Abstract
We previously reported that putative nuclear receptors for thyroid hormone can be demonstrated by incubation of hormone either with intact GH1 cells, a rat pituitary tumor cell line, or with isolated GH1 cell nuclei and rat liver nuclei in vitro.
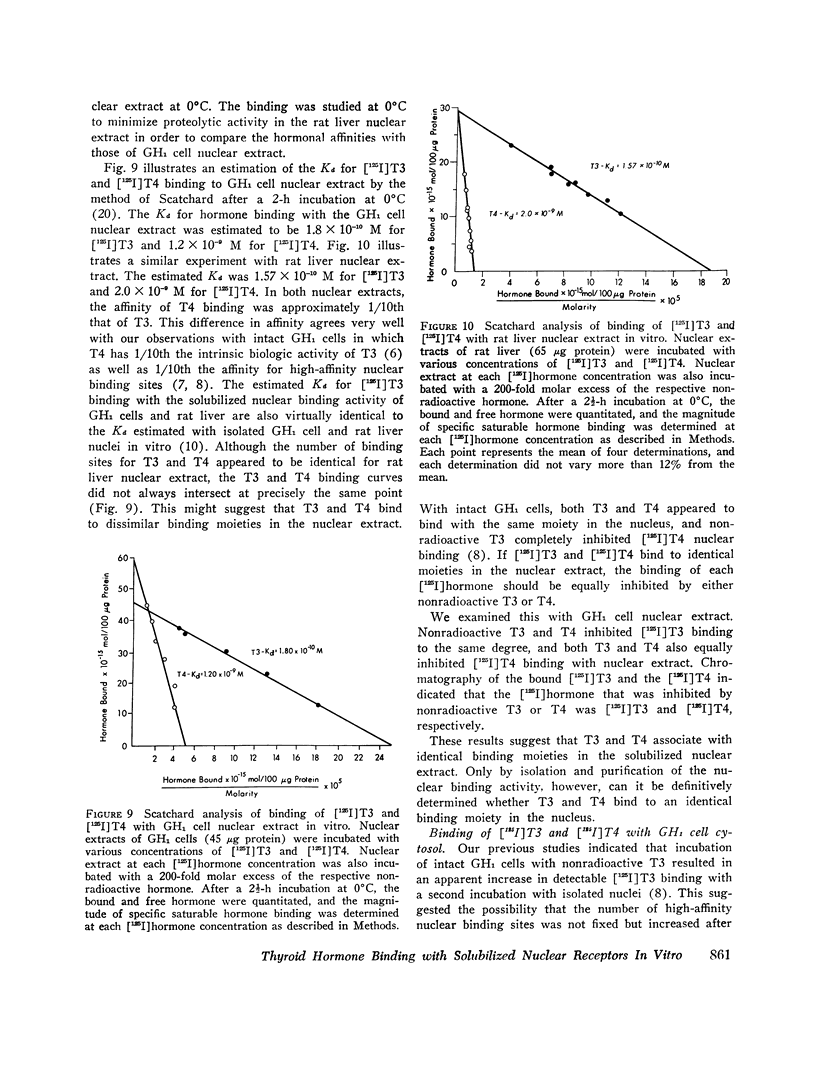
We characterized further the kinetics of triiodothyronine (T3) and thyroxine (T4) binding and the biochemical properties of the nuclear receptor after extraction to a soluble form with 0.4 M KCl. In vitro binding of [125I]T3 and [125I]T4 with GH1 cell and rat liver nuclear extract was examined at 0°C and 37°C. Equilibrium was attained within 5 min at 37°C and 2 h at 0°C. The binding activity from GH1 cells was stable for at least 1 h at 37°C and 10 days at - 20°C. Chromatography on a weak carboxylic acid column and inactivation by trypsin and Pronase, but not by DNase or RNase, suggested that the putative receptor was a nonhistone protein. The estimated equilibrium dissociation constants (Kd) for hormone binding to the solubilized nuclear binding activity was 1.80 × 10-10 M (T3) and 1.20 × 10-9 M (T4) for GH1 cells and 1.57 × 10-10 M (T3) and 2.0 × 10-9 M (T4) for rat liver. These Kd values for T3 are virtually identical to those which we previously reported with isolated rat liver nuclei and GH1 cell nuclei in vitro.
The 10-fold greater affinity for T3 compared to T4 in the nuclear extract is also identical to that observed with intact GH1 cells. In addition, the [125I]T3 and [125I]T4 high-affinity binding in the nuclear extract were inhibited by either nonradioactive T3 or T4, which suggests that the binding activity in nuclear extract was identical for T3 and T4.
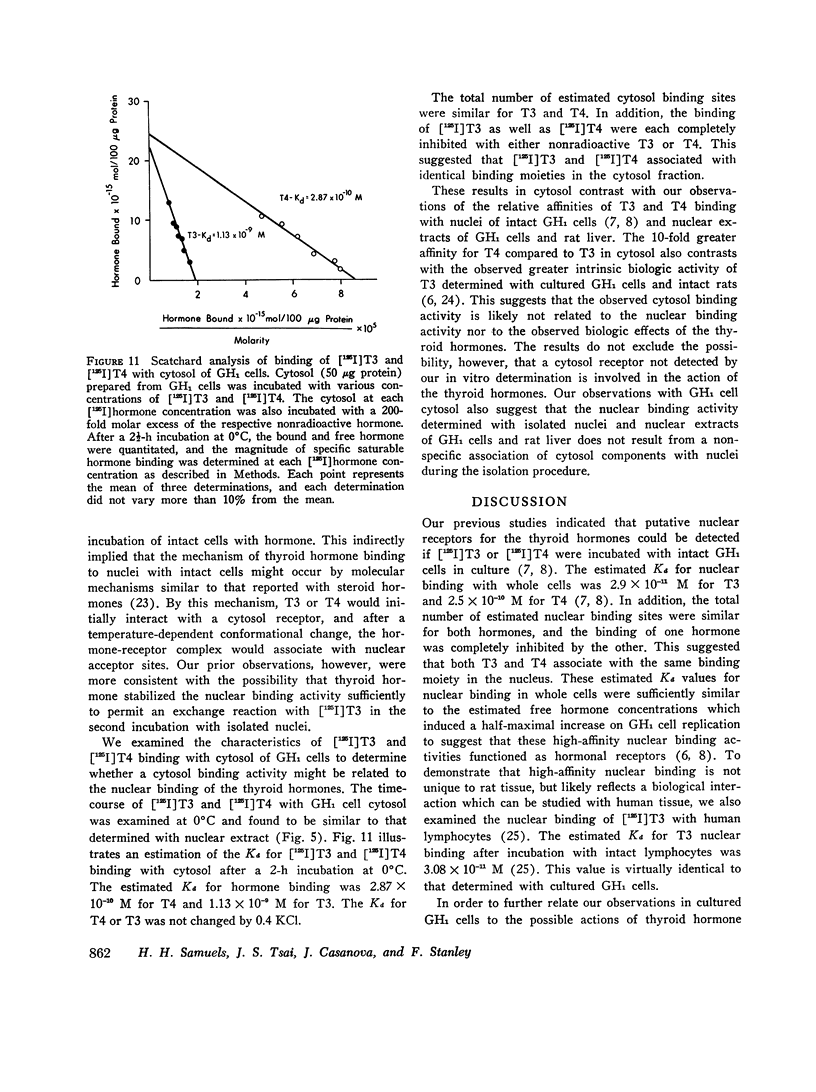
In contrast, the binding activity for T4 and T3 in GH1 cell cytosol was markedly different from that observed with nuclear extract (Kd values were 2.87 × 10-10 M for T4 and 1.13 × 10-9 M for T3).
Our results indicate that nuclear receptors for T3 and T4 can be isolated in a soluble and stable form with no apparent change in hormonal affinity. This should allow elucidation of the mechanisms of thyroid hormone action at the molecular level.
Full text
PDF

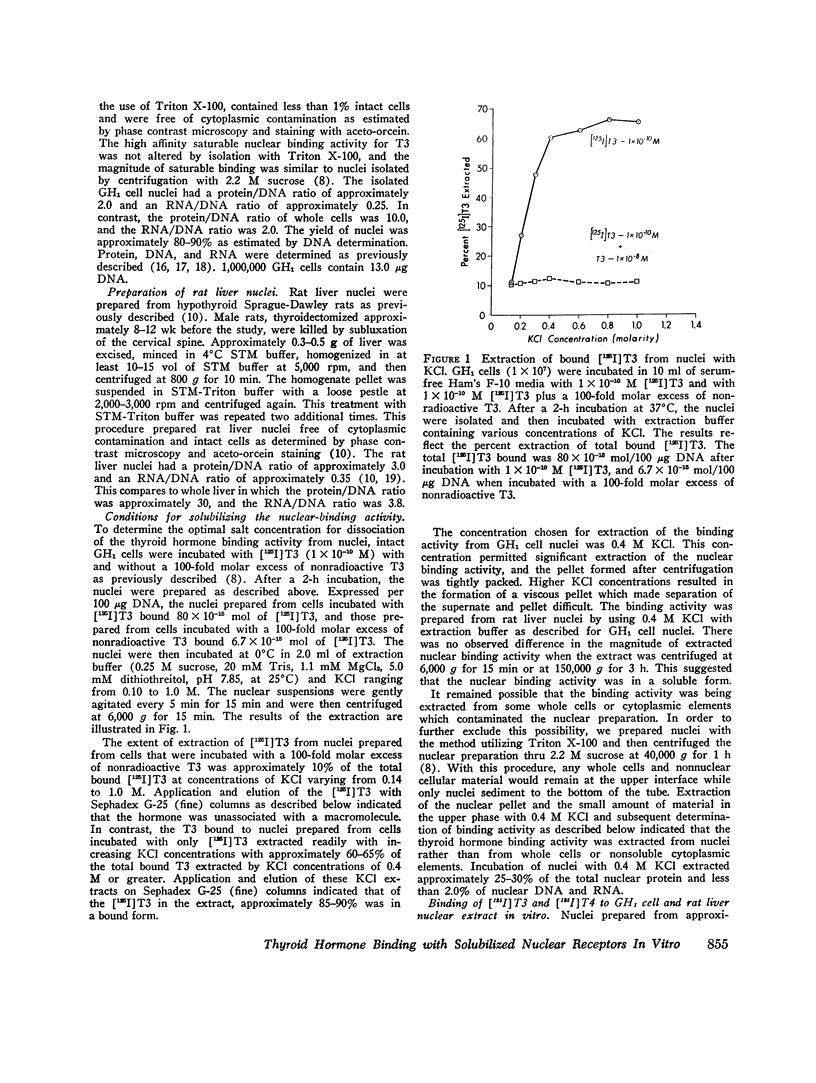

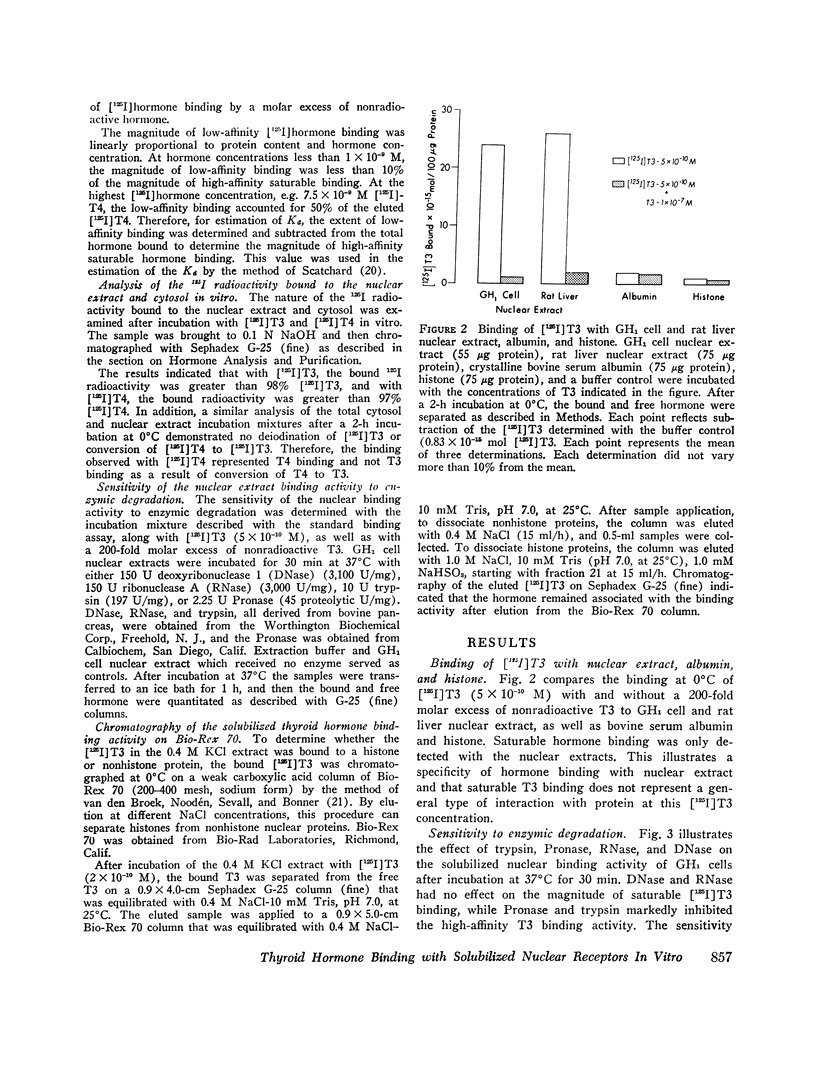
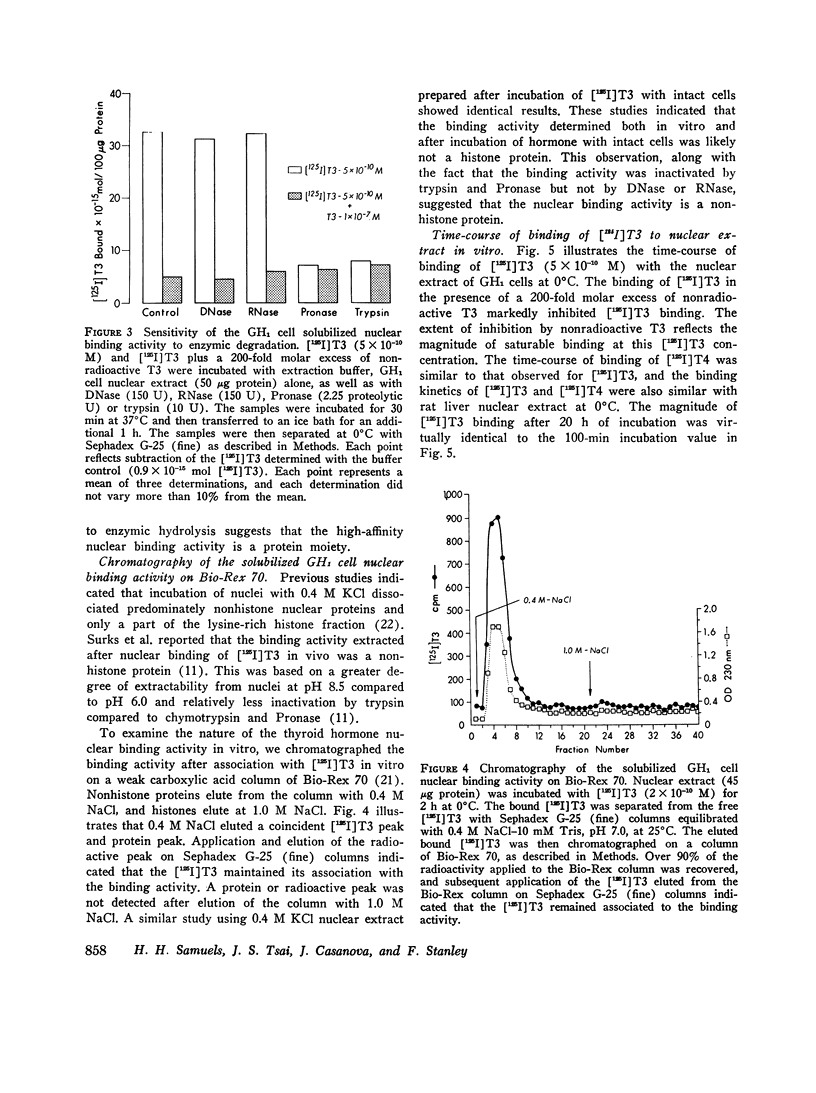
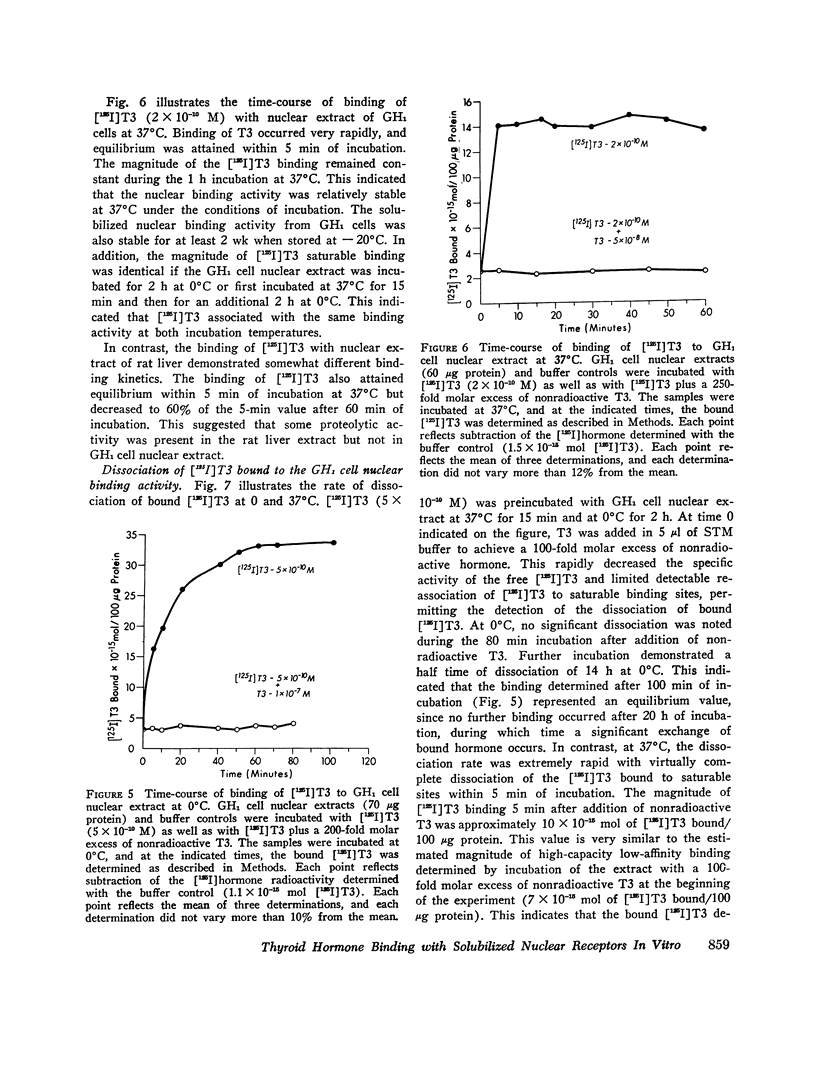
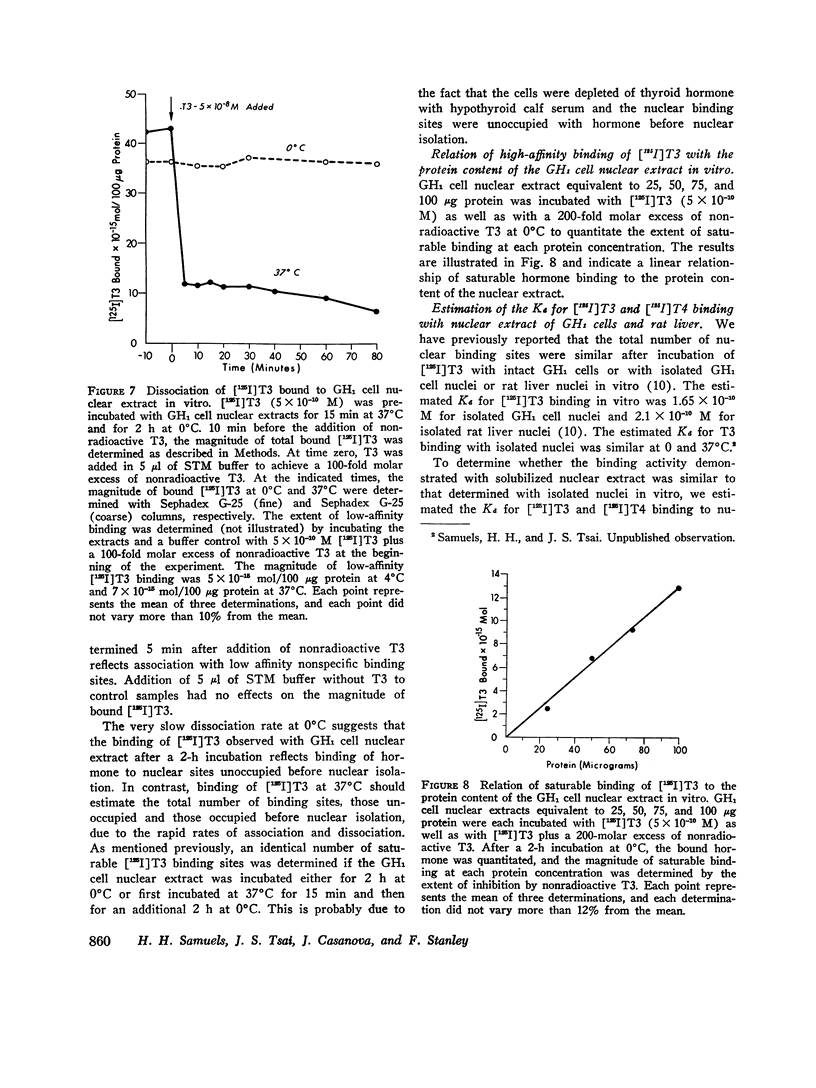



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ananieva L. N., Kozlov J. V. Stepwise removal of protein from a deoxyribonucleoprotein complex and de-repression of the genome. J Mol Biol. 1966 Dec 28;22(2):365–371. doi: 10.1016/0022-2836(66)90140-9. [DOI] [PubMed] [Google Scholar]
- Green W. L. Separation of iodo compounds in serum by chromatography on Sephadex columns. J Chromatogr. 1972 Oct 5;72(1):83–91. doi: 10.1016/0021-9673(72)80010-4. [DOI] [PubMed] [Google Scholar]
- HOCH F. L. Biochemical actions of thyroid hormones. Physiol Rev. 1962 Oct;42:605–673. doi: 10.1152/physrev.1962.42.4.605. [DOI] [PubMed] [Google Scholar]
- HYMER W. C., KUFF E. L. ISOLATION OF NUCLEI FROM MAMMALIAN TISSUES THROUGH THE USE OF TRITON X-100. J Histochem Cytochem. 1964 May;12:359–363. doi: 10.1177/12.5.359. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torizuka K., Miyake T., Fukase M. Specific binding proteins of thyroxine and triiodothyronine in liver soluble proteins. Biochim Biophys Acta. 1970 Mar 24;201(3):479–492. doi: 10.1016/0304-4165(70)90168-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONEY W. L., KUMAOKA S., RAWSON R. W., KROC R. L. Comparative effects of thyroxine analogues in experimental animals. Ann N Y Acad Sci. 1960 Apr 23;86:512–544. doi: 10.1111/j.1749-6632.1960.tb42827.x. [DOI] [PubMed] [Google Scholar]
- Mitsuma T., Colucci J., Shenkman L., Hollander C. S. Rapid simultaneous radioimmunoassay for triiodothyronine and thyroxine in unextracted serum. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2107–2113. doi: 10.1016/0006-291x(72)90766-8. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Koerner D., Schwartz H. L., Surks M. I. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972 Aug;35(2):330–333. doi: 10.1210/jcem-35-2-330. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Dillman W., Surks M. I. Effect of thyroid hormone analogues on the displacement of 125I-L-triiodothyronine from hepatic and heart nuclei in vivo: possible relationship to hormonal activity. Biochem Biophys Res Commun. 1973 Dec 10;55(3):544–550. doi: 10.1016/0006-291x(73)91177-7. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J. Thyroid hormone action: in vitro demonstration of putative receptors in isolated nuclei and soluble nuclear extracts. Science. 1974 Jun 14;184(4142):1188–1191. doi: 10.1126/science.184.4142.1188. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action. Demonstration of similar receptors in isolated nuclei of rat liver and cultured GH1 cells. J Clin Invest. 1974 Feb;53(2):656–659. doi: 10.1172/JCI107601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding S. W., Davis P. J. Thyroxine binding to soluble proteins in rat liver and its sex dependence. Biochim Biophys Acta. 1971 Jan 19;229(1):279–283. doi: 10.1016/0005-2795(71)90345-x. [DOI] [PubMed] [Google Scholar]
- Sufi S. B., Toccafondi R. S., Malan P. G., Ekins R. P. Binding of thyroid hormones to a soluble fraction from porcine anterior pituitary. J Endocrinol. 1973 Jul;58(1):41–52. doi: 10.1677/joe.0.0580041. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Koerner D., Dillman W., Oppenheimer J. H. Limited capacity binding sites for L-triiodothyronine in rat liver nuclei. Localization to the chromatin and partial characterization of the L-triiodothyronine-chromatin complex. J Biol Chem. 1973 Oct 25;248(20):7066–7072. [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J. S., Samuels H. H. Thyroid hormone action: demonstration of putative nuclear receptors in human lymphocytes. J Clin Endocrinol Metab. 1974 May;38(5):919–922. doi: 10.1210/jcem-38-5-919. [DOI] [PubMed] [Google Scholar]
- van den Broek H. W., Noodén L. D., Sevall J. S., Bonner J. Isolation, purification, and fractionation of nonhistone chromosomal proteins. Biochemistry. 1973 Jan 16;12(2):229–236. doi: 10.1021/bi00726a009. [DOI] [PubMed] [Google Scholar]



