions.110
Thus, it appears that there may be significant differences between parental responses to baby signals across sensory modalities. Preliminary pilot data suggests that these maternal brain responses to infant face cues may differ significantly in mothers with chronic cocaine exposure, particularly within the prefrontal cortex.113
Conclusion
Understanding basic neural mechanisms for failure in early parenting in substance abusing adults facilitates more refined and presumably earlier interventions during pregnancy and in the immediate postpartum period to help substance abusing parents invest in and provide sufficient and necessary care for their infant despite the earlier compromises they may bring to their parenting role. Elucidating these neural mechanisms in humans also has implications for understanding, first, how early childhood experience impacts an adult’s ability to be a caring and empathic parent, a failure that may be perpetuated across generations, and, second, how these failures in early parental care increase the intergenerational risk especially for depression, disorders of attachment, and addiction.
Acknowledgments
The work is also supported by the following grants: NICHD Grant K23 HD43097 (L.S.), GCRC MO1RR00188 (L.S.), the Irving Harris Foundation (L.C.M.), NIDA Grant RO1 DA-06025 (L.C.M.), a Research Scientist Development Award to L.C.M. (K05 DA020091), and by an award from the Yale Center for Clinical Investigation (L.C.M.).
References
- 1.STRATHEARN L, LI J, FONAGY P, MONTAGUE PR. What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.STRATHEARN L. Exploring the Neurobiology of Attachment. In: Mayes LC, Fonagy P, Target M, editors. Developmental Science and Psychoanalysis: Integration and Innovation. Karnac Press; London: 2006. pp. 117–130. [Google Scholar]
- 3.MAYES L, FELDMAN R, GRANGER R, HAYNES O, BORNSTEIN MH, SCHOTTENFELD R. The effects of polydrug use with and without cocaine on mother-infant interaction at 3 and 6 months. Infant Behavior and Development. 1997;20:489–502. [Google Scholar]
- 4.MAYES L, TRUMAN S. Substance abuse and parenting. Vol. 4. Social conditions and applied parenting. In: Bornstein M, editor. Handbook of parenting. Lawrence Erlbaum; Mahwah, NJ: 2002. pp. 329–359. [Google Scholar]
- 5.PORTER LS, PORTER BO. A blended infant massage-parenting enhancement program for recovering substance-abusing mothers. Pediatr Nurs. 2004;30:363–72. 401. [PubMed] [Google Scholar]
- 6.FEBO M, FERRIS CF. Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience. 2007;148:400–412. doi: 10.1016/j.neuroscience.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.JOHNS JM, JOYNER PW, MCMURRAY MS, ELLIOTT DL, HOFLER VE, MIDDLETON CL, KNUPP K, GREENHILL KW, LOMAS LM, WALKER CH. The effects of dopaminergic/serotonergic reuptake inhibition on maternal behavior, maternal aggression, and oxytocin in the rat. Pharmacol Biochem Behav. 2005;81:769–785. doi: 10.1016/j.pbb.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.JOHNS JM, ELLIOTT DL, HOFLER VE, JOYNER PW, MCMURRAY MS, JARRETT TM, HASLUP AM, MIDDLETON CL, ELLIOTT JC, WALKER CH. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behav Neurosci. 2005;119:1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.JOHNS JM, NELSON CJ, METER KE, LUBIN DA, COUCH CD, AYERS A, WALKER CH. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1998;20:525–532. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.JOHNS JM, NOONAN LR, ZIMMERMAN LI, LI L, PEDERSEN CA. Effects of chronic and acute cocaine on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav Neurosci. 1994;108:107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- 11.SARNYAI Z, BIRO E, BABARCZY E, VECSERNYES M, LACZI F, SZABO G, KRIVAN M, KOVACS GL, TELEGDY G. Oxytocin modulates behavioural adaptation to repeated treatment with cocaine in rats. Neuropharmacology. 1992;31:593–598. doi: 10.1016/0028-3908(92)90192-r. [DOI] [PubMed] [Google Scholar]
- 12.ELLIOTT JC, LUBIN DA, WALKER CH, JOHNS JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35:127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- 13.MCGREGOR IS, CALLAGHAN PD, HUNT GE. From ultrasocial to ntisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DUBE SR, FELITTI VJ, DONG M, CHAPMAN DP, GILES WH, ANDA RF. Childhood Abuse, Neglect, and Household Dysfunction and the Risk of Illicit Drug Use: The Adverse Childhood Experiences Study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 15.EGELAND B, BREITENBUCKER M, ROSENBERG D. Prospective study of the significance of life stress in etiology of child abuse. J Consult Clin Psychol. 1980;48:195. doi: 10.1037//0022-006x.48.2.195. [DOI] [PubMed] [Google Scholar]
- 16.WEAVER IC, CERVONI N, CHAMPAGNE FA, D’ALESSIO AC, SHARMA S, SECKL JR, DYMOV S, SZYF M, MEANEY MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 17.CHAMPAGNE FA, WEAVER ICG, DIORIO J, SHARMA S, MEANEY MJ. Natural Variations in Maternal Care are Associated with Estrogen Receptor Alpha Expression and Estrogen Sensitivity in the Medial Preoptic Area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- 18.CHAMPAGNE FA, CHRETIEN P, STEVENSON CW, ZHANG TY, GRATTON A, MEANEY MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UKEJE I, BENDERSKY M, LEWIS M. Mother-infant interaction at 12 months in prenatally cocaine-exposed children. American Journal of Drug and Alcohol Abuse. 2001;27:203–224. doi: 10.1081/ada-100103706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.BEEGHLY M, FRANK DA, ROSE-JACOBS R, CABRAL H, TRONICK EZ. Level of prenatal cocaine exposure and infant-caregiver attachment behavior. Neurotoxicology and Teratology. 2003;25:23–38. doi: 10.1016/s0892-0362(02)00323-9. [DOI] [PubMed] [Google Scholar]
- 21.MOLITOR A, MAYES LC, WARD A. Emotion regulation behavior during a separation procedure in 18-month-old children of mothers using cocaine and other drugs. Dev Psychopathol. 2003;15:39–54. doi: 10.1017/s0954579403000038. [DOI] [PubMed] [Google Scholar]
- 22.JOHNSON AL, MORROW CE, ACCORNERO VH, LIHUA X, ANTHONY JC, BANDSTRA ES. Maternal cocaine use: estimated effects on mother-child play interactions in the preschool period. Developmental and Behavioral Pediatrics. 2002;23:191–202. doi: 10.1097/00004703-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EIDEN RD, LEWIS A, CROFF S, YOUNG E. Maternal cocaine use and infant behavior. Infancy. 2002;3:77–96. [Google Scholar]
- 24.SWAIN JE, LORBERBAUM JP, KOSE S, STRATHEARN L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol & Psychiat. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.STRATHEARN L, LI J, FONAGY P, MONTAGUE PR. What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PAJULO M, SUCHMAN N, KALLAND M, MAYES L. Enhancing the effectiveness of residential treatment for substance abusing pregnant and parenting women: Focus on maternal reflective functioning and mother-child relationship. Infant Mental Health Journal. 2006;27:448–465. doi: 10.1002/imhj.20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.OLDS DL, ECKENRODE J, HENDERSON CR, KITZMAN H, POWERS J, COLE R, SIDORA K, MORRIS P, PETTITT LM, LUCKEY D. Long-term effects of home visitation on maternal life course and child abuse and neglect. JAMA. 1997;278:637–643. [PubMed] [Google Scholar]
- 28.NAQVI NH, RUDRAUF D, DAMASIO H, BECHARA A. Damage to the Insula Disrupts Addiction to Cigarette Smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KIRSCH P, ESSLINGER C, CHEN Q, MIER D, LIS S, SIDDHANTI S, GRUPPE H, MATTAY VS, GALLHOFER B, MEYER-LINDENBERG A. Oxytocin Modulates Neural Circuitry for Social Cognition and Fear in Humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.BAUMEISTER RF. Ego depletion and self-regulation failure: A resource model of self-control. Alcoholism: Clinical and Experimental Research. 2003;27:281–284. doi: 10.1097/01.ALC.0000060879.61384.A4. [DOI] [PubMed] [Google Scholar]
- 31.BAUMEISTER RF, HEATHERTON TF, TICE DM. Losing control: How and why people fail at self-regulation. Academic Press; San Diego, CA: 1994. [Google Scholar]
- 32.BRADY KT, SINHA R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- 33.MAYES LC. Developing brain and in-utero cocaine exposure: Effects on neural ontogeny. Development and Psychopathology. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- 34.MAYES LC, FAHY T. Prenatal drug exposure and cognitive development. In: Sternberg RJ, Grigorenko EL, editors. Environmental Effects on Cognitive Abilities. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. pp. 189–220. [Google Scholar]
- 35.LESTER BM, BOUKYDIS CZ, TWOMEY J. Maternal substance abuse and child outcome. In: Zeanah CH, editor. Handbook of infant mental health. Guilford Press; New York: 2000. pp. 161–175. [Google Scholar]
- 36.DENENBERG VH, ROSENBERG KM, PASCHKE R, ZARROW MX. Mice reared with rat aunts: Effects on plasma corticosterone and open-field activity. Nature. 1969;221:73–74. doi: 10.1038/221073a0. [DOI] [PubMed] [Google Scholar]
- 37.FRANCIS D, DIORIO J, LIU D, MEANEY MJ. Non-genomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 38.LEVINE S. Psychosocial factors in growth and development. In: Levi L, editor. Society, stress and disease. Oxford University Press; London: 1975. pp. 43–50. [Google Scholar]
- 39.PLOTSKY PM, MEANEY MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 40.LADD CO, HUOT RL, THRIVIKRAMAN KV, NEMEROFF CB, MEANEY MJ, PLOTSKY PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 41.LIU D, DIORIO J, DAY JC, FRANCIS DD, MEANEY MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 42.LIU D, DIORIO J, TANNENBAUM B, CALDJI C, FRANCIS D, FREEDMAN A, SHARMA S, PEARSON D, PLOTSKY PM, MEANEY MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 43.BARBAZANGES A, VALLEE M, MAYO W, DAY J, SIMON H, LE MOAL M, MACCARI S. Early and later adoptions have different long-term effects on male rat offspring. J Neurosci. 1996;16:7783–7790. doi: 10.1523/JNEUROSCI.16-23-07783.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CIRULLI F, BERRY A, ALLEVA E. Early disruption of the mother-infant relationship: effects on brain plasticity and implications for psychopathology. Neuroscience & Biobehavioral Reviews. 2003;27:73–82. doi: 10.1016/s0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 45.CALDJI C, FRANCIS D, SHARMA S, PLOTSKY PM, MEANEY MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 46.CALDJI C, TANNENBAUM B, SHARMA S, FRANCIS D, PLOTSKY PM, MEANEY MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci US A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FRANCIS DD, CHAMPAGNE FC, MEANEY MJ. Variations in maternal behavior are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 48.KINSLEY CH, TURCO D, BAUER A, BEVERLY M, WELLMAN J, GRAHAM AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacology Biochemistr. 1994;47:857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 49.HEYSER CJ, MOLINA VA, SPEAR LP. A fostering study of the effects of prenatal cocaine exposure: I. Maternal behaviors. Neurotoxicol Teratol. 1992;14:415–421. doi: 10.1016/0892-0362(92)90052-c. [DOI] [PubMed] [Google Scholar]
- 50.PEEKE HVS, DARK KA, SALAMY A, SALFI M, SHAH SN. Cocaine exposure prebreeding to weaning: Maternal and offspring effects. Pharmacol Biochem Behav. 1994;48:403–410. doi: 10.1016/0091-3057(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 51.LIGHT KC, GREWEN KM, AMICO JA, BOCCIA M, BROWNLEY KA, JOHNS JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29:1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.KENDRICK KM, KEVERNE EB, BALDWIN BA. Intracerebroventricular oxytocin stimulates maternal behavior in the sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- 53.PEDERSEN CA, PRANGE AJ. Evidence that central oxytocin plays a role in activation of maternal behavior. In: Krasnegor NA, Blass EM, Hofer MA, Smotherland WP, editors. Perinatal Development: A Psychobiological Perspective. Academic Press; Orlando: 1987. pp. 299–320. [Google Scholar]
- 54.PEDERSEN CA, ASCHER JA, MONROE YL, PRANGE AJ. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 55.VAN LEENGOED E, KERKER E, SWANSON HH. Inhibition of postpartum maternal behavior in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- 56.INSEL TR, HARBAUGH CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol Behav. 1989;45:1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 57.NELSON CJ, METER KE, WALKER CH, AYERS AA, JOHNS JM. A dose-response study of chronic cocaine on maternal behavior in rats. Neurotoxicol Teratol. 1998;20:657–660. doi: 10.1016/s0892-0362(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 58.CHILD WELFARE LEAGUE OF AMERICA. Alcohol and Other Drug Survey of State Child Welfare Agencies. Washington, DC: Child Welfare League of America; 1998. [Google Scholar]
- 59.DEPARTMENT OF HEALTH AND HUMAN SERVICES. Blending perspectives and building common ground: a report to congress on substance abuse and child protection. Washington, DC: Department of Health and Human Services; 1999. [Google Scholar]
- 60.BURNS KA, CHETHIK L, BURNS WJ, CLARK R. The early relationship of drug abusing mothers and their infants: An assessment at eight to twelve months of age. Journal of Clinical Psychology. 1997;53:279–287. doi: 10.1002/(sici)1097-4679(199704)53:3<279::aid-jclp11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 61.HANS LL, BERNSTEIN VJ, HENSON LG. The role of psychopathology in the parenting of drug-dependent women. Development & Psychopathology. 1999;11:957–977. doi: 10.1017/s0954579499002400. [DOI] [PubMed] [Google Scholar]
- 62.MURPHY S, ROSENBAUM M. Pregnant women on drugs: Combating stereotypes and stigma. Rutgers University Press; New Brunswick, NJ: 1999. [Google Scholar]
- 63.CHAFFIN M, KELLEHER K, HOLLENBERG J. Onset of physical abuse and neglect: Psychiatric, substance abuse, and social risk factors from prospective community data. Child Abuse and Neglect. 1996;20:191–203. doi: 10.1016/s0145-2134(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 64.MAYES LC, BORNSTEIN MH, CHAWARSKA K, HAYNES OM, GRANGER RH. Impaired regulation of arousal in three-month-old infants Exposed prenatally to cocaine and other drugs. Development and Psychopathology. 1996;8:29–42. [Google Scholar]
- 65.HARMER ALM, SANDERSON J, MERTIN P. Influence of negative childhood experiences on psychological functioning, social support, and parenting for mothers recovering from addiction. Child Abuse and Neglect. 1999;23:421–433. doi: 10.1016/s0145-2134(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 66.SUCHMAN NE, LUTHAR SS. Maternal addiction, child maladjustment, and sociodemographic risks: Implications for parenting behaviors. Addiction. 2000;95:1417–1428. doi: 10.1046/j.1360-0443.2000.959141711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.GOTTWALD SR, THURMAN SK. The effects of prenatal cocaine exposure on mother-infant interaction and infant arousal in the newborn period. Topics in Early Childhood Special Education. 1994;14:217–234. [Google Scholar]
- 68.EIDEN R. Maternal substance use and mother-infant feeding interactions. Infant Mental Health Journal. 2001;22:497–511. [Google Scholar]
- 69.BLACKWELL P, KIRKHART K, SCHMITT D, KAISER M. Cocaine/polydrug-affected dyads: implications for infant cognitive development and mother-infant interaction during the first six postnatal months. Journal of Applied Develomental Psychology. 1998;19:235–248. [Google Scholar]
- 70.BENDERSKY M, LEWIS M. Arousal modulation in cocaine-exposed infants. Developmental Psychology. 1998;34:555–564. [PMC free article] [PubMed] [Google Scholar]
- 71.MOLITOR M, MAYES L, WARD A. Emotion regulation behavior during a separation procedure in 18-month old children of mothers using cocaine and other drugs. Development and Psychopathology. 2003;15:39–45. doi: 10.1017/s0954579403000038. [DOI] [PubMed] [Google Scholar]
- 72.KOCHANSKA G. Emotional development in children with different attachment histories: the first three years. Child Development. 2001;72:474–490. doi: 10.1111/1467-8624.00291. [DOI] [PubMed] [Google Scholar]
- 73.BELSKY J, PASCO FEARON RM. Infant-mother attachment security, contextual risk, and early development: a moderational analysis. Development and Psychopathology. 2002;14:293–310. doi: 10.1017/s0954579402002067. [DOI] [PubMed] [Google Scholar]
- 74.HERTSGAARD L, GUNNAR M, ERICKSON MF, NACHMIAS M. Adrenocortical responses to the Strange Situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66:1100–1106. [PubMed] [Google Scholar]
- 75.CARLSON EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Development. 1998;69:1107–1128. [PubMed] [Google Scholar]
- 76.SHAW DS, OWENS EB, VONDRA JI, KEENAN K. Early risk factors and pathways in the development of early disruptive behavior problems. Development and Psychopathology. 1996;8:679–699. [Google Scholar]
- 77.RODNING C, BECKWITH L, HOWARD J. Quality of attachment and home environments in children prenatally exposed to PCP and cocaine. Special Issue: Attachment and developmental psychopathology. Development and Psychopathology. 1991;3:351–366. [Google Scholar]
- 78.ESPINOSA M, BECKWITH L, HOWARD J, TYLER R, SWANSON K. Maternal psychopathology and attachment in toddlers of heavy cocaine-using mothers. Infant Mental Health Journal. 2001;22:316–333. [Google Scholar]
- 79.MAYES LC. A developmental perspective on the regulation of arousal states. Seminars in Perinatology. 2000;24:267–279. doi: 10.1053/sper.2000.9121. [DOI] [PubMed] [Google Scholar]
- 80.MAYES LC, WARD A. Principles of neurobehavioral teratology. In: Cicchetti D, Walker E, editors. Neurodevelopmental mechanisms in psychopathology. Cambridge University Press; New York, NY: 2003. pp. 3–33. [Google Scholar]
- 81.FLEMING AS, STEINER M, CORTER C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm Behav. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- 82.LECKMAN JF, HERMAN A. Maternal behavior and developmental psychopathology. Biological Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- 83.NUMAN M. Maternal behavior. In: Knobil E, Neill JF, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 221–301. [Google Scholar]
- 84.NUMAN M, SHEEHAN TP. Neuroanatomical circuitry for mammalian maternal behavior. Annals of New York Academy of Science. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- 85.BRIDGES RS, NUMAN M, RONSHEIM PM, MANN PE, LUPINI CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proceedings of the National Academy of Science. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.NUMAN M, ROSENBLATT JS, KIMINSARUK BR. Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol. 1997;91:146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- 87.PEDERSEN CA, CALDWELL JD, WALKER C, AYERS G, MASON GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 88.SOFRONIEW MV, WEINDL A. Central nervous system distribution of vasporessin, oxytocin, and neurophysin. In: Martinex JL, Jensen RA, Messing RB, Rigter H, McGraugh JL, editors. Endogenous Peptides and Learning and Memory Processes. Academic Press; New York: 1981. [Google Scholar]
- 89.PEDERSEN CA, PRANGE AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci US A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.PEDERSEN CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann NY Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 91.SHEEHAN TP, CIRRITO J, NUMAN MJ, NUMAN M. Using c-Fos immunocyto-chemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav Neurosci. 2000;114:337–352. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- 92.FLEMING AS, VACCARINO F, LUEBKE C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731–743. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- 93.KOOB GF, LE MOAL M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 94.HANSEN S, HARTHON C, WALLIN E, LOFBERG L, SVENSSON K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxy-dopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105:588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- 95.LIU Y, WANG ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 96.PEDERSEN CA, CALDWELL JD, WALKER C, AYERS G, MASON GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 97.SWANSON LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 98.SQUIRE S, STEIN A. Functional MRI and parental responsiveness: a new avenue into parental psychopathology and early parent-child interactions? Br J Psychiatry. 2003;183:481–483. doi: 10.1192/bjp.183.6.481. [DOI] [PubMed] [Google Scholar]
- 99.LORBERBAUM JP, NEWMAN JD, HORWITZ AR, DUBNO JR, LYDIARD RB, HAMNER MB, BOHNING DE, GEORGE MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 100.NUMAN M, SHEEHAN TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann NY Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- 101.SEIFRITZ E, ESPOSITO F, NEUHOFF JG, LUTHI A, MUSTOVIC H, DAMMANN G, VON BU, RADUE EW, CIRILLO S, TEDESCHI G, DI SF. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 102.SWAIN J, LECKMAN JF, MAYES LC, FELDMAN R, CONSTABLE RT, SCHULTZ R. Neural substrates and psychology of human parent-infant attachment in the post-partum. Society for Biological Psychiatry 59th Annual Meeting; Society for Biological Psychiatry; 2004. [Google Scholar]
- 103.SWAIN JE, LECKMAN JF, MAYES LC, FELDMAN R, CONSTABLE RT, SCHULTZ RT. The neural circuitry of human parent-infant attachment in the early postpartum period. American College of Neuropsychopharmacology 42nd Annual Meeting; American College of Neuropsychopharmacology; 2003. [Google Scholar]
- 104.INSEL TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 105.STRATHEARN L, LI J, MONTAGUE PR. An fMRI study of maternal mentalization: Having the baby’s mind in mind. NeuroImage. 2005;26(Supp 1):S25. [Google Scholar]
- 106.STRATHEARN L, MCCLURE SM. 2002 Abstract Viewer/Itinerary Planner, Program No. 517.5. Online. Washington, DC: Society for Neuroscience; 2002. A functional MRI study of maternal responses of infant facial cues. Annual Scientific Meeting of the Society for Neuroscience. [Google Scholar]
- 107.BARTELS A, ZEKI S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 108.MCCLURE E, MONK C, NELSON E, ZARAHN E, LEIBENLUFT E, BILDER R, CHARNEY D, ERNST M, PINE D. A developmental examination of gender differences in brain engagement during evaluation of threat. Biological Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 109.NITSCHKE JB, NELSON EE, RUSCH BD, FOX AS, OAKES TR, DAVIDSON RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 110.RANOTE S, ELLIOTT R, ABEL KM, MITCHELL R, DEAKIN JF, APPLEBY L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- 111.NORIUCHI M, KIKUCHI Y, SENOO A. The Functional Neuroanatomy of Maternal Love: Mother’s Response to Infant’s Attachment Behaviors. Biological Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 112.STRATHEARN L, LI J, FONAGY P, MONTAGUE PR. What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.STRATHEARN L, KOSTEN TR. Does chronic cocaine use affect a mother’s brain response to baby face cues? A pilot fMRI study. The College on Problems of Drug Dependence 70th Annual Scientific Meeting.2008. [Google Scholar]