The mechanisms by which an electric shock terminates cardiac arrhythmias have been the subject of a large body of research (for review, see1). These studies have been driven by the understanding that a significant reduction in shock energy can only be achieved by full appreciation of the mechanisms by which a shock interacts with the heart and then exploiting them to devise novel low-voltage therapeutic approaches.
The research has demonstrated that the response of the myocardium to the shock involves simultaneous occurrence of positive and negative membrane polarization2–4. Detailed analysis of the etiology of this “virtual electrode polarization” (VEP) has demonstrated that tissue structure is responsible for its formation of VEP as well as for its shape, location, polarity, and intensity. The effect of tissue structure is two-fold. First, discontinuities in tissue structure (i.e. conductivities) force current to cross the membranes of neighboring cells, giving rise to VEP. Intercellular and interlaminar clefts5,6, or tissue lesions7 are possible factors in this process. Second, continuous tissue structure such as ventricular shape and fiber architecture also give rise to VEP8.
Action potential duration in the myocardium can be either extended by positive VEP or shortened by negative VEP, and strong negative VEP can completely abolish the action potential, creating a new, post-shock excitable area9. Propagation through the post-shock excitable area has proven to directly determine the outcome of a defibrillation shock. A shock succeeds in extinguishing fibrillatory wavefronts if excitations manage to traverse the newly-created post-shock excitable area before the rest of the myocardium recovers from refractoriness. Decreasing the post-shock excitable area could thus increase the likelihood of defibrillation success and lower the defibrillation voltage10; however, this has proven difficult since the post-shock excitable areas are often hidden deep in the ventricular wall10,11.
When the shock happens to affect resting cells (those that are part of the pre-shock, fibrillatory wavefront’s excitable gap), positive VEPs immediately depolarize these cells; these cells become “secondary sources” emitting new wavefronts, rapidly overwhelming any effects of negative VEP. The targeted activation of cells in the fibrillatory wavefront’s excitable gap by electric shocks thus has the potential to eliminate the reentrant circuit by rendering tissue refractory. Furthermore, since this is an excitation process (by positive VEP), the external current needs to only bring cells to the excitation threshold, requiring less energy as compared to de-excitation (by negative VEP) where an activated cell is forced into premature recovery.
The figure illustrates this process. It presents a simulation of a diastolic excitation of the right ventricular (RV) wall of a high-resolution rabbit heart model12, by a brief (5ms) low-voltage electric field directed across the RV wall; short- and long-axis views of the wall are shown. Positive VEP is generated at numerous locations, giving rise to subsequent activations. The endocardial deep valleys created by the trabeculations serve as “lenses”, focusing the external current, bringing the respective tissue regions to the excitation threshold, and rendering them as “secondary sources”. As simulations with this high-resolution 3D structure model12 demonstrate, this is an important mechanism by which low-voltage shocks generate numerous new activations in the excitable regions of the heart.
Figure.
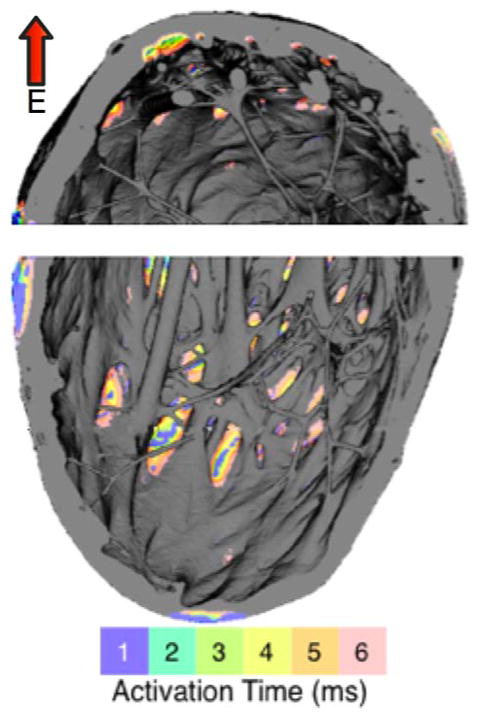
Simulated activation pattern on the endocardial surface of the rabbit heart RV wall resulting from the application of a brief uniform electric field (E) in direction perpendicular to the middle of the wall (red arrow).
The larger the number of positive VEPs resulting in excitation, the faster the activation of the entire excitable region occurs13. Even partial activation of the excitable gap by positive VEPs, particularly close to the crest of the wave, could result in defibrillation success since it could speed the wave by inducing a shift in the wave tip14, resulting in subsequent termination of reentry15.
The question is: how to best achieve such targeted activation of the reentrant wave’s excitable gap? A single low-voltage shock is unlikely to be effective since its ability to activate the gap depends on the timing of its delivery. Delivery of a train of several low-voltage pulses is the logical alternative, since it offers independence of the therapy outcome from the phase of the reentrant wave, as demonstrated by Ripplinger et al16 in the first low-voltage termination of ventricular tachycardia in a rabbit heart with chronic infarction. Interestingly, a train of monophasic pulses was found16 more effective in recruiting cells in the excitable gap than biphasic pulses, as the reversed phase of the biphasic pulse “undermined” the excitatory effect of the first phase, resulting in VEPs of decreased magnitude in the excitable gap..
What should the frequency of the low-voltage pulses be to achieve maximum efficacy in activating cells in the reentrant wave excitable gap? Fenton et al17 applied this concept to the termination of atrial reentrant arrhythmias; they used a train of monophasic pulses with a cycle length 5–10ms below the dominant cycle length of the arrhythmia. The choice of cycle length was possibly based on the practice of anti-tachycardia pacing, which delivers a train of stimuli coordinated with the arrhythmia cycle length in an attempt to disrupt the reentrant circuit by invading, with an outside wavefront, the excitable gap of the reentry. Fenton et al achieved atrial arrhythmia termination (flutter and fibrillation were not reported separately) at a low-voltage strength (0.9–1.4 V/cm) with a success rate of 93%.
The new study by Ambrosi et al18 in this issue of the Journal took low-voltage termination of atrial arrhythmias one step further towards an effective bioelectric therapy. Multiple monophasic pulses were applied within one or two cycle lengths of the arrhythmia. The benefit of the multiple pulses being delivered within one or two arrhythmia cycles is that at least one (or more) of the pulses will have a favorable timing for activating cells in the reentry’s excitable gap via VEP, rather than “falling on top” of the propagating wave. Ambrosi et al offered another important benefit of their approach: such a delivery of the pulse train would avoid the coincidental delivery of the atrial defibrillation/cardiovestion therapy on the T-wave, which could result in ventricular arrhythmia. The thresholds for atrial arrhythmia termination with the approach by Ambrosi et al were 0.86 (for shocks within one cycle length of the arrhythmia) and 0.28 V/cm (within two cycle lengths) for atrial flutter, and 3.46 V/cm (within one cycle) for atrial fibrillation, all for 100% success of termination. For atrial flutter, this is 2.5- and 7.6-fold decrease in shock strength compared to a single shock, respectively; for atrial fibrillation, the decrease is 2-fold. The difference in termination thresholds for atrial flutter and fibrillation is most likely due to the smaller excitable gap in fibrillation as well as the fact that the constant meander of reentrant wavefronts leads to dynamic changes in the locations of the excitable gaps, making excitation of the cells in these gaps by the same pulse train less likely to occur.
The new studies in low-voltage defibrillation, such as that by Ambrosi et al18, demonstrate that the quest to achieve termination of arrhythmias with electrical therapy, the strength of which is below the pain threshold, is in full swing. We thus anxiously await the new lows in defibrillation voltage.
Acknowledgments
Supported by National Institutes of Health grant HL082729.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trayanova N, Plank G, Zipes Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 5. W.B. Saunders Publishing Company; 2009. Modeling cardiac defibrillation. [Google Scholar]
- 2.Wikswo J, Lin S, Abbas R. Virtual electrodes in cardiac tissue: a common mechanism for anodal and cathodal stimulation. Biophys J. 1995;69:2195–2210. doi: 10.1016/S0006-3495(95)80115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efimov IR, Gray RA, Roth BJ. Virtual electrodes and deexcitation: new insights into fibrillation induction and defibrillation. J Cardiovasc Electrophysiol. 2000;11:339. doi: 10.1111/j.1540-8167.2000.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez B, Li L, Eason JC, Efimov IR, Trayanova N. Differences between left and right ventricular chamber geometry affect cardiac vulnerability to electric shocks. Circ Res. 2005;97:168. doi: 10.1161/01.RES.0000174429.00987.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plonsey R, Barr RC. Inclusion of junction elements in a linear cardiac model through secondary sources: application to defibrillation. Med Biol Eng Comput. 1986;24:137. doi: 10.1007/BF02443926. [DOI] [PubMed] [Google Scholar]
- 6.Hooks DA, Tomlinson KA, Marsden SG, et al. Cardiac microstructure: implications for electrical propagation and defibrillation in the heart. Circ Res. 2002;91:331. doi: 10.1161/01.res.0000031957.70034.89. [DOI] [PubMed] [Google Scholar]
- 7.White JB, Walcott GP, Pollard AE, Ideker RE. Myocardial discontinuities: a substrate for producing virtual electrodes that directly excite the myocardium by shocks. Circulation. 1998;97:1738. doi: 10.1161/01.cir.97.17.1738. [DOI] [PubMed] [Google Scholar]
- 8.Knisley S, Trayanova N, Aguel F. Roles of electric field and fiber structure in cardiac electric stimulation. Biophys J. 1999;77:1404–1417. doi: 10.1016/S0006-3495(99)76989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efimov IR, Cheng Y, Van Wagoner D, Mazgalev N, Tchou P. Virtual electrode-induced phase singularity: a basic mechanism of defibrillation failure. Circ Res. 1998;82:918–925. doi: 10.1161/01.res.82.8.918. [DOI] [PubMed] [Google Scholar]
- 10.Constantino J, Long Y, Ashihara T, Trayanova NA. Tunnel Propagation Following Defibrillation with ICD Shocks: Hidden Postshock Activations in the Left Ventricular Wall Underlie Isoelectric Window. Heart Rhythm. 2010 doi: 10.1016/j.hrthm.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashihara T, Constantino J, Trayanova NA. Tunnel propagation of postshock activations as a hypothesis for fibrillation induction and isoelectric window. Circ Res. 2008;102:737. doi: 10.1161/CIRCRESAHA.107.168112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadakkumpadan F, Arevalo H, Prassl AJ, et al. Image-based models of cardiac structure in health and disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2010;2:489–506. doi: 10.1002/wsbm.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maleckar MM, Woods MC, Sidorov VY, et al. Polarity reversal lowers activation time during diastolic field stimulation of the rabbit ventricles: insights into mechanisms. Am J Physiol Heart Circ Physiol. 2008;295:H1626. doi: 10.1152/ajpheart.00706.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pumir A, Nikolski V, Hörning M, et al. Wave Emission from Heterogeneities Opens a Way to Controlling Chaos in the Heart. Phys Rev Lett. 2007:99. doi: 10.1103/PhysRevLett.99.208101. [DOI] [PubMed] [Google Scholar]
- 15.Vadakkumpadan F, Rantner LJ, Tice B, et al. Image-based models of cardiac structure with applications in arrhythmia and defibrillation studies. J Electrocardiol. 2009;42:157, e151. doi: 10.1016/j.jelectrocard.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripplinger CM, Lou Q, Li W, Hadley J, Efimov IR. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm. 2009;6:87. doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton FH, Luther S, Cherry EM, et al. Termination of Atrial Fibrillation Using Pulsed Low-Energy Far-Field Stimulation. Circulation. 2009;120:467–476. doi: 10.1161/CIRCULATIONAHA.108.825091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosi C, Ripplinger CM, Efimov IR, Fedorov V. Termination of sustained atrial flutter and fibrillation using low voltage multiple shock therapy. Heart Rhythm. 2010 doi: 10.1016/j.hrthm.2010.10.018. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


