Abstract
Pancreatic endocrine tumors (PET) are rare neoplasms classified as functioning (F-PET) or non-functioning (NF-PET) according to the presence of a clinical syndrome due to hormonal hypersecretion. PETs show variable degrees of clinical aggressiveness and loss of chromosome 3p has been suggested to be associated with an advanced stage of disease. We assessed chromosome 3p copy number in 113 primary PETs and 32 metastases by fluorescence in situ hybridization (FISH) using tissue microarrays. The series included 56 well-differentiated endocrine tumors (WDET), 62 well-differentiated endocrine carcinomas (WDEC), and 6 poorly differentiated endocrine carcinomas (PDEC). Chromosome 3p alterations were found in 23/113 (20%) primary tumors, with losses being predominant over gains (14% vs. 6%). Loss of 3p was found in 5/55 (9%) WDET, 11/52 (21%) WDEC, and never in PDEC. Gains of 3p were detected in 4/55 (7%) WDET, no WDEC, but notably in 3/6 (50%) PDEC (OR 23.6; P = 0.003). Metastases were more frequently monosomic for 3p compared to primary tumors (OR 3.6; P = 0.005). Monosomy was significantly associated with larger tumor size, more advanced tumor stage, and metastasis. No association was found with survival. Chromosome 3p copy number alterations are frequent events in advanced stage PET, with gains prevailing in PDEC while losses are more frequent in WDEC, supporting the view that a specific pattern of alterations are involved in these diverse disease subtypes.
Keywords: FISH, Pancreatic endocrine tumors, Metastasis, 3p, Chromosomal aberrations
Introduction
Pancreatic endocrine tumors (PET) are rare neoplasms whose molecular pathogenesis is being unraveled but is still largely unknown [1–4]. PET are classified clinically as functioning (F-PET) or non-functioning (NF-PET) according to the presence of a syndrome due to hormonal hypersecretion. NF-PET and F-PET other than insulinoma are malignant in up to 70% of cases. The WHO classification distinguishes three categories: well-differentiated endocrine tumor having an indolent clinical course; well-differentiated endocrine carcinoma (WDEC) that is diagnosed based on the presence of invasion or metastasis; poorly differentiated endocrine carcinoma (PDEC) with a survival as poor as that of pancreatic adenocarcinoma [5]. However, the malignant potential of WDEC varies greatly and cannot be predicted by histological appearance alone. Although the recently proposed TNM staging and the proliferation rate of the neoplasia are valuable predictors of clinical outcome, additional prognostic parameters are warranted [6, 7].
Chromosomal alterations have been suggested to be prognostic parameters for tumor progression, recurrence, and tumor-specific death in these tumors. In PETs, alterations of 3p were investigated using two approaches: by LOH microsatellite analysis, allelic imbalance at 3p was observed in an average of 47% of cases [8–13] whereas by means of comparative genomic hybridization (CGH), loss of 3p was found in 21% of PETs [14–16]. Moreover, in almost all studies investigating this subject, these alterations were associated with an advanced stage of disease.
Potential candidate genes at this locus include VHL and RASSF1A, that have been recently associated with PET pathogenesis [12, 17–19]. Interestingly, recent data from Schmitt et al. suggest that VHL inactivation and consecutive hypoxia signals may be a mechanism for the development of sporadic PET with an adverse outcome [17]. Moreover, inactivation of the tumor suppressor gene RASSF1A by hypermethylation and 3p LOH has been proposed as a major event in PET tumorigenesis [12, 18, 19].
A large amount of evidences support the association of 3p alterations with prognosis, however the methods previously employed to detect this association are not easily applicable to clinical practice. Fluorescence in situ hybridization (FISH) is commonly used as a diagnostic device in cancer. Here, we explore the possibility to use FISH on chromosome 3p as a prognostic tool for PET by screening a large series composed of 113 PET and 32 metastases and correlating the results with clinical–pathological features of patients.
Materials and methods
Samples
The series comprises 113 primitive neoplasms and 32 metastases obtained from 124 PET patients operated on at the University Hospital of Verona, Italy. Of the metastases, 21 had a matched primary tumor in the series. Clinical–pathological features of the present series are shown in Table 1. Informed consent for the analysis of normal and neoplastic tissues was obtained from patients, in accordance with the approval of the Verona University and Hospital Trust’s Ethical Committee.
Table 1.
Clinical–pathological features of 124 PETs consisting of 113 primitive samples and 11 unmatched metastases
| Parameter | Categorya | Frequency (percent) |
|---|---|---|
| Gender | F | 67 (54%) |
| M | 57 (46%) | |
| Age | mean (1st quartile to 3rd quartile) | 56 (44–65) |
| PT | T1 | 34 (27%) |
| T2 | 33 (27%) | |
| T3 | 21 (17%) | |
| T4 | 36 (29%) | |
| PN | N0 | 74 (60%) |
| N1 | 50 (40%) | |
| PM | M0 | 85 (69%) |
| M1 | 39 (32%) | |
| WHO | PDEC | 6 (5%) |
| WDEC | 62 (50%) | |
| WDET | 56 (45%) | |
| Hormone secretion | Functioning | 26 (21%) |
| Non-functioning | 98 (79%) | |
| Proliferative index | Ki67 > 5% | 28 (23%) |
| Ki67 < 5% | 96 (77%) | |
| Tumor size | Mean (1st quartile to 3rd quartile) | 30 (15–45) |
a PDEC poorly differentiated endocrine carcinoma, WDEC well-differentiated endocrine carcinoma, WDET well-differentiated tumor
Tissue microarrays
Six tissue microarrays (TMAs) containing the 113 primary PETs and 32 metastases were constructed, using a tissue arrayer (Beecher Instruments, Silver Spring, MD) and including at least three cores of 1 mm diameter per sample [3].
Fluorescence in situ hybridization
FISH assay for chromosome 3p was performed on 4-μm-thick sections from TMAs, using a home-made SpectrumOrange labeled DNA probe for the relevant region (Abbott-Vysis, Downer Grove, IL, USA). Briefly, BAC clone specific for chromosome 3p (RP11-894C9) mapping at 3p21.31 and belonging to the Roswell Park Cancer Institute libraries (Peter J. de Jong at http://bacpac.chori.org/) was selected. Paraffin sections were hybridized with the probe labeled by nick translation [20], using 500 ng of probe labeled with Fluorolink Cy3-dUTP or Fluor-X-dCTP (Amersham, Buckinghamshire, UK). For each sample, at least 110 nuclei were analyzed for presence of 3p signals.
Statistical analysis
k-means algorithm using Hartigan–Wong method was used to classify PET samples into three classes, with no a priori centroid definition, based on the distribution of FISH counts. For sample assignation, 1,000 iterations were used. For association of samples locus status with clinical–pathological features, Fisher’s test was used. For correlation of percent of PET cells showing monosomy with clinical–pathological features, Kruskal–Wallis’ test was used for categorical covariates and Spearman’s correlation for numeric variables. Survival estimates were calculated with Kaplan–Meier’s method and compared by the log-rank test. P values less than 0.05 were considered significant. Where applicable, the tests were two-tailed. For all the calculations, the R statistical software package was used (http://www.r-project.org).
Results
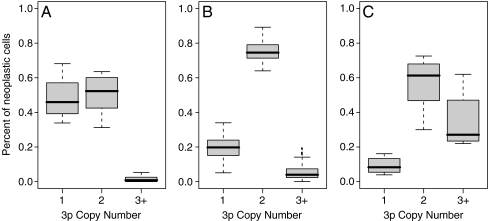
FISH was performed on six TMAs containing 113 primary PETs and 32 metastases. For each sample, we counted neoplastic cells having one, two or more than two signals per nucleus, for at least 110 cells per sample. Therefore, we used the cell counts to assign samples to three unsupervised classes, by the k-means algorithm. The centroids obtained and distributions of the three classes are shown in Fig. 1. The three classes were composed of 28 samples (19%) with an excess of monosomic signals, 108 samples (75%) with a predominant disomic state and 9 samples (6%) with evidence of gains (Table 2).
Fig. 1.
Distribution of frequency of neoplastic nuclei with one, two or at least three FISH signals of PET cases belonging to the classes of monosomic (a), disomic (b), gains (c), based on the frequency of signals per nucleus. The samples were assigned to the three classes using k-means algorithm
Table 2.
Frequency of DNA copy number status among 113 primitive PETs and 32 metastases
| Sample type | WHO classificationa | DNA copy number | ||
|---|---|---|---|---|
| 1 | 2 | 3+ | ||
| Primitive | PDEC | 0 | 3 (50%) | 3 (50%) |
| WDEC | 11 (21%) | 41 (79%) | 0 | |
| WDET | 5 (9%) | 46 (84%) | 4 (7%) | |
| Functioning | 2 (9%) | 18 (82%) | 2 (9%) | |
| Non-functioning | 14 (15%) | 72 (79%) | 5 (6%) | |
| Total | 16 (14%) | 90 (80%) | 7 (6%) | |
| Metastasis | PDEC | 0 | 2 (67%) | 1 (33%) |
| WDEC | 12 (41%) | 16 (55%) | 1 (3%) | |
| Functioning | 2 (40%) | 3 (60%) | 0 | |
| Non-functioning | 10 (37%) | 15 (56%) | 2 (7%) | |
| Total | 12 (38%) | 18 (56%) | 2 (6%) | |
a PDEC poorly differentiated endocrine carcinomas, WDEC well-differentiated endocrine carcinomas, WDET, well-differentiated tumors
Using such classification, we also found a high frequency of samples showing gains among PDEC (3/6; OR 23.6; 95% CI 2.4–243.4; Fisher’s test P = 0.003). In addition, we found that monosomic samples were significantly more represented in metastases (OR 3.6; 95% CI 1.3–9.6; Fisher’s test P = 0.005).
In order to investigate possible 3p alterations de novo acquired in metastases, we compared the 3p status of 21 metastases with their matched primary samples (Table 3). Thirteen patients had the same 3p copy number whereas in three samples, the copy number increased and five showed de novo losses.
Table 3.
DNA copy number of 3p in 21 primitive samples and their matched metastases
| Primitive copy number | Metastases copy number | ||
|---|---|---|---|
| 1 | 2 | 3+ | |
| 1 | 3 | 2 | 0 |
| 2 | 5 | 9 | 1 |
| 3+ | 0 | 0 | 1 |
As 3p loss was the most represented event, we then focused on the correlation between losses and other clinical–pathological covariates. In addition, to avoid potential misclassification, the percent of monosomic cells was directly used in the analysis instead of the categorization introduced above. The results of this analysis are summarized in Table 4. Percent of monosomic cells was higher in metastases and positively correlated with larger size, more advanced tumor invasion, carcinoma of the well-differentiated type and absence of hormone secretions. Although correlated with worse patients' outcome, we did not find a significant association between monosomy and survival, even when considering only the WDEC group. Similarly, we did not find any significant association between gains of 3p and survival.
Table 4.
Distribution of percent of monosomy according to clinical–pathological features of 113 primary pancreatic endocrine tumors
| Parameter | Class | Median (1st quartile to 3rd quartile) | P value |
|---|---|---|---|
| Primitives | 0.19 (0.14–0.25) | <0.001 | |
| Metastases | 0.26 (0.22–0.41) | ||
| pT | T1 | 0.15 (0.13–0.23) | 0.027 |
| T2 | 0.18 (0.15–0.22) | ||
| T3 | 0.22 (0.16–0.27) | ||
| T4 | 0.23 (0.17–0.32) | ||
| pN | N0 | 0.19 (0.14–0.25) | 0.704 |
| N1 | 0.20 (0.14–0.24) | ||
| pM | M0 | 0.19 (0.14–0.24) | 0.095 |
| M1 | 0.22 (0.16–0.28) | ||
| WHO | PDEC | 0.11 (0.08–0.13) | <0.001 |
| WDEC | 0.18 (0.13–0.21) | ||
| WDET | 0.16 (0.14–0.24) | ||
| Hormone secretion | Functioning | 0.15 (0.11–0.21) | 0.029 |
| Non-functioning | 0.20 (0.15–0.27) | ||
| Tumor size | 0.30 (Spearman’s rho) | 0.002 | |
| Ki67 | 0.05 (Spearman’s rho) | 0.596 |
PDEC poorly differentiated endocrine carcinoma, WDEC well-differentiated endocrine carcinoma, WDET well-differentiated tumor
Discussion
Several studies employing two main molecular approaches (LOH by microsatellites and CGH) have suggested a role for alterations of chromosome 3p in PET. A detailed survey of the relevant literature has been collected in Table 5. In particular, a relationship between LOH at 3p and locally advanced or metastatic PETs was found in all previous works. Of the two techniques used, LOH detected consistently a higher number of alterations [8–13] compared to CGH [14–16] (47% vs. 21%). This apparent discrepancy can be accounted for by the intrinsic features of the two methods. In fact, although LOH by microsatellites provides the ability to finely map the region where the alterations occur, it sums up gains and losses that are difficult to tell apart as both are seen as allelic imbalances. On the other hand, with CGH only true losses are reported.
Table 5.
Summary of 3p LOH data available in literature for PET
| No. of Cases | 3p LOH (total) | WHOa | Tumor typeb | Metastasis | Methodc | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| WDET | WDEC | F | NF | No | Yes | ||||
| 10 | 3/10 | 0/5 | 3/5 | nr | nr | 0/5 | 3/5 | ms | [12] |
| 30% | 0% | 60% | – | – | 0% | 60% | |||
| 82 | 47/82 | 7/25 | 40/57 | 24/47 | 23/35 | 17/41 | 28/38 | ms | [8] |
| 57% | 28% | 70% | 51% | 65% | 41.5% | 74% | |||
| 20 | 9/20 | 1/8 | 8/12 | 0/11 | 9/9 | 0/6 | 9/12 | ms | [10] |
| 45% | 12.5% | 67% | 0% | 100% | 0% | 75% | |||
| 43 | 14/43 | 2/22 | 12/21 | 11/29 | 3/14 | 2/22 | 12/21 | ms | [9] |
| 33% | 9% | 57% | 38% | 21% | 9% | 57% | |||
| 21 | 13/21 | 8/15 | 5/6 | 13/21 | none | 8/15 | 5/6 | ms | [11] |
| 62% | 53% | 83% | 62% | – | 53% | 83% | |||
| 16 | 5/16 | 2/8 | 3/8 | none | 5/16 | 2/9 | 3/7 | ms | [13] |
| 31% | 25% | 37.5% | – | 31% | 22% | 30% | |||
| 192 | 91/192 | 20/83 | 71/109 | 48/108 | 40/74 | 29/98 | 60/89 | Total LOH by ms | |
| 47% | 24% | 65% | 44% | 54% | 30% | 67% | |||
| 44 | 13/44 | 6/9 | 7/35 | nr | nr | 2/22 | 10/18 | CGH | [15] |
| 30% | 67% | 20% | – | – | 9% | 56% | |||
| 45 | 9/45 | 3/28 | 6/17 | 5/31 | 4/14 | 3/28 | 6/17 | CGH | [16] |
| 20% | 11% | 35% | 16% | 29% | 11% | 35% | |||
| 20 | 1/20 | 0/8 | 1/12 | none | 1/20 | 0/9 | 1/11 | CGH | [14] |
| 5% | 0% | 8% | – | 5% | 0% | 9% | |||
| 109 | 23/109 | 9/45 | 14/64 | 5/31 | 5/34 | 5/59 | 17/46 | Total LOH by CGH | |
| 21% | 20% | 22% | 16% | 15% | 8% | 37% | |||
a WDEC well-differentiated endocrine carcinoma, WDET well-differentiated endocrine tumor
b F functioning, NF non-functioning
c ms LOH by microsatellite analysis
nr not reported
Our study showed that a consistent number of chromosomal alterations, detected by FISH, involved the 3p locus with losses predominant over gains (14% vs. 6%). The use of FISH allows the characterization of chromosomal alterations at specific loci with a small cost per sample and avoids artifacts deriving from analysis with other molecular techniques such as LOH microsatellite analysis. Our results are more in line with previous CGH reports, probably due to the ability of both techniques to discern losses and gains.
In our series, we found that monosomic samples were significantly more represented in metastases than in primitive samples, in agreement with all previous studies reporting a higher frequency of 3p loss in association with metastatic event (see Table 5). Furthermore, in pair-wise analysis of 21 matched primaries and metastases, we observed that 3p copy number was conserved from primitive to metastasis in 13 samples. However, five metastases showed a decrease in 3p copy number; in two patients, primitive samples were monosomic and metastasis showed a disomic state, and one patient had a gain in metastasis. This suggests the occurrence of tumor heterogeneity in PET and as such a role in their metastasis formation mechanism.
In addition, we found that the proportion of monosomic cells was positively correlated with non-functioning status, larger size, a diagnosis of carcinoma of the well-differentiated type and a more advanced pT stage. The higher percent of monosomy found in non-functioning PET was already suggested by previous studies describing this class of PET as having both larger size and higher rate of chromosomal aberrations compared to the functioning ones [8, 10, 16]. These data suggest that 3p monosomy may occur later during the development of disease and may contribute to the progression of malignancy of PET. Although correlated with worse patient features, we did not find a significant association between monosomy and survival, also when considering only the WDEC group, probably due to the general high survival rate of PET.
Interestingly, several data support the role of genes mapping on 3p in PET pathogenesis and malignancy. Among these genes, VHL is a major candidate as patients with Von-Hippel Lindau syndrome may develop pancreatic endocrine tumors. Schmitt and colleagues have investigated the presence of genetic and epigenetic alterations at VHL locus in PETs [17]. By using FISH, they detected a frequency of 3p loss of 18%, a number that is very close to that found in our series. They suggest that VHL inactivation and consecutive hypoxia signals may be a mechanism for the development of sporadic PET with an adverse outcome. However, another study identified LOH in an area outside the VHL gene (3p14.2–3p21), in 83% of malignant insulinomas compared with 53% of benign insulinomas [11], suggesting that other candidates are also likely to be involved in PET onset and progression. Another hypothesis suggests the presence of a novel pancreatic endocrine tumor suppressor gene at 3p25, being the frequency of 3p loss about 33% in the region flanking the VHL gene. Allelic loss of this chromosomal region has been proposed to serve as a molecular marker discriminating benign from clinically malignant disease [9].
Also, Barghorn et al. identified a common deleted region (3p25-p24.2) in PETs that does not include VHL and propose a new member of the nuclear receptor superfamily, the peroxisome proliferator-activated receptor gamma (PPARγ), as a possible candidate tumor suppressor [8]. Indeed, this gene has been demonstrated to act as a regulator of differentiation and/or growth of many different cell types [21, 22] and loss-of-function of mutations in PPARγ have already been identified in human colon cancer [23]. Another candidate gene on chromosome 3p is the tumor suppressor gene RASSF1A that has been suggested to be inactivated by 3p LOH and hypermethylation in 30% of malignant well-differentiated carcinomas of the pancreas [12]. In light of the association of 3p loss with malignancy, we can underline the relevance of the pattern of 3p allelic losses during progression of PET. However, the specific targets of such alterations and their functional meaning in PET tumorigenesis remain to be further investigated.
Notably, we found a higher proportion of gains in PDEC with respect to WDEC, suggesting that this feature is characteristic of poorly differentiated tumors. We did not find any significant association between gains of 3p and survival, although it is worth noting that none of the seven patients showing gains (including the three PDECs) died of disease, suggesting that gain of 3p could be a positive prognostic factor.
Previous papers have demonstrated the presence of gain of 3p in different types of cancer such as hepatocarcinoma [24] and ductal carcinoma of the breast [25], but this feature has never been observed in pancreatic endocrine tumors. The reason may be probably due to the fact that poorly differentiated carcinomas are rarely included in PET studies. Therefore, based on our evidences, we cannot hypothesize any potential oncogene that could be a target for this kind of alteration.
As a methodological remark, we found that percent of monosomic cells showed more power in detecting association with clinical–pathological features, compared to a discrete classification based on 3p copy number. The reason for such a greater power may be due to the inherent higher precision of a quantitative measure as it can more finely reflect intermediate status, along the tumor development path, in which the neoplastic population is composed of genetically different clones with different selective advantages.
In conclusion, our data showed that alterations in 3p copy number may play an important role in tumor progression, malignant behavior and metastasis and may occur as a later event in cancer development. In addition, we found that losses and gains of 3p locus are associated with different forms of the disease: namely, gains with poorly differentiated endocrine carcinoma, losses with well-differentiated endocrine carcinoma. These data together confirm that 3p may contain genes important for metastatic development of PETs, but the specific targets as well as the prognostic potential of these alterations need to be further investigated.
Acknowledgments
This work was supported by grants from: Associazione Italiana Ricerca Cancro (AIRC, http://www.airc.it/), Fondazione CariParo (www.fondazionecariparo.it), Fondazione Cariverona (http://www.fondazionecariverona.org/); Italian Ministry of Health, Rome, Italy (http://www.salute.gov.it/).
Conflict of interest statement
We declare that we have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24(29):4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 2.Capelli P, Martignoni G, Pedica F, Falconi M, Antonello D, Malpeli G, Scarpa A. Endocrine neoplasms of the pancreas: pathologic and genetic features. Arch Pathol Lab Med. 2009;133(3):350–364. doi: 10.5858/133.3.350. [DOI] [PubMed] [Google Scholar]
- 3.Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A, delle Fave G, Pederzoli P, Croce CM, Scarpa A. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28(2):245–255. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbo V, Dalai I, Scardoni M, Barbi S, Beghelli S, Bersani S, Albarello L, Doglioni C, Schott C, Capelli P, Chilosi M, Boninsegna L, Becker KF, Falconi M, Scarpa A. MEN1 in pancreatic endocrine tumors: analysis of gene and protein status in 169 sporadic neoplasms reveals alterations in the vast majority of cases. Endocr Relat Cancer. 2010;17:771–783. doi: 10.1677/ERC-10-0028. [DOI] [PubMed] [Google Scholar]
- 5.Kloppel G, Perren A, Heitz P. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann NY Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 6.Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Korner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, Panzuto F, Pederzoli P, Fave GD, Falconi M. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–833. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 8.Barghorn A, Komminoth P, Bachmann D, Rutimann K, Saremaslani P, Muletta-Feurer S, Perren A, Roth J, Heitz PU, Speel EJ. Deletion at 3p25.3-p23 is frequently encountered in endocrine pancreatic tumours and is associated with metastatic progression. J Pathol. 2001;194(4):451–458. doi: 10.1002/path.886. [DOI] [PubMed] [Google Scholar]
- 9.Chung DC, Smith AP, Louis DN, Graeme-Cook F, Warshaw AL, Arnold A. A novel pancreatic endocrine tumor suppressor gene locus on chromosome 3p with clinical prognostic implications. J Clin Invest. 1997;100(2):404–410. doi: 10.1172/JCI119547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hessman O, Lindberg D, Einarsson A, Lillhager P, Carling T, Grimelius L, Eriksson B, Akerstrom G, Westin G, Skogseid B. Genetic alterations on 3p, 11q13, and 18q in nonfamilial and MEN 1-associated pancreatic endocrine tumors. Genes Chromosom Cancer. 1999;26(3):258–264. doi: 10.1002/(SICI)1098-2264(199911)26:3<258::AID-GCC11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforova MN, Nikiforov YE, Biddinger P, Gnepp DR, Grosembacher LA, Wajchenberg BL, Fagin JA, Cohen RM. Frequent loss of heterozygosity at chromosome 3p14.2-3p21 in human pancreatic islet cell tumours. Clin Endocrinol (Oxf) 1999;51(1):27–33. doi: 10.1046/j.1365-2265.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 12.Pizzi S, Azzoni C, Bottarelli L, Campanini N, D’Adda T, Pasquali C, Rossi G, Rindi G, Bordi C. Rassf1a promoter methylation and 3p21.3 loss of heterozygosity are features of foregut, but not midgut and hindgut, malignant endocrine tumours. J Pathol. 2005;206(4):409–416. doi: 10.1002/path.1784. [DOI] [PubMed] [Google Scholar]
- 13.Rigaud G, Missiaglia E, Moore PS, Zamboni G, Falconi M, Talamini G, Pesci A, Baron A, Lissandrini D, Rindi G, Grigolato P, Pederzoli P, Scarpa A. High resolution allelotype of nonfunctional pancreatic endocrine tumors: identification of two molecular subgroups with clinical implications. Cancer Res. 2001;61(1):285–292. [PubMed] [Google Scholar]
- 14.Floridia M, Bucciardini R, Fragola V, Galluzzo CM, Giannini G, Pirillo MF, Amici R, Andreotti M, Ricciardulli D, Tomino C, Vella S. Risk factors and occurrence of rash in HIV-positive patients not receiving nonnucleoside reverse transcriptase inhibitor: data from a randomized study evaluating use of protease inhibitors in nucleoside-experienced patients with very low cd4 levels (<50 cells/microl) HIV Med. 2004;5(1):1–10. doi: 10.1111/j.1468-1293.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 15.Speel EJ, Richter J, Moch H, Egenter C, Saremaslani P, Rutimann K, Zhao J, Barghorn A, Roth J, Heitz PU, Komminoth P. Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am J Pathol. 1999;155(6):1787–1794. doi: 10.1016/S0002-9440(10)65495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Moch H, Scheidweiler AF, Baer A, Schaffer AA, Speel EJ, Roth J, Heitz PU, Komminoth P. Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosom Cancer. 2001;32(4):364–372. doi: 10.1002/gcc.1201. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt AM, Schmid S, Rudolph T, Anlauf M, Prinz C, Kloppel G, Moch H, Heitz PU, Komminoth P, Perren A. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr Relat Cancer. 2009;16(4):1219–1227. doi: 10.1677/ERC-08-0297. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Broaddus RR, Yao JC, Xie S, White JA, Wu TT, Hamilton SR, Rashid A. Epigenetic alterations in neuroendocrine tumors: methylation of RAS-association domain family 1, isoform a and p16 genes are associated with metastasis. Mod Pathol. 2005;18(12):1632–1640. doi: 10.1038/modpathol.3800490. [DOI] [PubMed] [Google Scholar]
- 19.Dammann R, Schagdarsurengin U, Strunnikova M, Rastetter M, Seidel C, Liu L, Tommasi S, Pfeifer GP. Epigenetic inactivation of the Ras-association domain family 1 (RASSF1A) gene and its function in human carcinogenesis. Histol Histopathol. 2003;18(2):665–677. doi: 10.14670/HH-18.665. [DOI] [PubMed] [Google Scholar]
- 20.Lichter P, Jauch A, Cremer T, Ward DC. Detection of Down syndrome by in situ hybridization with chromosome 21 specific DNA probes. Prog Clin Biol Res. 1990;360:69–78. [PubMed] [Google Scholar]
- 21.Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95(15):8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 23.Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, Aaltonen LA, de la Chapelle A, Spiegelman BM, Eng C. Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol Cell. 1999;3(6):799–804. doi: 10.1016/S1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 24.Qin LX, Tang ZY, Sham JS, Ma ZC, Ye SL, Zhou XD, Wu ZQ, Trent JM, Guan XY. The association of chromosome 8p deletion and tumor metastasis in human hepatocellular carcinoma. Cancer Res. 1999;59(22):5662–5665. [PubMed] [Google Scholar]
- 25.Aubele M, Mattis A, Zitzelsberger H, Walch A, Kremer M, Hutzler P, Hofler H, Werner M. Intratumoral heterogeneity in breast carcinoma revealed by laser-microdissection and comparative genomic hybridization. Cancer Genet Cytogenet. 1999;110(2):94–102. doi: 10.1016/S0165-4608(98)00205-2. [DOI] [PubMed] [Google Scholar]