Abstract
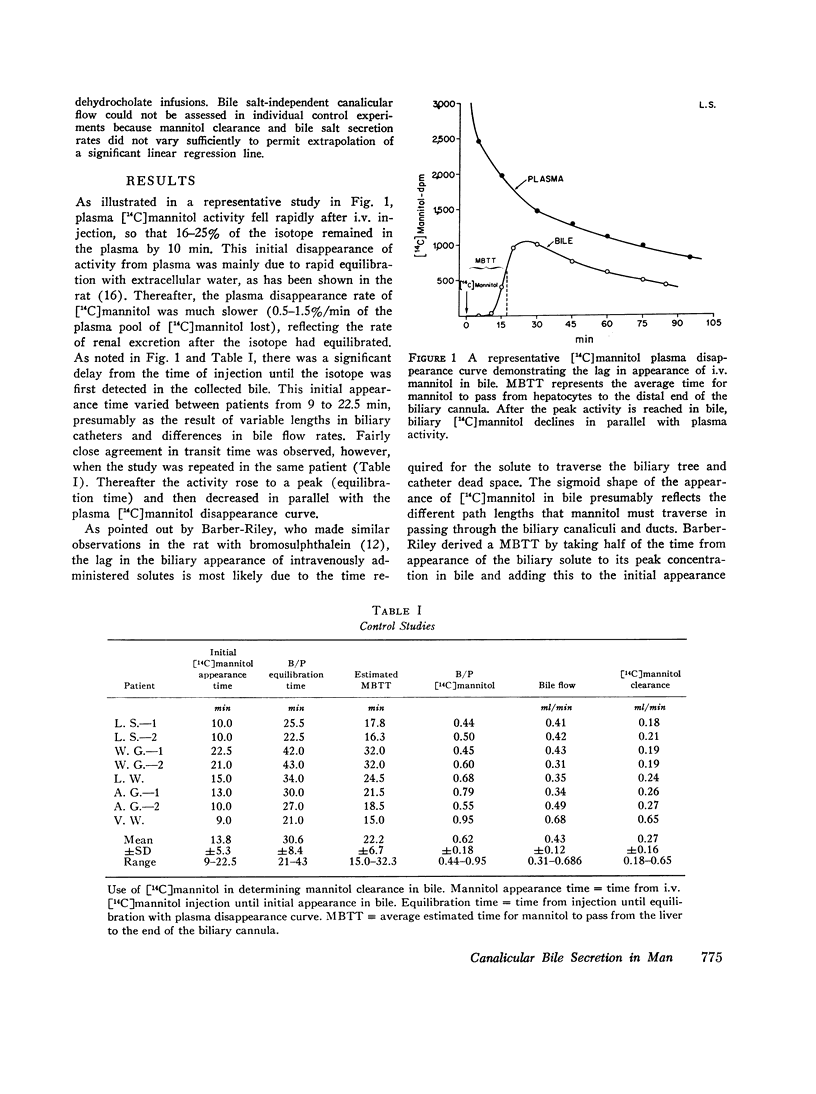
[14C]Mannitol was administered i.v. as a bolus injection to five postcholecystectomy patients with indwelling T-tubes and re-established enterohepatic circulations to evaluate the biliary clearance of [14C]mannitol as a means of estimating canalicular bile flow in man. [14C]Mannitol appeared in collections of bile 9-22.5 min after intravenous injection, rose to a peak, and thereafter paralleled the plasma [14C]mannitol disappearance curve. Bile-plasma [14C]mannitol ratios and [14C]mannitol clearances were determined during control and choleretic periods after correction of the bile [14C]mannitol points for the transit time of a given sample. After i.v. injection of sodium dehydrocholate in five studies, bile flow and mannitol clearance increased proportionately. However, when ductular secretion was stimulated with an i.v. bolus of secretin in three other studies, [14C]mannitol clearance remained essentially unchanged, indicating that [14C]mannitol entered bile at the level of the hepatocyte and could be utilized as a marker of canalicular flow in man.
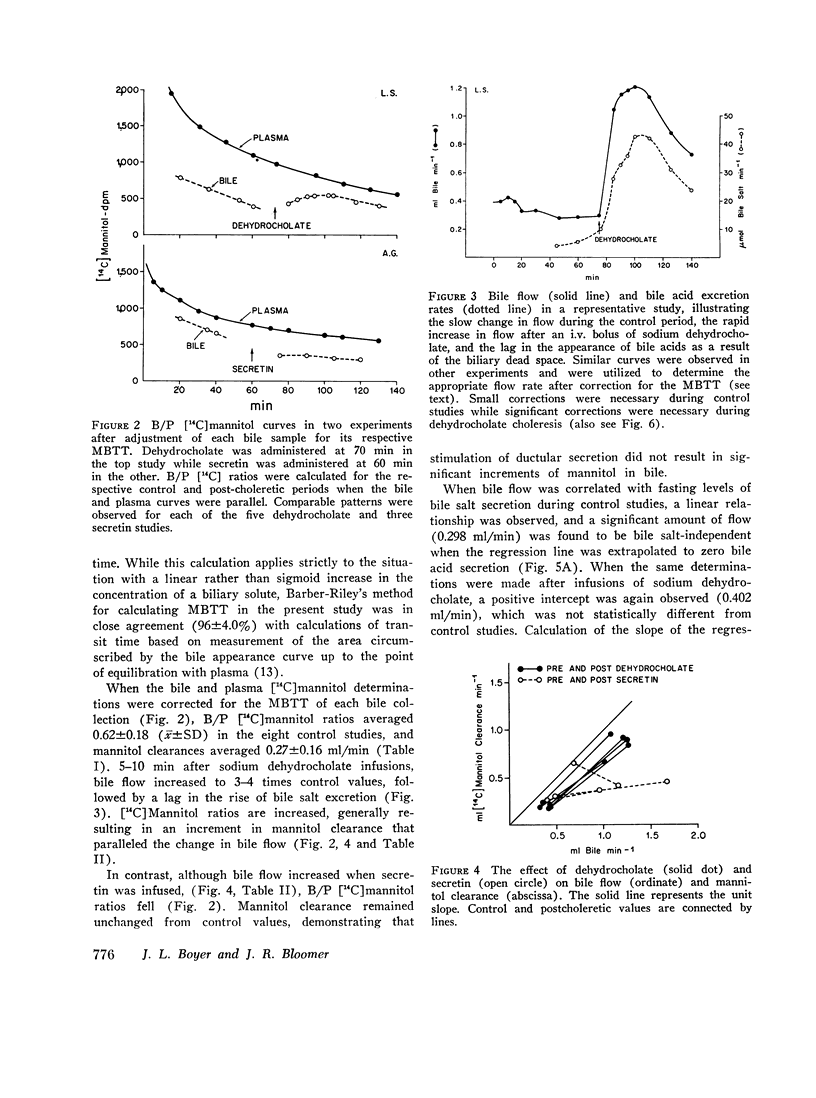
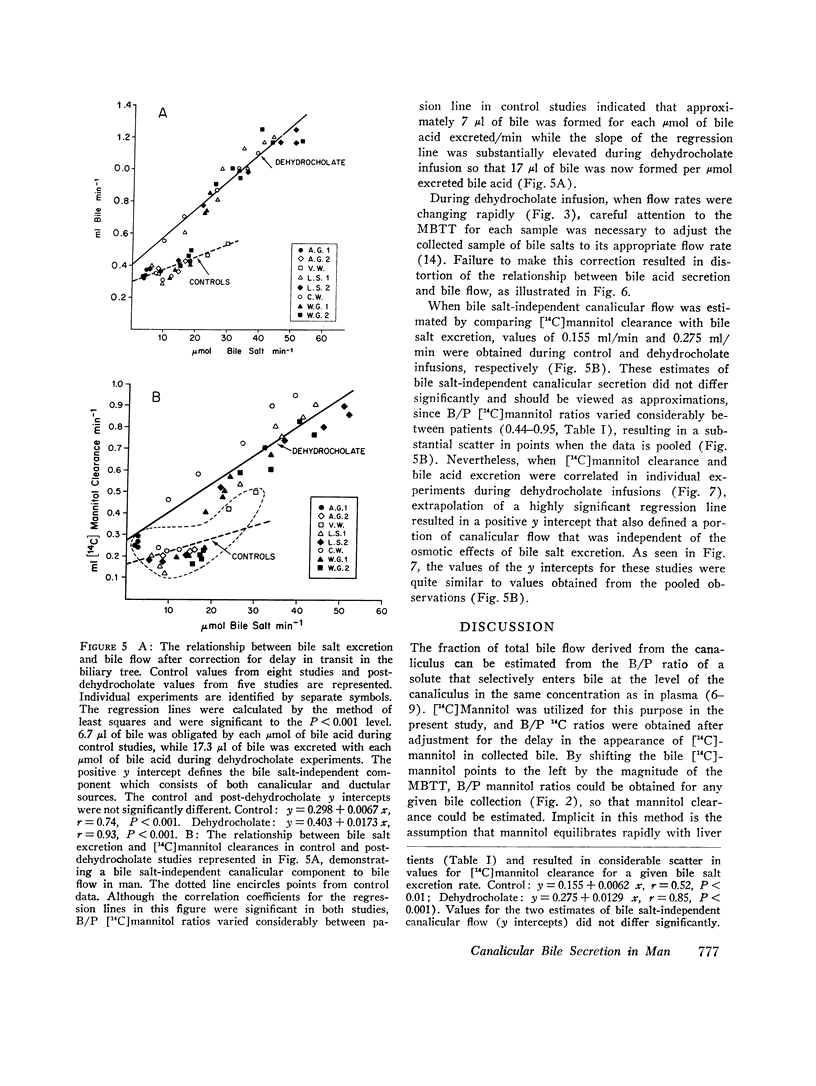
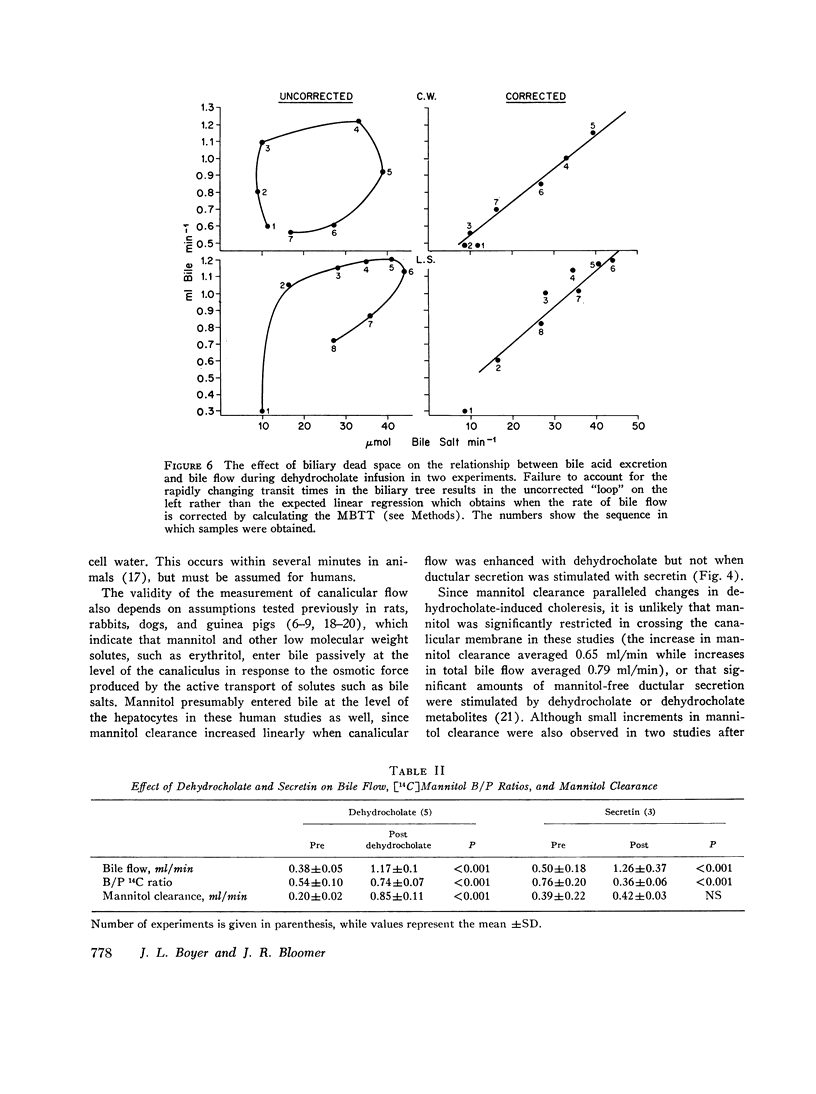
During control studies, when bile drained spontaneously from biliary fistulae in fasting patients, bileplasma [14C]mannitol ratios averaged 0.62±0.18 and canalicular flow, as estimated by [14C]mannitol clearance. (0.27±0.16 ml/min) accounted for 44-95% of total bile production (0.43±0.12 ml/min). When the rate of bile flow was plotted as a function of bile salt excretion after correction for the effects of biliary dead space, linear regression analysis revealed that approximately 7 μl of bile were secreted with each μmol of bile salt. Estimates of bile salt-independent canalicular flow accounted for at least one-third of the estimated 24-h bile production (604 ml) in these patients, indicating that this fraction of canalicular flow is a significant source of bile secretion in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER-RILEY G. MEASUREMENT OF CAPACITY OF BILIARY TREE IN RATS. Am J Physiol. 1963 Dec;205:1122–1126. doi: 10.1152/ajplegacy.1963.205.6.1122. [DOI] [PubMed] [Google Scholar]
- Berthelot P., Erlinger S., Dhumeaux D., Preaux A. M. Mechanism of phenobarbital-induced hypercholeresis in the rat. Am J Physiol. 1970 Sep;219(3):809–813. doi: 10.1152/ajplegacy.1970.219.3.809. [DOI] [PubMed] [Google Scholar]
- Boyer J. L. Canalicular bile formation in the isolated perfused rat liver. Am J Physiol. 1971 Oct;221(4):1156–1163. doi: 10.1152/ajplegacy.1971.221.4.1156. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Klatskin G. Canalicular bile flow and bile secretory pressure. Evidence for a non-bile salt dependent fraction in the isolated perfused rat liver. Gastroenterology. 1970 Dec;59(6):853–859. [PubMed] [Google Scholar]
- Boyer J. L., Scheig R. L., Klatskin G. The effect of sodium taurocholate on the hepatic metabolism of sulfobromophthalein sodium (BSP). The role of bile flow. J Clin Invest. 1970 Feb;49(2):206–215. doi: 10.1172/JCI106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinger S., Dhumeaux D., Berthelot P., Dumont M. Effect of inhibitors of sodium transport on bile formation in the rabbit. Am J Physiol. 1970 Aug;219(2):416–422. doi: 10.1152/ajplegacy.1970.219.2.416. [DOI] [PubMed] [Google Scholar]
- Erlinger S. Physiology of bile flow. Prog Liver Dis. 1972;4:63–82. [PubMed] [Google Scholar]
- Forker E. L. Bile formation in guinea pigs: analysis with inert solutes of graded molecular radius. Am J Physiol. 1968 Jul;215(1):56–62. doi: 10.1152/ajplegacy.1968.215.1.56. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Hepatocellular uptake of inulin, sucrose, and mannitol in rats. Am J Physiol. 1970 Dec;219(6):1568–1573. doi: 10.1152/ajplegacy.1970.219.6.1568. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Hicklin T., Sornson H. The clearance of mannitol and erythritol in rat bile. Proc Soc Exp Biol Med. 1967 Oct;126(1):115–119. doi: 10.3181/00379727-126-32380. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967 Jul;46(7):1189–1195. doi: 10.1172/JCI105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio J. J., Valdivieso V. D. Studies on the mechanism of the ethynylestradiol impairment of bile flow and bile salt excretion in the rat. Gastroenterology. 1971 Sep;61(3):339–344. [PubMed] [Google Scholar]
- Guzelian P., Boyer J. L. Glucose reabsorption from bile. Evidence for a biliohepatic circulation. J Clin Invest. 1974 Feb;53(2):526–535. doi: 10.1172/JCI107586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. E., Schoenfield L. J. Cholestasis induced by sodium taurolithocholate in isolated hamster liver. J Clin Invest. 1971 Nov;50(11):2305–2312. doi: 10.1172/JCI106728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D. Does bile acid secretion determine canalicular bile production in rats? Am J Physiol. 1971 Mar;220(3):667–673. doi: 10.1152/ajplegacy.1971.220.3.667. [DOI] [PubMed] [Google Scholar]
- LINDSTEDT S. The turnover of cholic acid in man: bile acids and steroids. Acta Physiol Scand. 1957 Sep 17;40(1):1–9. doi: 10.1111/j.1748-1716.1957.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Macarol V., Morris T. Q., Baker K. J., Bradley S. E. Hydrocortisone choleresis in the dog. J Clin Invest. 1970 Sep;49(9):1714–1723. doi: 10.1172/JCI106389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf W. H., Kitano M. The early disappearance of extracellular tracers from plasma after intravenous injection. Proc Soc Exp Biol Med. 1972 Dec;141(3):940–943. doi: 10.3181/00379727-141-36906. [DOI] [PubMed] [Google Scholar]
- Preisig R., Bucher H., Stirnemann H., Tauber J. Postoperative choleresis following bile duct obstruction in man. Rev Fr Etud Clin Biol. 1969 Feb;14(2):151–158. [PubMed] [Google Scholar]
- Scherstén T., Nilsson S., Cahlin E., Filipson M., Brodin-Persson G. Relationship between the biliary excretion of bile acids and the excretion of water, lecithin, and cholesterol in man. Eur J Clin Invest. 1971 Jan;1(4):242–247. doi: 10.1111/eci.1971.1.4.242. [DOI] [PubMed] [Google Scholar]
- Soloway R. D., Clark M. L., Powell K. M., Senior J. R., Brooks F. P. Effects of secretin and bile salt infusions on canine bile composition and flow. Am J Physiol. 1972 Mar;222(3):681–686. doi: 10.1152/ajplegacy.1972.222.3.681. [DOI] [PubMed] [Google Scholar]
- Soloway R. D., Hofmann A. F., Thomas P. J., Schoenfield L. J., Klein P. D. Triketocholanoic (dehydrocholic) acid. Hepatic metabolism and effect on bile flow and biliary lipid secretion in man. J Clin Invest. 1973 Mar;52(3):715–724. doi: 10.1172/JCI107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H. O., Ramos O. L. DETERMINANTS OF THE FLOW AND COMPOSITION OF BILE IN THE UNANESTHETIZED DOG DURING CONSTANT INFUSIONS OF SODIUM TAUROCHOLATE. J Clin Invest. 1960 Jan;39(1):161–170. doi: 10.1172/JCI104015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H. O., Ross E. D., Bradley S. E. Canalicular bile production in dogs. Am J Physiol. 1968 Apr;214(4):866–874. doi: 10.1152/ajplegacy.1968.214.4.866. [DOI] [PubMed] [Google Scholar]



