Abstract
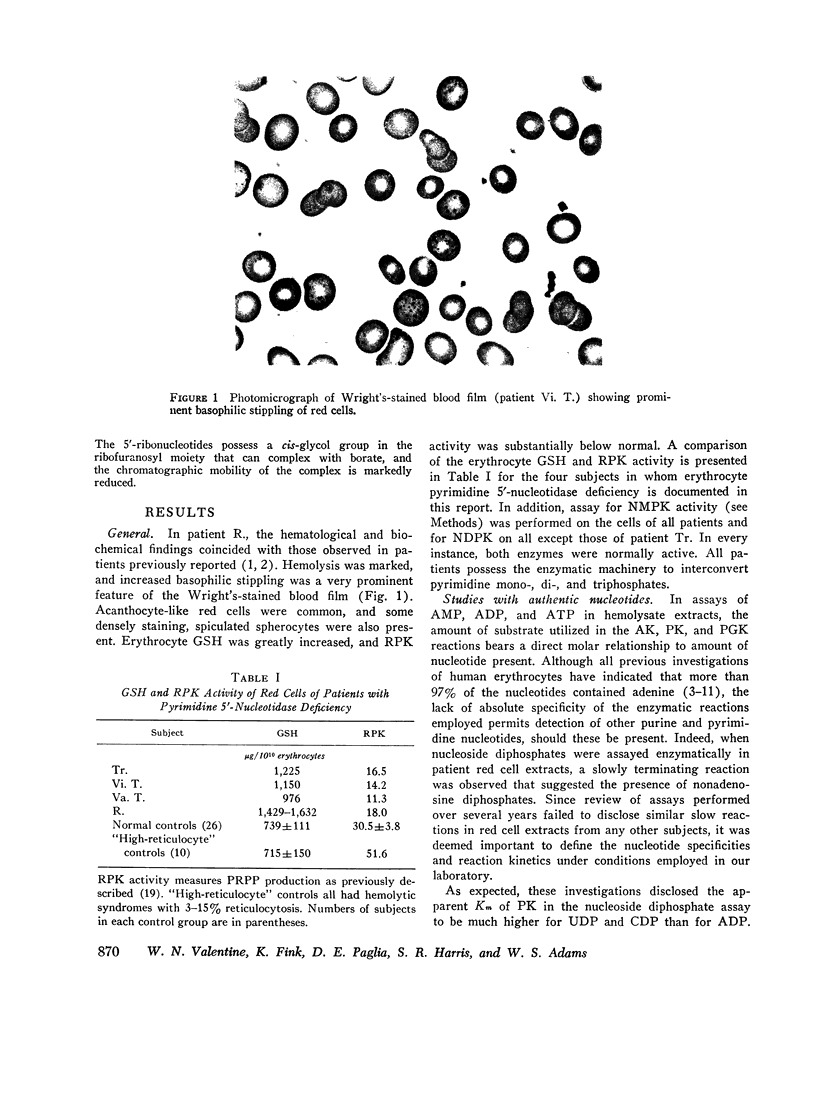
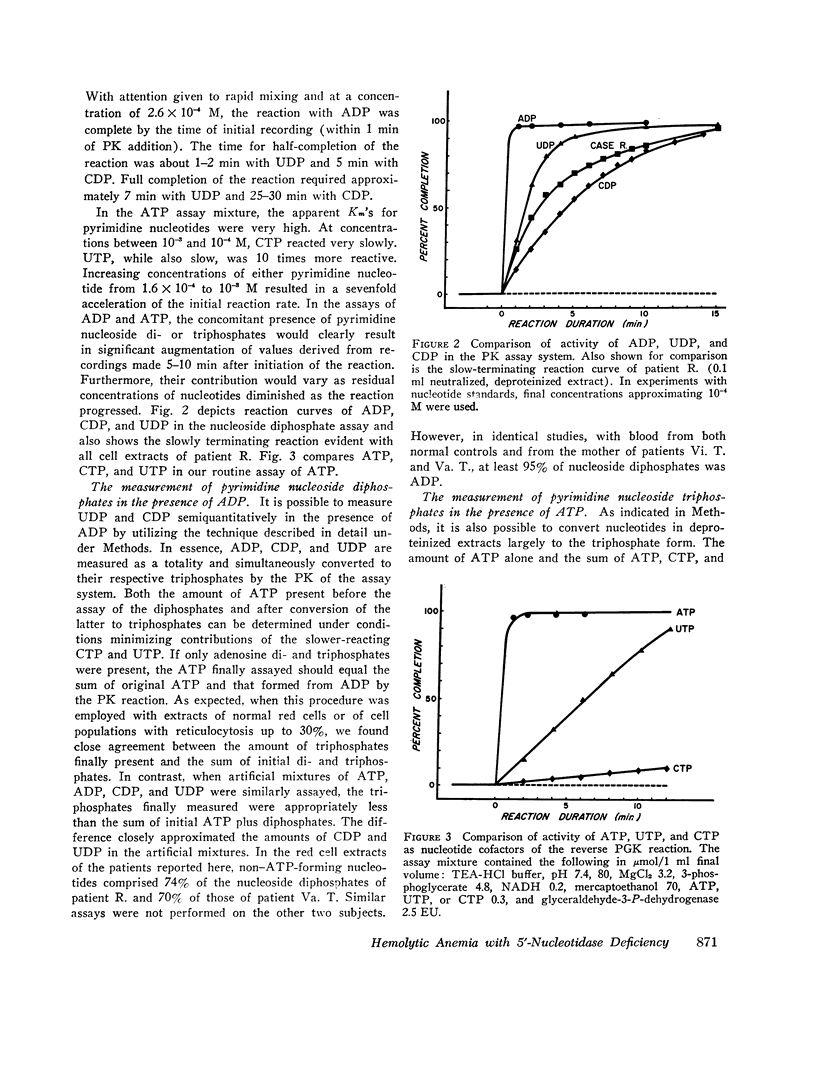
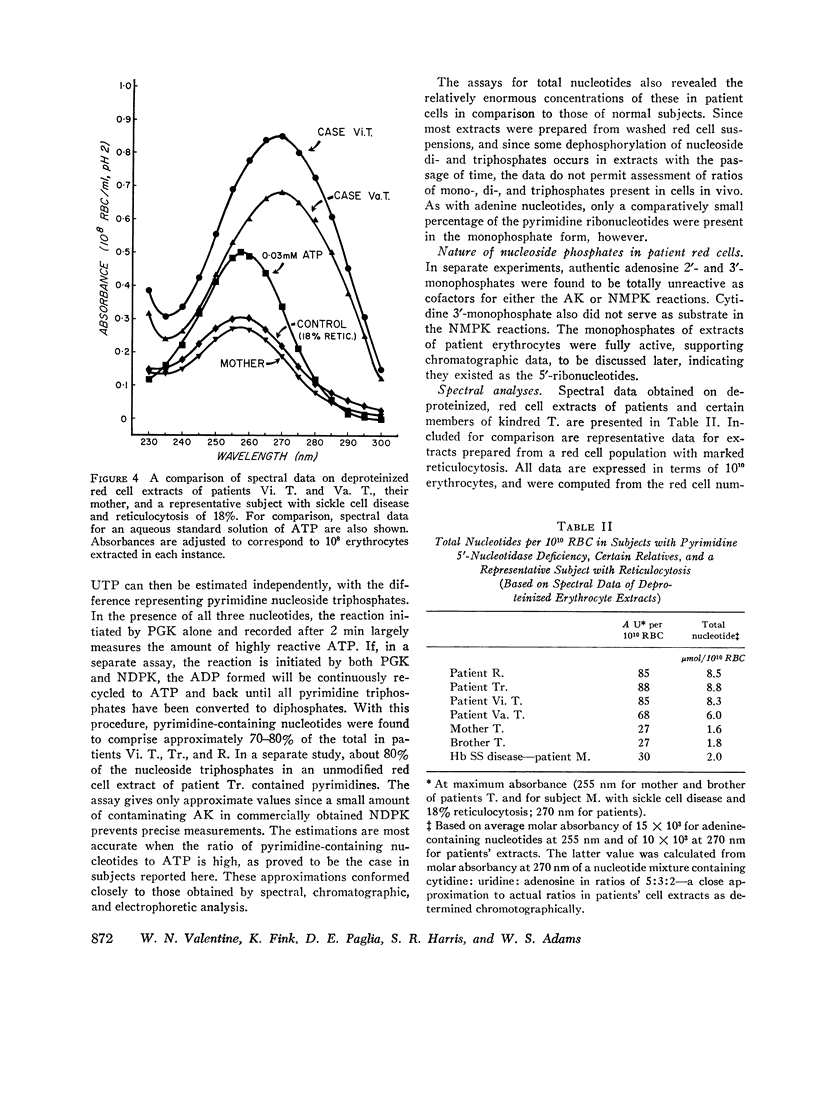
A severe deficiency of a red cell pyrimidine 5′-nucleotidase was found to be associated with hereditary hemolytic anemia in four members of three kindreds. The syndrome was characterized by marked increases above normal in red cell basophilic stippling, total nucleotides, and GSH and by a fairly severe deficiency of ribosephosphate pyrophosphokinase (EC 2.7.6.1.). Patient erythrocytes uniquely contained large amounts of pyrimidine 5′-ribonucleotides. In earlier studies, these were erroneously considered to be adenosine phosphates, since all previous investigations of the nucleotides of human red cells and reticulocytes have shown 97% or more to contain adenine. Total nucleotides in patient cells were present in amounts 3-6 times greater than normal, and approximately 80% contained pyrimidine. The ultraviolet spectral curves of deproteinized red cell extracts exhibited a shift in maximum absorbance from the usual 256-257 nm to approximately 266-270 nm, and absorbance at 250, 270, 280, and 290 nm, expressed as a ratio of that at 260 nm, differed greatly from normal. The spectral characteristics of extracts provide the basis of a readily performed screening procedure, which does not require enzyme assay. The nucleotidase activity in deficient red cells assayed less than 14%, and usually less than 10%, of normal and much less in terms of reticulocyte-rich blood, where it was consistently found to be increased. The enzyme has a pH optimum of 7.5-8.0, is inhibited by EDTA, and does not utilize purine 5′-ribonucleotides or β-glycerophosphate as substrates. While comparatively few family members have been available thus far for study, initial data are compatible with an autosomal, recessive mode of transmission of the deficiency. The pyrimidine 5′-ribonucleotides are presumably derived from RNA degradation and, not being diffusible, accumulate when the enzyme catalyzing their dephosphorylation is deficient. It is postulated that the prominent basophilic stippling results from retarded ribosomal RNA degradation secondary to accumulation of degradation products, namely pyrimidine 5′-ribonucleotides. Ribosephosphate pyrophosphokinase deficiency is considered to be an epiphenomenon. The mechanism responsible for increased red cell GSH is unknown.
Full text
PDF



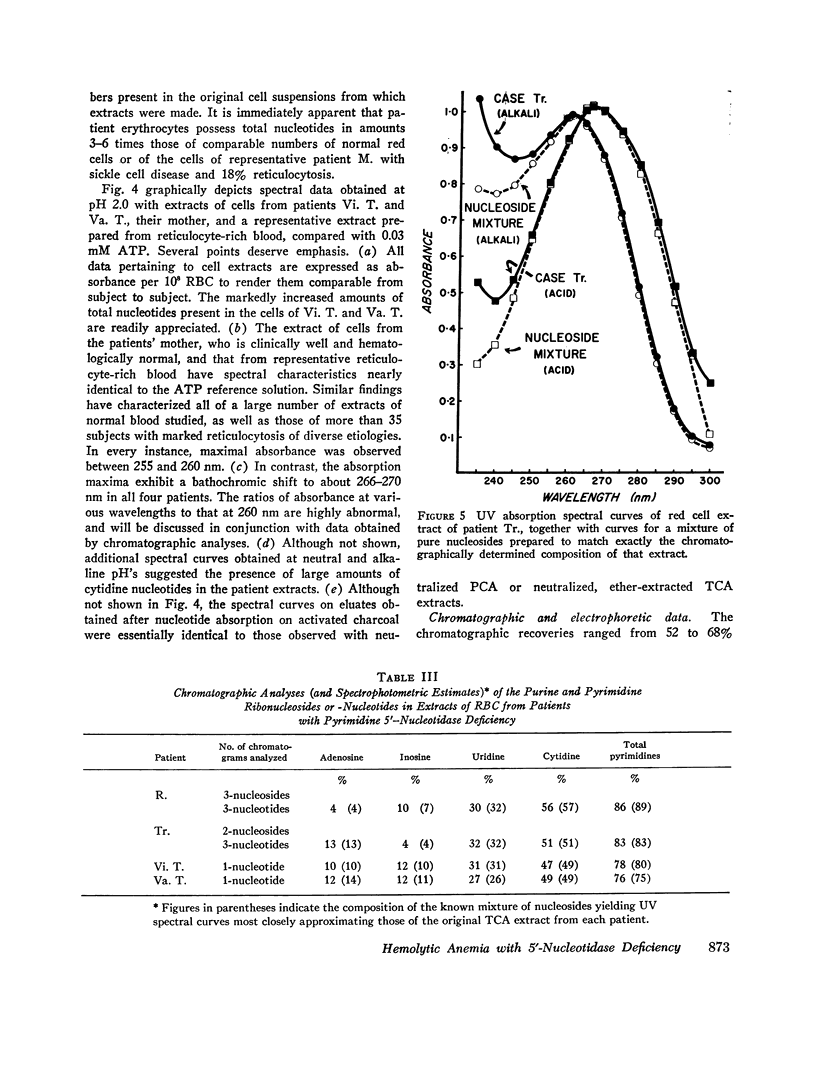
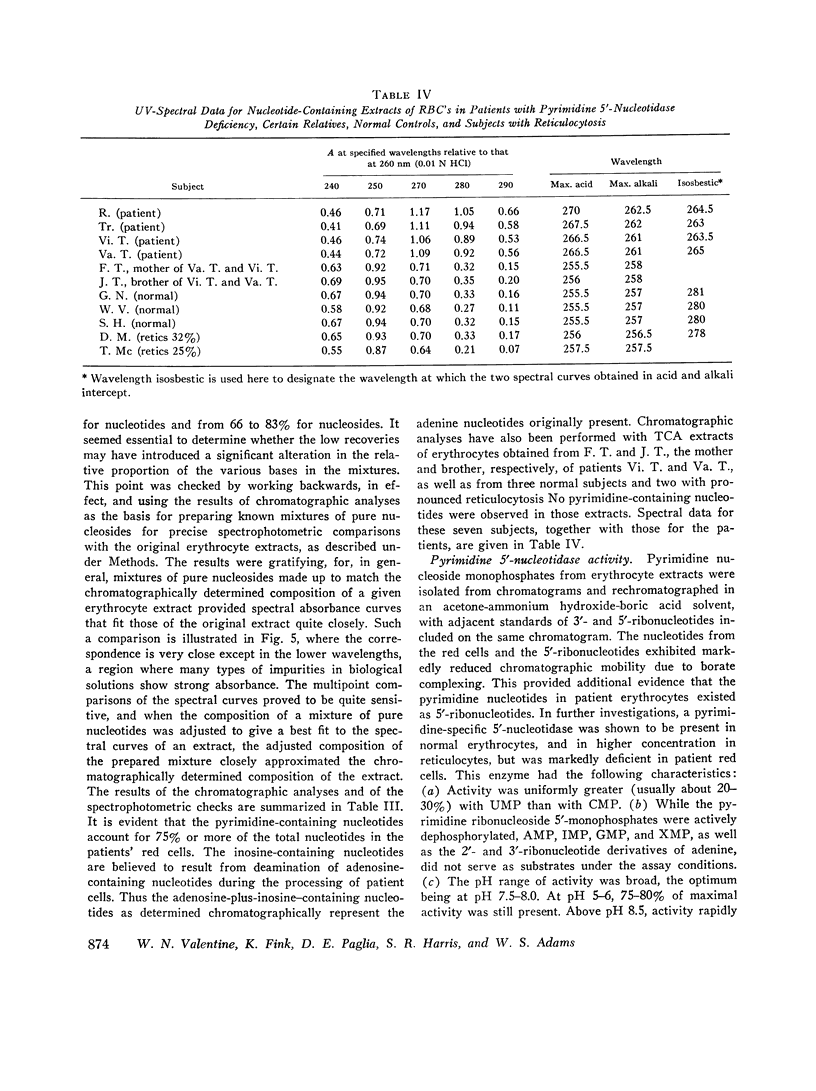





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Human red cell glycolytic intermediates. J Biol Chem. 1959 Mar;234(3):449–458. [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- BISHOP C. Changes in the nucleotides of stored or incubated human blood. Transfusion. 1961 Nov-Dec;1:349–354. doi: 10.1111/j.1537-2995.1961.tb00073.x. [DOI] [PubMed] [Google Scholar]
- BISHOP C., RANKINE D. M., TALBOTT J. H. The nucleotides in normal human blood. J Biol Chem. 1959 May;234(5):1233–1237. [PubMed] [Google Scholar]
- BREWER G. J. A NEW INHERITED ABNORMALITY OF HUMAN ERYTHROCYTES: ELEVATED ERYTHROCYTIC ADENOSINE TRIPHOSPHATE. Biochem Biophys Res Commun. 1965 Feb 3;18:430–434. doi: 10.1016/0006-291x(65)90726-6. [DOI] [PubMed] [Google Scholar]
- Baughan M. A., Valentine W. N., Paglia D. E., Ways P. O., Simons E. R., DeMarsh Q. B. Hereditary hemolytic anemia associated with glucosephosphate isomerase (GPI) deficiency--a new enzyme defect of human erythrocytes. Blood. 1968 Aug;32(2):236–249. [PubMed] [Google Scholar]
- Fink K., Adams W. S. Paper chromatographic data for purines, pyrimidines and derivatives in a variety of solvents. J Chromatogr. 1966 Apr;22(1):118–129. doi: 10.1016/s0021-9673(01)97077-3. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Mills D. C. The adenylate kinase of human plasma, erythrocytes and platelets in relation to the degradation of adenosine diphosphate in plasma. Biochem J. 1967 Jun;103(3):773–784. doi: 10.1042/bj1030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- JENSEN W. N., MORENO G. D., BESSIS M. C. AN ELECTRON MICROSCOPIC DESCRIPTION OF BASOPHILIC STIPPLING IN RED CELLS. Blood. 1965 Jun;25:933–943. [PubMed] [Google Scholar]
- KOUTRAS G. A., HATTORI M., SCHNEIDER A. S., EBAUGH F. G., Jr, VALENTINE W. N. STUDIES ON CHROMATED ERYTHROCYTES. EFFECT OF SODIUM CHROMATE ON ERYTHROCYTE GLUTATHIONE REDUCTASE. J Clin Invest. 1964 Feb;43:323–331. doi: 10.1172/JCI104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEL P., CHAMBON P., KARON H., KULIC I., SERTER M. [Free nucleotides in red blood cells and reticulocytes]. Folia Haematol Int Mag Klin Morphol Blutforsch. 1962;78:525–543. [PubMed] [Google Scholar]
- MILLS G. C., SUMMERS L. B. The metabolism of nucleotides and other phosphate esters in erythrocytes during in vitro incubation at 37 degrees. Arch Biochem Biophys. 1959 Sep;84:7–14. doi: 10.1016/0003-9861(59)90548-x. [DOI] [PubMed] [Google Scholar]
- Minakami S., Suzuki C., Saito T., Yoshikawa H. Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem. 1965 Dec;58(6):543–550. doi: 10.1093/oxfordjournals.jbchem.a128240. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- SCHNEIDER A. S., VALENTINE W. N., HATTORI M., HEINS H. L., Jr HEREDITARY HEMOLYTIC ANEMIA WITH TRIOSEPHOSPHATE ISOMERASE DEFICIENCY. N Engl J Med. 1965 Feb 4;272:229–235. doi: 10.1056/NEJM196502042720503. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L. Enzymic synthesis of guanosine and cytidine triphosphates: a note on the nucleotide specificity of the pyruvate phosphokinase reaction. Biochim Biophys Acta. 1955 Apr;16(4):616–618. doi: 10.1016/0006-3002(55)90302-4. [DOI] [PubMed] [Google Scholar]
- Scholar E. M., Brown P. R., Parks R. E., Jr, Calabresi P. Nucleotide profiles of the formed elements of human blood determined by high-pressure liquid chromatography. Blood. 1973 Jun;41(6):927–936. [PubMed] [Google Scholar]
- Scott E. M. Kinetic comparison of genetically different acid phosphatases of human erythrocytes. J Biol Chem. 1966 Jul 10;241(13):3049–3052. [PubMed] [Google Scholar]
- TSUBOI K. K., HUDSON P. B. Acid phosphatase. VI. Kinetic properties of purified yeast and erythrocyte phosphomonoesterase. Arch Biochem Biophys. 1956 Mar;61(1):197–210. doi: 10.1016/0003-9861(56)90332-0. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Anderson H. M., Paglia D. E., Jaffé E. R., Konrad P. N., Harris S. R. Studies on human erythrocyte nucleotide metabolism. II. Nonspherocytic hemolytic anemia, high red cell ATP, and ribosephosphate pyrophosphokinase (RPK, E.C.2.7.6.1) deficiency. Blood. 1972 May;39(5):674–684. [PubMed] [Google Scholar]
- Valentine W. N., Bennett J. M., Krivit W., Konrad P. N., Lowman J. T., Paglia D. E., Wakem C. J. Nonspherocytic haemolytic anaemia with increased red cell adenine nucleotides, glutathione and basophilic stippling and ribosephosphate pyrophosphokinase (RPK) deficiency: studies on two new kindreds. Br J Haematol. 1973 Feb;24(2):157–167. doi: 10.1111/j.1365-2141.1973.tb05736.x. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Kürschner K. K. Studies on human erythrocyte nucleotide metabolism. I. Monisotopic methodologies. Blood. 1972 May;39(5):666–673. [PubMed] [Google Scholar]
- Valentine W. N., Oski F. A., Paglia D. E., Baughan M. A., Schneider A. S., Naiman J. L. Hereditary hemolytic anemia with hexokinase deficiency. Role of hexokinase in erythrocyte aging. N Engl J Med. 1967 Jan 5;276(1):1–11. doi: 10.1056/NEJM196701052760101. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Paglia D. E., Neerhout R. C., Konrad P. N. Erythrocyte glyoxalase II deficiency with coincidental hereditary elliptocytosis. Blood. 1970 Dec;36(6):797–808. [PubMed] [Google Scholar]