Abstract
Cystinosis is the major cause of inherited Fanconi syndrome, and should be suspected in young children with failure to thrive and signs of renal proximal tubular damage. The diagnosis can be missed in infants, because not all signs of renal Fanconi syndrome are present during the first months of life. In older patients cystinosis can mimic idiopathic nephrotic syndrome due to focal and segmental glomerulosclerosis. Measuring elevated white blood cell cystine content is the corner stone for the diagnosis. The diagnosis is confirmed by molecular analysis of the cystinosin gene. Corneal cystine crystals are invariably present in all patients with cystinosis after the age of 1 year. Treatment with the cystine depleting drug cysteamine should be initiated as soon as possible and continued lifelong to prolong renal function survival and protect extra-renal organs. This educational feature provides practical tools for the diagnosis and treatment of cystinosis.
Keywords: Cystinosis, Cystinosin, Renal Fanconi syndrome, Proximal tubule, Cysteamine
Introduction
During the last few decades, significant progress has been made in elucidating the genetic background and pathogenesis of numerous metabolic disorders, allowing earlier diagnosis and better treatment. However, because many of these disorders are relatively rare and can have various presentations, recognizing them in patients still poses problems. Furthermore, individual physicians frequently lack experience in adequate management of the patients. The objective of this teaching article is to provide pediatric nephrologists with practical tools for the diagnosis and treatment of nephropathic cystinosis. The pathogenesis of cystinosis, which is still not completely elucidated, remains beyond the scope of this review.
Definition and clinical forms of cystinosis
Cystinosis is an autosomal recessive disorder characterized by an accumulation of the amino acid cystine in lysosomes throughout the body. The responsible gene CTNS, encoding the lysosomal cystine carrier cystinosin, has been cloned in 1998 and is located on the short arm of the chromosome 17 (p13) [1, 2].
Depending on the age at presentation and the degree of disease severity, three clinical forms of cystinosis are distinguished:
Nephropathic infantile form (MIM #219800), which is the most frequent and most severe form of the disease
Nephropathic juvenile form (MIM #219900); synonyms: intermediate cystinosis, late-onset form, adolescent form
Non-nephropathic adult form (MIM #219750); synonyms: benign non-nephropathic cystinosis, ocular non-nephropathic cystinosis
All three forms of the disease are caused by mutations of the CTNS gene and have phenotypic overlap.
Clinical presentation of renal disease in cystinosis
Nephropathic infantile cystinosis
Patients with infantile cystinosis are generally born from uneventful pregnancies and have normal birth weight and length. Despite cystine accumulation starting in utero, clinical symptoms are absent at birth and gradually develop during the first months of life. The kidneys are the first affected organs, and progressively lose function of their proximal tubular transporters, resulting in urinary loss of water, Na+, K+, bicarbonate, Ca2+, Mg2+, phosphate, amino acids, glucose, proteins, and many other solutes reabsorbed in this nephron segment. This generalized proximal tubular dysfunction is called “deToni–Debré–Fanconi syndrome” or “renal Fanconi syndrome” for short, named after the pediatricians who first described the disorder in the last century [3]. Asymptomatic aminoaciduria can already appear during the first weeks of life and is followed by glucosuria, phosphaturia, and urinary bicarbonate losses during the first months of infancy [4, 5]. In one sibling of a known patient with cystinosis, longitudinally followed from birth, the excretion of the low molecular weight (LMW) protein alpha-1 microglobulin was increased only at the age of 6 months [5]. This observation indicates that diverse proximal tubular transporters have differential sensitivity to cystinosin dysfunction, and that the diagnosis of cystinosis can be missed during the first months of life, especially when only a limited number of urinary markers are used to identify renal Fanconi syndrome. At the age of 6 months, full-blown Fanconi syndrome is usually present and causes clinical symptoms of polyuria, thirst, failure to thrive, growth retardation, vomiting, periods of dehydration, constipation, developmental delay, and rickets in some patients. Biochemically, the patients present with hypokalemia, hypophosphatemia, metabolic acidosis, low serum uric acid, low carnitine, and, sometimes, hyponatremia [2]. Occasionally, hypokalemia in combination with hypochloremic metabolic alkalosis and elevated plasma renin activity can mimic Bartter syndrome [6, 7]. Proteinuria can reach grams per day, and consists of LMW proteins, albumin, and high molecular weight proteins [8]. Excessive losses of calcium and phosphate can cause the development of nephrocalcinosis and the formation of renal stones [9].
Because the clinical condition of most patients remains quite satisfactory for several months and not all characteristic symptoms are present in the same young patient, the current approach of adapting the feeding scheme and screening for malabsorption syndromes or food allergy frequently results in several months' delay in correct diagnosis.
In most patients the glomerular filtration rate (GFR) remains normal for up to 2 years and then progressively deteriorates towards end stage renal disease (ESRD) at the end of the first decade [10].
Both hemodialysis and peritoneal dialysis are suitable for renal replacement therapy (RRT) in cystinosis patients. The choice for the dialysis mode is made comparably to patients with other renal disorders.
Renal transplantation is the treatment of choice in patients with ESRD, as the disease does not recur in the grafted organ. Cystine crystals can be observed in graft biopsies, but are originating from the host mononuclear cells, and are of no pathological value [11]. Two independent studies demonstrated superior renal graft survival in cystinosis, compared with other renal diseases [12, 13]. However, analyzing data from the ERA-EDTA registry failed to demonstrate this advantage [14]. Renal Fanconi syndrome can persist after initiation of dialysis or after renal transplantation, but only rarely necessitates a nephrectomy of the native kidneys, because excessive fluid and electrolyte losses generally decrease during RRT.
Nephropathic juvenile form
The nephropathic juvenile form of the disease is diagnosed in the minority of the patients (~5%) and manifests with a spectrum of symptoms, varying from milder (compared with the infantile form) proximal tubulopathy to an apparent nephrotic syndrome [2, 15]. In terms of the age at presentation, there is a continuum between the infantile and the late-onset form; however, most of the patients described were older than 10 years. The deterioration of renal function also occurs in the late-onset form, but the rate of renal disease progression is mostly slower compared with the infantile form of cystinosis.
Non-nephropathic adult form
Ocular non-nephropathic cystinosis manifests only with complaints of photophobia due to cystine accumulation in the cornea of the eye, which is also present in nephropathic cystinosis. The kidney, retina, and other organs are spared in these patients [16, 17]. Recently, the coexistence of juvenile and ocular forms of cystinosis was described in one family [18], suggesting that there might be a continuum between mild forms of cystinosis and thus warranting the follow-up of renal function in patients with adult cystinosis.
Renal pathology
Renal biopsy is not required for the diagnosis of cystinosis, and therefore the descriptions of renal histology at early stages of the disease are limited. Serial renal biopsy in 2 cystinosis patients demonstrated an atrophy of the proximal convoluted tubules, called a “swan neck deformity”, which appeared after 6 months of life [19]. In a larger series of kidney specimens the most striking features were the irregularities of the tubular brush border and very large cells with a prominent and hyperchromatic cytoplasm [20]. Cystine crystals are mostly seen within the interstitial cells and rarely in the podocytes, but not in the tubular cells. Giant multinucleated podocytes and parietal epithelial cells are frequently observed and are pathognomonic for the disorder [21]. Electron microscopy demonstrates podocyte foot process retraction, especially in patients with pronounced proteinuria [8].
The deterioration of renal function is accompanied by progressive tubulo-interstitial and glomerular lesions, consisting of interstitial fibrosis, tubular atrophy, segmental or global collapsing of the glomerular tuft and an accumulation of mesangial matrix material [20].
Renal histology in patients with late-onset cystinosis may demonstrate glomerular lesions, undistinguishable from idiopathic focal and segmental glomerulosclerosis (FSGS) [18], and therefore the diagnosis can be missed in older patients presenting with nephrotic syndrome.
Extra-renal symptoms of cystinosis
Eye involvement
Corneal cystine crystals are absent at birth and generally can be observed by an experienced ophthalmologist with slit lamp examination at the age of 1 year. These crystals cause reflections of light and result in photophobia with substantial discomfort. Untreated teenagers may develop painful corneal erosions, punctate, filamentous or band keratopathy, iris crystals, and peripheral corneal neovascularization [22, 23]. The degeneration of the retinal pigment epithelium, resulting in patchy depigmentation of the retina (beginning in the periphery and extending in time), may cause visual impairment mostly starting from the second decade of life [24]. However, retinal epithelium vacuolization has already described in an 18-week-old fetus and fundoscopic retinal changes were observed in two siblings with cystinosis as early as 5 and 10 weeks of age [25, 26].
Endocrine organ involvement
The continuing multi-organ accumulation of cystine crystals leads to impairment of endocrine organs. Hypothyroidism is found in up to 70% of untreated cystinosis patients older than 10 years [27]. Impaired insulin production can be exacerbated by steroid therapy after renal transplantation and results in insulin-dependent diabetes mellitus [28]. Puberty generally proceeds normally in females, and several have given birth. Males may have primary hypogonadism and do not always complete pubertal development [29]. So far, no male cystinotic patients are known to have induced pregnancy, even while under adequate cysteamine treatment. Recently, azoospermia was demonstrated in cystinotic males treated with cysteamine, although spermatogenesis was observed in a testis biopsy of one patient, opening new possibilities for in vitro fertilization [30].
Central nervous system and muscle involvement
Two major types of cystinotic encephalopathy, developing predominantly during the third decade of life, are distinguished: encephalopathy presenting with cerebellar and pyramidal signs, mental deterioration and pseudo-bulbar palsy; or encephalopathy associated with stroke-like episodes [31]. The most common imaging finding in these patients is cerebral cortical atrophy, as visualized by computed tomography (CT) [32]; however, non-absorptive hydrocephalus, demyelinization of the internal capsule and brachium pontis, mineralization of the hemispheres and basal ganglia have also been reported [33]. Moreover, benign intracranial hypertension presenting with headaches and papilledema has been described as well [34]. Although general intelligence is normal in cystinosis patients, an alteration of specific neurocognitive functions such as visual–motor integration, visual memory, sustained attention, planning, and motor speed have been observed [35]. These intellectual deficits are present already in young children and are suggested to originate from a developmental abnormality of the cerebral white matter [36].
Vacuolar myopathy, resulting from excessive cystine accumulation in the muscle, manifests generally after the 10th birthday [37]. Patients suffer from progressive muscle wasting and reduced strength with restrictive ventilatory defects [38]. High prevalence of swallowing difficulty (>50%), including oral, pharyngeal and esophageal swallowing phases, has been recently reported in a study population of 101 patients with cystinosis aged 6 to 45 years [39]. The severity of swallowing dysfunction had a direct relation to the severity of muscular disease and decreased with the number of years on cysteamine therapy [39].
Other organ involvement
Other reported extra-renal symptoms include decreased skin and hair pigmentation (in patients of European descent), impaired sweating, exocrine pancreas deficiency, portal hypertension, hypersplenism and cystinotic bone disease [2, 38]. The latter is multifactorial in nature and is attributed to losses of calcium, phosphate, and vitamin D, cystine accumulation in the bone, and uremic osteodystrophy [40]. Notably, dual-energy X-ray absorptiometry (DEXA) does not predict the risk of fractures in cystinosis patients possibly due to false elevation of bone mineral composition by bone cystine crystals [41] .
Patients of African or Middle Eastern origin can have dark hair and dark skin, which should not exclude the diagnosis.
Diagnosis of cystinosis
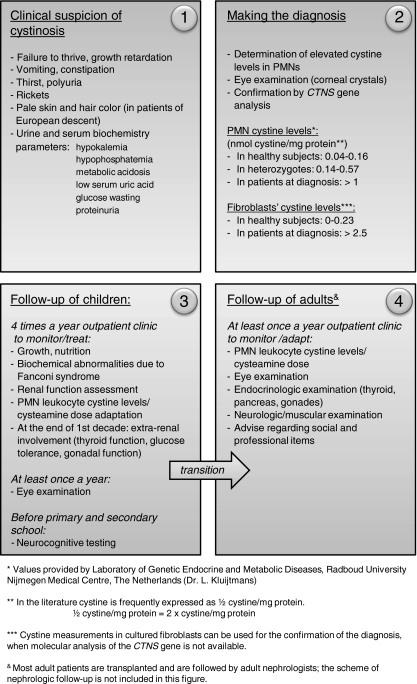
A flow chart that can be used for the diagnosis and subsequent follow-up of patients with cystinosis is presented in Fig. 1. Cystinosis should be suspected in all patients with failure to thrive and signs of renal Fanconi syndrome, as it is the most common cause of inherited Fanconi syndrome in children. The detection of elevated intracellular cystine content is the cornerstone for the diagnosis. The methods for cystine determination differ depending on the cell type: mixed leukocyte preparation or polymorphonuclear (PMN) leukocytes. Furthermore, several biochemical methods are currently used for cystine measurement, such as a cystine-binding assay, amino acid chromatography or high performance liquid chromatography, making it difficult to compare the results of different laboratories [42–44]. In our opinion, cystine measurement should be performed in isolated PMN leukocytes, as these cells preferentially accumulate cystine in blood. This method increases the sensitivity of cystine detection during the monitoring of cysteamine treatment [45]. The cystine-binding assay has been a gold standard for cystine measurements for years; however, at present, most laboratories have switched to the other methods of detection, because of lower price and avoidance of radioactivity. In this respect, tandem mass spectrometry (tandem MS) is the most sensitive method and is currently widely used for cystine determination in cystinosis [46]. Each laboratory performing cystine measurements should provide their own reference values in patients at the time of diagnosis, and also for heterozygote and healthy subjects. Reference values, provided by our laboratory are indicated in Fig. 1. In Europe, the quality of cystine measurements in individual laboratories is monitored by ERNDIM (European Research Network for evaluation and improvement of screening, Diagnosis and treatment of Inherited disorders of Metabolism) [47].
Fig. 1.
Flowchart for the diagnosis and follow-up of patients with cystinosis
Molecular analysis of the cystinosin gene allows early diagnosis and can be used for prenatal diagnosis of the disease. Prenatal diagnosis of cystinosis can also be made by measuring 35S-labeled cystine accumulation in cultured amniocytes or chorionic villi samples (CVS); and by a direct measurement of cystine in uncultured CVS [48]. Since the cloning of CTNS in 1998, over 90 mutations have been reported, with a detection ratio close to 100% [49–53]. The most common mutation accounting for approximately 75% of the affected alleles in Northern Europe is a 57-kb deletion, affecting the first 10 exons of CTNS, the 5’ region upstream encoding the CARKL gene [50] and the first two non-coding exons of TRPV1. While renal phenotype in infantile cystinosis is comparable between patients with homozygous 57-kb deletion and those with other truncating mutations, this is unknown with regard to extra-renal phenotype. A genotype–phenotype correlation related to the clinical forms of cystinosis is observed, with severe truncating mutations mostly found in patients with the infantile form of the disease and at least one mutation allowing the residual function of cystinosin in patients with intermediate or adult cystinosis. However, several unexplained exceptions were reported [54].
Differential diagnosis
Cystinosis should be distinguished from other inherited causes of renal Fanconi syndrome, which are summarized in Table 1. After 1 year of age, the observation of cystine crystals in the cornea is pathognomonic for cystinosis, whereas the absence of the crystals excludes the diagnosis after the age of 2 years. Patients with the juvenile form of cystinosis also invariably demonstrate corneal cystine crystals.
Table 1.
Causes of renal Fanconi syndrome to be considered for differential diagnoses of cystinosis
| Disorder (OMIM) | Gene | Inheritance | Protein | Key clinical/biochemical features |
|---|---|---|---|---|
| ARC syndrome | VPS33B | autosomal recessive | Vps33 | Arthrogryposis, cholestasis, dysmorphic features, ichtiosis, abnormal platelets, severe infections |
| Cystinosis | CTNS | autosomal recessive | Cystinosin | Failure to thrive, rickets, metabolic, acidosis, renal failure, photophobia |
| Dent’s diseasea | CLCN5 | X-linked recessive | Chloride channel 5 | LMW proteinuria, hypercalciuria, nephrocalcinosis, nephrolithiasis, renal failure |
| Fanconi–Bickel syndrome | SLC2A2 | autosomal recessive | Facilitative glucose transporter 2 (GLUT2) | Hepatorenal glycogen accumulation, hepatomegaly, rachitic and osteomalacia, mental retardation |
| Galactosaemia | GALT | autosomal recessive | Galactose-1-phosphate-uridyl-transferase | Hepatomegaly, liver disease, cataract, mental retardation |
| Glycogen Storage Disease Type 1 | G6PC | autosomal recessive | Glucose-6-phosphatase | Hypoglycemia, lactic acidosis and hyperlipidemia, hepatomegaly |
| Hereditary fructose intolerance | ALDOB | autosomal recessive | Aldose B | Fructose intolerance, growth retardation |
| I-cell disease (mucolipidosis II) | GNPTAB | autosomal recessive | N-acetylglucosamine-1-phosphotransferase | Dwarfism, contractures of large joints, coarse facial features, thickend skin and mucosae, mental retardation |
| Idiopathic Fanconi syndrome | Unknown | autosomal dominant autosomal recessive | Unknown | Isolated Fanconi syndrome |
| Lowe syndromea | OCRL1 | X-linked recessive | Phosphatidyl-inositol 4,5-biphosphate-5-phosphatase | Short stature, congenital cataract, mental retardation, seizures, cryptorchidism, arthropathy, elevated transaminases and creatine kinase |
| Metachromatic leukodystrophy | ARSA; PSAP | autosomal recessive | Arylsulfatase A; Prosaposin | Muscle wasting and weakness, spasticity, developmental regression |
| Mitochondrial diseaseb | Diverse | Diverse | Diverse | Diverse |
| Tyrosinaemia | FAHD2A | autosomal recessive | Fumaryl-acetoacetate hydrolase | Glomerulosclerosis, nephrocalcinosis, hepatomegaly, cirrhosis, rickets, growth retardation |
| Wilson disease | ATP7B | autosomal recessive | Copper-transporting ATPase (β subunit) | Kayser–Fleischer rings (cornea), hepatitis, cirrhosis CNS abnormalities |
aGlucosuria can be absent
bPatients with mitochondrial diseases can present with Fanconi syndrome. Among other reported genes, affected proteins involve cytochrome C oxidase, phosphoenolpyruvate carboxykinase or Acyl-CoA dehydrogenase
It is noteworthy that a second lysosomal cystine storage disease, namely mucolipidosis type II, or I-cell disease, has been identified [55]. Clinical symptoms are very heterogeneous, but usually include dwarfism, coarse facial features, and mental retardation [56]. Additionally, proximal tubular dysfunction was reported with LMW proteinuria, aminoaciduria, hyperphosphaturia, and high urinary calcium excretion [57]. This autosomal recessive disorder is caused by a deficiency of N-acetylglucosamine-1-phosphotransferase, resulting in defective mannose-6-phosphorylation [58]. As a consequence, lysosomal enzymes are not transported to the lysosomes upon post-translational modification in the Golgi apparatus. Similar to cystinosis, lysosomal cystine levels are elevated in fibroblasts isolated from patients with I-cell disease, but hepatic or white blood cell cystine levels are within the normal range [59].
In patients with Bartter-like presentation, the concomitant presence of symptoms of proximal tubular dysfunction such as aminoaciduria, glucosuria, and phosphaturia should not allow the diagnosis of cystinosis to be missed. Patients with intermediate cystinosis presenting with pronounced proteinuria and FSGS in renal biopsies can be easily overlooked. Eye examination demonstrating corneal cystine crystals and possibly mild tubulopathy should point to the diagnosis of cystinosis in these patients.
Treatment
Life style matters
All patients with cystinosis should have free access to water and toilet, because of pronounced polyuria and polydipsia. Prolonged exposure to heat and sun should be avoided because of photophobia, the risk of dehydration and/or heat stroke due to impaired sweating.
Symptomatic therapy
The aim of symptomatic therapy in patients presenting with Fanconi syndrome is the maintenance of fluid and electrolyte balance, good nutrition and prevention of rickets. The dose of potassium, sodium, bicarbonate, and phosphate varies substantially and shall be regularly adapted according to serum values. 1,25-dihydroxycholecalciferol supplementation should be used starting from early childhood [60]. However, excessive administration of phosphate, 1,25-dihydroxycholecalciferol and bicarbonate may aggravate the development of nephrocalcinosis or stimulate renal stone formation [60, 61]. Calcium supplementation is generally not indicated. Carnitine replacement normalizes plasma and muscular carnitine levels; however, it is not established whether carnitine administration results in improved muscular performance [62, 63].
Poor appetite, vomiting, and oral motor dysfunction often require a nasogastric tube or gastrostomy feeding, especially in young children [64, 65]. Treatment with recombinant growth hormone results in catch-up growth and further maintenance of growth velocity [66]. Growth hormone is frequently not required in patients treated with cysteamine, especially when started at an early age, as cysteamine by itself improves growth [67].
Other complications, such as hypothyroidism, diabetes, or hypogonadism, are considered for treatment with levothyroxin, insulin, and testosterone respectively.
Inhibition of the renin–angiotensin system (RAS) results in a decrease of albuminuria in cystinosis [68]; however, this class of drugs should be used with caution, because of the risk of hypotension and renal function decline in cystinosis patients already suffering from extracellular volume and/or salt depletion.
Specific treatment with cysteamine
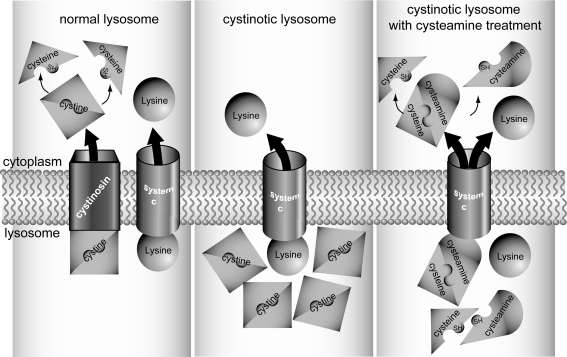
The amino thiol cysteamine depletes lysosomal cystine content by a disulfide exchange reaction with cystine, resulting in the formation of cysteine–cysteamine mixed disulfide and cysteine (Fig. 2). Cysteine–cysteamine mixed disulfide exits lysosomes via a “system c” transporter and the remaining cysteine via a cysteine carrier. The administration of cysteamine at 1.3–1.9 g/m2 in four daily doses drastically lowers the cystine content of the lysosomes, postpones or even prevents the deterioration of renal function and the development of extra-renal complications [69, 70]. Furthermore, cysteamine treatment improves growth [71]. Cysteamine should be administered as soon as the diagnosis of cystinosis is made, and continued lifelong, even after renal transplantation to protect the extra-renal organs.
Fig. 2.
Cystine depleting action of cysteamine. In the left panel, a normal lysosome is presented; cystine located in the lysosomes is exported via cystinosin. In the cytosol, cystine is reduced to two cysteine residues. The middle panel shows a cystinotic lysosome, where cystinosin is absent (or dysfunctional), resulting in increased levels of lysosomal cystine. Upon cysteamine treatment in the cystinotic lysosome, cystine is degraded into cysteine and cysteine–cysteamine, as presented in the right panel. Both degradation products can be exported via cysteine and as yet unidentified “system c” transporters, encompassing the defective cystinosin
The side effects of cysteamine are mostly restricted to gastrointestinal discomfort (due to the release of gastrin and the resulting stimulation of H+ secretion in the stomach [72]), and bad breath and sweat odor (due to formation of dimethylsulfide and methanethiol, which are metabolites of cysteamine [73]). Gastric acid hypersecretion and ulcerogenicity of cysteamine can be improved by the administration of proton pump inhibitors [70]. Allergic reactions, fever, seizures, and neutropenia are also reported, especially when the dose of the drug is abruptly increased. Recently, 8 European patients treated with high cysteamine doses were reported to exhibit endothelial proliferative lesions on the elbows, skin striae, and bone and muscular pain, which improved or disappeared after lowering the cysteamine dosing (personal communication from Orphan Europe). Because of these adverse events, using cysteamine doses above the recommended 1.9 g/m2 should be discouraged. Cysteamine dose calculation per kg body weight (60–90 mg/kg/day) results in dosing above the recommended range in children > 20 kg.
As the target tissue cystine levels necessary to prevent the progression of renal disease and the occurrence of extra-renal complications are still unknown, the 0.9 percentile of heterozygote values in the PMN cells is mostly recommended as an upper cystine limit before the next dose of cysteamine is given (<0.5 nmol cystine per mg protein) [74]. Because leukocyte cystine content returns to the initial high levels 6 h after cysteamine administration [75], the drug should be taken every 6 h including through the night [76]. For monitoring cysteamine therapy blood should be drawn 6 h after the last intake of the drug. The current development of enteric-coated cysteamine formulation will remarkably ameliorate compliance with cysteamine therapy and improve the quality of life, when its efficiency and safety have been confirmed in ongoing clinical trials [77].
The regular measurements of cystine in PMN leukocytes are required in order to adjust cysteamine dose. Unfortunately, in the majority of patients cysteamine cannot reverse Fanconi syndrome and only postpones the development of renal failure.
Cysteamine toxicity with the development of cleft palate and kyphosis, as well as intrauterine growth retardation and fetal death, without signs of maternal toxicity, was observed in rats treated with high cysteamine dose (100–150 mg/kg/day) [78]. Although the effect in humans treated with lower doses is unknown, it is generally recommended to discontinue cysteamine in female patients who are planning a pregnancy.
Because systemic cysteamine treatment has no effect on corneal cystine crystals, topical 0.5 % cysteamine eye drops are indicated. These drops are highly effective and when administered 6 to 12 times daily are able to dissolve corneal cystine crystals completely within 8 to 41 months, even at a later age [79]. Hopefully, commercially available cysteamine eye drops, gel formulation or ointment can be developed to decrease the required frequency of application and to improve compliance [80].
A recent pioneering study using CTNS-knockout mice demonstrated a beneficial effect of syngeneic bone marrow and hematopoietic stem cell transplantation on cystine accumulation in various organs and on renal function survival [81], emphasizing novel potential therapeutic possibilities for cystinosis patients.
Follow-up of cystinosis patients
Follow-up recommendations for cystinosis patients, applied in our institutions, are summarized in Fig. 1. Infants and children with cystinosis who have renal Fanconi syndrome should be examined frequently (at least four times a year) to monitor growth and the nutritional state, metabolic control of Fanconi syndrome, and white blood cell cystine measurements for adapting cysteamine dose. Ophthalmic control, including slit lamp examination and funduscopy, at a minimum of once a year, is mandatory. Children in pre- and school age should undergo neurocognitive testing to detect specific deficiencies and to adapt the school program (if required). Children after renal transplantation are usually followed according to the rules in the transplantation clinic; however, cystine measurements and eye examination should be performed with the same frequency as in children with renal Fanconi syndrome. Starting from the end of the first decade of life, special attention should be paid to the possible appearance of extra-renal complications. Adolescents should be informed about the influence of cystinosis on their sexual development and fertility. Qualified advice regarding study and job choices should be performed by psychologists and social workers. Transition to the adult multi-disciplinary clinics, preferably experienced in treatment of cystinosis patients, should be carefully prepared. In stable adult patients, cystine white blood cells levels should be monitored at least once a year. Additionally, yearly check-ups by an ophthalmologist, endocrinologist, and neurologist are required to monitor the extra-renal complications of the disease.
Take-home message
Despite the awareness of the medical community, the diagnosis of cystinosis is frequently delayed, because of the initial incomplete presentation and the rarity of the disorder. In all patients presenting with failure to thrive during the first years of life, renal Fanconi syndrome should be excluded. The true diagnosis of cystinosis is based on the measurement of elevated cystine content in blood cells, and molecular analysis of the CTNS gene. Cysteamine is currently the only available treatment interfering with the disease pathogenesis; however, new treatment modalities may become available in the future.
Multiple choice questions
Answers appear following the reference list
- Prenatal diagnosis of cystinosis is possible by:
- Measuring leukocyte cystine content in the mother
- Measuring the 35S cystine accumulation in a chorionic villi sample or cultured amniocytes
- Molecular analysis of the CTNS gene in fetal tissue
- Measuring alfa-fetoprotein in the amniotic fluid
- In a newborn cystinosis can be diagnosed by:
- Measuring leukocyte cystine content
- Screening for symptoms of renal Fanconi syndrome (urinary excretion of LMW proteins, phosphate, amino acids, glucose)
- Molecular analysis of the CTNS gene
- Detection of cystine crystals in the cornea
- All of the above
- After making diagnosis of cystinosis, treatment with cysteamine should be started:
- Immediately upon confirmation of diagnosis
- As soon as symptoms of renal Fanconi syndrome are present
- When GFR is decreased
- After renal transplantation cysteamine treatment should be:
- Discontinued, because of possible side-effects
- Discontinued, because of interaction with immunosuppressive treatment
- Continued, but only if renal graft function is decreased
- Continued, to protect the extra renal-organs
- An adolescent with cystinosis complains about the side-effects of cysteamine medication (vomiting, bad odor, frequent intake), because they interfere with his social life. Therefore, he sometimes skips one of the intakes, but uses a higher dose of cysteamine on the other occasions. Given the circumstances, what is advisable?
- Instead of four daily doses, divide the same cysteamine dose over three intakes
- Explain that cysteamine is active for only 6 h and therefore should be taken four times daily. Occasionally taking high doses increases the risk of side-effects
- Administer proton pump inhibitors to treat gastric discomfort
Footnotes
Answers
1. b and c
2. a and c
3. a
4. d
5. b and c
References
- 1.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, Van't Hoff W, Antignac C. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 2.Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 3.Fanconi G, Bickel H. Die chronische Aminoacidurie (Aminosäurediabetes oder nephrotisch-glukosurischer Zwergwuchs) bei der Glykogenose und der Cystinkrankheit. Helv Paediatr Acta. 1949;4:359–396. [PubMed] [Google Scholar]
- 4.Brodehl J, Hagge W, Gellissen K. Changes in kidney function in cystinosis. I. Inulin, PAH and electrolyte clearance in various stages of the disease. Ann Paediatr. 1965;205:131–154. [PubMed] [Google Scholar]
- 5.Levtchenko E, Monnens L. Development of Fanconi syndrome during infancy in a patient with cystinosis. Acta Paediatr. 2006;95:379–380. doi: 10.1080/08035250500369601. [DOI] [PubMed] [Google Scholar]
- 6.Pennesi M, Marchetti F, Crovella S, Boaretto F, Travan L, Lazzerini M, Neri E, Ventura A. A new mutation in two siblings with cystinosis presenting with Bartter syndrome. Pediatr Nephrol. 2005;20:217–219. doi: 10.1007/s00467-004-1702-y. [DOI] [PubMed] [Google Scholar]
- 7.Caltik A, Akyuz SG, Erdogan O, Bulbul M, Demircin G. Rare presentation of cystinosis mimicking Bartter's syndrome: reports of two patients and review of the literature. Ren Fail. 2010;32:277–280. doi: 10.3109/08860221003592804. [DOI] [PubMed] [Google Scholar]
- 8.Wilmer MJ, Christensen EI, Heuvel LP, Monnens LA, Levtchenko EN. Urinary protein excretion pattern and renal expression of megalin and cubilin in nephropathic cystinosis. Am J Kidney Dis. 2008;51:893–903. doi: 10.1053/j.ajkd.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Saleem MA, Milford DV, Alton H, Chapman S, Winterborn MH. Hypercalciuria and ultrasound abnormalities in children with cystinosis. Pediatr Nephrol. 1995;9:45–47. doi: 10.1007/BF00858968. [DOI] [PubMed] [Google Scholar]
- 10.Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med. 1993;328:1157–1162. doi: 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- 11.Spear GS, Gubler MC, Habib R, Broyer M. Renal allografts in cystinosis and mesangial demography. Clin Nephrol. 1989;32:256–261. [PubMed] [Google Scholar]
- 12.Ehrich JH, Brodehl J, Byrd DI, Hossfeld S, Hoyer PF, Leipert KP, Offner G, Wolff G. Renal transplantation in 22 children with nephropathic cystinosis. Pediatr Nephrol. 1991;5:708–714. doi: 10.1007/BF00857880. [DOI] [PubMed] [Google Scholar]
- 13.Kashtan CE, McEnery PT, Tejani A, Stablein DM. Renal allograft survival according to primary diagnosis: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol. 1995;9:679–684. doi: 10.1007/BF00868709. [DOI] [PubMed] [Google Scholar]
- 14.Rigden SP. Data from the ERA-EDTA registry. In: Broyer M, editor. Cystinosis. 1. Amsterdam: Elsevier; 1999. pp. 20–27. [Google Scholar]
- 15.Langman CB, Moore ES, Thoene JG, Schneider JA. Renal failure in a sibship with late-onset cystinosis. J Pediatr. 1985;107:755–756. doi: 10.1016/S0022-3476(85)80410-8. [DOI] [PubMed] [Google Scholar]
- 16.Schneider JA, Wong V, Bradley K, Seegmiller JE. Biochemical comparisons of the adult and childhood forms of cystinosis. N Engl J Med. 1968;279:1253–1257. doi: 10.1056/NEJM196812052792303. [DOI] [PubMed] [Google Scholar]
- 17.Anikster Y, Lucero C, Guo J, Huizing M, Shotelersuk V, Bernardini I, McDowell G, Iwata F, Kaiser-Kupfer MI, Jaffe R, Thoene J, Schneider JA, Gahl WA. Ocular nonnephropathic cystinosis: clinical, biochemical, and molecular correlations. Pediatr Res. 2000;47:17–23. doi: 10.1203/00006450-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Servais A, Moriniere V, Grunfeld JP, Noel LH, Goujon JM, Chadefaux-Vekemans B, Antignac C. Late-onset nephropathic cystinosis: clinical presentation, outcome, and genotyping. Clin J Am Soc Nephrol. 2008;3:27–35. doi: 10.2215/CJN.01740407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahoney CP, Striker GE. Early development of the renal lesions in infantile cystinosis. Pediatr Nephrol. 2000;15:50–56. doi: 10.1007/PL00013448. [DOI] [PubMed] [Google Scholar]
- 20.Gubler MC, Lacoste M, Sich M, Broyer M. The pathology of the kidney in cystinosis. In: Broyer M, editor. Cystinosis. 1. Amsterdam: Elsevier; 1999. pp. 42–48. [Google Scholar]
- 21.Stokes MB, Jernigan S, D'Agati VD. Infantile nephropathic cystinosis. Kidney Int. 2008;73:782–786. doi: 10.1038/sj.ki.5002730. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser-Kupfer MI, Caruso RC, Minkler DS, Gahl WA. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol. 1986;104:706–711. doi: 10.1001/archopht.1986.01050170096030. [DOI] [PubMed] [Google Scholar]
- 23.Tsilou ET, Rubin BI, Reed GF, Iwata F, Gahl W, Kaiser-Kupfer MI. Age-related prevalence of anterior segment complications in patients with infantile nephropathic cystinosis. Cornea. 2002;21:173–176. doi: 10.1097/00003226-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Tsilou ET, Rubin BI, Reed G, Caruso RC, Iwata F, Balog J, Gahl WA, Kaiser-Kupfer MI. Nephropathic cystinosis: posterior segment manifestations and effects of cysteamine therapy. Ophthalmology. 2006;113:1002–1009. doi: 10.1016/j.ophtha.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JA, Verroust FM, Kroll WA, Garvin AJ, Horger EO, III, Wong VG, Spear GS, Jacobson C, Pellett OL, Becker FL. Prenatal diagnosis of cystinosis. N Engl J Med. 1974;290:878–882. doi: 10.1056/NEJM197404182901604. [DOI] [PubMed] [Google Scholar]
- 26.Schneider JA, Wong V, Seegmiller JE. The early diagnosis of cystinosis. J Pediatr. 1969;74:114–116. doi: 10.1016/S0022-3476(69)80017-X. [DOI] [PubMed] [Google Scholar]
- 27.Chan AM, Lynch MJ, Bailey JD, Ezrin C, Fraser D. Hypothyroidism in cystinosis. A clinical, endocrinologic and histologic study involving sixteen patients with cystinosis. Am J Med. 1970;48:678–692. doi: 10.1016/S0002-9343(70)80002-X. [DOI] [PubMed] [Google Scholar]
- 28.Fivush B, Green OC, Porter CC, Balfe JW, O'Regan S, Gahl WA. Pancreatic endocrine insufficiency in posttransplant cystinosis. Am J Dis Child. 1987;141:1087–1089. doi: 10.1001/archpedi.1987.04460100065027. [DOI] [PubMed] [Google Scholar]
- 29.Winkler L, Offner G, Krull F, Brodehl J. Growth and pubertal development in nephropathic cystinosis. Eur J Pediatr. 1993;152:244–249. doi: 10.1007/BF01956154. [DOI] [PubMed] [Google Scholar]
- 30.Besouw MT, Kremer JA, Janssen MC, Levtchenko EN. Fertility status in male cystinosis patients treated with cysteamine. Fertil Steril. 2010;93:1880–1883. doi: 10.1016/j.fertnstert.2008.12.113. [DOI] [PubMed] [Google Scholar]
- 31.Broyer M, Tete MJ, Guest G, Bertheleme JP, Labrousse F, Poisson M. Clinical polymorphism of cystinosis encephalopathy. Results of treatment with cysteamine. J Inherit Metab Dis. 1996;19:65–75. doi: 10.1007/BF01799350. [DOI] [PubMed] [Google Scholar]
- 32.Nichols SL, Press GA, Schneider JA, Trauner DA. Cortical atrophy and cognitive performance in infantile nephropathic cystinosis. Pediatr Neurol. 1990;6:379–381. doi: 10.1016/0887-8994(90)90004-K. [DOI] [PubMed] [Google Scholar]
- 33.Vogel DG, Malekzadeh MH, Cornford ME, Schneider JA, Shields WD, Vinters HV. Central nervous system involvement in nephropathic cystinosis. J Neuropathol Exp Neurol. 1990;49:591–599. doi: 10.1097/00005072-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Dogulu CF, Tsilou E, Rubin B, Fitzgibbon EJ, Kaiser-Kupper MI, Rennert OM, Gahl WA. Idiopathic intracranial hypertension in cystinosis. J Pediatr. 2004;145:673–678. doi: 10.1016/j.jpeds.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 35.Delgado G, Schatz A, Nichols S, Appelbaum M, Trauner D. Behavioral profiles of children with infantile nephropathic cystinosis. Dev Med Child Neurol. 2005;47:403–407. doi: 10.1017/S0012162205000782. [DOI] [PubMed] [Google Scholar]
- 36.Bava S, Theilmann RJ, Sach M, May SJ, Frank LR, Hesselink JR, Vu D, Trauner DA. Developmental changes in cerebral white matter microstructure in a disorder of lysosomal storage. Cortex. 2010;46:206–216. doi: 10.1016/j.cortex.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gahl WA, Dalakas MC, Charnas L, Chen KT, Pezeshkpour GH, Kuwabara T, Davis SL, Chesney RW, Fink J, Hutchison HT. Myopathy and cystine storage in muscles in a patient with nephropathic cystinosis. N Engl J Med. 1988;319:1461–1464. doi: 10.1056/NEJM198812013192206. [DOI] [PubMed] [Google Scholar]
- 38.Anikster Y, Lacbawan F, Brantly M, Gochuico BL, Avila NA, Travis W, Gahl WA. Pulmonary dysfunction in adults with nephropathic cystinosis. Chest. 2001;119:394–401. doi: 10.1378/chest.119.2.394. [DOI] [PubMed] [Google Scholar]
- 39.Sonies BC, Almajid P, Kleta R, Bernardini I, Gahl WA. Swallowing dysfunction in 101 patients with nephropathic cystinosis—benefit of long-term cysteamine therapy. Medicine. 2005;84:137–146. doi: 10.1097/01.md.0000164204.00159.d4. [DOI] [PubMed] [Google Scholar]
- 40.Nesterova G, Gahl W. Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr Nephrol. 2008;23:863–878. doi: 10.1007/s00467-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 41.Zimakas PJA, Sharma AK, Rodd CJ. Osteopenia and fractures in cystinotic children post renal transplantation. Pediatr Nephrol. 2003;18:384–390. doi: 10.1007/s00467-003-1093-5. [DOI] [PubMed] [Google Scholar]
- 42.Oshima RG, Willis RC, Furlong CE, Schneider JA. Binding assays for amino acids. The utilization of a cystine binding protein from Escherichia coli for the determination of acid-soluble cystine in small physiological samples. J Biol Chem. 1974;249:6033–6039. [PubMed] [Google Scholar]
- 43.Ged C, Jean G, Tete MJ, Broyer M, Kamoun P. Cystine leukocyte content in cystinotic patients receiving cysteamine. Ann Biol Clin. 1991;49:482–486. [PubMed] [Google Scholar]
- 44.Graaf-Hess A, Trijbels F, Blom H. New method for determining cystine in leukocytes and fibroblasts. Clin Chem. 1999;45:2224–2228. [PubMed] [Google Scholar]
- 45.Levtchenko E, Graaf-Hess A, Wilmer M, Heuvel L, Monnens L, Blom H. Comparison of cystine determination in mixed leukocytes vs polymorphonuclear leukocytes for diagnosis of cystinosis and monitoring of cysteamine therapy. Clin Chem. 2004;50:1686–1688. doi: 10.1373/clinchem.2004.031872. [DOI] [PubMed] [Google Scholar]
- 46.Chabli A, Aupetit J, Raehm M, Ricquier D, Chadefaux-Vekemans B. Measurement of cystine in granulocytes using liquid chromatography-tandem mass spectrometry. Clin Biochem. 2007;40:692–698. doi: 10.1016/j.clinbiochem.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Fowler B, Burlina A, Kozich V, Vianey-Saban C. Quality of analytical performance in inherited metabolic disorders: the role of ERNDIM. J Inherit Metab Dis. 2008;31:680–689. doi: 10.1007/s10545-008-1025-4. [DOI] [PubMed] [Google Scholar]
- 48.Jackson M, Young E. Prenatal diagnosis of cystinosis by quantitative measurement of cystine in chorionic villi and cultured cells. Prenat Diagn. 2005;25:1045–1047. doi: 10.1002/pd.1249. [DOI] [PubMed] [Google Scholar]
- 49.Forestier L, Jean G, Attard M, Cherqui S, Lewis C, Van't Hoff W, Broyer M, Town M, Antignac C. Molecular characterization of CTNS deletions in nephropathic cystinosis: development of a PCR-based detection assay. Am J Hum Genet. 1999;65:353–359. doi: 10.1086/302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Touchman JW, Anikster Y, Dietrich NL, Maduro VVB, McDowell G, Shotelersuk V, Bouffard GG, Beckstrom-Sternberg SM, Gahl WA, Green ED. The genomic region encompassing the nephropathic cystinosis gene (CTNS): complete sequencing of a 200-kb segment and discovery of a novel gene within the common cystinosis-causing deletion. Genome Res. 2000;10:165–173. doi: 10.1101/gr.10.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anikster Y, Shotelersuk V, Gahl WA. CTNS mutations in patients with cystinosis. Hum Mutat. 1999;14:454–458. doi: 10.1002/(SICI)1098-1004(199912)14:6<454::AID-HUMU2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Valero EM, Ballart A, Iturriaga C, Lluch M, Macias J, Vanier MT, Pineda M, Coll MJ. Identification of 25 new mutations in 40 unrelated Spanish Niemann-Pick type C patients: genotype-phenotype correlations. Clin Genet. 2005;68:245–254. doi: 10.1111/j.1399-0004.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 53.Alcantara-Ortigoza MA, Belmont-Martinez L, Vela-Amieva M, Gonzalez-Del AA. Analysis of the CTNS gene in nephropathic cystinosis Mexican patients: report of four novel mutations and identification of a false positive 57-kb deletion genotype with LDM-2/exon 4 multiplex PCR assay. Genet Test. 2008;12:409–414. doi: 10.1089/gte.2008.0014. [DOI] [PubMed] [Google Scholar]
- 54.Kalatzis V, Nevo N, Cherqui S, Gasnier B, Antignac C. Molecular pathogenesis of cystinosis: effect of CTNS mutations on the transport activity and subcellular localization of cystinosin. Hum Mol Genet. 2004;13:1361–1371. doi: 10.1093/hmg/ddh152. [DOI] [PubMed] [Google Scholar]
- 55.Tietze F, Butler JD. Elevated cystine levels in cultured skin fibroblasts from patients with I-cell disease. Pediatr Res. 1979;13:1350–1355. doi: 10.1203/00006450-197912000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Beck M, Barone R, Hoffmann R, Kratzer W, Rakowsky T, Nigro F, Fiumara A. Inter- and intrafamilial variability in mucolipidosis II (I-cell disease) Clin Genet. 1995;47:191–199. doi: 10.1111/j.1399-0004.1995.tb03958.x. [DOI] [PubMed] [Google Scholar]
- 57.Bocca G, Monnens LA. Defective proximal tubular function in a patient with I-cell disease. Pediatr Nephrol. 2003;18:830–832. doi: 10.1007/s00467-003-1213-2. [DOI] [PubMed] [Google Scholar]
- 58.Kornfeld S, Sly WS. I-cell disease and pseudo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation and localization. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 1995. pp. 2495–2508. [Google Scholar]
- 59.Greene AA, Jonas AJ, Harms E, Smith ML, Pellett OL, Bump EA, Miller AL, Schneider JA. Lysosomal cystine storage in cystinosis and mucolipidosis type II. Pediatr Res. 1985;19:1170–1174. doi: 10.1203/00006450-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Loirat C. Symptomatic therapy. In: Broyer M, editor. Cystinosis. 1. Amsterdam: Elsevier; 1999. pp. 97–102. [Google Scholar]
- 61.Theodoropoulos DS, Shawker TH, Heinrichs C, Gahl WA. Medullary nephrocalcinosis in nephropathic cystinosis. Pediatr Nephrol. 1995;9:412–418. doi: 10.1007/BF00866713. [DOI] [PubMed] [Google Scholar]
- 62.Gahl WA, Bernardini I, Dalakas M, Rizzo WB, Harper GS, Hoeg JM, Hurko O, Bernar J. Oral carnitine therapy in children with cystinosis and renal Fanconi syndrome. J Clin Invest. 1988;81:549–560. doi: 10.1172/JCI113353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gahl WA, Bernardini IM, Dalakas MC, Markello TC, Krasnewich DM, Charnas LR. Muscle carnitine repletion by long-term carnitine supplementation in nephropathic cystinosis. Pediatr Res. 1993;34:115–119. doi: 10.1203/00006450-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Elenberg E, Norling LL, Kleinman RE, Ingelfinger JR. Feeding problems in cystinosis. Pediatr Nephrol. 1998;12:365–370. doi: 10.1007/s004670050467. [DOI] [PubMed] [Google Scholar]
- 65.Trauner DA, Fahmy RF, Mishler DA. Oral motor dysfunction and feeding difficulties in nephropathic cystinosis. Pediatr Neurol. 2001;24:365–368. doi: 10.1016/S0887-8994(01)00268-5. [DOI] [PubMed] [Google Scholar]
- 66.Wuhl E, Haffner D, Gretz N, Offner G, Hoff WG, Broyer M, Mehls O. Treatment with recombinant human growth hormone in short children with nephropathic cystinosis: no evidence for increased deterioration rate of renal function. Pediatr Res. 1998;43:484–488. doi: 10.1203/00006450-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Besouw M, Levtchenko E. Growth retardation in children with cystinosis. Minerva Pediatr. 2010;62:307–313. [PubMed] [Google Scholar]
- 68.Levtchenko E, Blom H, Wilmer M, Heuvel L, Monnens L. ACE inhibitor enalapril diminishes albuminuria in patients with cystinosis. Clin Nephrol. 2003;60:386–389. doi: 10.5414/cnp60386. [DOI] [PubMed] [Google Scholar]
- 69.Kimonis VE, Troendle J, Rose SR, Yang ML, Markello TC, Gahl WA. Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis. J Clin Endocrinol Metab. 1995;80:3257–3261. doi: 10.1210/jc.80.11.3257. [DOI] [PubMed] [Google Scholar]
- 70.Kleta R, Bernardini I, Ueda M, Varade WS, Phornphutkul C, Krasnewich D, Gahl WA. Long-term follow-up of well-treated nephropathic cystinosis patients. J Pediatr. 2004;145:555–560. doi: 10.1016/j.jpeds.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 71.Gahl WA, Reed GF, Thoene JG, Schulman JD, Rizzo WB, Jonas AJ, Denman DW, Schlesselman JJ, Corden BJ, Schneider JA. Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med. 1987;316:971–977. doi: 10.1056/NEJM198704163161602. [DOI] [PubMed] [Google Scholar]
- 72.Dohil R, Fidler M, Barshop B, Newbury R, Sellers Z, Deutsch R, Schneider J. Esomeprazole therapy for gastric acid hypersecretion in children with cystinosis. Pediat Nephrol. 2005;20:1786–1793. doi: 10.1007/s00467-005-2027-1. [DOI] [PubMed] [Google Scholar]
- 73.Besouw M, Blom H, Tangerman A, Graaf-Hess A, Levtchenko E. The origin of halitosis in cystinotic patients due to cysteamine treatment. Mol Genet Metab. 2007;91:228–233. doi: 10.1016/j.ymgme.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Middleton R, Bradbury M, Webb N, O'Donoghue D, Hoff W. Cystinosis. A clinicopathological conference. 'From toddlers to twenties and beyond'. Adult-Paediatric Nephrology Interface Meeting, Manchester 2001. Nephrol Dial Transplant. 2003;18:2492–2495. doi: 10.1093/ndt/gfg445. [DOI] [PubMed] [Google Scholar]
- 75.Belldina EB, Huang MY, Schneider JA, Brundage RC, Tracy TS. Steady-state pharmacokinetics and pharmacodynamics of cysteamine bitartrate in paediatric nephropathic cystinosis patients. Br J Clin Pharmacol. 2003;56:520–525. doi: 10.1046/j.1365-2125.2003.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levtchenko EN, Dael CM, Graaf-Hess AC, Wilmer MJ, Heuvel LP, Monnens LA, Blom HJ. Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr Nephrol. 2006;21:110–113. doi: 10.1007/s00467-005-2052-0. [DOI] [PubMed] [Google Scholar]
- 77.Dohil R, Gangoiti JA, Cabrera BL, Fidler M, Schneider JA, Barshop BA. Long-term treatment of cystinosis in children with twice-daily cysteamine. J Pediatr. 2010;156:823–827. doi: 10.1016/j.jpeds.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 78.Beckman DA, Mullin JJ, Assadi FK. Developmental toxicity of cysteamine in the rat: effects on embryo-fetal development. Teratology. 1998;58:96–102. doi: 10.1002/(SICI)1096-9926(199809/10)58:3/4<96::AID-TERA5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 79.Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000;71:100–120. doi: 10.1006/mgme.2000.3062. [DOI] [PubMed] [Google Scholar]
- 80.Buchan B, Kay G, Heneghan A, Matthews KH, Cairns D. Gel formulations for treatment of the ophthalmic complications in cystinosis. Int J Pharm. 2010;392:192–197. doi: 10.1016/j.ijpharm.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 81.Syres K, Harrison F, Tadlock M, Jester JV, Simpson J, Roy S, Salomon DR, Cherqui S. Successful treatment of the murine model of cystinosis using bone marrow cell transplantation. Blood. 2009;114:2542–2552. doi: 10.1182/blood-2009-03-213934. [DOI] [PubMed] [Google Scholar]