Abstract
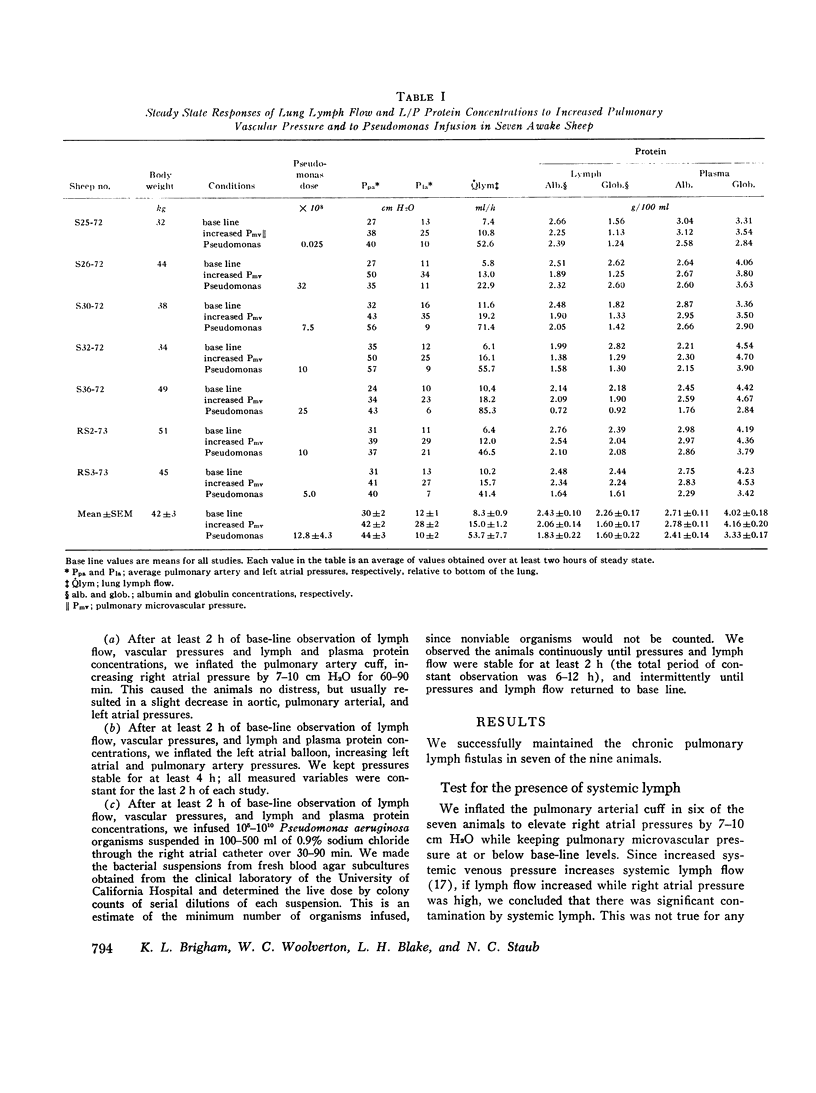
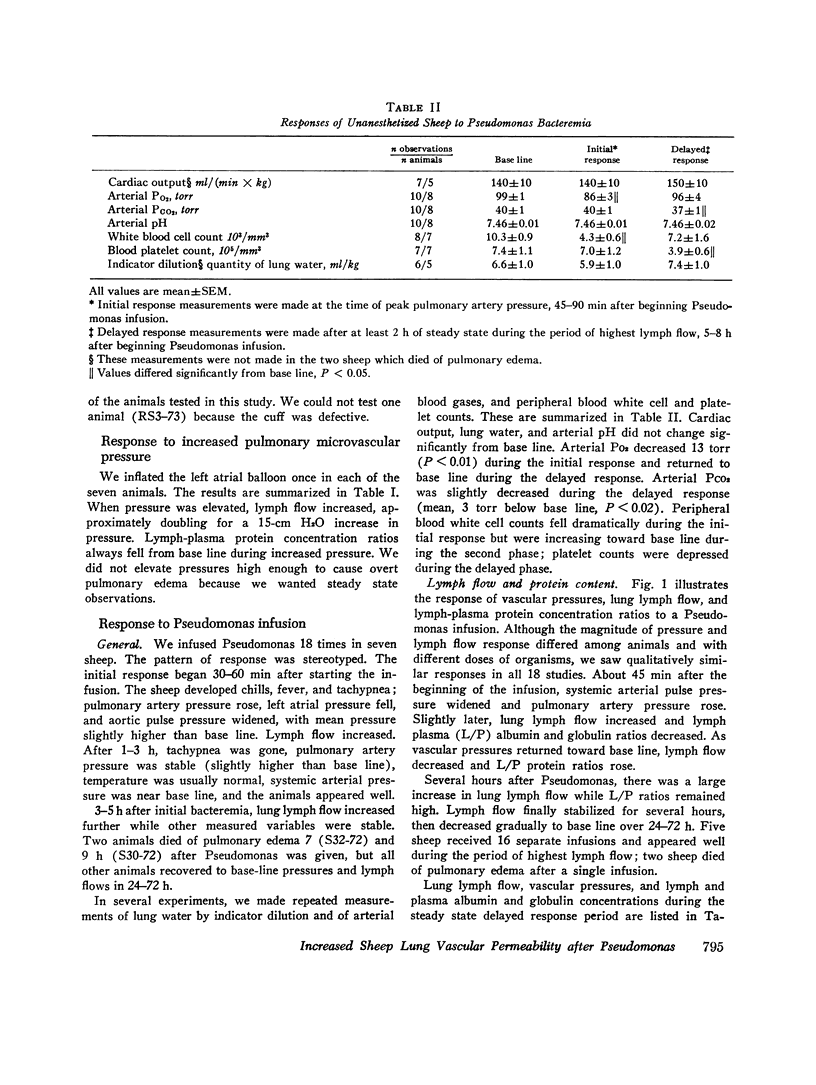
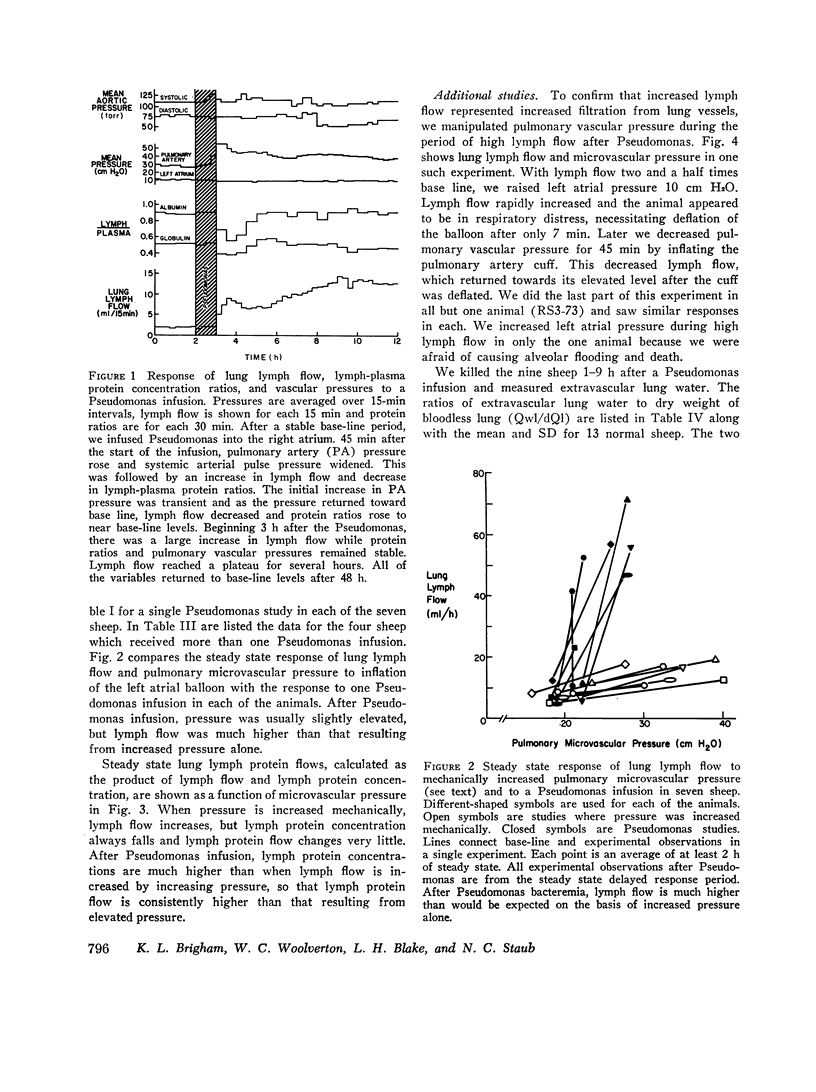
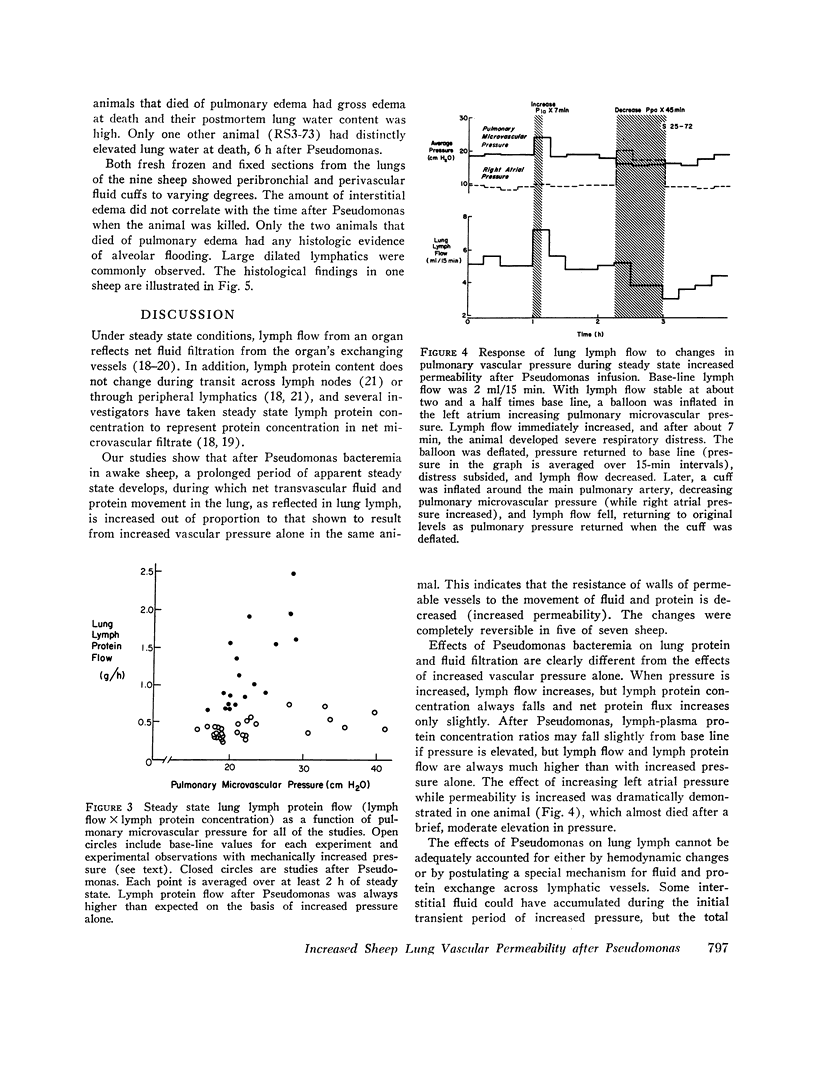
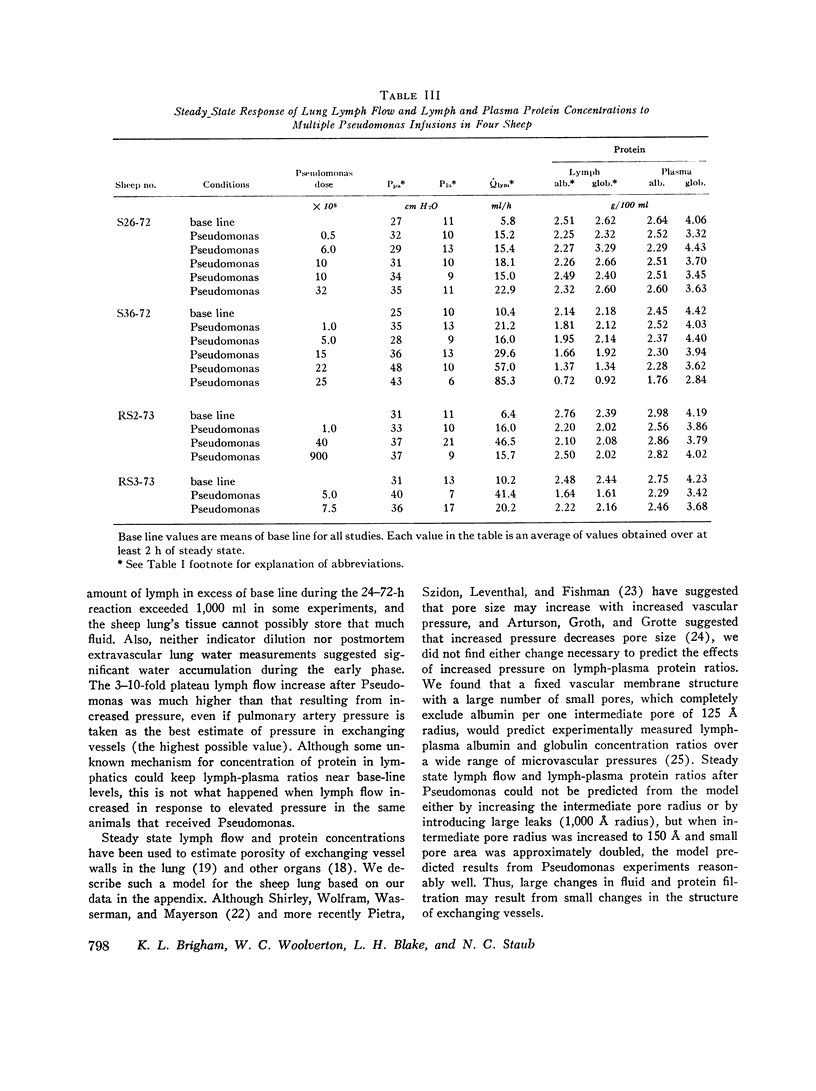
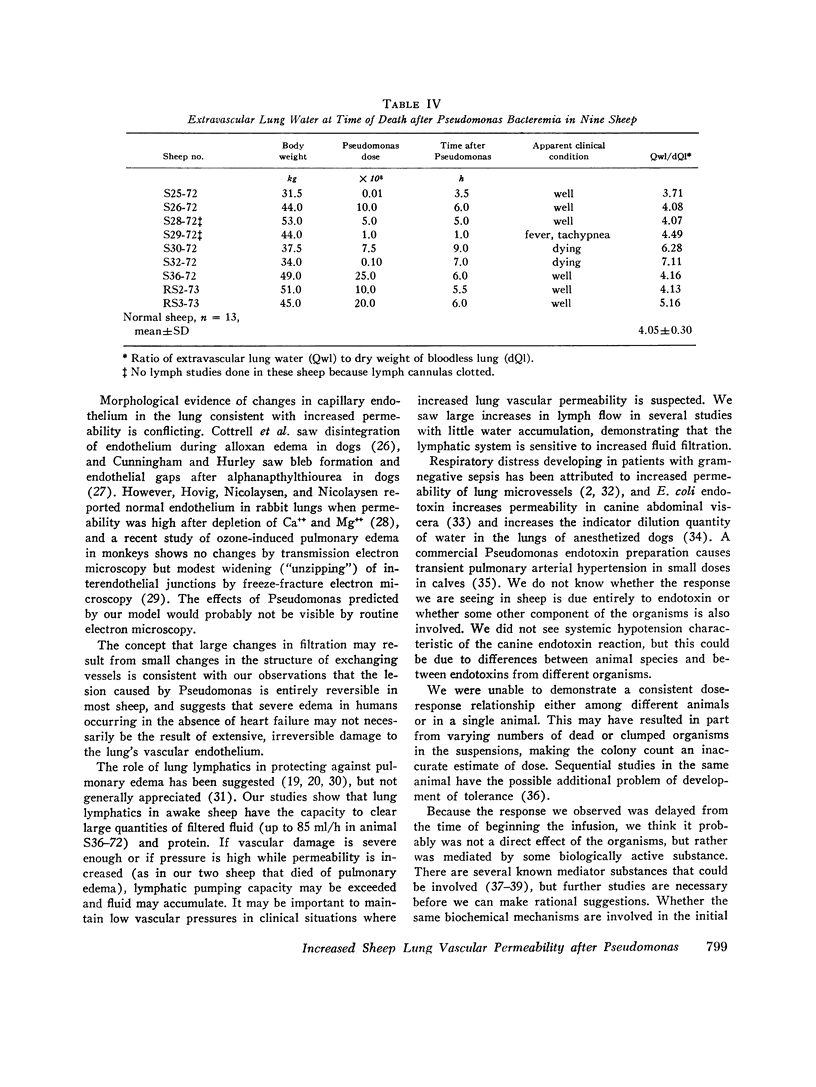
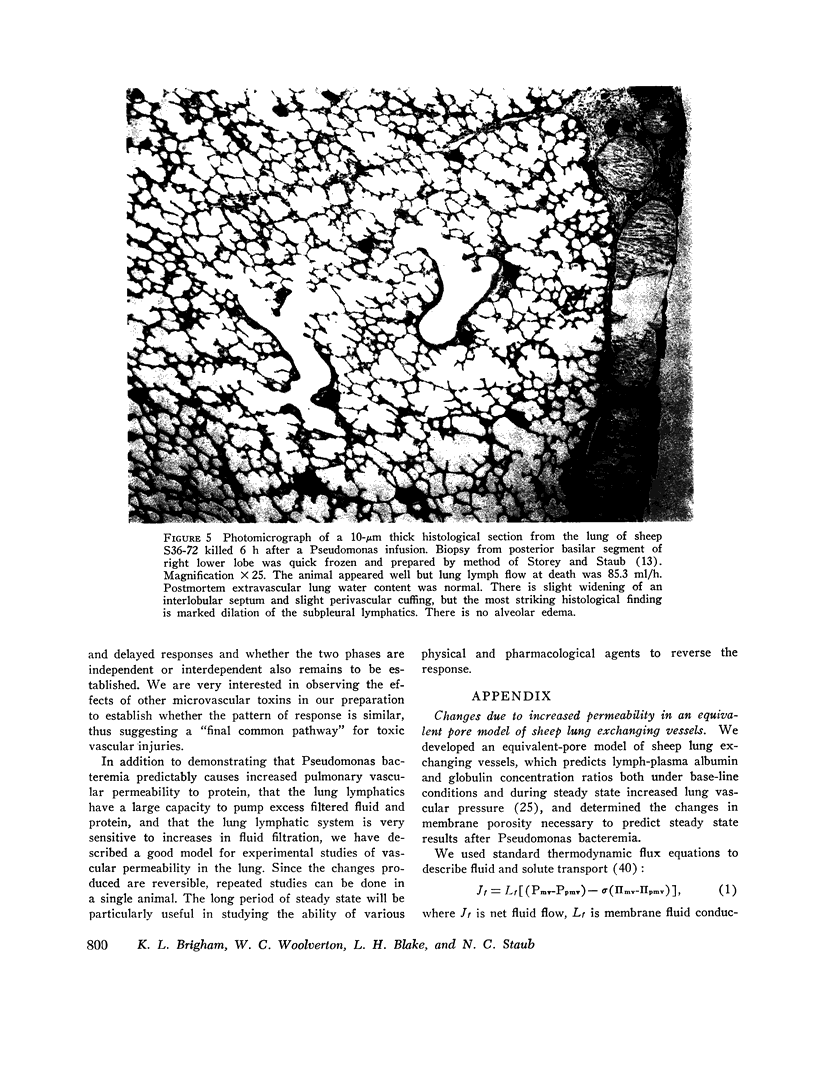
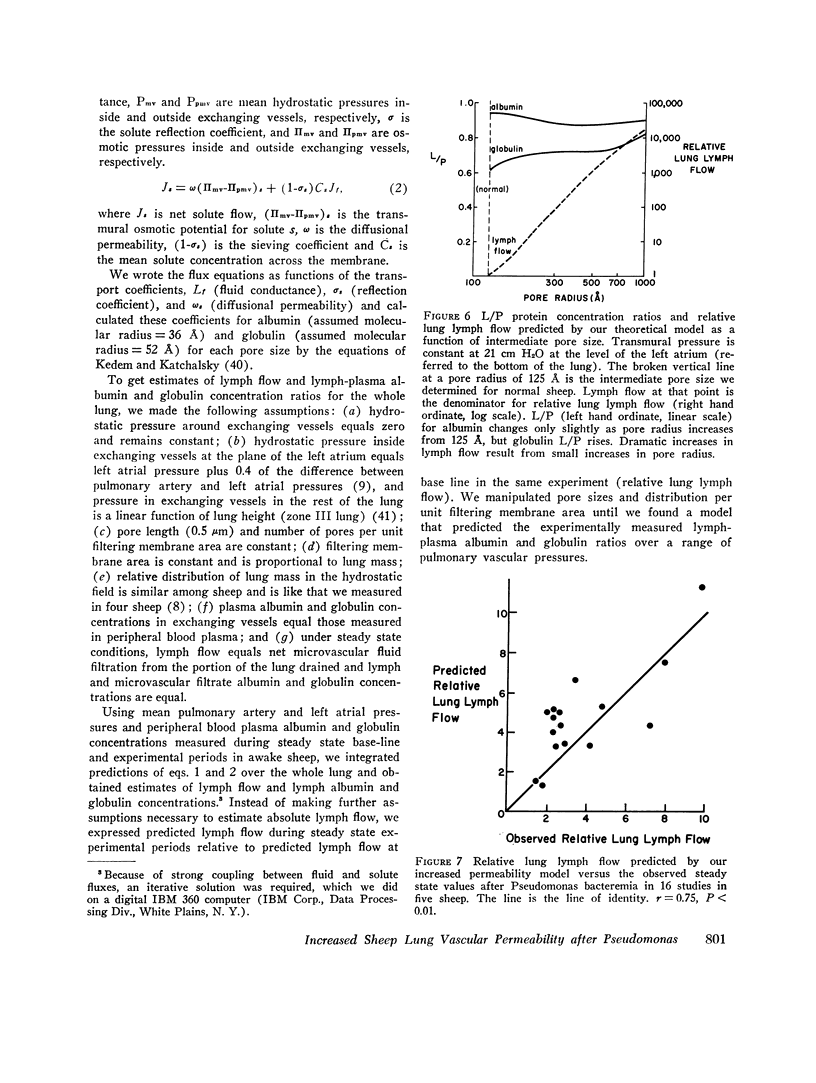
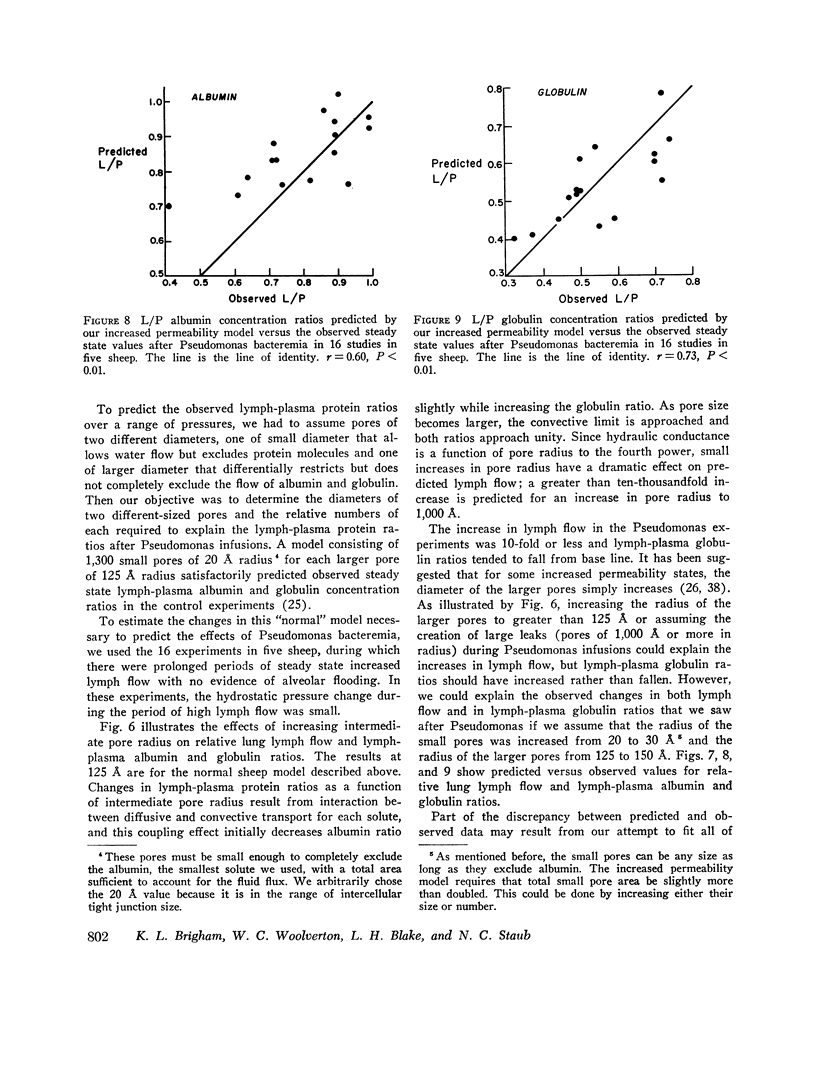
In awake sheep, we compared the responses of lung lymph flow and lymph and plasma protein concentrations to steady state elevations of pulmonary vascular pressures made by inflating a left atrial balloon with those after an intravenous infusion of 105-1010Pseudomonas aeruginosa. Lymph flow increased when pressure was increased, but lymph-plasma protein concentration ratios always fell and lymph protein flow (lymph flow × lymph protein concentration) increased only slightly. After Pseudomonas, sheep had transient chills, fever, leukopenia, hypoxemia, increased pulmonary artery pressure and lymph flow and decreased left atrial pressure and lymph protein concentration, 3-5 h after Pseudomonas, when vascular pressures and lymph protein concentrations had returned to near base line, lymph flow increased further to 3-10 times base line and remained at a steady level for many hours. During this steady state period, lymph-plasma protein concentration ratios were similar to base line and lymph protein flow was higher than in the increased pressure studies. Two sheep died of pulmonary edema 7 and 9 h after Pseudomonas, but in 16 studies, five other sheep appeared well during the period of highest lymph flow and all variables returned to base line in 24-72 h. Six serial indicator dilution lung water studies in five sheep changed insignificantly from base line after Pseudomonas. Postmortem lung water was high in the two sheep dead of pulmonary edema and one other, but six sheep killed 1-6 h after Pseudomonas had normal lung water. Because of the clear difference between the effects of increased pressure and Pseudomonas on lymphplasma protein concentration ratios and lymph protein flow, we conclude that Pseudomonas causes a prolonged increase in lung vessel permeability to protein. Because we saw lung lymph flow as high as 10 times base line without pulmonary edema, we conclude that lung lymphatics are a sensitive high-capacity mechanism for removing excess filtered fluid. An equivalent pore model of sheep lung vessels suggests that the changes we saw after Pseudomonas could result from small changes in the structure of exchanging vessel walls.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. Lymphatics and lymphoid tissues. Annu Rev Physiol. 1967;29:197–224. doi: 10.1146/annurev.ph.29.030167.001213. [DOI] [PubMed] [Google Scholar]
- Anas P., Neely W. A., Hardy J. D. Interstitial fluid pressure changes in endotoxin shock. Surgery. 1968 Jun;63(6):938–941. [PubMed] [Google Scholar]
- Arturson G., Groth T., Grotte G. The functional ultrastructure of the blood-lymph barrier. Computer analysis of data from dog heart-lymph experiments using theoretical models. Acta Physiol Scand Suppl. 1972;374:1–30. doi: 10.1111/j.1748-1716.1972.tb05297.x. [DOI] [PubMed] [Google Scholar]
- Body R. D., Hill J. R., Humphreys P. W., Normand I. C., Reynolds E. O., Strang L. B. Permeability of lung capillaries to macromolecules in foetal and new-born lambs and sheep. J Physiol. 1969 May;201(3):567–588. doi: 10.1113/jphysiol.1969.sp008773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L., Snell J. D., Jr In vivo assessment of pulmonary vascular integrity in experimental pulmonary edema. J Clin Invest. 1973 Aug;52(8):2041–2052. doi: 10.1172/JCI107388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIEN S., SINCLAIR D. G., DELLENBACK R. J., CHANG C., PERIC B., USAMI S., GREGERSEN M. I. EFFECT OF ENDOTOXIN ON CAPILLARY PERMEABILITY TO MACROMOLECULES. Am J Physiol. 1964 Sep;207:518–522. doi: 10.1152/ajplegacy.1964.207.3.518. [DOI] [PubMed] [Google Scholar]
- CROSBY W. H., MUNN J. I., FURTH F. W. Standardizing a method for clinical hemoglobinometry. U S Armed Forces Med J. 1954 May;5(5):693–703. [PubMed] [Google Scholar]
- Cottrell T. S., Levine O. R., Senior R. M., Wiener J., Spiro D., Fishman A. P. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res. 1967 Dec;21(6):783–797. doi: 10.1161/01.res.21.6.783. [DOI] [PubMed] [Google Scholar]
- Cunningham A. L., Hurley J. V. Alpha-naphthyl-thiourea-induced pulmonary oedema in the rat: a topographical and electron-microscope study. J Pathol. 1972 Jan;106(1):25–35. doi: 10.1002/path.1711060103. [DOI] [PubMed] [Google Scholar]
- GUYTON A. C., LINDSEY A. W. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. 1959 Jul;7(4):649–657. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- Gaar K. A., Jr, Taylor A. E., Owens L. J., Guyton A. C. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am J Physiol. 1967 Oct;213(4):910–914. doi: 10.1152/ajplegacy.1967.213.4.910. [DOI] [PubMed] [Google Scholar]
- Garlick D. G., Renkin E. M. Transport of large molecules from plasma to interstitial fluid and lymph in dogs. Am J Physiol. 1970 Dec;219(6):1595–1605. doi: 10.1152/ajplegacy.1970.219.6.1595. [DOI] [PubMed] [Google Scholar]
- Goresky C. A., Cronin R. F., Wangel B. E. Indicator dilution measurements of extravascular water in the lungs. J Clin Invest. 1969 Mar;48(3):487–501. doi: 10.1172/JCI106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADDY F. J., STEPHENS G., VISSCHER M. B. The physiology and pharmacology of lung edema. Pharmacol Rev. 1956 Sep;8(3):389–434. [PubMed] [Google Scholar]
- Hovig T., Nicolaysen A., Nicolaysen G. Ultrastructural studies of the alveolar-capillary barrier in isolated plasma-perfused rabbit lungs. Effects of EDTA and of increased capillary pressure. Acta Physiol Scand. 1971 Jul;82(3):417–432. doi: 10.1111/j.1748-1716.1971.tb04985.x. [DOI] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- MAYERSON H. S., PATTERSON R. M., McKEE A., LEBRIE S. J., MAYERSON P. Permeability of lymphatic vessels. Am J Physiol. 1962 Jul;203:98–106. doi: 10.1152/ajplegacy.1962.203.1.98. [DOI] [PubMed] [Google Scholar]
- Majno G., Gilmore V., Leventhal M. On the mechanism of vascular leakage caused by histaminetype mediators. A microscopic study in vivo. Circ Res. 1967 Dec;21(6):833–847. doi: 10.1161/01.res.21.6.833. [DOI] [PubMed] [Google Scholar]
- Nicolaysen G. Increase in capillary filtration rate resulting from reduction in the intravascular calcium ion-concentration. Acta Physiol Scand. 1971 Apr;81(4):517–527. doi: 10.1111/j.1748-1716.1971.tb04929.x. [DOI] [PubMed] [Google Scholar]
- PEARCE M. L., YAMASHITA J., BEAZELL J. MEASUREMENT OF PULMONARY EDEMA. Circ Res. 1965 May;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- Pietra G. G., Szidon J. P., Leventhal M. M., Fishman A. P. Hemoglobin as a tracer in hemodynamic pulmonary edema. Science. 1969 Dec 26;166(3913):1643–1646. doi: 10.1126/science.166.3913.1643. [DOI] [PubMed] [Google Scholar]
- Pietra G. G., Szidon J. P., Leventhal M. M., Fishman A. P. Histamine and interstitial pulmonary edema in the dog. Circ Res. 1971 Oct;29(4):323–337. doi: 10.1161/01.res.29.4.323. [DOI] [PubMed] [Google Scholar]
- Reeves J. T., Daoud F. S., Estridge M. Pulmonary hypertension caused by minute amounts of endotoxin in calves. J Appl Physiol. 1972 Dec;33(6):739–743. doi: 10.1152/jappl.1972.33.6.739. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., Walters G. Pulmonary oedema in bacterial shock. Lancet. 1968 Apr 6;1(7545):719–721. doi: 10.1016/s0140-6736(68)92165-x. [DOI] [PubMed] [Google Scholar]
- Robin E. D., Carey L. C., Grenvik A., Glauser F., Gaudio R. Capillary leak syndrome with pulmonary edema. Arch Intern Med. 1972 Jul;130(1):66–71. [PubMed] [Google Scholar]
- SHIRLEY H. H., Jr, WOLFRAM C. G., WASSERMAN K., MAYERSON H. S. Capillary permeability to macromolecules: stretched pore phenomenon. Am J Physiol. 1957 Aug;190(2):189–193. doi: 10.1152/ajplegacy.1957.190.2.189. [DOI] [PubMed] [Google Scholar]
- STOREY W. F., STAUB N. C. Ventilation of terminal air units. J Appl Physiol. 1962 May;17:391–397. doi: 10.1152/jappl.1962.17.3.391. [DOI] [PubMed] [Google Scholar]
- Snell J. D., Jr, Ramsey L. H. Pulmonary edema as a result of endotoxemia. Am J Physiol. 1969 Jul;217(1):170–175. doi: 10.1152/ajplegacy.1969.217.1.170. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Nagano H., Pearce M. L. Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J Appl Physiol. 1967 Feb;22(2):227–240. doi: 10.1152/jappl.1967.22.2.227. [DOI] [PubMed] [Google Scholar]
- Staub N. C. The pathophysiology of pulmonary edema. Hum Pathol. 1970 Sep;1(3):419–432. doi: 10.1016/s0046-8177(70)80075-2. [DOI] [PubMed] [Google Scholar]
- WEST J. B., DOLLERY C. T., NAIMARK A. DISTRIBUTION OF BLOOD FLOW IN ISOLATED LUNG; RELATION TO VASCULAR AND ALVEOLAR PRESSURES. J Appl Physiol. 1964 Jul;19:713–724. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]



