Abstract
Fourteen novel conjugates of 3,28-di-O-acylbetulins with AZT were prepared as anti-HIV agents, based on our previously reported potent anti-HIV triterpene leads, including 3-O-acyl and 3,28-di-O-acylbetulins. Nine of the conjugates (49–53, 55, 56, 59, 60) exhibited potent anti-HIV activity at the submicromolar level, with EC50 values ranging from 0.040 to 0.098 µM in HIV-1NL4-3 infected MT-4 cells. These compounds were equipotent or more potent than 3-O-(3',3'-dimethylsuccinyl)betulinic acid (2), which is currently in Phase IIb anti-AIDS clinical trial.
Keywords: HIV-1, Betulin, AZT, Conjugate
1. Introduction
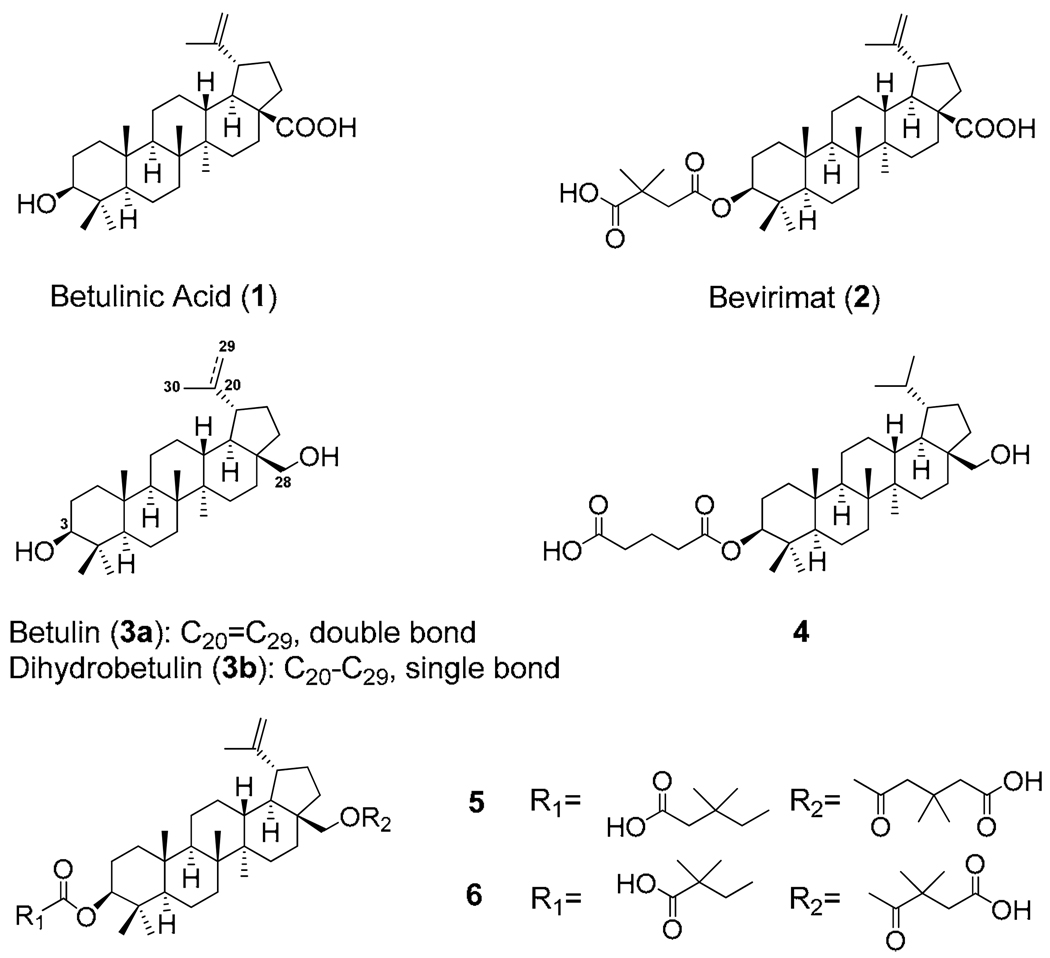
Betulinic acid (1), a lupane-type triterpene, is widely distributed throughout the plant kingdom. Various biological activities1–5 have been reported for 1, including anti-HIV, anti-cancer, and anti-inflammatory properties. Our previous modification study on 1 led to the discovery of 3-O-(3',3'-dimethylsuccinyl)betulinic acid (2, bevirimat, now also known as MPC-4326). Compound 2 exhibits potent anti-HIV activity and is currently in phase IIb clinical trials in the US.6–9 It is a first-in-class drug candidate as a viral maturation inhibitor, which disrupts the processing of capsid precursor p25 (CA-SP1) to mature capsid protein p24 (CA), resulting in generation of non-infectious HIV-1 virions.10
In subsequent research, various derivatives of betulin (3a) and dihydrobetulin (3b) were investigated based on the structural similarity of 1 and 3a,3b.11–14 Several analogs, including 3-O-glutaryldihydrobetulin (4),14 3,28-di-O-(3',3'-dimethylglutaryl)betulin (5), and 3-O-(3',3'-dimethylsuccinyl)-28-O-(2",2"-dimethylsuccinyl)betulin (6)12,13 exhibited comparable or greater anti-HIV activity compared with the structurally related 2(Figure 1).15
Figure1.
Betulinic acid, betulin and their derivatives which demonstrated potent anti-HIV activities.
In a continuing study of potent anti-HIV agents based on the betulin scaffold, we wanted to explore the conjugation of our unique HIV-1 maturation inhibitors with other classes of anti-HIV agents, because the strategy of multi-target therapeutics could be more efficacious and less prone to resistance than monotherapies.16,17 Although it is more challenging to create multi-target therapeutics by building multi-target actions into one single chemical entity rather than mixing monotherapies,16,18 the multi-targeted single agent could greatly simplify treatment regimens and reduce the risk of drug-drug interactions.16 Herein, we report our preliminary research on conjugation of 3'-azido-3'-deoxythymidine (AZT),19 the first clinically approved nucleoside reverse transcriptase inhibitor (NRTI), with betulin derivatives. The hybrid conjugates were formed through a linker with ester bonds, which are easy to hydrolyze and subsequently release the parent compounds, betulin derivative and AZT, to exert their functions.
Design
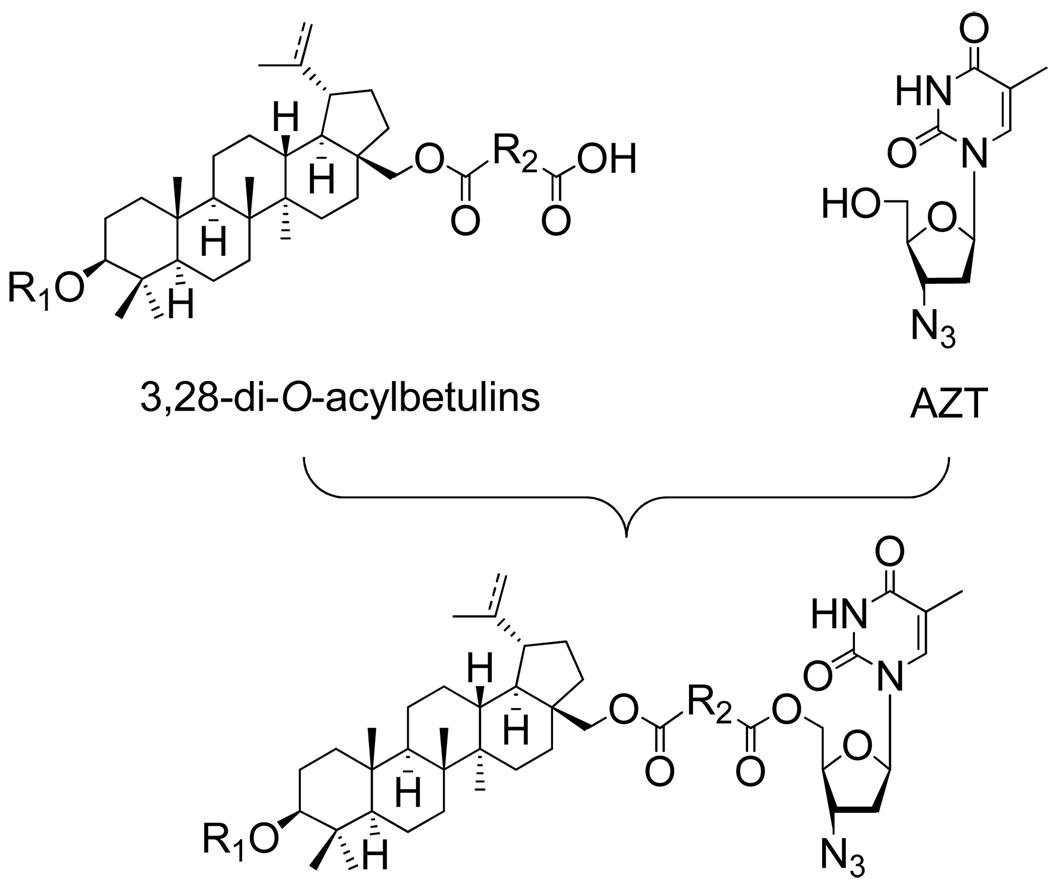
Initially, we considered where to link AZT to the triterpene skeleton. 3,28-Di-O-acylbetulin derivatives contain two free carboxylic acid groups, which appear to be ideal fragments for constructing conjugates. Previous studies suggested that the free terminal carboxylic acid in the 3-O-acyl group is essential for anti-maturation activity; thus, the other –COOH group in the 28-O-acyl moiety was considered to be the appropriate conjugating site (Fig. 2.).
Figure 2.
General structure of hybrid-type anti-HIV agents.
Secondly, we considered the identity of the 3-O-acyl group (>R1). Our prior modification studies on 1, 3a and 3b showed that the esterification of the 3-OH with 3',3'-dimethylglutaryl or 3',3'-dimethylsuccinyl fragments produced significantly active derivatives (e.g., 5 and 6).6,12,14,20–22 However, the 3a-derivatives bearing a 3-O-glutaryl group exhibited only weak or moderate anti-HIV activity.14 In contrast, introduction of a glutaryl group onto the 3-OH of 3b led to a dramatic increase of anti-HIV potency as seen in compound 4. Therefore, in this study, triterpenes with the lup-20(29)-ene scaffold of 3a were acylated with 3,3-dimethylglutaryl (54) and 3,3-dimethylsuccinyl (49–53) moieties, while those with the lupine scaffold of 3b were also acylated with a glutaryl group (58–61), as well as the two preceding moieties (55–57 and 62).
Thirdly, we considered the identity of the 28-O-acyl group (R2). Although previous biological data for betulin derivatives suggested that the group at this position might be not essential for the anti-HIV activity of this compound type, it could still have some influence on potency.12 In addition, here the 28-O-acyl group is also being used as a linker in the conjugates. Therefore, the identity of the linking ester group might play a vital part in the anti-HIV activity of hybrid conjugates. Among the 3,28-di-O-acylbetulin derivatives previously studied, compounds 5 and 6 exhibited the greatest potency, and thus the 3,3-dimethylglutaryl and 2,2-dimethylsuccinyl groups found in these two compounds were used for constructing the triterpene-AZT conjugates. Succinyl, glutaryl, and 3,3-dimethylsuccinyl esters were also investigated as the 28-O-acyl moiety in the conjugate compounds.
The preparation of fourteen new conjugates of 3,28-di-O-acylbetulins with AZT and evaluation of anti-HIV activity are described in this paper. Table 1 lists the structural identities of the 3- and 28-O-acyl side chains, and the degree of saturation of the C20-C29 bond for all intermediates (21–48) and final conjugates (49–62).
Table 1.
Ester Side Chains of 21–62.
| Compound | C20-C29 | R1 | R2 |
|---|---|---|---|
| 21, 35, 49 | C=C | Bb | Aa |
| 22, 36, 50 | B | B | |
| 23, 37, 51 | B | B2 c | |
| 24, 38, 52 | B | Cd | |
| 25, 39, 53 | B | De | |
| 26, 40, 54 | D | D | |
| 27, 41, 55 | C-C | B | B |
| 28, 42, 56 | B | B2 | |
| 29, 43, 57 | B | D | |
| 30, 44, 58 | C | B | |
| 31, 45, 59 | C | B2 | |
| 32, 46, 60 | C | C | |
| 33. 47, 61 | C | D | |
| 34, 48, 62 | D | D |
A: succinyl 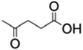 ;
;
B: 3,3-dimethylsuccinyl 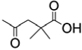 ;
;
B2: 2,2-dimethylsuccinyl 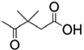 ;
;
C: glutaryl 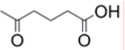 ;
;
D: 3,3-dimethylglutaryl 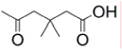
2. Chemistry
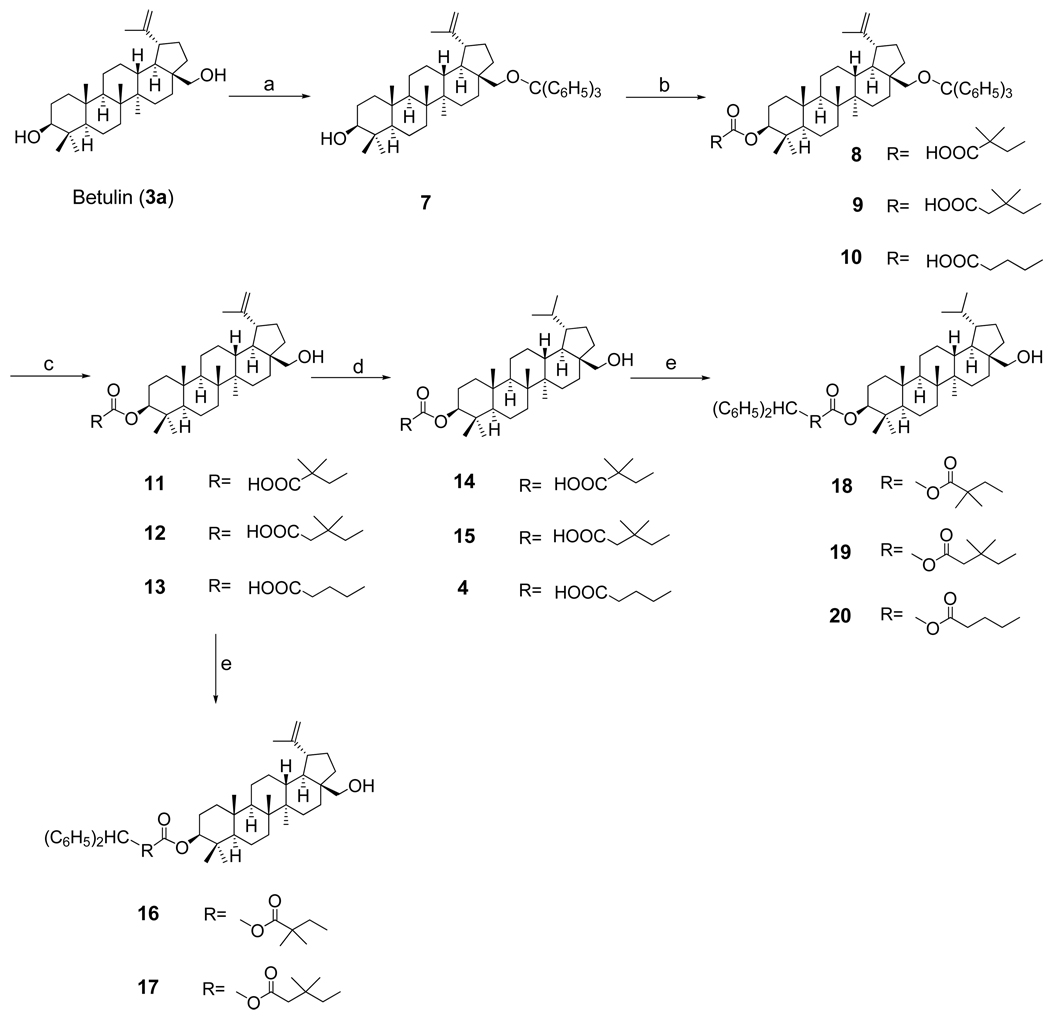
The synthetic route to conjugated products (49–62) is outlined in Scheme 1 and Scheme 2. The key intermediate 3-O-acylbetulins (11–13) were successfully prepared by the methods described previously14 as shown in Scheme 1. Protection of the 28-OH group with triphenylmethyl (or trityl) chloride yielded betulin 28-O-trityl ether (7), which was further treated with an appropriate dicarboxylic acid or anhydride in anhydrous pyridine in the presence of 4-dimethylaminopyridine (DMAP) to furnish the corresponding 3-O-acyl-28-O-tritylbetulins (8–10). As was found in previous studies, the reaction of 7 with 2,2-dimethylsuccinic acid gave a mixture of 3-O-2',2'-dimethylsuccinyl- and −3',3'-dimethylsuccinylbetulin derivatives, in which the latter isomer (8) was the major product. The isomers were separated by silica gel chromatography, and their structures were assigned by 2D–NMR analysis (HMBC). For 8 and 9, the protecting trityl group was subsequently cleaved with pyridium p-toluenesulfonate (PPTS) in CH2Cl2-EtOH to yield 3-O-acylbetulins (11 and 12, respectively). An ethyl ester side-product was obtained predominately by removing the 28-O-trityl group from 3-O-glutaryl-28-O-tritylbetulin (10) with PPTS; however, the desired product (13) was obtained exclusively by refluxing 10 with Lewis acid FeCl3 in 95% THF.
Scheme 1.
Reagents and conditions: (a) trityl chloride, DAMP, DMF, reflux; (b) dicarboxylic acid (or anhydride), DMAP, pyridine, reflux; (c) PPTS, CH2Cl2-EtOH or FeCl3, 95%THF, reflux; (d) H2, 10%Pd/C, EtOAc-EtOH, r.t.; (e) diphenyldiazomethane, MeOH, r.t.
Scheme 2.
General synthetic route to conjugates of betulin derivatives with AZT.
Hydrogenation of 3-O-acylbetulins (11–13) with H2/Pd-C in EtOAc-EtOH afforded the 3-O-acyldihydrobetulins (14, 15, and 4, respectively) almost quantitatively. Before a second acyl moiety was introduced at the C-28 position, the free terminal carboxylic acid in the 3-O-acyl group was first protected as the diphenyldiazomethyl ester. Treatment of 3-O-acylbetulins (11, 12) and –dihydrobetulins (14, 15, 4) with freshly prepared diphenyldiazomethane in MeOH produced the protected derivatives (16–20) in quantitative yield.
The 3,28-di-O-acylbetulins (21–34) were readily prepared by heating the protected 3-O-acylbetulin derivatives with an appropriate dicarboxylic acid or anhydride overnight in the presence of DMAP and dry pyridine (Scheme 2). The two isomeric products obtained when a dimethylsuccinyl group was introduced at C-28 of 16, 18, and 20 were successfully separated by preparative HPLC, yielding corresponding pure derivatives. The orientation of the dimethylsuccinyl group attached to the C-28 hydroxy group of 22/23, 27/28 and 30/31 was established by 2D NMR analyses (HSQC and HMBC). The 28-O-(3",3"-dimethylsuccinyl)betulin derivative was the major product in each case. The electron-donating effect of the dimethyl groups on the adjacent carboxyl group would cause decreased electrophilicity, resulting in different yields of the two isomers in acylation reactions either at C-3 or C-28.
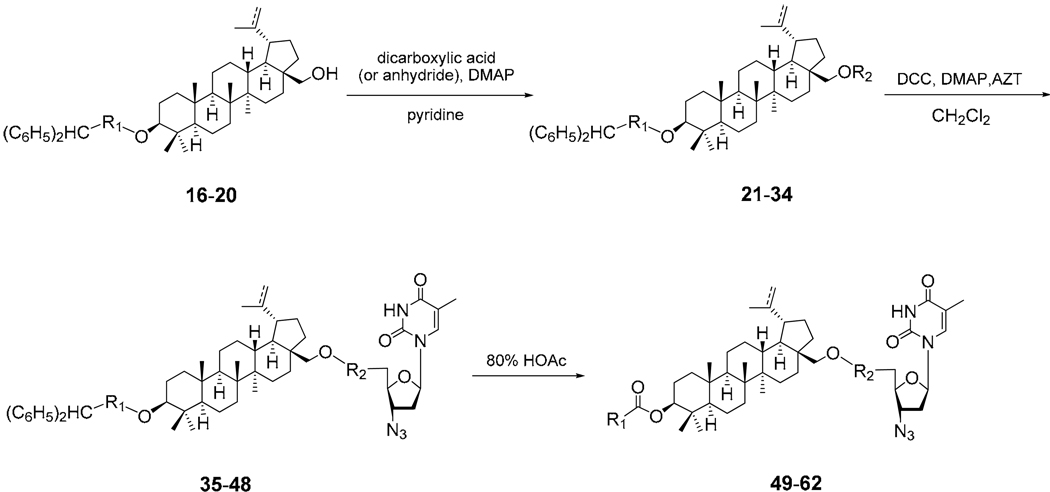
Conjugation of the 3,28-di-O-acylbetulin (21–26) and –dihydrobetulin derivatives (27–34) with AZT in the presence of dicyclohexylcarbodiimide (DCC) and DMAP in CH2Cl2 gave the corresponding conjugates (35–48). However, the corresponding anhydride was also obtained in each case, resulting in only a moderate yield of the desired conjugate. Finally, the terminal protecting group on the 3-O-acyl moiety was removed with 80% acetic acid to afford the target conjugates (49–62).
3. Results and Discussion
The anti-HIV-1 replication activities of the newly synthesized conjugates (49–62) were evaluated in HIV-1NL4-3 infected MT-4 cells in parallel with AZT. The bioassay results are summarized in Table 2.
Table 2.
Anti-HIV-1 Data of 49–62 Against HIV-1NL4-3 Infected MT-4 Cellsa
| Compound | EC50b (µM) | IC50c (µM) | TId |
|---|---|---|---|
| 49 | 0.045 | 7.1 | 158 |
| 50 | 0.098 | 7.0 | 71 |
| 51 | 0.040 | 5.4 | 135 |
| 52 | 0.060 | 8.7 | 145 |
| 53 | 0.063 | 8.1 | 129 |
| 54 | 0.18 | 7.7 | 43 |
| 55 | 0.087 | 9.0 | 103 |
| 56 | 0.056 | 7.7 | 138 |
| 57 | 0.29 | 9.0 | 31 |
| 58 | 0.26 | 7.2 | 28 |
| 59 | 0.073 | 8.0 | 110 |
| 60 | 0.093 | 9.0 | 97 |
| 61 | 1.21 | 8.5 | 7 |
| 62 | 0.36 | 9.8 | 27 |
| 2 | 0.096e | >5e | >52e |
| AZT | 0.027 | >37.4 | >1385 |
Data presented are averages of at least two separate experiments;
Concentration that inhibits HIV-1NL4-3 replication by 50%;
Concentration that inhibits mock-infected MT-4 cell growth by 50%;
TI = IC50/EC50;
Data are taken from reference 23
In the MT-4 screening system, nine conjugates (49–53, 55, 56, 59, 60) exhibited potent anti-HIV activity at a submicromolar level (EC50 values ranging from 0.040 µM to 0.098 µM), and thus, were equipotent or more potent than 2. Compounds 51 and 49, which have a 3-O-(3′,3′-dimethylsuccinyl) moiety together with a 28-O-(2″,2″-dimethylsuccinyl) or 28-O-succinyl group, respectively, linked to AZT, exhibited the highest potency, with EC50 values of 0.040 and 0.045 µM, respectively. Interestingly, all three conjugates (51, 56 and 59) with a 2,2-dimethylsuccinyl linking group at C-28 generally showed higher potency than their corresponding congeners with the same 3-O-acyl group, but a different 28-O-acyl group (51 compared with 50, 52 and 53; 56 compared with 55 and 57; 59 compared with 58, 60, and 61). Of particular note, the conjugates (50, 55, and 58) with a 3,3-dimethylsuccinyl moiety as the linking unit at C-28 showed decreased activity in HIV-1 infected MT-4 cells relative to those with a 2,2-dimethylsuccinyl group (51, 56, and 59). These results confirmed our previous finding that a 2,2-dimethylsuccinyl is superior to a 3,3-dimethylsuccinyl side chain at the C-28 position of anti-HIV triterpene analogs. For example, compound 6 was reported to be 230-fold more potent against HIV replication in H9 lymphocytes assay than its isomer with a 3,3-dimethylsuccinyl side chain at C-28.13
Among conjugates containing glutaryl or dimethyglutaryl esters, 59 and 60 [3-O-glutaryl, 28-O -(2″,2″-dimethylsuccinyl), EC50 0.073 µM and 3,28-di-O-glutaryl, EC50 0.093 µM] were equipotent with 2. However, 54 and 62 [3,28-di-O-(3',3'-dimethylglutaryl)] showed only moderate activity, and 61 [3-O-glutaryl, 28-O-(3″,3″-glutaryl)] was the weakest compound.
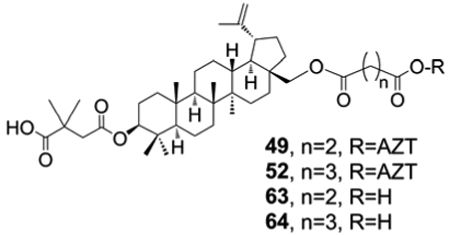
Comparison of the anti-HIV potency of dihydrobetulin conjugates 55–57 with that of betulin conjugates 50, 51, and 53 suggested that hydrogenation of the isopropylidene group at C-19 did not help to increase the anti-HIV potency. Finally, comparison of data for 49/63 and 52/64 in Table 3 showed that hybrid conjugate compounds were four- and five-fold more active, respectively, than the corresponding compounds not linked to AZT.
Table 3.
Anti-HIV-1 Data of Conjugates 49, 52 and Analogous Carboxylic Acids 63, 64 Against HIV-1NL4-3 Infected MT-4 Cellsa
 | |||
|---|---|---|---|
| Compound | EC50b (µM) | IC50c (µM) | TId |
| 49 | 0.045 | 7.1 | 158 |
| 63 | 0.18 | 11.3 | 63 |
| 52 | 0.060 | 8.7 | 145 |
| 64 | 0.310 | >14.6 | >47 |
| 2 | 0.096e | >5e | >52e |
| AZT | 0.027 | >37.4 | >1385 |
Data presented are averages of at least two separate experiments;
Concentration that inhibits HIV-1NL4-3 replication by 50%;
Concentration that inhibits mock-infected MT-4 cell growth by 50%;
TI = IC50/EC50;
Data are taken from reference 23.
In conclusion, fourteen novel conjugates of 3,28-di-O-acyl-betulins with AZT were prepared as anti-HIV-1 agents. In MT-4 cells, nine of the conjugates demonstrated potent anti-HIV activity at a submicromolar level with EC50 values ranging from 0.040 µM to 0.098 µM, and thus, were equipotent or more potent than 2. A 2,2-dimethylsuccinyl group at the C-28 position served as the best linker between the triterpene scaffold and AZT in this study.
4. Experimental Section
4.1 General Experimental Procedures
Optical rotations were measured with a JASCO P-2200 digital polarimeter. NMR spectra (400 MHz for 1H, 100 MHz for 13C, using TMS as internal standard) were recorded on a Bruker AVANCE 400 spectrometer. HRESIMS was obtained on a Waters LCT Premier. Column chromatography was performed with silica gel 60N (63–210 nm, Merck); Preparative HPLC was carried out on Mightysil RP-18 GP column (250 × 20mm i.d., Kanto Chemical Co., Inc.) or Cosmosil column (Cholester, 250 × 20mm i.d.; π NAP, 250 × 20mm i.d.; Nacalai Tesque). Unless otherwise indicated, all reagents were purchased from commercial suppliers and used without further purification.
4.2 Chemical preparation
4.2.1 28-O-Tritylbetulin (7)
To a solution of 3a (1.02 g, 2.30 mmol) in DMF (15 mL) were added dimethylaminopyridine (DMAP, 0.33 g, 2.70 mmol) and triphenylmethyl chloride (1.26 g, 4.52 mmol). After refluxing for 6 h, the reaction mixture was poured into ice water, and extracted with CHCl3. The organic layer was washed with 2N HCl solution and brine, dried over Na2SO4 and concentrated. The obtained residue was chromatographed over silica gel column (hexane/EtOAc = 10:1) to afford 7 as a white solid (1.16 g, 73.5% yield). − 2.1° (c 1.49, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.54 (3H, s, CH3-26), 0.77 (3H, s, CH3-24), 0.78 (3H, s, CH3-25), 0.91 (3H, s, CH3-27), 0.97 (3H, s, CH3-23), 1.65 (3H, s, CH3-30), 2.15–2.24 (3H, m, H-22, H-16 and H-19), 2.92 (1H, d, J = 8.8 Hz, H-28), 3.15–3.19 (2H, m, H-28 and H-3), 4.53, 4.59 (each 1H, br s, H2-29), 7.23 (3H, t, J = 7.2 Hz, trityl H-4'), 7.31 (6H, t, J = 7.6, 7.2 Hz, trityl H-3',5'), 7.49 (6H, d, J = 7.6 Hz, trityl H-2',6'); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 15.3 (C-24), 15.9 (C-26), 16.1 (C-25), 18.3 (C-6), 19.1 (C-30), 20.7 (C-11), 25.2 (C-12), 26.9 (C-15), 27.4 (C-2), 28.0 (C-23), 29.9 (C-21), 30.2 (C-16), 34.2 (C-7), 35.2 (C-22), 37.1 (C-10), 37.3 (C-13), 38.7 (C-1), 38.8 (C-4), 40.6 (C-8), 42.5 (C-14), 47.6 (C-17), 47.7 (C-19), 48.9 (C-18), 50.3 (C-9), 55.3 (C-5), 59.6 (C-28), 79.0 (C-3), 85.9 (trityl C(Ph)3), 109.3 (C-29), 126.8 (trityl C-4'), 127.7 (trityl C-2', 6'), 128.8 (trityl C-3', 5'), 144.5 (trityl C-1'), 150.8 (C-20). HRESIMS (positive) m/z 707.4781 [M+Na]+ (calcd for C49H64O2Na, 707.4804).
4.2.2 General procedure for preparation of 3-O-acyl-28-O-tritylbetulins (8–10)
3-O-Acyl-28-O-tritylbetulins were prepared by refluxing a solution of 7 (1 equiv), appropriate dicarboxylic acid (4 equiv) or anhydride (4–5 equiv) and DMAP (1–2 equiv) in anhydrous pyridine (10–20 mL) overnight. After cooling to room temperature, the reaction mixture was diluted with ice-water, and extracted with CHCl3. The organic layer was washed with water, 2N HCl solution and brine, dried over Na2SO4, and concentrated. The crude product was purified by silica gel column chromatography (hexane/EtOAc = 10:1).
3-O-(3',3'-Dimethylsuccinyl)-28-O-tritylbetulin (8)
Yield 38.9% (starting from 4.48 g of 7 and 2,2-dimethylsuccinic acid); white solid; 3-O-(2',2'-dimethylsuccinyl)-28-O-tritylbetulin was also separated as by-product (11.1% yield). +2.1° (c 1.25, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.53 (3H, s, CH3-26), 0.79 (3H, s, CH3-25), 0.81 (3H, s, CH3-24), 0.84 (3H, s, CH3-23), 0.90 (3H, s, CH3-27), 1.30, 1.31 (each 3H, s, dimethylsuccinyl CH3), 1.65 (3H, s, CH3-30), 2.15–2.24 (3H, m, H-22, H-16 and H-19), 2.57, 2.68 (each 1H, d, J = 15.6 Hz, dimethylsuccinyl H2-2'), 2.92, 3.15 (each 1H, d, J = 8.8 Hz, H2-28), 4.48 (1H, dd, J = 5.2, 11.2 Hz, H-3), 4.53, 4.59 (each 1H, br s, H2-29), 7.23 (3H, t, J = 7.2 Hz, trityl H-4'), 7.31 (6H, t, J = 8.0, 7.2 Hz, trityl H-3',5'), 7.50 (6H, d, J = 8.0 Hz, trityl H-2',6'); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 15.9 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 19.1 (C-30), 20.7 (C-11), 23.6 (C-2), 25.1 (C-12), 25.0, 25.6 (dimethylsuccinyl CH3), 26.9 (C-15), 27.9 (C-23), 29.9 (C-21), 30.1 (C-16), 34.1 (C-7), 35.2 (C-22), 37.0 (C-10), 37.2 (C-13), 37.7 (C-4), 38.3 (C-1), 40.5 (dimethylsuccinyl C-3'), 40.6 (C-8), 42.5 (C-14), 44.7 (dimethylsuccinyl C-2'), 47.6 (C-17), 47.8 (C-19), 48.9 (C-18), 50.2 (C-9), 55.3 (C-5), 59.5 (C-28), 81.6 (C-3), 85.8 (trityl C(Ph)3), 109.4 (C-29), 126.8 (trityl C-4'), 127.7 (trityl C-2', 6'), 128.8 (trityl C-3', 5'), 144.5 (trityl C-1'), 150.8 (C-20), 170.9 (dimethylsuccinyl COO−), 182.8 (dimethylsuccinyl COOH). HRESIMS (positive) m/z 835.5286 [M+Na]+ (calcd for C55H72O5Na, 835.5277).
3-O-(3',3'-Dimethylglutaryl)-28-O-tritylbetulin (9)
Yield 86.6% (starting from 956 mg of 7 and 3,3-dimethylglutaric anhydride); white solid. +7.5° (c 0.52, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.55 (3H, s, CH3-26), 0.81 (3H, s, CH3-25), 0.85 (3H, s, CH3-24), 0.86 (3H, s, CH3-23), 0.91 (3H, s, CH3-27), 1.16 (6H, s, dimethylglutaryl CH3), 1.65 (3H, s, CH3-30), 2.15–2.24 (3H, m, H-22, H-16 and H-19), 2.40, 2.47 (each 1H, d, J = 14.0 Hz, dimethylglutaryl H2-2'), 2.48 (2H, s, dimethylglutaryl H2-4'), 2.92, 3.15 (each 1H, d, J = 8.8 Hz, H2-28), 4.49 (1H, dd, J = 4.8, 10.8 Hz, H-3), 4.54, 4.60 (each 1H, br s, H2-29), 7.23 (3H, t, J = 7.6Hz, trityl H-4'), 7.31 (6H, t, J = 7.6Hz, trityl H-3',5'), 7.50 (6H, d, J = 7.6Hz, trityl H-2',6'); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 15.9 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 19.1 (C-30), 20.8 (C-11), 23.8 (C-2), 25.2 (C-12), 27.0 (C-15), 27.9, 28.0 (dimethylglutaryl CH3), 28.0 (C-23), 30.0 (C-21), 30.2 (C-16), 32.7 (dimethylglutaryl C-3'), 34.2 (C-7), 35.2 (C-22), 37.1 (C-10), 37.3 (C-13), 37.7 (C-4), 38.4 (C-1), 40.7 (C-8), 42.5 (C-14), 45.2 (dimethylglutaryl C-4'), 45.7 (dimethylglutaryl C-2'), 47.6 (C-17), 47.8 (C-19), 49.0 (C-18), 50.3 (C-9), 55.4 (C-5), 59.7 (C-28), 81.5 (C-3), 85.9 (trityl C(Ph)3), 109.4 (C-29), 126.8 (trityl C-4'), 127.7 (trityl C-2', 6'), 128.8 (trityl C-3', 5'), 144.5 (trityl C-1'), 150.8 (C-20), 172.3 (dimethylglutaryl COO−), 175.9 (dimethylglutaryl COOH). HRESIMS (positive) m/z 849.5403 [M+Na]+ (calcd for C56H74O5Na, 849.5434).
3-O-Glutaryl-28-O-tritylbetulin (10)
Yield 84.1% (starting from 1.07 g of 7 and glutaric anhydride); white solid. +5.1° (c 1.96, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.53 (3H, s, CH3-26), 0.79 (3H, s, CH3-25), 0.83 (3H, s, CH3-24), 0.83 (3H, s, CH3-23), 0.90 (3H, s, CH3-27), 1.65 (3H, s, CH3-30), 1.97 (2H, quint, J = 7.2 Hz, glutary H2-3'), 2.15–2.24 (3H, m, H-22, H-16 and H-19), 2.39 (2H, t, J = 7.2 Hz, glutary H2-2'), 2.43 (2H, s, glutaryl H2-4'), 2.92, 3.15 (each 1H, d, J = 8.8 Hz, H2-28), 4.48 (1H, dd, J = 5.6, 10.8 Hz, H-3), 4.53, 4.59 (each 1H, br s, H2-29), 7.23 (3H, t, J = 7.2Hz, trityl H-4'), 7.31 (6H, t, J = 8.0, 7.2Hz, trityl H-3',5'), 7.50 (6H, d, J = 8.0Hz, trityl H-2',6'); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 15.8 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 19.1 (C-30), 20.0 (glutaryl C-3'), 20.8 (C-11), 23.7 (C-2), 25.1 (C-12), 27.0 (C-15), 28.0 (C-23), 29.9 (C-21), 30.1 (C-16), 32.9 (glutaryl C-4'), 33.6 (glutaryl C-2'), 34.1 (C-7), 35.2 (C-22), 37.0 (C-10), 37.2 (C-13), 37.8 (C-4), 38.3 (C-1), 40.6 (C-8), 42.5 (C-14), 47.6 (C-17), 47.8 (C-19), 48.9 (C-18), 50.2 (C-9), 55.3 (C-5), 59.5 (C-28), 81.1 (C-3), 85.8 (trityl C(Ph)3), 109.4 (C-29), 126.8 (trityl C-4'), 127.7 (trityl C-2', 6'), 128.8 (trityl C-3', 5'), 144.5 (trityl C-1'), 150.8 (C-20), 172.6 (glutaryl COO−), 178.1 (glutaryl COOH). HRESIMS (positive) m/z 821.5139 [M+Na]+ (calcd for C54H70O5Na, 821.5121).
4.2.3 General procedure for preparation of 3-O-acylbetulins (11–12)
3-O-Acyl-28-O-tritylbetulin (1 equiv) was dissolved in EtOH-CH2Cl2 and then pyridium p-toluenesulfonate (PPTS) (3–5 equiv) was added. The reaction mixture was refluxed at 70°C overnight. After evaporation under reduced pressure, ice water was added to the residue, and extraction was performed with CHCl3. The organic layer was washed with water and brine, dried over Na2SO4, and concentrated. The residue was subjected over silica gel column (hexane/EtOAc=1:1) to give pure product.
3-O-(3',3'-Dimethylsuccinyl)betulin (11)
Yield 58.9% (starting from 1.51 g of 8); white solid; +24.1° (c 1.95, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.81 (3H, s, CH3-24), 0.84 (6H, s, CH3-23, 25), 0.97 (3H, s, CH3-27), 1.02 (3H, s, CH3-26), 1.28, 1.30 (each 3H, s, dimethylsuccinyl CH3), 1.73 (3H, s, CH3-30), 2.38 (1H, dt, J = 5.6, 10.4 Hz, H-19), 2.56, 2.67 (each 1H, d, J = 15.6 Hz, dimethylsuccinyl H2-2'), 3.34, 3.80 (each 1H, d, J = 10.8 Hz, H2-28), 4.49 (1H, dd, J = 5.2, 10.4 Hz, H-3), 4.58, 4.68 (each 1H, br s, H2-29); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 19.0 (C-30), 20.8 (C-11), 23.6 (C-2), 25.1 (C-12), 25.0, 25.6 (dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.1 (C-16), 29.7 (C-21), 33.9 (C-22), 34.1 (C-7), 37.0 (C-10), 37.3 (C-13), 37.7 (C-4), 38.4 (C-1), 40.5 (dimethylsuccinyl C-3'), 40.9 (C-8), 42.7 (C-14), 44.7 (dimethylsuccinyl C-2'), 47.7 (C-17), 47.8 (C-19), 48.7 (C-18), 50.3 (C-9), 55.4 (C-5), 60.5 (C-28), 81.5 (C-3), 109.7 (C-29), 150.4 (C-20), 170.9 (dimethylsuccinyl COO−), 182.6 (dimethylsuccinyl COOH). HRESIMS (positive) m/z 593.4171 [M+Na]+ (calcd for C36H58O5Na, 593.4182).
3-O-(3',3'-Dimethylglutaryl)betulin (12)
Yield 67.2% (starting from 1.0 g of 9); white solid. +24.0° (c 1.88, CHCl3). 1H NMR (C5D5N, 400 MHz) δ 0.79 (3H, s, CH3-25), 0.92 (3H, s, CH3-24), 0.94 (3H, s, CH3-23), 0.96 (3H, s, CH3-26), 1.03 (3H, s, CH3-27), 1.36, 1.37 (each 3H, s, dimethylglutaryl CH3), 1.76 (3H, s, CH3-30), 2.11–2.17 (1H, m, H-21), 2.37–2.45 (2H, m, H-16 and H-22), 2.61 (1H, dt, J = 5.6, 10.8Hz, H-19), 2.74, 2.79 (each 1H, d, J = 16.0 Hz, dimethylglutaryl H2-2'), 2.76 (2H, s, dimethylglutaryl H2-4'), 3.65, 4.06 (each 1H, d, J = 10.8 Hz, H2-28), 4.71–4.74 (1H, m, H-3), 4.74, 4.88 (each 1H, br s, H2-29); 13C NMR (C5D5N, 100 MHz) δ 14.9 (C-27), 16.1 (C-26), 16.2 (C-25), 16.9 (C-24), 18.4 (C-6), 19.3 (C-30), 21.0 (C-11), 24.2 (C-2), 25.6 (C-12), 27.5 (C-15), 28.0 (dimethylglutaryl CH3), 28.1 (C-23), 30.0 (C-16), 30.4 (C-21), 32.7 (dimethylglutaryl C-3'), 34.4 (C-7), 34.9 (C-22), 37.2 (C-10), 37.5 (C-13), 37.9 (C-4), 38.5 (C-1), 41.2 (C-8), 43.0 (C-14), 45.9 (dimethylglutaryl C-4', 2'), 48.3 (C-19), 48.5 (C-17), 49.1 (C-18), 50.5 (C-9), 55.5 (C-5), 59.4 (C-28), 80.6 (C-3), 109.9 (C-29), 151.2 (C-20), 171.9 (dimethylglutaryl COO−), 174.4 (dimethylglutaryl COOH). HRESIMS (positive) m/z 607.4351 [M+Na]+ (calcd for C37H60O5Na, 607.4338).
4.2.4 3-O-Glutarylbetulin (13)
To a solution of 10 (1.50 g, 1.88 mmol) in 95% THF (50 mL) was added anhydrous FeCl3 (1.22 g, 7.52 mmol). The solution was refluxed overnight. After cooling to room temperature, the solvent was evaporated in vacuo and the residue was dissolved in CHCl3. The CHCl3 solution was washed with water and brine, dried over Na2SO4, filtrated and concentrated. The crude product was purified by silica column chromatography (hexane/EtOAc = 2:1) to give 13 as a white solid (600 mg, yield 57.4 %). +25.1° (c 3.45, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.84 (3H, s, CH3-24), 0.85 (3H, s, CH3-23), 0.86 (3H, s, CH3-25), 0.99 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.69 (3H, s, CH3-30), 1.98 (2H, quint, J = 7.2 Hz, glutary H2-3'), 2.35–2.44 (1H, m, H-19), 2.39 (2H, t, J = 7.2 Hz, glutary H2-2'), 2.43 (2H, s, glutaryl H2-4'), 3.35, 3.81 (each 1H, d, J = 10.8 Hz, H2-28), 4.49 (1H, dd, J = 5.6, 10.4 Hz, H-3), 4.59, 4.69 (each 1H, br s, H2-29); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 19.1 (C-30), 20.1 (glutaryl C-3'), 20.9 (C-11), 23.7 (C-2), 25.2 (C-12), 27.1 (C-15), 28.0 (C-23), 29.2 (C-16), 29.8 (C-21), 32.9 (glutaryl C-4'), 33.7 (glutaryl C-2'), 34.0 (C-22), 34.2 (C-7), 37.1 (C-10), 37.3 (C-13), 37.8 (C-4), 38.4 (C-1), 41.0 (C-8), 42.7 (C-14), 47.8 (C-17), 47.8 (C-19), 48.8 (C-18), 50.3 (C-9), 55.4 (C-5), 60.6 (C-28), 81.1 (C-3), 109.7 (C-29), 150.4 (C-20), 172.6 (glutaryl COO−), 177.6 (glutaryl COOH). HRESIMS (positive) m/z 579.4027 [M+Na]+ (calcd for C35H56O5Na, 579.4025).
4.2.5 General procedure of hydrogenation
3-O-Acyl-dihydrobetulin derivatives (4, 14, and 15) were prepared by treating a solution of 3-O-acylbetulin derivative (250–400 mg) in EtOAc-EtOH with 10% palladium catalyst (Pd-C, 240–800 mg) under hydrogen atmosphere overnight with stirring. The reaction mixture was filtered, and the filtrate was concentrated under reduced pressure to give a white solid. The purity of the product was confirmed by NMR analyses and used in the next reaction without further purification.
3-O-(3',3'-Dimethylsuccinyl)dihydrobetulin (14)
Yield 100% (starting from 395 mg of 11 and 240 mg of 10% Pd-C); white solid; −9.3° (c 1.5, CHCl3). 1H NMR (C5D5N, 400 MHz) δ 0.79 (6H, s, CH3-25), 0.82, 0.89 (each 2H, d, J = 8.0 Hz, CH3-29, 30), 0.94 (3H, s, CH3-24), 0.96 (3H, s, CH3–23, 26), 0.98 (3H, s, CH3-27), 1.53 (6H, s, dimethylsuccinyl CH3), 2.38–2.41 (2H, m, H-16 and H-22), 2.88, 2.94 (each 1H, d, J = 15.6 Hz, dimethylsuccinyl H2-2'), 3.58, 4.04 (each 1H, d, J = 10.4 Hz, H2-28), 4.76 (1H, dd, J = 4.4, 11.6 Hz, H-3); 13C NMR (C5D5N, 100 MHz) δ 14.8 (C-27), 15.2 (C-29), 16.1 (C-26), 16.2 (C-25), 16.9 (C-24), 18.4 (C-6), 21.1 (C-11), 22.3 (C-21), 23.2 (C-30), 24.1 (C-2), 25.9, 26.2 (dimethylsuccinyl CH3), 27.2 (C-12), 27.4 (C-15), 28.1 (C-23), 29.8 (C-20), 30.1 (C-16), 34.5 (C-7), 34.9 (C-22), 37.0 (C-13), 37.2 (C-10), 38.0 (C-4), 38.5 (C-1), 40.9 (dimethylsuccinyl C-3'), 41.2 (C-8), 43.1 (C-14), 45.0 (C-19), 45.2 (dimethylsuccinyl C-2'), 48.6 (C-17), 48.4 (C-18), 50.1 (C-9), 55.5 (C-5), 59.4 (C-28), 80.9 (C-3), 171.6 (dimethylsuccinyl COO−), 179.3 (dimethylsuccinyl COOH). HRESIMS (positive) m/z 595.4327 [M+Na]+ (calcd for C36H60O5Na, 595.4338).
3-O-(3',3'-Dimethylglutaryl)dihydrobetulin (15)
Yield 97.4% (starting from 250 mg of 12 and 250 mg of 10% Pd-C); white solid. −8.9° (c 1.54, CHCl3). 1H NMR (C5D5N, 400 MHz) δ 0.82 (3H, s, CH3-25), 0.83, 0.90 (each 2H, d, J = 8.0 Hz, CH3-29, 30), 0.93 (3H, s, CH3-24), 0.95 (3H, s, CH3-23), 0.97 (3H, s, CH3-26), 1.00 (3H, s, CH3-27), 1.37, 1.37 (each 3H, s, dimethylglutaryl CH3), 2.37–2.41 (2H, m, H-16 and H-22), 2.75, 2.79 (each 1H, d, J = 14.4 Hz, dimethylglutaryl H2-2'), 2.77 (2H, s, dimethylglutaryl H2-4'), 3.59, 4.05 (each 1H, d, J = 10.8 Hz, H2-28), 4.74 (1H, dd, J = 4.4, 11.6 Hz, H-3); 13C NMR (C5D5N, 100 MHz) δ 14.8 (C-27), 15.2 (C-29), 16.1 (C-26), 16.2 (C-25), 16.9 (C-24), 18.4 (C-6), 21.1 (C-11), 22.3 (C-21), 23.2 (C-30), 24.2 (C-2), 27.2 (C-12), 27.4 (C-15), 28.0 (dimethylglutaryl CH3), 28.1 (C-23), 29.8 (C-20), 30.1 (C-16), 32.7 (dimethylglutaryl C-3'), 34.5 (C-7), 34.9 (C-22), 37.0 (C-13), 37.2(C-10), 37.9 (C-4), 38.5 (C-1), 41.2 (C-8), 43.1 (C-14), 45.0 (C-19), 45.9 (dimethylglutaryl C-4', 2'), 48.4 (C-18), 48.6 (C-17), 50.2 (C-9), 55.5 (C-5), 59.4 (C-28), 80.7 (C-3), 172.0 (dimethylglutaryl COO−), 174.4 (dimethylglutaryl COOH). HRESIMS (positive) m/z 609.4493 [M+Na]+ (calcd for C37H62O5Na, 609.4495).
3-O-Glutaryl-dihydrobetulin (4)
Yield 91.1% (starting from 387 mg of 13 and 800 mg of 10% Pd-C); white solid. −5.4° (c 2.40, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.85 (each 2H, d, J = 8.0 Hz, CH3-29, 30), 0.85 (3H, s, CH3-24), 0.85 (3H, s, CH3-23), 0.87 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.04 (3H, s, CH3-26), 1.99 (2H, quint, J = 7.2 Hz, glutary H2-3'), 2.40 (2H, t, J = 7.2 Hz, glutary H2-2'), 2.43 (2H, s, glutaryl H2-4'), 3.32, 3.79 (each 1H, d, J = Hz, H2-28), 4.50 (1H, dd, J = 6.4, 10.0 Hz, H-3); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27),14.9 (C-29), 16.0 (C-26), 16.1 (C-25), 16.6 (C-24), 18.2 (C-6), 20.1 (glutaryl C-3'), 20.9 (C-11), 21.7 (C-21), 22.9 (C-30), 23.7 (C-2), 26.9 (C-12), 26.9 (C-15), 28.0 (C-23), 29.3 (C-16), 29.5 (C-20), 33.0 (glutaryl C-4'), 33.7 (glutaryl C-2'), 34.0 (C-22), 34.3 (C-7), 36.9 (C-13), 37.1 (C-10), 37.8 (C-4), 38.4 (C-1), 41.0 (C-8), 42.9 (C-14), 44.6 (C-19), 47.9 (C-17), 48.1 (C-18), 50.0 (C-9), 55.4 (C-5), 60.6 (C-28), 81.1 (C-3), 172.7 (glutaryl COO−), 177.9 (glutaryl COOH). HRESIMS (positive) m/z 581.4180 [M+Na]+ (calcd for C35H58O5Na, 581.4182).
4.2.6 General procedure for syntheses of 3-O-acylbetulin benzhydryl ester derivatives (16–20)
To a solution of 3-O-acylbetulin derivatives (1 equiv) in CH2Cl2-CH3OH, freshly prepared diphenyldiazomethane was added until the reaction solution turned pink. The resulting mixture was kept stirring at room temperature overnight. After evaporated solvent in vacuo, the residue was subjected over silica gel column (hexane/EtOAc = 10:1) to give the desired product.
3-O-(3',3'-Dimethylsuccinyl)betulin benzhydryl ester (16)
Yield 100% (starting from 265 mg of 11); white solid; +16.9° (c 1.74, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.75 (3H, s, CH3-24), 0.79 (3H, s, CH3-23), 0.80 (3H, s, CH3-25), 0.99 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.32, 1.32 (each 3H, s, dimethylsuccinyl CH3), 1.70 (3H, s, CH3-30), 2.40 (1H, dt, J = 5.6, 10.4 Hz, H-19), 2.66, 2.70 (each 1H, d, J = 16.0 Hz, dimethylsuccinyl H2-2'), 3.34, 3.80 (each 1H, d, J = 10.8 Hz, H2-28), 4.45 (1H, dd, J = 5.2, 10.8 Hz, H-3), 4.60, 4.70 (each 1H, br s, H2-29), 6.87 (1H, s, CH(Ph)2), 7.25–7.34 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 15.9 (C-26), 16.0 (C-25), 16.4 (C-24), 18.1 (C-6), 19.0 (C-30), 20.8 (C-11), 23.5 (C-2), 25.1 (C-12), 25.1, 25.4 (dimethylsuccinyl CH3), 27.0 (C-15), 27.8 (C-23), 29.1 (C-16), 29.7 (C-21), 33.9 (C-22), 34.1 (C-7), 37.0 (C-10), 37.2 (C-13), 37.6 (C-4), 38.3 (C-1), 40.6 (dimethylsuccinyl C-3'), 40.9 (C-8), 42.6 (C-14), 44.5 (dimethylsuccinyl C-2'), 47.7 (C-17), 47.8 (C-19), 48.7 (C-18), 50.2 (C-9), 55.3 (C-5), 60.4 (C-28), 76.9 (CH(Ph)2), 81.2 (C-3), 109.7 (C-29), 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.3 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 150.4 (C-20), 170.8 (dimethylsuccinyl 1'-COO−), 175.3 (dimethylsuccinyl 4'-COO−). HRESIMS (positive) m/z 759.4958 [M+Na]+ (calcd for C49H68O5Na, 759.4964).
3-O-(3',3'-Dimethyglutaryl)betulin benzhydryl ester (17)
Yield 100% (starting from 207 mg of 12); white solid. +18.6° (c 1.29, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.81 (3H, s, CH3-24), 0.84 (6H, s, CH3-23, 25), 0.99 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.09 (6H, s, dimethylglutaryl CH3), 1.70 (3H, s, CH3-30), 2.34–2.45 (1H, m, H-19), 2.36, 2.43 (each 1H, d, J = 14.4 Hz, dimethylglutaryl H2-2'), 2.55, 2.58 (each 1H, d, J = 14.4 Hz, dimethylglutaryl H2-4'), 3.34, 3.81 (each 1H, d, J = 10.8 Hz, H2-28), 4.47 (1H, dd, J = 4.8, 11.2 Hz, H-3), 4.60, 4.70 (each 1H, br s, H2-29), 6.89 (1H, s, CH(Ph)2), 7.25–7.39 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.6 (C-24), 18.2 (C-6), 19.0 (C-30), 20.8 (C-11), 23.8 (C-2), 25.2 (C-12), 27.0 (C-15), 27.6, 27.7 (dimethylglutaryl CH3), 28.0 (C-23), 29.2 (C-16), 29.7 (C-21), 32.8 (dimethylglutaryl C-3'), 33.9 (C-22), 34.1 (C-7), 37.0 (C-10), 37.3 (C-13), 37.7 (C-4), 38.4 (C-1), 40.9 (C-8), 42.7 (C-14), 45.5 (dimethylsuccinyl C-4'), 45.8 (dimethylsuccinyl C-2'), 47.8 (C-17), 47.8 (C-19), 48.7 (C-18), 50.3 (C-9), 55.4 (C-5), 60.5 (C-28), 76.7 (CH(Ph)2), 80.9 (C-3), 109.7 (C-29), 127.1 (benzhydryl C-2', 6'), 127.8 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.2, 140.3 (benzhydryl C-1'), 150.4 (C-20), 170.8 (dimethylglutaryl 5'-COO−), 171.6 (dimethylglutaryl 1'-COO−). HRESIMS (positive) m/z 773.5124 [M+Na]+ (calcd for C50H70O5Na, 773.5121).
3-O-(3',3'-Dimethylsuccinyl)dihydrobetulin benzhydryl ester (18)
Yield 100% (starting from 400 mg of 14); white solid. −7.9° (c 5.27, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.75 (3H, s, CH3-24), 0.79 (3H, s, CH3-23), 0.78, 0.85 (each 2H, d, J = 8.0 Hz, CH3-29, 30), 0.81 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.31, 1.32 (each 3H, s, dimethylsuccinyl CH3), 2.66, 2.70 (each 1H, d, J = 15.6 Hz, dimethylsuccinyl H2-2'), 3.31, 3.78 (each 1H, d, J = 10.8 Hz, H2-28), 4.45 (1H, dd, J = 5.0, 10.8 Hz, H-3), 6.87 (1H, s, CH(Ph)2), 7.27–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 15.9 (C-26), 16.0 (C-25), 16.5 (C-24), 18.1 (C-6), 20.8 (C-11), 21.7 (C-21), 22.9 (C-30), 23.5 (C-2), 25.2, 25.4 (dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 27.9 (C-23), 29.3 (C-16), 29.5 (C-20), 34.0 (C-22), 34.2 (C-7), 36.8 (C-13), 37.0 (C-10), 37.6 (C-4), 38.3 (C-1), 40.7 (dimethylsuccinyl C-3'), 40.9 (C-8), 42.8 (C-14), 44.5 (C-19 and dimethylsuccinyl C-2'), 47.9 (C-17), 48.0 (C-18), 49.9 (C-9), 55.3 (C-5), 60.6 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 170.8 (dimethylsuccinyl 1'-COO−), 175.4 (dimethylsuccinyl 4'-COO−). HRESIMS (positive) m/z 761.5118 [M+Na]+ (calcd for C49H70O5Na, 761.5121).
3-O-(3',3'-Dimethylglutaryl)dihydrobetulin benzhydryl ester (19)
Yield 85.3% (starting from 228 mg of 15); white solid. − 7.7° (c 1.26, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.79 (3H, s, CH3-24), 0.82 (3H, s, CH3-23, 25), 0.76, 0.83 (each 2H, d, J = 8.0 Hz, CH3-29, 30), 0.94 (3H, s, CH3-27), 1.00 (3H, s, CH3-26), 1.07 (6H, s, dimethylglutaryl CH3), 2.34, 2.41 (each 1H, d, J = 14.4 Hz, dimethylglutaryl H2-2'), 2.53, 2.56 (each 1H, d, J = 14.4 Hz, dimethylglutaryl H2-4'), 3.30, 3.76 (each 1H, d, J = 10.8 Hz, H2-28), 4.46 (1H, dd, J = 4.8, 10.8 Hz, H-3), 6.87 (1H, s, CH(Ph)2), 7.23–7.34 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.5 (C-27), 14.8 (C-29), 15.9 (C-26), 16.0 (C-25), 16.5 (C-24), 18.1 (C-6), 20.7 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 26.8 (C-12), 26.8 (C-15), 27.5, 27.6 (dimethylglutaryl CH3), 27.9 (C-23), 29.3 (C-16), 29.4 (C-20), 32.7 (dimethylglutaryl C-3'), 34.0 (C-22), 34.1 (C-7), 36.7 (C-13), 36.9 (C-10), 37.6 (C-4), 38.3 (C-1), , 40.9 (C-8), 42.8 (C-14), 44.5 (C-19), 45.3 (dimethylglutaryl C-4'), 45.7 (dimethylglutaryl C-2'), 47.8 (C-17), 48.0 (C-18), 49.9 (C-9), 55.2 (C-5), 60.1 (C-28), 76.6 (CH(Ph)2), 80.8 (C-3), 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.3 (benzhydryl C-3', 5'), 140.2 (benzhydryl C-1'), 170.7 (dimethylglutaryl 5'-COO−), 171.5 (dimethylglutaryl 1'-COO–). hReSIMS (positive) m/z 775.5303 [M+Na]+ (calcd for C50H72O5Na, 775.5277).
3-O-Glutaryl-dihydrobetulin benzhydryl ester (20)
Yield 96.4% (starting from 332 mg of 4); white solid. −4.2° (c 3.84, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.84 (3H, s, CH3-24), 0.85 (3H, s, CH3-23), 0.79, 0.85 (each 2H, d, J = 8.0 Hz, CH3-29, 30), 0.87 (3H, s, CH3-25), 0.97 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 2.00 (2H, t, J = 7.2 Hz, glutaryl H2-3'), 2.36 (2H, t, J = 7.2 Hz, glutaryl H2-2'), 2.51 (2H, t, J = 7.2 Hz, glutaryl H2-4'), 3.32, 3.78 (each 1H, d, J = 10.8 Hz, H2-28), 4.50 (1H, dd, J = 5.6, 10.4 Hz, H-3), 6.90 (1H, s, CH(Ph)2), 7.26–7.34 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 20.4 (glutaryl C-3'), 20.9 (C-11), 21.7 (C-21), 22.9 (C-30), 23.7 (C-2), 26.9 (C-12), 26.9 (C-15), 28.0 (C-23), 29.3 (C-16), 29.5 (C-20), 33.6 (glutaryl C-4'), 33.7 (glutaryl C-2'), 34.0 (C-22), 34.3 (C-7), 36.8 (C-13), 37.1 (C-10), 37.8 (C-4), 38.4 (C-1), 41.0 (C-8), 42.9 (C-14), 44.6 (C-19), 47.9 (C-17), 48.1 (C-18), 50.0 (C-9), 55.3 (C-5), 60.6 (C-28), 76.9 (CH(Ph)2), 81.0 (C-3), 127.1 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 140.2 (benzhydryl C-1'), 171.9 (glutaryl 5'-COO−), 172.6 (glutaryl 1'-COO−). HRESIMS (positive) m/z 747.4962 [M+Na]+ (calcd for C48H68O5Na, 747.4964).
4.2.7 General procedure for syntheses of 3,28-di-O-acylbetulin derivatives (21–34)
3,28-Di-O-acylbetulin derivatives were prepared by refluxing a solution of 3-O-acylbetulin benzhydryl ester derivatives (1 equiv), DMAP (1.5–4.5 equiv) and appropriate dicarboxylic acid (6–9 equiv) or anhydride (3–6 equiv) in anhydrous pyridine (5–8 mL) overnight. After cooling to room temperature, the reaction mixture was poured into ice-water, and extracted with CHCl3. The organic layer was washed with water, 2N HCl solution and brine in turn, dried over Na2SO4, and concentrated. The residue was chromatographed over silica gel column (CHCl3 or hexane/EtOAc = 5:1) or purified by preparative HPLC.
3-O-(4'-Benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-succinylbetulin (21)
Yield 76.8% (starting from 504.6 mg of 16 and succinic anhydride); white solid. +5.6° (c 0.5, CHCl3). 1H NMR (CDCl3, 500 MHz) δ 0.73 (3H, s, CH3-24), 0.77 (6H, s, CH3-23 and CH3-25), 0.96 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.30, 1.31 (each 3H, s, dimethylsuccinyl CH3), 1.68 (3H, s, CH3-30), 2.43 (1H, dt, J = 5.5, 10.9 Hz, H-19), 2.64, 2.69 (each 1H, d, J = 15.8 Hz, 3-O-dimethylsuccinyl H2-2'), 2.68 (4H, t, J = 7.3 Hz, 28-O-succinyl H2-2", H2-3"), 3.88, 4.30 (each 1H, d, J = 10.8 Hz, H2-28), 4.43 (1H, dd, J = 5.2, 11.2 Hz, H-3), 4.59, 4.68 (each 1H, br s, H2-29), 6.85 (1H, s, CH(Ph)2), 7.24–7.32 (10H, m, aromatic-H); 13C NMR (CDCl3, 125 MHz) δ 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.7 (C-11), 23.5 (C-2), 25.1 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 28.8 (28-O-succinyl C-3"), 29.0 (28-O-succinyl C-2"), 29.5 (C-21), 29.7 (C-16), 34.0 (C-7), 34.5 (C-22), 37.0 (C-10), 37.5 (C-13), 37.6 (C-4), 38.3 (C-1), 40.7 (dimethylsuccinyl C-3'), 40.8 (C-8), 42.6 (C-14), 44.5 (3-O-dimethylsuccinyl C-2'), 46.4 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.3 (C-5), 63.2 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 109.9 (C-29), 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 150.1 (C-20), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 172.5 (28-O-succinyl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 176.8 (28-O-succinyl COOH). HRESIMS (positive) m/z 859.5112 [M+Na]+ (calcd for C53H72O8Na, 859.5125).
3-O-(4'-Benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-(3",3"-dimethylsuccinyl)betulin (22) and 3-O-(4'-benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-(2",2"-dimethylsuccinyl)betulin (23)
Yield 68.4% and 31.6%, respectively (starting from 114 mg of 16 and 2,2-dimethylsuccinic acid); separated by preparative HPLC (πNAP, MeOH/2% HOAc = 97:3) for spectroscopic analysis. For synthesis, mixture was used for next reaction without purification.
Compound 22
+6.0° (c 6.81, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.75 (3H, s, CH3-24), 0.79 (3H, s, CH3-23), 0.80 (3H, s, CH3-25), 0.97 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.32 (12H, s, dimethylsuccinyl CH3), 1.70 (3H, s, CH3-30), 2.43 (1H, dt, J = 5.6, 10.8 Hz, H-19), 2.64, 2.70 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 2.66 (2H, s, 28-O-dimethylsuccinyl H2-2"), 3.88, 4.29 (each 1H, d, J = 10.8 Hz, H2-28), 4.45 (1H, dd, J = 5.2, 10.8 Hz, H-3), 4.61, 4.70 (each 1H, br s, H2-29), 6.87 (1H, s, CH(Ph)2), 7.25–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.8 (C-27), 16.1 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 19.2 (C-30), 20.9 (C-11), 23.6 (C-2), 25.2 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 25.3, 25.4 (28-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.6 (C-21), 29.8 (C-16), 34.2 (C-7), 34.6 (C-22), 37.1 (C-10), 37.7 (C-4, C-13), 38.4 (C-1), 40.7 (dimethylsuccinyl C-3', C-3"), 40.9 (C-8), 42.7 (C-14), 44.4 (28-O-dimethylsuccinyl C-2"), 44.6 (3-O-dimethylsuccinyl C-2'), 46.3 (C-17), 47.7 (C-19), 48.9 (C-18), 50.3 (C-9), 55.4 (C-5), 63.1 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 109.8 (C-29), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.4 (benzhydryl C-1'), 150.1 (C-20), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 171.5 (28-O-dimethylsuccinyl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 182.4 (28-O-dimethylsuccinyl COOH). HRESIMS (positive) m/z 887.5421 [M+Na]+ (calcd for C55H76O8Na, 887.5438).
Compound 23
+6.3° (c 3.15, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.75 (3H, s, CH3-24), 0.79 (3H, s, CH3-23), 0.80 (3H, s, CH3-25), 0.98 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.31, 1.32 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.32 (6H, s, 28-O-dimethylsuccinyl CH3), 1.70 (3H, s, CH3-30), 2.44 (1H, dt, J = 5.6, 10.8 Hz, H-19), 2.64, 2.70 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 2.66 (2H, s, 28-O-dimethylsuccinyl H2-3"), 3.85, 4.32 (each 1H, d, J = 10.8 Hz, H2-28), 4.45 (1H, dd, J = 5.2, 10.8 Hz, H-3), 4.61, 4.70 (each 1H, br s, H2-29), 6.87 (1H, s, CH(Ph)2), 7.24–7.32 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.8 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 19.2 (C-30), 20.8 (C-11), 23.6 (C-2), 25.2 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 25.3, 25.5 (28-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.6 (C-21), 29.9 (C-16), 34.1 (C-7), 34.5 (C-22), 37.1 (C-10), 37.7 (C-4, 13), 38.4 (C-1), 40.7 (dimethylsuccinyl C-3', C-2"), 40.9 (C-8), 42.7 (C-14), 44.0 (28-O-dimethylsuccinyl C-3"), 44.6 (3-O-dimethylsuccinyl C-2'), 46.6 (C-17), 47.8 (C-19), 48.9 (C-18), 50.3 (C-9), 55.4 (C-5), 63.3 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 109.8 (C-29), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.4 (benzhydryl C-1'), 150.1 (C-20), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−, 28-O-dimethylsuccinyl COOH), 177.0 (28-O-dimethylsuccinyl 1"-COO−). HRESIMS (positive) m/z 887.5440 [M+Na] + (calcd for C55H76O8Na, 887.5438).
3-O-(4'-Benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-glutarylbetulin (24)
Yield 59.5% (starting from 406 mg of 16 and glutaric anhydride); white solid. +10.8° (c 0.5, CHCl3). 1H NMR (CDCl3, 500 MHz) δ 0.73 (3H, s, CH3-24), 0.77 (6H, s, CH3-23 and CH3-25), 0.96 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.30, 1.31 (each 3H, s, dimethylsuccinyl CH3), 1.68 (3H, s, CH3-30), 1.97 (2H, quint, J = 7.2 Hz, 28-O-glutaryl H2-3"), 2.41–2.46 (1H, m, J = 5.5, 10.9 Hz, H-19), 2.43 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-2"), 2.44 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-4"), 2.64, 2.69 (each 1H, d, J = 15.8 Hz, 3-O-dimethylsuccinyl H2-2'), 3.85, 4.28 (each 1H, d, J = 10.8 Hz, H2-28), 4.43 (1H, dd, J = 5.2, 11.4 Hz, H-3), 4.59, 4.68 (each 1H, br s, H2-29), 6.85 (1H, s, CH(Ph)2), 7.24–7.32 (10H, m, aromatic-H); 13C NMR (CDCl3, 125 MHz) δ 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.0 (28-O-glutaryl C-3"), 20.8 (C-11), 23.5 (C-2), 25.1 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.5 (C-21), 29.7 (C-16), 32.8 (28-O-glutaryl C-4"), 33.3 (28-O-glutaryl C-2"), 34.1 (C-7), 34.5 (C-22), 37.0 (C-10), 37.6 (2C, C-13 and C-4), 38.4 (C-1), 40.7 (3-O-dimethylsuccinyl C-3'), 40.9 (C-8), 42.7 (C-14), 44.5 (3-O-dimethylsuccinyl C-2'), 46.4 (C-17), 47.7 (C-19), 48.8 (C-18), 50.2 (C-9), 55.3 (C-5), 62.8 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 109.9 (C-29), 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 150.1 (C-20), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 173.3 (28-O-glutaryl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 177.7 (28-O-glutaryl COOH). HRESIMS (positive) m/z 873.5286 [M+Na]+ (calcd for C54H74O8Na, 873.5281).
3-O-(4'-Benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-(3",3"-dimethylglutaryl)betulin (25)
Yield 87.0% (starting from 222 mg of 16 and 3,3-dimethylglutaric acid); white solid. +6.8° (c 3.59, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.74 (3H, s, CH3-24), 0.79 (3H, s, CH3-23), 0.79 (3H, s, CH3-25), 0.98 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.16 (6H, s, dimethylglutaryl CH3), 1.31, 1.32 (each 3H, s, dimethylsuccinyl CH3), 1.70 (3H, s, CH3-30), 2.40 – 2.50 (1H, m, H-19), 2.47, 2.48 (each 2H, s, dimethylglutaryl H2-2", 4"), 2.65, 2.70 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.86, 4.29 (each 1H, d, J = 10.8 Hz, H2-28), 4.44 (1H, dd, J = 5.2, 10.8 Hz, H-3), 4.60, 4.70 (each 1H, br s, H2-29), 6.87 (1H, s, CH(Ph)2), 7.24–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.8 (C-11), 23.5 (C-2), 25.2 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 26.9 (C-15), 27.8 (28-O-dimethylsuccinyl CH3), 27.9 (C-23), 29.5 (C-21), 29.8 (C-16), (dimethylglutaryl C-3"), 34.0 (C-7), 34.6 (C-22), 37.0 (C-10), 37.6 (C-4, 13), 38.3 (C-1), (dimethylsuccinyl C-3'), 40.8 (C-8), 42.6 (C-14), 44.5 (3-O-dimethylsuccinyl C-2'), 45.1 (dimethylglutaryl C-4"), 45.3 (dimethylglutaryl C-2"), 46.2 (C-17), 47.7 (C-19), 48.8 (C-18), 50.2 (C-9), 55.3 (C-5), 62.8 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 109.9 (C-29), 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 150.0 (C-20), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 172.7 (28-O-dimethylglutaryl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 176.9 (28-O-dimethylglutaryl COOH). HRESIMS (positive) m/z 901.5583 [M+Na]+ (calcd for C56H78O8Na, 901.5594).
3-O-(5'-Benzhydryloxy-3',3'-dimethylglutaryl)-28-O-(3",3"-dimethylglutaryl)betulin (26)
Yield 100% (starting from 150 mg of 17 and 3,3-dimethylglutaric anhydride); white solid. +7.3° (c 3.36, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.81 (3H, s, CH3-24), 0.84 (3H, s, CH3-23, 25), 0.98 (3H, s, CH3-27), 1.04 (3H, s, CH3-26), 1.09 (6H, s, 3-O-dimethylglutaryl CH3), 1.16 (6H, s, 28-O-dimethylglutaryl CH3), 1.70 (3H, s, CH3-30), 2.43–2.45 (1H, m, H-19), 2.36, 2.43 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-2'), 2.47, 2.48 (each 2H, s, 28-O-dimethylglutaryl H2-2", 4"), 2.55, 2.58 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-4'), 3.86, 4.29 (each 1H, d, J = 11.2 Hz, H2-28), 4.47 (1H, dd, J = 4.8, 11.2 Hz, H-3), 4.60, 4.70 (each 1H, br s, H2-29), 6.89 (1H, s, CH(Ph)2), 7.25–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.6 (C-24), 18.1 (C-6), 19.1 (C-30), 20.8 (C-11), 23.8 (C-2), 25.2 (C-12), 27.0 (C-15), 27.6, 27.7 (3-O-dimethylglutaryl CH3), 27.8 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.5 (C-21), 29.8 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 32.8 (28-O-dimethylglutaryl C-3'), 34.1 (C-7), 34.6 (C-22), 37.0 (C-10), 37.6 (C-4, 13), 38.4 (C-1), 40.9 (C-8), 42.7 (C-14), 45.1 (28-O-dimethylglutaryl C-4"), 45.3 (28-O-dimethylglutaryl C-2"), 45.4 (3-O-dimethylglutaryl C-4'), 45.8 (3-O-dimethylglutaryl C-2'), 46.2 (C-17), 47.7 (C-19), 48.8 (C-18), 50.2 (C-9), 55.4 (C-5), 62.9 (C-28), 76.7 (CH(Ph)2), 80.9 (C-3), 109.9 (C-29), 127.1 (benzhydryl C-2', 6'), 127.8 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.2, 140.3 (benzhydryl C-1'), 150.0 (C-20), 170.8 (3-O-dimethylglutaryl 5'-COO−), 171.7 (3-O-dimethylglutaryl 1'-COO−), 172.8 (28-O-dimethylglutaryl 1"-COO−), 176.5 (28-O-dimethylglutaryl COOH). HRESIMS (positive) m/z 915.5771 [M+Na]+ (calcd for C57H80O8Na, 915.5751).
3-O-(4'-Benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-(3",3"-dimethylsuccinyl)dihydrobetulin (27) and 3-O-(4'-benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-(2",2"-dimethylsuccinyl)dihydrobetulin (28)
Total yield 96.3% (starting from 330 mg of 18 and 2,2-dimethylsuccinic acid); used in next reaction without purification. For spectroscopic analysis, a small amount of sample was further purified by preparative HPLC (πNAP, MeOH/2% HOAc = 97:3); white solid.
Compound 27
−12.3° (c 3.50, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.75 (3H, s, CH3-24), 0.77, 0.85 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.79 (3H, s, CH3-23), 0.80 (3H, s, CH3-25), 0.94 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.31, 1.32 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.31 (6H, s, 28-O-dimethylsuccinyl CH3), 2.65 (2H, s, 28-O-dimethylsuccinyl H2-2"), 2.66, 2.70 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 3.85, 4.26 (each 1H, d, J = 11.2 Hz, H2-28), 4.45 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.87 (1H, s, CH(Ph)2), 7.25–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 16.0 (C-25, C-26), 16.5 (C-24), 18.1 (C-6), 20.8 (C-11), 21.5 (C-21), 22.9 (C-30), 23.5 (C-2), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 25.3, 25.4 (28-O-dimethylsuccinyl CH3), 26.8 (C-12, C-15), 27.9 (C-23), 29.4 (C-20), 29.8 (C-16), 34.1 (C-7), 34.6 (C-22), 36.9 (C-10), 37.1 (C-13), 37.6 (C-4), 38.3 (C-1), 40.5 (28-O-dimethylsuccinyl C-3"), 40.7 (3-O-dimethylsuccinyl C-3'), 40.9 (C-8), 42.8 (C-14), 44.4 (28-O-dimethylsuccinyl C-2"), 44.5 (C-19, 3-O-dimethylsuccinyl C-2'), 46.4 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 63.1 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 171.6 (28-O-dimethylsuccinyl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 182.9 (28-O-dimethylsuccinyl COOH). HRESIMS (positive) m/z 889.5585 [M+Na]+ (calcd for C55H78O8Na, 889.5594).
Compound 28
− 11.4° (c 2.30, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.75 (3H, s, CH3-24), 0.78, 0.85 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.79 (3H, s, CH3-23), 0.80 (3H, s, CH3-25), 0.95 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.32, 1.31 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.31 (each 3H, s, 28-O-dimethylsuccinyl CH3), 2.66, 2.70 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 2.65 (2H, s, 28-O-dimethylsuccinyl H2-3"), 3.79, 4.31 (each 1H, d, J = 10.8 Hz, H2-28), 4.45 (1H, dd, J = 5.2, 11.2 Hz, H-3), 6.87 (1H, s, CH(Ph)2), 7.25–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 16.0 (C-25, C-26), 16.5 (C-24), 18.1 (C-6), 20.8 (C-11), 21.5 (C-21), 22.9 (C-30), 23.5 (C-2), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 25.4, 25.5 (28-O-dimethylsuccinyl CH3), 26.8 (C-12, C-15), 27.9 (C-23), 29.4 (C-20), 29.9 (C-16), 34.1 (C-7), 34.7 (C-22), 37.0 (C-10), 37.1 (C-13), 37.7 (C-4), 38.4 (C-1), 40.7 (3-O-dimethylsuccinyl C-3', 28-O-dimethylsuccinyl 2"), 40.9 (C-8), 42.8 (C-14), 43.9 (28-O-dimethylsuccinyl C-3"), 44.5 (C-19, 3-O-dimethylsuccinyl C-2'), 46.6 (C-17), 48.2 (C-18), 49.9 (C-9), 55.3 (C-5), 63.3 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−, 28-O-dimethylsuccinyl 1"-COOH), 177.0 (28-O-dimethylsuccinyl 1"-COO−). HRESIMS (positive) m/z 889.5590 [M+Na]+ (calcd for C55H78O8Na, 889.5594).
3-O-(4'-Benzhydryloxy-3',3'-dimethylsuccinyl)-28-O-(3",3"-dimethylglutaryl)dihydrobetulin (29)
Yield 100% (starting from 110 mg of 18 and 3,3-dimethylglutaric anhydride); white solid. −10.2° (c 2.04, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.74 (3H, s, CH3-24), 0.78 (3H, s, CH3-23), 0.78, 0.85 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.80 (3H, s, CH3-25), 0.95 (3H, s, CH3-27), 1.04 (3H, s, CH3-26), 1.15 (6H, s, dimethylglutaryl CH3), 1.31, 1.32 (each 3H, s, dimethylsuccinyl CH3), 2.46, 2.48 (each 2H, s, dimethylglutaryl H2-2", 4"), 2.65, 2.71 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.83, 4.28 (each 1H, d, J = 10.8 Hz, H2-28), 4.45 (1H, dd, J = 5.2, 11.2 Hz, H-3), 6.86 (1H, s, CH(Ph)2), 7.25–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 16.0 (C-25, C-26), 16.5 (C-24), 18.1 (C-6), 20.7(C-11), 21.6 (C-21), 22.9 (C-30), 23.5 (C-2), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 27.9 (C-23, 28-O-dimethylsuccinyl CH3), 29.4 (C-20), 29.9 (C-16), 32.7 (dimethylglutaryl C-3"), 34.1 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.6 (C-4), 38.4 (C-1), 40.7 (dimethylsuccinyl C-3'), 40.9 (C-8), 42.8 (C-14), 44.5 (C-19, 3-O-dimethylsuccinyl C-2'), 45.1 (dimethylglutaryl C-4"), 45.4 (dimethylglutaryl C-2"), 46.4 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 63.0 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.3 (benzhydryl C-1'), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 173.0 (28-O-dimethylglutaryl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 176.0 (28-O-dimethylglutaryl COOH). HRESIMS (positive) m/z 903.5748 [M+Na]+ (calcd for C56H80O8Na, 903.5751).
3-O-(5'-Benzhydryloxyglutaryl)-28-O-(3",3"-dimethylsuccinyl)dihydrobetulin (30) and 3-O-(5'-benzhydryloxyglutaryl)-28-O-(2",2"-dimethylsuccinyl)dihydrobetulin (31)
Total yield 98.9% (starting from 225 mg of 20 and 2,2-dimethylsuccinic acid); used for next reaction without purification. For spectroscopic analysis, a small amount of sample was further purified by preparative HPLC (πNAP, CH3OH/H2O/CH3COOH = 95:4:1); colorless oil.
Compound 30
−7.1° ( c 3.50, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.85 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.84 (each 3H, s, CH3-23, 24), 0.87 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.05 (3H, s, CH3-26), 1.32 (6H, s, 28-O-dimethylsuccinyl CH3), 2.00 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 2.36 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.51 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 2.65 (2H, s, 28-O-dimethylsuccinyl H2-2"), 3.85, 4.28 (each 1H, d, J = 10.8 Hz, H2-28), 4.49 (1H, dd, J = 6.0, 10.0 Hz, H-3), 6.90 (1H, s, CH(Ph)2), 7.26–7.34 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 16.1 (C-25, C-26), 16.5 (C-24), 18.2 (C-6), 20.3 (glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 25.3 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.8 (C-16), 33.6 (glutaryl C-4'), 33.7 (glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.8 (C-4), 38.4 (C-1), 40.5 (28-O-dimethylsuccinyl C-3"), 40.9 (C-8), 42.9 (C-14), 44.4 (28-O-dimethylsuccinyl C-2"), 44.5 (C-19), 46.4 (C-17), 48.2 (C-18), 50.0 (C-9), 55.3 (C-5), 63.1 (C-28), 76.9 (CH(Ph)2), 81.0 (C-3), 127.0 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 140.2 (benzhydryl C-1'), 171.5 (28-O-dimethylsuccinyl 1"-COO−), 171.9 (3-O-glutaryl 5'-COO−), 172.6 (3-O-glutaryl 1'-COO−), 182.6 (28-O-dimethylsuccinyl COOH). HRESIMS (positive) m/z 875.5408 [M+Na]+ (calcd for C54H76O8Na, 875.5438).
Compound 31
−7.4° (c 1.26, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.79, 0.85 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.84 (each 3H, s, CH3-23, 24), 0.87 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.05 (3H, s, CH3-26), 1.31 (6H, s, 28-O-dimethylsuccinyl CH3), 2.00 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 2.36 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.50 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 2.65 (2H, s, 28-O-dimethylsuccinyl H2-3"), 3.81, 4.32 (each 1H, d, J = 10.8 Hz, H2-28), 4.49 (1H, dd, J = 6.0, 10.0 Hz, H-3), 6.90 (1H, s, CH(Ph)2), 7.26–7.34 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 14.9 (C-29), 16.1 (C-25, C-26), 16.6 (C-24), 18.2 (C-6), 20.4 (glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 25.4, 25.5 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 33.6 (glutaryl C-4'), 33.7 (glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.8 (C-4), 38.4 (C-1), 40.8 (28-O-dimethylsuccinyl C-2"), 40.9 (C-8), 42.9 (C-14), 43.9 (28-O-dimethylsuccinyl C-3"), 44.6 (C-19), 46.7 (C-17), 48.2 (C-18), 50.0 (C-9), 55.4 (C-5), 63.4 (C-28), 77.0 (CH(Ph)2), 81.0 (C-3), 127.0 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 140.2 (benzhydryl C-1'), 171.9 (3-O-glutaryl 5'-COO−), 172.6 (3-O-glutaryl 1'-COO−), 175.8 (28-O-dimethylsuccinyl 1"-COOH), 177.1 (28-O-dimethylsuccinyl COO−). HRESIMS (positive) m/z 875.5450 [M+Na]+ (calcd for C54H76O8Na, 875.5438).
3-O-(5'-Benzhydryloxyglutaryl)-28-O-glutaryldihydrobetulin (32)
Yield 94.9% (starting from 89 mg of 20 and glutaric anhydride); colorless oil. −1.2° (c 1.04, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.84 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.83 (3H, s, CH3-24), 0.84 (3H, s, CH3-24), 0.87 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.06 (3H, s, CH3-26), 1.98 (2H, quint, J = 72 Hz, 28-O-glutaryl H2-3"), 2.00 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 2.36 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.43 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-2"), 2.44 (2H, t, J = 72 Hz, 28-O-glutaryl H2-4"), 2.50 (2H, t, J = 72 Hz, 3-O-glutaryl H2-4'), 3.84, 4.30 (each 1H, d, J = 10.8 Hz, H2-28), 4.49 (1H, dd, J = 6.0, 10.4 Hz, H-3), 6.90 (1H, s, CH(Ph)2), 7.26–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 16.1 (C-25, C-26), 16.6 (C-24), 18.2 (C-6), 20.0 (28-O-glutaryl C-3"), 20.4 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 26.8 (C-12), 27.0 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.8 (28-O-glutaryl C-4"), 33.3 (28-O-glutaryl C-2"), 33.6 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.1 (C-10), 37.2 (C-13), 37.8 (C-4), 38.4 (C-1), 41.0 (C-8), 42.9 (C-14), 44.6 (C-19), 46.6 (C-17), 48.2 (C-18), 50.0 (C-9), 55.4 (C-5), 62.9 (C-28), 77.0 (CH(Ph)2), 81.0 (C-3), 127.1 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 140.2 (benzhydryl C-1'), 171.9 (3-O-glutaryl 5'-COO−), 172.6 (3-O-glutaryl 1'-COO−), 173.3 (28-O-glutaryl 1"-COO−), 177.3 (28-O-glutaryl COOH). HRESIMS (positive) m/z 861.5292 [M+Na]+ (calcd for C53H74O8Na, 861.5281).
3-O-(5'-Benzhydryloxyglutaryl)-28-O-(3",3"-dimethylglutaryl)dihydrobetulin (33)
Yield 91.6% (starting from 82 mg of 20 and 3,3-dimethylglutaric anhydride); colorless oil. −5.4° (c 1.43, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.84 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.83 (3H, s, CH3-24), 0.84 (3H, s, CH3-24), 0.87 (3H, s, CH3-25), 0.97 (3H, s, CH3-27), 1.06 (3H, s, CH3-26), 1.16 (6H, s, 28-O-dimethylglutaryl CH3), 2.00 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 2.36 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.46 (2H, s, 28-O-dimthylglutaryl H2-2"), 2.49 (2H, s, 28-O-glutaryl H2-4"), 2.50 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 3.84, 4.30 (each 1H, d, J = 10.8 Hz, H2-28), 4.49 (1H, dd, J = 5.6, 10.8 Hz, H-3), 6.90 (1H, s, CH(Ph)2), 7.26–7.33 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.7 (C-27), 14.9 (C-29), 16.1 (C-25, C-26), 16.6 (C-24), 18.2 (C-6), 20.4 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 26.8 (C-12), 26.9 (C-15), 27.9 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.7 (28-O-dimethylglutaryl C-3"), 33.6 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.1 (C-10), 37.2 (C-13), 37.8 (C-4), 38.4 (C-1), 41.0 (C-8), 42.9 (C-14), 44.6 (C-19), 45.1 (28-O-dimethylglutaryl C-4"), 45.4 (28-O-dimethylglutaryl C-2"), 46.4 (C-17), 48.2 (C-18), 50.0 (C-9), 55.4 (C-5), 63.1 (C-28), 77.0 (CH(Ph)2), 81.0 (C-3), 127.1 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 140.2 (benzhydryl C-1'), 171.9 (3-O-glutaryl 5'-COO−), 172.6 (3-O-glutaryl 1'-COO−), 173.0 (28-O-dimethylglutaryl 1"-COO−), 175.6 (28-O-dimethylglutaryl COOH). HRESIMS (positive) m/z 889.5605 [M+Na]+ (calcd for C55H78O8Na, 889.5594).
3-O-(5'-Benzhydryloxy-3',3'-dimethylglutaryl)-28-O-(3",3"-dimethylglutaryl)dihydrobetulin (34)
Yield 97% (starting from 137 mg of 19 and 3,3-dimethylglutaric anhydride); white solid. –9.3° (c 2.94, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.83 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.81 (3H, s, CH3-24), 0.84 (3H, s, CH3-24), 0.85 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.05 (3H, s, CH3-26), 1.09 (6H, s, 3-O-dimethylglutaryl CH3), 1.15 (6H, s, 28-O-dimethylglutaryl CH3), 2.36, 2.43 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-2'), 2.46 (2H, s, 28-O-dimthylglutaryl H2-2"), 2.48 (2H, s, 28-O-dimethylglutaryl H2-4"), 2.55, 2.58 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-4'), 3.83, 4.29 (each 1H, d, J = 11.2 Hz, H2-28), 4.47 (1H, dd, J = 4.8, 10.8 Hz, H-3), 6.89 (1H, s, CH(Ph)2), 7.25–7.34 (10H, m, aromatic-H); 13C NMR (CDCl3, 100 MHz) δ 14.6 (C-27), 14.9 (C-29), 16.0 (C-25, C-26), 16.6 (C-24), 18.1 (C-6), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 26.8 (C-12, C-15), 27.6, 27.7 (3-O-dimethylglutaryl CH3), 27.9 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 32.8 (3-O-dimethylglutaryl C-3'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.6 (C-4), 38.4 (C-1), 41.0 (C-8), 42.8 (C-14), 44.5 (C-19), 45.1 (28-O-dimethylglutaryl C-4"), 45.3 (28-O-dimethylglutaryl C-2"), 45.4 (3-O-dimethylglutaryl C-4'), 45.8 (3-O-dimethylglutaryl C-2'), 46.4 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 63.0 (C-28), 76.7 (CH(Ph)2), 80.9 (C-3), 127.1 (benzhydryl C-2', 6'), 127.8 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 140.2, 140.3 (benzhydryl C-1'), 170.8 (3-O-dimethylglutaryl 5'-COO−), 171.7 (3-O-dimethylglutaryl 1'-COO−), 172.9 (28-O-dimethylglutaryl 1"-COO−), 176.4 (28-O-dimethylglutaryl COOH). HRESIMS (positive) m/z 917.5843 [M+Na]+ (calcd for C57H82O8Na, 917.5907).
4.2.8 General procedure for coupling of 3,28-di-O-acylbetulin derivatives with AZT (35–48)
To a solution of 3,28-di-O-acylbetulin derivatives (1 equiv) in CH2Cl2 (4–10 mL) was added DMAP (2 equiv), DCC (2 equiv) and 3'-azido-3'-dexoythymidine (AZT, 2 equiv). The reaction mixture was kept stirring at room temperature for overnight. After filtration, the filtrate was concentrated and the residue was chromatographed over silica gel column (hexane/EtOAc = 2:1) or purified by HPLC.
3'-Azido-3'-deoxythymidine 5'-yl 3-O-(4'-benzhydryloxy-3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl succinate (35)
Yield 41.7% (starting from 80 mg of 21); white solid. +6.2° (c 0.53, CHCl3). 1H NMR (CDCl3, 500 MHz) δ 0.72 (3H, s, CH3-24), 0.76 (3H, s, CH3-23), 0.77 (3H, s, CH3-25), 0.95 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.29, 1.30 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.68 (3H, s, CH3-30), 1.95 (3H, br s, AZT-CH3), 2.33–2.50 (3H, m, AZT-H2-2' and H-19), 2.62–2.79 (4H, m, 28-O-succinyl H2-2", 3"), 2.64, 2.68 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.86, 4.30 (each 1H, d, J = 11.2 Hz, H2-28), 4.05 (1H, q-like, AZT-H-4'), 4.21–4.24 (1H, m, AZT-H-3'),4.30 (1H, dd, J = 3.4, 12.0 Hz, AZT-H2-5a'), 4.52 (1H, dd, J = 4.0, 12.0 Hz, AZT-H2-5b'), 4.42 (1H, dd, J = 5.2, 11.2 Hz, H-3), 4.59, 4.68 (each 1H, br s, H2-29), 6.15 (1H, t-like, J = 6.3 Hz, AZT-H-1'), 6.84 (1H, s, CH(Ph)2), 7.24–7.32 (11H, m, aromatic-H and AZT-H-6), 8.13 (1H, br s, AZT-NH); 13C NMR (CDCl3, 125 MHz) δ 12.6 (AZT-CH3), 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.7 (C-11), 23.5 (C-2), 25.1 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.0 (28-O-succinyl C-2", 3"), 29.5 (C-21), 29.7 (C-16), 34.1 (C-7), 34.5 (C-22), 37.0 (C-10), 37.6 (AZT-C-2'), 37.6 (C-13), 37.7 (C-4), 38.3 (C-1), 40.7 (dimethylsuccinyl C-3'), 40.8 (C-8), 42.7 (C-14), 44.5 (3-O-dimethylsuccinyl C-2'), 46.4 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.3 (C-5), 60.1 (AZT-C-3'), 63.1 (AZT-C-5'), 63.3 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 81.9 (AZT-C-4'), 85.1 (AZT-C-1'), 110.0 (C-29), 111.3 (AZT-C-5), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.3 (AZT-C-6), 140.3 (benzhydryl C-1'), 149.8 (AZT-C-2), 150.0 (C-20), 163.1 (AZT-C-4), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 172.0 (28-O-succinyl 4"-COO-AZT), 172.6 (28-O-succinyl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−). HRESIMS (positive) m/z 1108.6014 [M+Na]+ (calcd for C63H83N5O11Na,1108.5987).
1-(3'-Azido-3'-deoxythymidine-5'-yl)-4-[3-O-(4'-benzhydryloxy-3',3'-dimethylsuccinyl)-lu p-20(29)en-28-yl] 2,2-dimethylsuccinate (36) and 4-(3'-Azido-3'-deoxythymidine-5'-yl)-1-[3-O-(4'-benzhydryloxy-3',3'-dimethylsuccinyl)-lup-2 0(29)en-28-yl] 2,2-dimethylsuccinate (37)
Total yield 49.3% (starting from 296 mg of mixture of 22 and 23); used for next reaction without separation. A small amount of sample mixture was separated by HPLC (cholester, CH3OH/H2O = 97:3) for spectroscopic analyses.
Compound 36
+7.2° (c 1.77 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.74 (3H, s, CH3-24), 0.78 (each 3H, s, CH3-23, 25), 0.96 (3H, s, CH3-27), 1.02 (3H, s, CH3-26), 1.30, 1.31 (each 3H, s, 28- O -dimethylsuccinyl CH3), 1.31, 1.32 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.68 (3H, s, CH3–30), 1.94 (3H, br s, AZT-CH3), 2.26–2.33, 2.44–2.50 (each 1H, m, AZT-H2-2'), 2.37–2.44 (1H, m, H-19), 2.65, 2.69 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 2.61, 2.75 (each 1H, d, J = 16.4 Hz, 28-O-dimethylsuccinyl H2-2"), 3.84, 4.29 (each 1H, d, J = 10.8 Hz, H2-28), 4.09 (1H, q-like, AZT-H-4'), 4.26–4.30 (1H, m, AZT-H-3'), 4.30, 4.52 (each 1H, dd, J = 4.0, 12.0 Hz, AZT-H2-5'), 4.43 (1H, dd, J = 5.2, 10.8 Hz, H-3), 4.60, 4.69 (each 1H, br s, H2-29), 6.18 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.86 (1H, s, CH(Ph)2), 7.24–7.33 (11H, m, aromatic-H and AZT-H-6), 8.56 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.7 (C-27), 15.9 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.7 (C-11), 23.5 (C-2), 25.2 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 25.1, 25.9 (28-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.5 (C-21), 29.7 (C-16), 34.1 (C-7), 34.5 (C-22), 37.0 (C-10), 37.4 (AZT-C-2'), 37.6 (C-4, 13), 38.3 (C-1), 40.7 (dimethylsuccinyl C-3', C-3"), 40.8 (C-8), 42.7 (C-14), 44.5 (3-O-dimethylsuccinyl C-2'), 44.6 (28-O-dimethylsuccinyl C-2"), 46.3 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.3 (C-5), 60.4 (AZT-C-3'), 63.1 (C-28), 63.0 (AZT-C-5'), 77.0 (CH(Ph)2), 81.2 (C-3), 81.9 (AZT-C-4'), 84.8 (AZT-C-1'), 110.0 (C-29), 111.4 (AZT-C-5), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.0 (AZT-C-6), 140.4 (benzhydryl C-1'), 149.9 (C-20 and AZT-C-2), 163.3 (AZT-C-4), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 171.7 (28-O-dimethylsuccinyl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 176.4 (28-O-dimethylsuccinyl 4"-COO-AZT). HRESIMS (positive) m/z 1136.6310 [M+Na]+ (calcd for C65H87N5O11Na, 1136.6300).
Compound 37
+11.6° (c 3.64 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.73 (3H, s, CH3–24), 0.77 (3H, s, CH3-23), 0.78 (3H, s, C-25), 0.96 (3H, s, CH3-27), 1.02 (3H, s, CH3-26), 1.30, 1.32 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.31, 1.34 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.69 (3H, s, CH3-30), 1.95 (3H, br s, AZT-CH3), 2.33–2.50 (3H, m, AZT-H2-2' and H-19), 2.62, 2.66 (each 1H, d, J = 16.4 Hz, 28-O-dimethylsuccinyl H2-3"), 2.64, 2.69 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.85, 4.29 (each 1H, d, J = 10.8 Hz, H2-28), 4.04 (1H, q-like, AZT-H-4'), 4.18–4.22(1H, m, AZT-H-3'), 4.26 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5a'), 4.42 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.43 (1H, dd, J = 4.8, 10.4 Hz, H-3), 4.60, 4.69 (each 1H, br s, H2-29), 6.15 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.85 (1H, s, CH(Ph)2), 7.24–7.32 (11H, m, aromatic-H and AZT-H-6), 8.94 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.6 (AZT-CH3), 14.7 (C-27), 15.9 (C-26), 16.0 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.7 (C-11), 23.5 (C-2), 25.2 (C-12), 25.2, 25.5 (3-O-dimethylsuccinyl CH3), 25.4, 25.8 (28-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.5 (C-21), 29.8 (C-16), 34.1 (C-7), 34.5 (C-22), 37.0 (C-10), 37.5 (AZT-C-2'), 37.6 (C-4, 13), 38.3 (C-1), 40.7 (3-O-dimethylsuccinyl C-3'), 40.8 (C-8), 41.0 (28-O-dimethylsuccinyl C-2"), 42.7 (C-14), 43.8 (28-O-dimethylsuccinyl C-3"), 44.5 (3-O-dimethylsuccinyl C-2'), 46.5 (C-17), 47.7 (C-19), 48.8 (C-18), 50.2 (C-9), 55.3 (C-5), 60.3 (AZT-C-3'), 62.9 (AZT-C-5'), 63.2 (C-28), 77.0 (CH(Ph)2), 81.2 (C-3), 81.7 (AZT-C-4'), 85.2 (AZT-C-1'), 109.9 (C-29), 111.3 (AZT-C-5), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.3 (AZT-C-6), 140.3 (benzhydryl C-1'), 150.0 (C-20 and AZT-C-2), 163.5 (AZT-C-4), 170.7 (28-O-dimethylsuccinyl 4"-COO-AZT), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 176.9 (28-O-dimethylsuccinyl 1"-COO−). HRESIMS (positive) m/z 1136.6299 [M+Na]+ (calcd for C65H87N5O11Na, 1136.6300).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(4'-benzhydryloxy-3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl glutarate (38)
Yield 47.4% (starting from 180 mg of 24); white solid. +24.2° (c 0.5 CHCl3). 1H NMR (CDCl3, 500 MHz) δ 0.73 (3H, s, CH3-24), 0.77 (3H, s, CH3-23), 0.78 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.30, 1.31 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.68 (3H, s, CH3–30), 1.94 (3H, s, AZT-CH3), 1.99 (2H, quint, J = 7.0 Hz, 28-O-glutaryl H2-3"), 2.42 (2H, t, J = 7.0 Hz, 28-O-glutaryl H2-2"), 2.47 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-4"), 2.33–2.50 (3H, m, AZT-H2-2' and H-19), 2.64, 2.69 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.85, 4.28 (1H, d, J = 11.2 Hz, H2-28a), 4.06 (1H, m, AZT-H-4'), 4.23 (1H, m, AZT-H-3'), 4.32 (1H, dd, J = 3.5, 12.2 Hz, AZT-H-5a'), 4.39 (1H, dd, J = 4.8, 12.2 Hz, AZT-H-5b'), 4.43 (1H, dd, J = 4.8, 11.2 Hz, H-3), 4.59, 4.69 (each 1H, br s, H2-29), 6.10 (1H, t-like, J = 5.5 Hz, AZT-H-1'), 6.85 (1H, s, CH(Ph)2), 7.21–7.32 (11H, m, aromatic-H and AZT-H-6), 9.37 (1H, br s, AZT-NH); 13C NMR (CDCl3, 125 MHz) 13C NMR (CDCl3, 125 MHz) δ 12.6 (AZT-CH3), 14.7 (C-27), 15.9 (C-26), 16.0 (C-25), 16.4 (C-24), 18.0 (C-6), 19.0 (C-30), 19.9 (28-O-glutaryl C-3"), 20.7 (C-11), 23.5 (C-2), 25.0 (C-12), 25.1, 25.3 (3-O-dimethylsuccinyl CH3), 26.9 (C-15), 27.8 (C-23), 29.4 (C-21), 29.7 (C-16), 33.0 (28-O-glutaryl C-4"), 33.1 (28-O-glutaryl C-2"), 34.0 (C-7), 34.4 (C-22), 36.9 (C-10), 37.4 (AZT-C-2'), 37.5 (C-13), 37.6 (C-4), 38.3 (C-1), 40.6 (dimethylsuccinyl C-3'), 40.8 (C-8), 42.6 (C-14), 44.4 (3-O-dimethylsuccinyl C-2'), 46.3 (C-17), 47.6 (C-19), 48.7 (C-18), 50.1 (C-9), 55.2 (C-5), 60.5 (AZT-C-3'), 62.8 (C-28), 63.3 (AZT-C-5'), 76.9 (CH(Ph)2), 81.2 (C-3), 81.6 (AZT-C-4'), 85.6 (AZT-C-1'), 109.9 (C-29), 111.2 (AZT-C-5), 126.9, 127.0 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.3 (benzhydryl C-3', 5'), 135.4 (AZT-C-6), 140.2, 140.3 (benzhydryl C-1'), 149.9 (C-20), 150.0 (AZT-C-2), 163.4 (AZT-C-4), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 172.3 (28-O-glutaryl 5"-COO-AZT), 173.1 (28-O-glutaryl 1"-COO−), 175.3 (3-O-dimethylsuccinyl 4'-COO−). HRESIMS (positive) m/z 1122.6141 [M+Na]+ (calcd for C64H85N5O11Na, 1122.6143).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(4'-benzhydryloxy-3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl 3,3-dimethylglutarate (39)
Yield 74.2% (starting from 120 mg of 25); white solid. +14.1° (c 2.55 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.74 (3H, s, CH3-24), 0.78 (each 3H, s, CH3-23, 25), 0.96 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.13, 1.16 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.30, 1.31 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.69 (3H, s, CH3-30), 1.95 (3H, br s, AZT-CH3), 2.30–2.35, 2.40–2.51 (2H, m, AZT-H2-2'), 2.35–2.42 (1H, m, H-19), 2.43, 2.50 (each 1H, d, J = 16.0 Hz, 28-O-dimethylglutaryl H2-2"), 2.49, 2.56 (each 1H, d, J = 16.0 Hz, 28-O-dimethylglutaryl H2-4"), 2.65, 2.69 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.82, 4.26 (each 1H, d, J = 10.8 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.25 (1H, m, AZT-H-3'), 4.27 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5a'), 4.44 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.44 (1H, dd, J = 4.4, 12.0 Hz, H-3), 4.60, 4.69 (each 1H, br s, H2-29), 6.15 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.86 (1H, s, CH(Ph)2), 7.24–7.33 (11H, m, aromatic-H and AZT-H-6), 8.78 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.6 (AZT-CH3), 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.8 (C-11), 23.5 (C-2), 25.2 (C-12), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.5 (C-21), 29.8 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 34.1 (C-7), 34.6 (C-22), 37.0 (C-10), 37.5 (AZT-C-2'), 37.6 (C-4, 13), 38.3 (C-1), 40.7 (3-O-dimethylsuccinyl C-3'), 40.8 (C-8), 42.6 (C-14), 44.5 (3-O-dimethylsuccinyl C-2', 28-O-dimethylglutaryl C-4"), 45.0 (28-O-dimethylglutaryl C-2"), 46.2 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.3 (C-5), 60.4 (AZT-C-3'), 62.6 (C-28), 62.7 (AZT-C-5'), 77.0 (CH(Ph)2), 81.2 (C-3), 81.8 (AZT-C-4'), 85.2 (AZT-C-1'), 109.9 (C-29), 111.3 (AZT-C-5), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.2 (AZT-C-6), 140.3 (benzhydryl C-1'), 150.0 (C-20 and AZT-C-2), 163.4 (AZT-C-4), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 171.1 (28-O-dimethylglutaryl 5"-COO-AZT), 172.3 (28-O-dimethylglutaryl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−). HRESIMS (positive) m/z 1150.6467 [M+Na]+ (calcd for C66H89N5O11Na, 1150.6456).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(4'-benzhydryloxy-3',3'-dimethylglutaryl)-lup-20(29)en-28-yl 3,3-dimethylglutarate (40)
Yield 58.8% (starting from 143 mg of 26); white solid. +13.5° (c 4.13 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.80 (3H, s, CH3-24), 0.82 (6H, s, CH3-23, 25), 0.97 (3H, s, CH3-27), 1.02 (3H, s, CH3-26), 1.08 (6H, s, 3-O-dimethylglutaryl CH3), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.68 (3H, s, CH3-30), 1.95 (3H, br s, AZT-CH3), 2.32–2.50 (3H, m, AZT-H2-2' and H-19), 2.34, 2.41 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-2'), 2.44, 2.50 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.49, 2.56 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-3"), 2.54, 2.57 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-4'), 3.82, 4.26 (each 1H, d, J = 11.2 Hz, H2-28), 4.06 (1H, q-like, AZT-H-4'), 4.21–4.28 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5a'), 4.43 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.46 (1H, dd, J = 4.8, 11.2 Hz, H-3), 4.59, 4.69 (each 1H, br s, H2-29), 6.15 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 6.88 (1H, s, CH(Ph)2), 7.24–7.33 (11H, m, aromatic-H and AZT-H-6), 9.07 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.7 (C-27), 15.9 (C-26), 16.1 (C-25), 16.6 (C-24), 18.1 (C-6), 19.1 (C-30), 20.7 (C-11), 23.7 (C-2), (C-12), 26.9 (C-15), 27.6, (3-O-dimethylglutaryl CH3), 27.8, 27.9 (28-O-dimethylglutaryl CH3), 27.9 (C-23), 29.5 (C-21), 29.8 (C-16), 32.5 (28-O-dimethylglutaryl C-3"), 32.8 (3-O-dimethylglutaryl C-3'), 34.1 (C-7), 34.6 (C-22), 37.0 (C-10), 37.5 (AZT-C-2', C-13), 37.6 (C-4), 38.3 (C-1), 40.8 (C-8), 42.6 (C-14), 44.5 (28-O-dimethylglutaryl C-4"), 45.0 (28-O-dimethylsuccinyl C-2"), 45.4 (3-O-dimethylglutaryl C-4'), 45.7 (3-O-dimethylglurayl C-2'), (C-17), 47.6 (C-19), 48.7 (C-18), 50.2 (C-9), 55.3 (C-5), 60.4 (AZT-C-3'), 62.6 (C-28), 62.7 (AZT-C-5'), 76.6 (CH(Ph)2), 80.8 (C-3), 81.7 (AZT-C-4'), 85.2 (AZT-C-1'), 109.9 (C-29), 111.3 (AZT-C-5), 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.2 (AZT-C-6), 140.2 (benzhydryl C-1'), 149.9 (C-20), 150.0 (AZT-C-2), 163.5 (AZT-C-4), 170.8 (3-O-dimethylglutaryl 5'-COO−), 171.1 (28-O-dimethylglutaryl 5"-COO-AZT), 171.6 (3-O-dimethylglutaryl 1'-COO−), 172.2 (28-O-dimethylglutaryl 1"-COO−). HRESIMS (positive) m/z 1164.6608 [M+Na]+ (calcd for C67H91N5O11Na, 1164.6613).
1-(3'-Azido-3'-deoxythymidine-5'-yl)-4-[3-O-(4'-benzhydryl-3',3'-dimethylsuccinyl)-lup-2 8-yl] 2,2-dimethylsuccinate (41) and 4-(3'-Azido-3'-deoxythymidine-5'-yl)-1-[3-O-(4'-benzhydryl-3',3'-dimethylsuccinyl)-lup-28-y l] 2,2-dimethylsuccinate (42)
Total yield 36.0% (starting from 285 mg of mixture of 27 and 28); used for next reaction without purification. For spectroscopic analysis, a small amount of sample was further purified by preparative HPLC (cholester, CH3OH/H2O = 97:3); white solid.
Compound 41
−6.7° (c 1.69 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0Hz, CH3–29, 30), 0.74 (3H, s, CH3-24), 0.78 (3H, s, CH3-23), 0.79 (3H, s, CH3-25), 0.94 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.31 (12H, s, 3-O, 28-O-dimethylsuccinyl CH3), 1.94 (3H, br s, AZT-CH3), 2.25–2.32, 2.44–2.50 (each 1H, m, AZT-H2-2'), 2.65, 2.70 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 2.59, 2.75 (each 1H, d, J = 16.4 Hz, 28-O-dimethylsuccinyl H2-2"), 3.81, 4.28 (each 1H, d, J = 11.2 Hz, H2-28), 4.09 (1H, q-like, AZT-H-4'), 4.25–4.29 (1H, m, AZT-H-3'), 4.29 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5a'), 4.52 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5b'), 4.44 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.18 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.86 (1H, s, CH(Ph)2), 7.24–7.33 (11H, m, aromatic-H and AZT-H-6), 8.59 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.4 (AZT-CH3), 14.6 (C-27), 14.8 (C-29), 15.9 (C-26), 16.0 (C-25), 16.5 (C-24), 18.1 (C-6), 20.7 (C-11), 21.5 (C-21), 22.9 (C-30), 23.5 (C-2), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 25.0, 25.9 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.8 (C-15), 27.9 (C-23), 29.4 (C-20), 29.8 (C-16), 34.1 (C-7), 34.6 (C-22), 36.9 (C-10), 37.1 (C-13), 37.4 (AZT-C-2'), 37.6 (C-4), 38.3 (C-1), 40.7 (dimethylsuccinyl C-3', C-3"), 40.9 (C-8), 42.8 (C-14), 44.5 (3-O-dimethylsuccinyl C-2', C-19), 44.6 (28-O-dimethylsuccinyl C-2"), 46.5 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 60.4 (AZT-C-3'), 63.1 (C-28), 63.3 (AZT-C-5'), 77.0 (CH(Ph)2), 81.3 (C-3), 81.9 (AZT-C-4'), 84.8 (AZT-C-1'), 111.4 (AZT-C-5), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.0 (AZT-C-6), 140.3 (benzhydryl C-1'), 150.0 (AZT-C-2), 163.3 (AZT-C-4), 170.9 (3-O-dimethylsuccinyl 1'-COO−), 171.7 (28-O-dimethylsuccinyl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 176.5 (28-O-dimethylsuccinyl 4"-COO-AZT). HRESIMS (positive) m/z 1116.6604 [M+H]+ (calcd for C65H90N5O11, 1116.6637).
Compound 42
−1.8° (c 2.69 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0Hz, CH3–29, 30), 0.74 (3H, s, CH3-24), 0.78 (3H, s, CH3-23), 0.80 (3H, s, CH3-25), 0.94 (3H, s, CH3–27), 1.02 (3H, s, CH3-26), 1.31 (6H, s, 3-O-dimethylsuccinyl CH3), 1.31, 1.34 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.96 (3H, br s, AZT-CH3), 2.32–2.39, 2.44–2.50 (each 1H, m, AZT-H2-2'), 2.65, 2.70 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 2.61, 2.66 (each 1H, d, J = 16.0 Hz, 28-O-dimethylsuccinyl H2-3"), 3.81, 4.29 (each 1H, d, J = 10.8 Hz, H2-28), 4.04 (1H, q-like, AZT-H-4'), 4.18–4.22 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.4 Hz, AZT-H-5a'), 4.42 (1H, dd, J = 4.4, 12.4 Hz, AZT-H-5b'), 4.44 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.15 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.86 (1H, s, CH(Ph)2), 7.24–7.33 (11H, m, aromatic-H and AZT-H-6), 8.93 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26, 25), 16.5 (C-24), 18.1 (C-6), 20.7 (C-11), 21.5 (C-21), 22.9 (C-30), 23.5 (C-2), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 25.4, 25.8 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 27.9 (C-23), 29.4 (C-20), 29.9 (C-16), 34.1 (C-7), 34.6 (C-22), 36.9 (C-10), 37.1 (C-13), 37.5 (AZT-C-2', 37.6 (C-4), 38.3 (C-1), 40.7 (3-O-dimethylsuccinyl C-3'), 40.9 (C-8), 41.0 (28-O-dimethylsuccinyl C-2"), 42.8 (C-14), 43.8 (28-O-dimethylsuccinyl C-3"), 44.5 (3-O-dimethylsuccinyl C-2', C-19), 46.7 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 60.2 (AZT-C-3'), 62.8 (AZT-C-5'), 63.3 (C-28), 77.0 (CH(Ph)2), 81.3 (C-3), 81.7 (AZT-C-4'), 85.1 (AZT-C-1'), 111.3 (AZT-C-5), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.3 (AZT-C-6), 140.3 (benzhydryl C-1'), 150.0 (AZT-C-2), 163.5 (AZT-C-4), 170.7 (28-O-dimethylsuccinyl 4"-COO−AZT), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−), 176.9 (28-O-dimethylsuccinyl 1"-COO−). HRESIMS (positive) m/z 1138.6445 [M+Na]+ (calcd for C65H89N5O11Na, 1138.6456).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-4'-benzhydryloxy-3',3'-dimethylsuccinyl)-lup-28-yl 3,3-dimethylglutarate (43)
Yield 66.1% (starting from 118 mg of 29); white solid. −0.32° (c 1.53 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.76, 0.83 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.73 (3H, s, CH3-24), 0.77 (3H, s, CH3–23), 0.78 (3H, s, CH3- 25), 0.93 (3H, s, CH3-27), 1.00 (3H, s, CH3-26), 1.11, 1.14 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.29, 1.30 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.94 (3H, br s, AZT-CH3), 2.31–2.38, 2.43–2.50 (2H, m, AZT-H2-2'), 2.41, 2.48 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.47, 2.55 (each 1H, d, J = 15.2 Hz, 28-O-dimethylglutaryl H2-4"), 2.63, 2.68 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.77, 4.24 (each 1H, d, J = 10.8 Hz, H2-28), 4.05 (1H, q-like, AZT-H-4'), 4.19–4.23 (1H, m, AZT-H-3'), 4.25 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5a'), 4.43 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.43 (1H, dd, J = 4.4, 12.0 Hz, H-3), 6.14 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.85 (1H, s, CH(Ph)2), 7.23–7.31 (11H, m, aromatic-H and AZT-H-6), 8.48 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.6 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26, 25), 16.5 (C-24), 18.1 (C-6), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.5 (C-2), 25.2, 25.4 (3-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 27.9 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 34.2(C-7), 34.7 (C-22), 37.0 (C-10), 37.1 (C-13), 37.5 (AZT-C-2'), 37.7 (C-4), 38.3 (C-1), 40.7 (3-O-dimethylsuccinyl C-3'), 40.9 (C-8), 42.8 (C-14), 44.5 (3-O-dimethylsuccinyl C-2', 28-O-dimethylglutaryl C-4", C-19), 45.0 (28-O-dimethylsuccinyl C-2"), 46.4 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 60.4 (AZT-C-3'), 62.7 (C-28, AZT-C-5'), 77.0 (CH(Ph)2), 81.3 (C-3), 81.8 (AZT-C-4'), 85.2 (AZT-C-1'), 111.3 (AZT-C-5), 127.0, 127.1 (benzhydryl C-2', 6'), 127.7 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.2 (AZT-C-6), 140.3 (benzhydryl C-1'), 149.9 (AZT-C-2), 163.2 (AZT-C-4), 170.8 (3-O-dimethylsuccinyl 1'-COO−), 171.2 (28-O-dimethylglutaryl 5"-COO-AZT), 172.3 (28-O-dimethylglutaryl 1"-COO−), 175.4 (3-O-dimethylsuccinyl 4'-COO−). HRESIMS (positive) m/z 1130.6781 [M+H]+ (calcd for C66H92N5O11, 1130.6793).
1-(3'-Azido-3'-deoxythymidine-5'-yl)-4-[3-O-(4'-benzhydryloxyglutaryl)-lup-28-yl] 2,2-dimethylsuccinate (44) and 4-(3'-Azido-3'-deoxythymidine-5'-yl)-1-[3-O-(4'-benzhydryloxyglutaryl)-lup-28-yl] 2,2-dimethylsuccinate (45)
Yield 15.8% and 10.5% respectively (starting from 208 mg of mixture of 30 and 31); separated by preparative HPLC (cholester, MeOH/H2O = 97:3); colorless oil.
Compound 44
−1.96° (c 0.72 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.85 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.83 (3H, s, CH3-24), 0.84 (3H, s, CH3-23), 0.86 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.31, 1.32 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.94 (3H, br s, AZT-CH3), 2.00 (2H, quint, J = 7.2Hz, 3-O-glutaryl H2-3'), 2.26–2.35, 2.44–2.50 (each 1H, m, AZT-H2-2'), 2.35 (2H, t, J = 7.2Hz, 3-O-glutaryl H2-2'), 2.50 (2H, t, J = 7.2Hz, 3-O-glutaryl H2-4'), 2.60, 2.74 (each 1H, d, J = 16.0 Hz, 28-O-dimethylsuccinyl H2-2"), 3.82, 4.28 (each 1H, d, J = 10.8 Hz, H2-28), 4.09 (1H, q-like, AZT-H-4'), 4.25–4.30 (1H, m, AZT-H-3'), 4.28 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5a'), 4.51 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 6.0, 10.0 Hz, H-3), 6.17 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.89 (1H, s, CH(Ph)2), 7.25–7.34 (11H, m, aromatic-H and AZT-H-6), 8.71 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.4 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.2 (C-6), 20.4 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 25.1, 25.8 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 33.6 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.6 (C-22), 37.0 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.8 (C-4), 38.4 (C-1), 40.7 (28-O-dimethylsuccinyl C-3"), 40.9 (C-8), 42.9 (C-14), 44.5 (C-19), 44.6 (28-O-dimethylsuccinyl C-2"), 46.5 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 60.4 (AZT-C-3'), 63.1 (C-28), 63.3 (AZT-C-5'), 76.9 (CH(Ph)2), 81.0 (C-3), 82.0 (AZT-C-4'), 84.9 (AZT-C-1'), 111.4 (AZT-C-5), 127.0 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 135.0 (AZT-C-6), 140.2 (benzhydryl C-1'), 150.0 (AZT-C-2), 163.4 (AZT-C-4), 171.7 (28-O-dimethylsuccinyl 1"-COO−), 171.9 (3-O-glutaryl 5'-COO−), 172.6 (3-O-glutaryl 1'-COO−), 176.5 (28-O-dimethylsuccinyl 4"-COO-AZT). HRESIMS (positive) m/z 1102.6483 [M+H]+ (calcd for C64H88N5O11, 1102.6480).
Compound 45
+1.4° (c 0.99 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.85 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.83 (3H, s, CH3-24), 0.84 (3H, s, CH3-23), 0.86 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.05 (3H, s, CH3-26), 1.31, 1.34 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.96 (3H, br s, AZT-CH3), 2.00 (2H, quint, J = 7.2Hz, 3-O-glutaryl H2-3'), 2.33–2.52 (2H, m, AZT-H2-2'), 2.35 (2H, t, J = 7.2Hz, 3-O-glutaryl H2-2'), 2.50 (2H, t, J = 7.2Hz, 3-O-glutaryl H2-4'), 2.62, 2.66 (each 1H, d, J = 16.0 Hz, 28-O-dimethylsuccinyl H2-2"), 3.82, 4.30 (each 1H, d, J = 10.8 Hz, H2-28), 4.05 (1H, q-like, AZT-H-4'), 4.17–4.22 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5a'), 4.42 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 6.0, 10.0 Hz, H-3), 6.15 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.89 (1H, s, CH(Ph)2), 7.25–7.34 (11H, m, aromatic-H and AZT-H-6), 8.74 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 20.4 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 25.5, 25.8 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 33.6 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.8 (C-4), 38.4 (C-1), 40.9 (C-8), 41.0 (28-O-dimethylsuccinyl C-2"), 42.9 (C-14), 43.8 (28-O-dimethylsuccinyl C-3"), 44.6 (C-19), 46.7 (C-17), 48.2 (C-18), 50.0 (C-9), 55.3 (C-5), 60.3 (AZT-C-3'), 62.9 (AZT-C-5'), 63.3 (C-28), 76.9 (CH(Ph)2), 81.0 (C-3), 81.8 (AZT-C-4'), 85.2 (AZT-C-1'), 111.3 (AZT-C-5), 127.0 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 135.3 (AZT-C-6), 140.2 (benzhydryl C-1'), 150.0 (AZT-C-2), 163.4 (AZT-C-4), 170.7 (28-O-dimethylsuccinyl 4"-COO-AZT), 171.9 (3-O-glutaryl 5'-COO−), 172.6 (3-O-glutaryl 1'-COO−), 176.8 (28-O-dimethylsuccinyl 1"-COO−). HRESIMS (positive) m/z 1102.6475 [M+H]+ (calcd for C64H88N5O11, 1102.6480).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(4'-benzhydryloxyglutaryl)-lup-28-yl glutarate(46)
Yield 49.8% (starting from 79 mg of 32); colorless oil. +4.4° (c 0.93, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.84 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.83 (each 3H, s, CH3-24, CH3-23), 0.86 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.05 (3H, s, CH3-26), 1.94 (3H, d, J = 1.2 Hz, AZT-CH3), 1.99 (4H, quint, J = 7.2 Hz, 3-O, 28-O-glutaryl H2-3'), 2.32–2.52 (2H, m, AZT-H2-2'), 2.35 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.41 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-2"), 2.46 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-4"), 2.50 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 3.83, 4.30 (each 1H, d, J = 11.2 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.24 (1H, m, AZT-H-3'), 4.32 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5a'), 4.40 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 6.0, 10.4 Hz, H-3), 6.10 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 6.89 (1H, s, CH(Ph)2), 7.25–7.33 (11H, m, aromatic-H and AZT-H-6), 9.04 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.8 (C-29), 16.0 (C-26, −25), 16.5 (C-24), 18.1 (C-6), 20.0 (28-O-glutaryl C-3"), 20.3 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.8 (C-30), 23.7 (C-2), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 33.1 (28-O-glutaryl C-4"), 33.2 (28-O-glutaryl C-2"), 33.6 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.6 (C-22), 37.0 (C-10), 37.2 (C-13), 37.4 (AZT-C-2'), 37.8 (C-4), 38.3 (C-1), 40.9 (C-8), 42.8 (C-14), 44.5 (C-19), 46.5 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 60.6 (AZT-C-3'), 62.9 (C-28), 63.3 (AZT-C-5'), 76.8 (CH(Ph)2), 81.0 (C-3), 81.7 (AZT-C-4'), 85.6 (AZT-C-1'), 111.3 (AZT-C-5), 127.0 (benzhydryl C-2', 6'), 127.8 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.3 (AZT-C-6), 140.2 (benzhydryl C-1'), 150.0 (AZT-C-2), 163.5 (AZT-C-4), 171.8 (3-O-glutaryl 5'-COO−), 172.3 (28-O-glutaryl 5"-COO-AZT), 172.6 (3-O-glutaryl 1'-COO−), 173.1 (28-O-glutaryl 1"-COO−). HRESIMS (positive) m/z 1112.6271 [M+Na]+ (calcd for C63H87N5O11Na, 1112.6300).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(4'-benzhydryloxyglutaryl)-lup-28-yl 3,3-dimethylglutarate (47)
Yield 48.7% (starting from 76 mg of 33); colorless oil. +4.1° (c 0.54, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.84 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.83 (3H, s, CH3-24), 0.84(3H, s, CH3-23), 0.86 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.04 (3H, s, CH3-26), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.95 (3H, d, J = 1.2 Hz, AZT-CH3), 2.00 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 2.32–2.52 (2H, m, AZT-H2-2'), 2.35 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.43, 2.49 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.48, 2.56 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-4"), 2.50 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 3.79, 4.27 (each 1H, d, J = 11.2 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.28 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5a'), 4.43 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 6.0, 10.4 Hz, H-3), 6.15 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 6.89 (1H, s, CH(Ph)2), 7.25–7.33 (11H, m, aromatic-H and AZT-H-6), 8.91 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.8 (C-29), 16.0 (C-26, −25), 16.5 (C-24), 18.1 (C-6), 20.3 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.8 (C-30), 23.7 (C-2), 26.8 (C-12), 26.9 (C-15), 27.8, 27.9 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.5 (28-O-dimethylglutaryl C-3"), 33.6 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.8 (C-4), 38.3 (C-1), 40.9 (C-8), 42.8 (C-14), 44.5 (28-O-dimethylglutaryl C-4" and C-19), 45.1 (28-O-dimethylglutaryl C-2"), 46.4 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 60.5 (AZT-C-3'), 62.7 (C-28, and AZT-C-5'), 76.9 (CH(Ph)2), 81.0 (C-3), 81.8 (AZT-C-4'), 85.2 (AZT-C-1'), 111.3 (AZT-C-5), 127.0 (benzhydryl C-2', 6'), 127.9 (benzyhydryl C-4'), 128.5 (benzhydryl C-3', 5'), 135.2 (AZT-C-6), 140.2 (benzhydryl C-1'), 150.1 (AZT-C-2), 163.5 (AZT-C-4), 171.1 (28-O-dimethylglutaryl 5"-COO-AZT), 171.8 (3-O-glutaryl 5'-COO−), 172.2 (28-O-dimethylglutaryl 1"-COO−), 172.6 (3-O-glutaryl 1'-COO−). HRESIMS (positive) m/z 1138.6449 [M+Na]+ (calcd for C65H89N5O11Na, 1138.6456).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(4'-benzhydryloxy-3',3'-dimethylglutaryl)-lup-28-yl 3,3-dimethylglutarate (48)
Yield 68.3% (starting from 126 mg of 34); colorless oil. +0.97° (c 2.37, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.84 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.81 (3H, s, CH3-24), 0.83 (6H, s, CH3-23, CH3-25), 0.95 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.09 (6H, s, 3-O-dimethylglutaryl CH3), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.95 (3H, br s, AZT-CH3), 2.32–2.52 (2H, m, AZT-H2-2'), 2.35, 2.42 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-2'), 2.43, 2.49 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.48, 2.56 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-4"), 2.55, 2.58 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-4'), 3.79, 4.26 (each 1H, d, J = 11.2 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.21–4.28 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.4 Hz, AZT-H-5a'), 4.44 (1H, dd, J = 4.4, 12.4 Hz, AZT-H-5b'), 4.47 (1H, dd, J = 4.8, 10.8 Hz, H-3), 6.15 (1H, t-like, J = 6.4Hz, AZT-H-1'), 6.89 (1H, s, CH(Ph)2), 7.22–7.34 (11H, m, aromatic-H and AZT-H-6), 8.97 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.6 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26, −25), 16.6 (C-24), 18.1 (C-6), 20.7 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 26.8 (C-12), 26.9 (C-15), 27.6 (3-O-dimethylglutaryl CH3), 27.9, 28.0 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 32.8 (3-O-dimethylglutaryl C-3'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.1 (C-13), 37.5 (AZT-C-2'), 37.6 (C-4), 38.3 (C-1), 40.9 (C-8), 42.8 (C-14), 44.5 (28-O-dimethylglutaryl C-4" and C-19), 45.0 (28-O-dimethylglutaryl C-2"), 45.5 (3-O-glutaryl C-4'), 45.8 (3-O-glutaryl C-2'), 46.4 (C-17), 48.1 (C-18), 49.9 (C-9), 55.3 (C-5), 60.4 (AZT-C-3'), 62.7 (C-28, and AZT-C-5'), 76.7 (CH(Ph)2), 80.9 (C-3), 81.8 (AZT-C-4'), 85.2 (AZT-C-1'), 111.3 (AZT-C-5), 127.1 (benzhydryl C-2', 6'), 127.8 (benzyhydryl C-4'), 128.4 (benzhydryl C-3', 5'), 135.3 (AZT-C-6), 140.2 (benzhydryl C-1'), 150.0 (AZT-C-2), 163.6 (AZT-C-4), 170.8 (3-O-dimethylglutaryl 5'-COO−), 171.2 (28-O-dimethylglutaryl 5"-COO-AZT), 171.6 (3-O-dimethylglutaryl 1'-COO−), 172.3 (28-O-dimethylglutaryl 1"-COO−). HRESIMS (positive) m/z 1144.6917 [M+H]+ (calcd for C67H94N5O11, 1144.6950).
4.2.9 General procedure for removing benzhydryl group (49–62)
The final products were prepared by refluxing of a solution of benzdryl ester derivative in 80% HOAc (2–6 mL) overnight. After cooling to room temperature, the reaction mixture was extracted with CHCl3, and the organic layer was washed with water and brine, dried over Na2SO4 and concentrated. The residue was purified by preparative HPLC (cholester, MeOH/H2O/HOAc = 93:6:1).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl succinate (49)
Yield 46.5% (starting from 62 mg of 35); white solid. +7.2° (c 0.57, CHCl3). 1H NMR (CDCl3, 500 MHz) δ 0.80 (3H, s, CH3-24), 0.82 (3H, s, CH3-23), 0.83 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.00 (3H, s, CH3-26), 1.29, 1.31 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.68 (3H, s, CH3-30), 1.94 (3H, d, J = 0.9 Hz, AZT-CH3), 2.33–2.49 (3H, m, AZT-H2-2' and H-19), 2.57, 2.68 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 2.67–2.77 (4H, m, 28-O-succinyl H2-2", 3"), 3.86, 4.30 (each 1H, d, J = 11.2 Hz, H2-28), 4.05 (1H, q-like, AZT-H-4'), 4.21–4.25 (1H, m, AZT-H-3'), 4.30 (1H, dd, J = 3.4, 12.3 Hz, AZT-H2-5a'), 4.49 (1H, dd, J = 5.2, 11.2 Hz, H-3), 4.52 (1H, dd, J = 4.3, 12.3 Hz, AZT-H2-5b'), 4.58, 4.68 (each 1H, br s, H2-29), 6.14 (1H, t-like, J = 6.3 Hz, AZT-H-1'), 7.30 (1H, d, J = 1.2 Hz, AZT-H-6), 8.68 (1H, br s, AZT-NH); 13C NMR (CDCl3, 125 MHz) δ 12.6 (AZT-CH3), 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.8 (C-11), 23.6 (C-2), 25.1 (C-12), 25.1, 25.6 (3-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.0 (28-O-succinyl C-2", 3"), 29.5 (C-21), 29.7 (C-16), 34.1 (C-7), 34.6 (C-22), 37.0 (C-10), 37.6 (AZT-C-2' and C-13), 37.7 (C-4), 38.3 (C-1), 40.4 (dimethylsuccinyl C-3'), 409 (C-8), 42.7 (C-14), 44.7 (3-O-dimethylsuccinyl C-2'), 46.4 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.4 (C-5), 60.1 (AZT-C-3'), 63.1 (AZT-C-5'), 63.4 (C-28), 81.5 (C-3), 81.9 (AZT-C-4'), 85.2 (AZT-C-1'), 110.0 (C-29), 111.3 (AZT-C-5), 135.4 (AZT-C-6), 149.9 (AZT-C-2), 150.0 (C-20), 163.5 (AZT-C-4), 171.1 (3-O-dimethylsuccinyl 1'-COO−), 172.0 (28-O-succinyl 4"-COO-AZT), 172.6 (28-O-succinyl 1"-COO−), 180.8 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 942.5218 [M+Na]+ (calcd for C50H73N5O11Na, 942.5204).
1-(3'-Azido-3'-deoxythymidine-5'-yl)-4-[3-O-(3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl] 2,2-dimethylsuccinate (50) and 4-(3'-Azido-3'-deoxythymidine-5'-yl)-1-[3-O-(3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl] 2,2-dimethylsuccinate (51)
Total yield 78.9% (starting from 105 mg of mixture of 36 and 37); in a ratio of 2:1; white solid.
Compound 50
+8.2° (c 2.38 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.81 (3H, s, CH3-24), 0.83 (each 3H, s, CH3-23, 25), 0.96 (3H, s, CH3-27), 1.00 (3H, s, CH3-26), 1.29, 1.30 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.30, 1.31 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.68 (3H, s, CH3-30), 1.93 (3H, br s, AZT-CH3), 2.26–2.33, 2.44–2.50 (each 1H, m, AZT-H2-2'), 2.37–2.44 (1H, m, H-19), 2.56, 2.68 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 2.60, 2.74 (each 1H, d, J = 16.4 Hz, 28-O-dimethylsuccinyl H2-2"), 3.84, 4.27 (each 1H, d, J = 11.2 Hz, H2-28), 4.09 (1H, q-like, AZT-H-4'), 4.25–4.31 (2H, m, AZT-H-3' and AZT-H-5a'), 4.51 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5b'), 4.48 (1H, dd, J = 5.6, 11.2 Hz, H-3), 4.59, 4.68 (each 1H, br s, H2-29), 6.16 (1H, t-like, J = 6.4Hz, AZT-H-1'), 7.28 (1H, d, J = 0.8Hz, AZT-H-6), 9.38 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.4 (AZT-CH3), 14.7 (C-27), 15.9 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.7 (C-11), 23.6 (C-2), 25.1 (C-12), 25.1, 25.6 (3-O-dimethylsuccinyl CH3), 25.1, 25.9 (28-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.5 (C-21), 29.7 (C-16), 34.1 (C-7), 34.5 (C-22), 37.0 (C-10), 37.5 (AZT-C-2'), 37.6 (C-13), 37.7 (C-4), 38.4 (C-1), 40.5 (3-O-dimethylsuccinyl C-3'), 40.7 (28-O-dimethylsuccinyl C-3"), 40.8 (C-8), 42.7 (C-14), 44.6 (28-O-dimethylsuccinyl C-2"), 44.7 (3-O-dimethylsuccinyl C-2'), 46.3 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.4 (C-5), 60.4 (AZT-C-3'), 63.1 (C-28), 63.0 (AZT-C-5'), 81.5 (C-3), 82.0 (AZT-C-4'), 84.9 (AZT-C-1'), 110.0 (C-29), 111.3 (AZT-C-5), 135.2 (AZT-C-6), 149.9 (C-20), 150.1 (AZT-C-2), 164.0 (AZT-C-4), 171.1 (3-O-dimethylsuccinyl 1'-COO−), 171.7 (28-O-dimethylsuccinyl 1"-COO−), 176.5 (28-O-dimethylsuccinyl 4"-COO-AZT), 182.2 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 970.5511 [M+Na]+ (calcd for C52H77N5O11Na, 970.5517).
Compound 51
+15.6° (c 4.68 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.80 (3H, s, CH3-24), 0.82 (6H, s, CH3–23, C-25), 0.96 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.28, 1.29 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.30, 1.33 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.67 (3H, s, CH3–30), 1.94 (3H, br s, AZT-CH3), 2.33–2.50 (3H, m, AZT-H2-2' and H-19), 2.61, 2.65 (each 1H, d, J = 16.4 Hz, 28-O-dimethylsuccinyl H2-3"), 2.56, 2.67 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 3.83, 4.27 (each 1H, d, J = 10.8 Hz, H2-28), 4.03 (1H, q-like, AZT-H-4'), 4.18–4.22(1H, m, AZT-H-3'), 4.25 (1H, dd, J = 4.0, 12.4 Hz, AZT-H-5a'), 4.41 (1H, dd, J = 4.4, 12.4 Hz, AZT-H-5b'), 4.48 (1H, dd, J = 5.2, 10.8 Hz, H-3), 4.58, 4.67 (each 1H, br s, H2-29), 6.12 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.31 (1H, d, J = 0.8 Hz, AZT-H-6), 9.95 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.7 (C-27), 15.9 (C-26), 16.0 (C-25), 16.4 (C-24), 18.1 (C-6), 19.1 (C-30), 20.7 (C-11), 23.5 (C-2), 25.1 (C-12), 24.9, 25.6 (3-O-dimethylsuccinyl CH3), 25.4, 25.7 (28-O-dimethylsuccinyl CH3), 26.9 (C-15), 27.8 (C-23), 29.5 (C-21), 29.8 (C-16), 34.0 (C-7), 34.5 (C-22), 37.0 (C-10), 37.5 (AZT-C-2' and C-13), 37.6 (C-4), 38.3 (C-1), 40.4 (3-O-dimethylsuccinyl C-3'), 40.8 (C-8), 41.0 (28-O-dimethylsuccinyl C-2"), 42.6 (C-14), 43.8 (28-O-dimethylsuccinyl C-3"), 44.7 (3-O-dimethylsuccinyl C-2'), 46.5 (C-17), 47.7 (C-19), 48.8 (C-18), 50.2 (C-9), 55.3 (C-5), 60.2 (AZT-C-3'), 62.9 (AZT-C-5'), 63.2 (C-28), 81.5 (C-3), 81.8 (AZT-C-4'), 85.3 (AZT-C-1'), 109.9 (C-29), 111.2 (AZT-C-5), 135.6 (AZT-C-6), 149.9 (C-20), 150.2 (AZT-C-2), 164.4 (AZT-C-4), 170.7 (28-O-dimethylsuccinyl 4"-COO-AZT), 171.0 (3-O-dimethylsuccinyl 1'-COO−), 176.9 (28-O-dimethylsuccinyl 1"-COO−), 182.7 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 970.5490 [M+Na]+ (calcd for C52H77N5O11Na, 970.5517).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl glutarate (52)
Yield 42.7% (starting from 90 mg of 38); white solid. +18.8° (c 0.6, CHCl3). 1H NMR (CDCl3, 500 MHz) δ 0.81 (3H, s, CH3-24), 0.83 (3H, s, CH3-23), 0.83 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.02 (3H, s, CH3-26), 1.29, 1.31 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.68 (3H, s, CH3-30), 1.94 (3H, d, J = 1.2 Hz, AZT-CH3), 1.99 (2H, quint, J = 7.2 Hz, 28-O-glutaryl H2-3"), 2.33–2.49 (3H, m, AZT-H2-2' and H-19), 2.42 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-2"), 2.46 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-4"), 2.57, 2.67 (each 1H, d, J = 15.8 Hz, 3-O-dimethylsuccinyl H2-2'), 3.85, 4.28 (each 1H, d, J = 10.8 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.24 (1H, m, AZT-H-3'), 4.32 (1H, dd, J = 3.9, 12.3 Hz, AZT-H2-5a'), 4.40 (1H, dd, J = 4.7, 12.3 Hz, AZT-H2-5b'), 4.49 (1H, dd, J = 5.2, 11.2 Hz, H-3), 4.59, 4.68 (each 1H, br s, H2-29), 6.09 (1H, t-like, J = 6.3 Hz, AZT-H-1'), 7.21 (1H, d, J = 1.1 Hz, AZT-H-6); 13C NMR (CDCl3, 125 MHz) δ 12.6 (AZT-CH3), 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.0 (28-O-glutaryl C-3"), 20.8 (C-11), 23.6 (C-2), 25.1 (C-12), 25.1, 25.6 (3-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (C-23), 29.5 (C-21), 29.8 (C-16), 33.1 (28-O-glutaryl C-4"), 33.2 (28-O-glutaryl C-2"), 34.1 (C-7), 34.6 (C-22), 37.0 (C-10), 37.5 (AZT-C-2'), 37.6 (C-13), 37.7 (C-4), 38.4 (C-1), 40.4 (dimethylsuccinyl C-3'), 40.9 (C-8), 42.7 (C-14), 44.8 (3- O -dimethylsuccinyl C-2'), 46.4 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.4 (C-5), 60.6 (AZT-C-3'), 63.0 (C-28), 63.3 (AZT-C-5'), 81.6 (C-3), 81.7 (AZT-C-4'), 85.7 (AZT-C-1'), 110.0 (C-29), 111.3 (AZT-C-5), 135.5 (AZT-C-6), 149.8 (C-20), 149.9 (AZT-C-2), 163.5 (AZT-C-4), 171.2 (3-O-dimethylsuccinyl 1'-COO−), 172.3 (28-O-glutaryl 5"-COO-AZT), 173.1 (28-O-glutaryl 1"-COO−), 180.8 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 956.5361 [M+Na]+ (calcd for C51H75N5O11Na, 956.5361).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(3',3'-dimethylsuccinyl)-lup-20(29)en-28-yl 3,3-dimethylglutarate (53)
Yield 87.3% (starting from 114 mg of 39); white solid. +15.3° (c 2.84, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.80 (3H, s, CH3-24), 0.83 (each 3H, s, CH3-23, 25), 0.96 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.29, 1.30 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.68 (3H, s, CH3-30), 1.94 (3H, br s, AZT-CH3), 2.30–2.35, 2.40–2.51 (2H, m, AZT-H2-2'), 2.35–2.42 (1H, m, H-19), 2.44, 2.49 (each 1H, d, J = 16.0 Hz, 28-O-dimethylglutaryl H2-2"), 2.49, 2.55 (each 1H, d, J = 16.0 Hz, 28-O-dimethylglutaryl H2-4"), 2.56, 2.68 (each 1H, d, J = 16.0 Hz, 3-O-dimethylsuccinyl H2-2'), 3.82, 4.26 (each 1H, d, J = 11.2 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.25 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5a'), 4.43 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.48 (1H, dd, J = 5.2, 10.8 Hz, H-3), 4.60, 4.69 (each 1H, br s, H2-29), 6.14 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.29 (1H, d, J = 1.2 Hz, AZT-H-6), 9.43 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.5 (C-24), 18.1 (C-6), 19.1 (C-30), 20.8 (C-11), 23.6 (C-2), 25.1 (C-12), 25.0, 25.6 (3-O-dimethylsuccinyl CH3), 27.0 (C-15), 27.9 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.5 (C-21), 29.8 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 34.1 (C-7), 34.6 (C-22), 37.0 (C-10), 37.5 (AZT-C-2', C-13), 37.7 (C-4), 38.4 (C-1), 40.4 (3-O-dimethylsuccinyl C-3'), 40.8 (C-8), 42.7 (C-14), 44.5 (28-O-dimethylglutaryl C-4"), 44.7 (3-O-dimethylsuccinyl C-2'), 45.0 (28-O-dimethylglutaryl C-2"), 46.2 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.4 (C-5), 60.4 (AZT-C-3'), 62.7 (C-28), 62.8 (AZT-C-5'), 81.5 (C-3), 81.8 (AZT-C-4'), 85.3 (AZT-C-1'), 109.9 (C-29), 111.2 (AZT-C-5), 135.4 (AZT-C-6), 150.0 (C-20), 150.1 (AZT-C-2), 163.9 (AZT-C-4), 171.0 (3-O-dimethylsuccinyl 1'-COO−), 171.2 (28-O-dimethylglutaryl 5"-COO-AZT), 172.3 (28-O-dimethylglutaryl 1"-COO−), 182.1 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 962.5881 [M+H]+ (calcd for C53H80N5O11, 962.5854).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(3',3'-dimethylglutaryl)-lup-20(29)en-28-yl 3,3-dimethylglutarate (54)
Yield 84.2% (starting from 107 mg of 40); white solid. +15.6° (c 3.09 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.84 (each 3H, s, CH3-24, 25), 0.85 (6H, s, CH3-23), 0.97 (3H, s, CH3-27), 1.02 (3H, s, CH3-26), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.15 (6H, s, 3-O-dimethylglutaryl CH3), 1.68 (3H, s, CH3-30), 1.94 (3H, br s, AZT-CH3), 2.32–2.51 (3H, m, AZT-H2-2' and H-19), 2.40, 2.47 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-2'), 2.46 (2H, s, 3-O-dimethylglutaryl H2-4'), 2.44, 2.49 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.48, 2.56 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-3"), 3.81, 4.26 (each 1H, d, J = 11.2 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.28 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 4.0, 12.4 Hz, AZT-H-5a'), 4.43 (1H, dd, J = 4.0, 12.4 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 5.2, 11.6 Hz, H-3), 4.60, 4.69 (each 1H, br s, H2-29), 6.14 (1H, t-like, J = 6.4Hz, AZT-H-1'), 7.29 (1H, br s, AZT-H-6), 9.41 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.7 (C-27), 16.0 (C-26), 16.1 (C-25), 16.6 (C-24), 18.1 (C-6), 19.1 (C-30), 20.8 (C-11), 23.7 (C-2), 25.1 (C-12), 27.0 (C-15), 27.9 (3-O-dimethylglutaryl CH3), 27.9, 28.0 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.5 (C-21), 29.8 (C-16), 32.6 (28-O-dimethylglutaryl C-3", 3-O-dimethylglutaryl C-3'), 34.1 (C-7), 34.6 (C-22), 37.0 (C-10), 37.5 (AZT-C-2', C-13), 37.6 (C-4), 38.3 (C-1), 40.8 (C-8), 42.7 (C-14), 44.5 (28-O-dimethylglutaryl C-4"), 45.0 (28-O-dimethylsuccinyl C-2"), 45.2 (3-O-dimethylglutaryl C-4'), 45.6 (3-O-dimethylglurayl C-2'), 46.2 (C-17), 47.7 (C-19), 48.7 (C-18), 50.2 (C-9), 55.4 (C-5), 60.4 (AZT-C-3'), 62.6 (C-28), 62.7 (AZT-C-5'), 81.3 (C-3), 81.8 (AZT-C-4'), 85.3 (AZT-C-1'), 109.9 (C-29), 111.2 (AZT-C-5), 135.4 (AZT-C-6), 150.0 (C-20), 150.1 (AZT-C-2), 164.0 (AZT-C-4), 171.2 (28-O-dimethylglutaryl 5"-COO-AZT), 172.3 (3-O-dimethylglutaryl 1'-COO−, 28-O-dimethylglutaryl 1"-COO−), 176.0 (3-O-dimethylglutaryl 5'-COOH). HRESIMS (positive) m/z 998.5824 [M+Na]+ (calcd for C54H81N5O11Na, 998.5830).
1-(3'-Azido-3'-deoxythymidine-5'-yl)-4-[3-O-(3',3'-dimethylsuccinyl)-lup-28-yl] 2,2-dimethylsuccinate (55) and 4-(3'-Azido-3'-deoxythymidine-5'-yl)-1-[3-O-(3',3'-dimethylsuccinyl)-lup-28-yl] 2,2-dimethylsuccinate (56)
Total yield 56.7% (starting from 148 mg of mixture of 41 and 42); in a ratio of 1.1:1; white solid.
Compound 55
−4.2° (c 2.99 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.76, 0.83 (each 3H, d, J = 8.0 Hz, CH3–29, 30), 0.82 (3H, s, CH3-24), 0.84 (6H, s, CH3-23, CH3-25), 0.94 (3H, s, CH3-27), 1.01 (3H, s, CH3-26), 1.31 (6H, s, 3-O-dimethylsuccinyl CH3), 1.30, 1.31 (each 1H, s, 28-O-dimethylsuccinyl CH3), 1.93 (3H, br s, AZT-CH3), 2.25–2.32, 2.44–2.50 (each 1H, m, AZT-H2-2'), 2.57, 2.68 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 2.59, 2.73 (each 1H, d, J = 16.4 Hz, 28-O-dimethylsuccinyl H2-2"), 3.81, 4.27 (each 1H, d, J = 10.8 Hz, H2-28), 4.09 (1H, q-like, AZT-H-4'), 4.25–4.29 (1H, m, AZT-H-3'), 4.28 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5a'), 4.52 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.16 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.27 (1H, br s, AZT-H-6), 9.31 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.4 (AZT-CH3), 14.6 (C-27), 14.8 (C-29), 16.0 (C-26, C-25), 16.5 (C-24), 18.1 (C-6), 20.8 (C-11), 21.6 (C-21), 22.8 (C-30), 23.6 (C-2), 25.1, 25.6 (3-O-dimethylsuccinyl CH3), 25.0, 25.8 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 27.9 (C-23), 29.4 (C-20), 29.8 (C-16), 34.2 (C-7), 34.6 (C-22), 37.0 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.7 (C-4), 38.4 (C-1), 40.4 (3-O-dimethylsuccinyl C-3'), 40.7 (28-O-dimethylsuccinyl C-3"), 40.9 (C-8), 42.8 (C-14), 44.5 (C-19), 44.6 (28-O-dimethylsuccinyl C-2"), 44.7 (3-O-dimethylsuccinyl C-2'), 46.5 (C-17), 48.1 (C-18), 49.9 (C-9), 55.4 (C-5), 60.4 (AZT-C-3'), 63.1 (C-28), 63.3 (AZT-C-5'), 81.5 (C-3), 82.0 (AZT-C-4'), 84.9 (AZT-C-1'), 111.3 (AZT-C-5), 135.2 (AZT-C-6), 150.2 (AZT-C-2), 164.0 (AZT-C-4), 171.0 (3-O-dimethylsuccinyl 1'-COO−), 171.7 (28-O-dimethylsuccinyl 1"-COO−), 176.5 (28-O-dimethylsuccinyl 4"-COO-AZT), 182.0 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 950.5848 [M+H]+ (calcd for C52H80N5O11, 950.5854).
Compound 56
−2.5° (c 1.74 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0 Hz, CH3–29, 30), 0.82 (3H, s, CH3-24), 0.85 (6H, s, CH3-23, CH3-25), 0.95 (3H, s, CH3-27), 1.00 (3H, s, CH3-26), 1.31 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.30, 1.33 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.95 (3H, br s, AZT-CH3), 2.32–2.39, 2.44–2.50 (each 1H, m, AZT-H2-2'), 2.57, 2.68 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 2.61, 2.66 (each 1H, d, J = 16.0 Hz, 28-O-dimethylsuccinyl H2-3"), 3.81, 4.29 (each 1H, d, J = 10.8 Hz, H2-28), 4.04 (1H, q-like, AZT-H-4'), 4.17–4.22 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5a'), 4.42 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.50 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.13 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.30 (1H, d, br s, AZT-H-6), 9.16 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.7 (C-27), 14.9 (C-29), 16.0 (C-26, 25), 16.5 (C-24), 18.2 (C-6), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.6 (C-2), 25.1, 25.6 (3-O-dimethylsuccinyl CH3), 25.5, 25.8 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 27.9 (C-23), 29.4 (C-20), 30.0 (C-16), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.7 (C-4), 38.4 (C-1), 40.4 (3-O-dimethylsuccinyl C-3'), 40.9 (C-8), 41.1 (28-O-dimethylsuccinyl C-2"), 42.9 (C-14), 43.9 (28-O-dimethylsuccinyl C-3"), 44.6 (C-19), 44.7 (3-O-dimethylsuccinyl C-2'), 46.7 (C-17), 48.2 (C-18), 50.0 (C-9), 55.4 (C-5), 60.3 (AZT-C-3'), 63.0 (AZT-C-5'), 63.4 (C-28), 81.5 (C-3), 81.8 (AZT-C-4'), 85.3 (AZT-C-1'), 111.3 (AZT-C-5), 135.4 (AZT-C-6), 150.0 (AZT-C-2), 163.8 (AZT-C-4), 170.8 (28-O-dimethylsuccinyl 4"-COO−AZT), 171.0 (3-O-dimethylsuccinyl 1'-COO−), 176.9 (28-O-dimethylsuccinyl 1"-COO-), 181.7 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 950.5873 [M+H]+ (calcd for C52H80N5O11, 950.5854).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(3',3'-dimethylsuccinyl)-lup-28-yl 3,3-dimethylglutarate (57)
Yield 45.6% (starting from 84 mg of 43); white solid. −0.02° (c 2.12, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.82 (3H, s, CH3-24), 0.85 (6H, s, CH3-23, CH3- 25), 0.95 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.30, 1.31 (each 3H, s, 3-O-dimethylsuccinyl CH3), 1.95 (3H, br s, AZT-CH3), 2.33–2.40, 2.43–2.50 (2H, m, AZT-H2-2'), 2.42, 2.48 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.49, 2.56 (each 1H, d, J = 15.2 Hz, 28-O-dimethylglutaryl H2-4"), 2.57, 2.68 (each 1H, d, J = 15.6 Hz, 3-O-dimethylsuccinyl H2-2'), 3.79, 4.26 (each 1H, d, J = 10.8 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.23 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5a'), 4.44 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.50 (1H, dd, J = 5.2, 10.4 Hz, H-3), 6.14 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.29 (1H, br s, AZT-H-6), 9.15 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26, 25), 16.5 (C-24), 18.2 (C-6), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.6 (C-2), 25.1, 25.6 (3-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 27.9 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 34.2(C-7), 34.8 (C-22), 37.0 (C-10), 37.2 (C-13), 37.6 (AZT-C-2'), 37.7 (C-4), 38.4 (C-1), 40.4 (3-O-dimethylsuccinyl C-3'), 40.9 (C-8), 42.9 (C-14), 44.5 (28-O-dimethylglutaryl C-4", C-19), 44.7 (3-O-dimethylsuccinyl C-2'), 45.1 (28-O-dimethylsuccinyl C-2"), 46.4 (C-17), 48.1 (C-18), 50.0 (C-9), 55.4 (C-5), 60.5 (AZT-C-3'), 62.8 (C-28, AZT-C-5'), 81.5 (C-3), 81.9 (AZT-C-4'), 85.3 (AZT-C-1'), 111.3 (AZT-C-5), 135.4 (AZT-C-6), 150.1 (AZT-C-2), 163.8 (AZT-C-4), 171.0 (3-O-dimethylsuccinyl 1'-COO−), 171.2 (28-O-dimethylglutaryl 5"-COO-AZT), 172.3 (28-O-dimethylglutaryl 1"-COO−), 181.8 (3-O-dimethylsuccinyl 4'-COOH). HRESIMS (positive) m/z 964.6037 [M+H]+ (calcd for C53H82N5O11, 964.6011).
1-(3'-Azido-3'-deoxythymidine-5'-yl)-4-(3-O-glutaryl-lup-28-yl) 2,2-dimethylsuccinate (58)
Yield 82.5% (starting from 20.4 mg of 44); white solid. −1.9° (c 1.11 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.85 (6H, s, CH3-24, CH3-23), 0.87 (3H, s, CH3-25), 0.95 (3H, s, CH3-27), 1.02 (3H, s, CH3-26), 1.30, 1.31 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.94 (3H, br s, AZT-CH3), 1.98 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 2.26–2.35, 2.44–2.51 (each 1H, m, AZT-H2-2'), 2.40 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.44 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 2.60, 2.74 (each 1H, d, J = 16.0 Hz, 28-O-dimethylsuccinyl H2-2"), 3.82, 4.28 (each 1H, d, J = 10.8 Hz, H2-28), 4.09 (1H, q-like, AZT-H-4'), 4.25–4.30 (1H, m, AZT-H-3'), 4.28 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5a'), 4.51 (1H, dd, J = 4.0, 12.0 Hz, AZT-H2-5b'), 4.49 (1H, dd, J = 5.2, 10.0 Hz, H-3), 6.16 (1H, t-like, J = 6.4Hz, AZT-H-1'), 7.28 (1H, d, J = 1.2Hz, AZT-H-6), 8.96 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.4 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26), 16.1 (C-25), 16.6 (C-24), 18.2 (C-6), 20.1 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 25.1, 25.9 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.9 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.1 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.8 (C-4), 38.4 (C-1), 40.7 (28-O-dimethylsuccinyl C-3"), 40.9 (C-8), 42.9 (C-14), 44.6 (C-19, 28-O-dimethylsuccinyl C-2"), 46.5 (C-17), 48.2 (C-18), 50.0 (C-9), 55.4 (C-5), 60.4 (AZT-C-3'), 63.2 (C-28), 63.3 (AZT-C-5'), 81.1 (C-3), 82.0 (AZT-C-4'), 84.9 (AZT-C-1'), 111.3 (AZT-C-5), 135.2 (AZT-C-6), 150.0 (AZT-C-2), 163.7 (AZT-C-4), 171.7 (28-O-dimethylsuccinyl 1"-COO−), 172.7 (3-O-glutaryl 1'-COO−), 176.5 (28-O-dimethylsuccinyl 4"-COO-AZT), 177.1 (3-O-glutaryl 5'-COOH). HRESIMS (positive) m/z 936.5716 [M+H]+ (calcd for C51H78N5O11, 936.5698).
4-(3'-Azido-3'-deoxythymidine-5'-yl)-1-(3-O-glutaryl-lup-28-yl) 2,2-dimethylsuccinate (59)
Yield 66.1% (starting from 32.6 mg of 45); white solid. +2.3° (c 1.66 CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.85 (6H, s, CH3-24, CH3-23), 0.87 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.04 (3H, s, CH3-26), 1.31, 1.34 (each 3H, s, 28-O-dimethylsuccinyl CH3), 1.96 (3H, br s, AZT-CH3), 1.98 (2H, quint, J = 7.2Hz, 3-O-glutaryl H2-3'), 2.32–2.51 (2H, m, AZT-H2-2'), 2.40 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.43 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 2.61, 2.66 (each 1H, d, J = 16.4 Hz, 28-O-dimethylsuccinyl H2-2"), 3.81, 4.30 (each 1H, d, J = 10.8 Hz, H2-28), 4.05 (1H, q-like, AZT-H-4'), 4.17–4.23 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.4 Hz, AZT-H-5a'), 4.42 (1H, dd, J = 4.4, 12.4 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.14 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.31 (1H, d, J = 0.8 Hz, AZT-H-6), 9.14 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.7 (C-27), 14.9 (C-29), 16.0 (C-26), 16.1 (C-25), 16.6 (C-24), 18.2 (C-6), 20.1 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 25.5, 25.8 (28-O-dimethylsuccinyl CH3), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.9 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.8 (C-4), 38.4 (C-1), 40.9 (C-8), 41.1 (28-O-dimethylsuccinyl C-2"), 42.9 (C-14), 43.9 (28-O-dimethylsuccinyl C-3"), 44.6 (C-19), 46.7 (C-17), 48.2 (C-18), 50.0 (C-9), 55.4 (C-5), 60.3 (AZT-C-3'), 62.9 (AZT-C-5'), 63.3 (C-28), 81.1 (C-3), 81.8 (AZT-C-4'), 85.2 (AZT-C-1'), 111.3 (AZT-C-5), 135.5 (AZT-C-6), 150.1 (AZT-C-2), 163.8 (AZT-C-4), 170.8 (28-O-dimethylsuccinyl 4"-COO-AZT), 172.7 (3-O-glutaryl 1'-COO−), 176.9 (28-O-dimethylsuccinyl 1"-COO−), 177.3 (3-O-glutaryl 5'-COOH). HRESIMS (positive) m/z 936.5694 [M+H]+ (calcd for C51H78N5O11, 936.5698).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-glutaryl-lup-28-yl glutarate (60)
Yield 58 7% (starting from 41.2 mg of 46); white solid. +7.0° (c 1.40, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.78, 0.84 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.84 (6H, s, CH3-24, CH3-23), 0.87 (3H, s, CH3-25), 0.96 (3H, s, CH3-27), 1.05 (3H, s, CH3-26), 1.94 (3H, d, J = 1.2 Hz, AZT-CH3), 1.97 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 1.99 (2H, quint, J = 7.2 Hz, 28-O-glutaryl H2-3"), 2.34–2.51 (2H, m, AZT-H2-2'), 2.40 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.41 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-2"), 2.43 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 2.46 (2H, t, J = 7.2 Hz, 28-O-glutaryl H2-4"), 3.83, 4.29 (each 1H, d, J = 11.2 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.24 (1H, m, AZT-H-3'), 4.32 (1H, dd, J = 4.0, 12.0 Hz, AZT-H-5a'), 4.40 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 6.0, 10.4 Hz, H-3), 6.09 (1H, t-like, J = 6.4Hz, AZT-H-1'), 7.23 (1H, br s, AZT-H-6), 9.10 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26, −25), 16.6 (C-24), 18.2 (C-6), 20.1 (28-O-glutaryl C-3", 3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 26.8 (C-12), 26.9 (C-15), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.9 (3-O-glutaryl C-4'), 33.1 (28-O-glutaryl C-4"), 33.2 (28-O-glutaryl C-2"), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.5 (AZT-C-2'), 37.8 (C-4), 38.4 (C-1), 41.0 (C-8), 42.9 (C-14), 44.6 (C-19), 46.6 (C-17), 48.2 (C-18), 50.0 (C-9), 55.4 (C-5), 60.6 (AZT-C-3'), 63.0 (C-28), 63.3 (AZT-C-5'), 81.1 (C-3), 81.8 (AZT-C-4'), 85.7 (AZT-C-1'), 111.3 (AZT-C-5), 135.5 (AZT-C-6), 150.0 (AZT-C-2), 163.7 (AZT-C-4), 172.3 (28- O-glutaryl 5"-COO-AZT), 172.7 (3-O-glutaryl 1'-COO−), 173.1 (28-O-glutaryl 1"-COO−), 177.2 (3-O-glutaryl 5'-COOH). HRESIMS (positive) m/z 922.5557 [M+H]+ (calcd for C60H76N5O11, 922.5541).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-glutaryl-lup-28-yl 3,3-dimethylglutarate (61)
Yield 75.6% (starting from 39 mg of 47); white solid. +3.5° (c 2.13, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0Hz, CH3-29, 30), 0.85 (6H, s, CH3-24, CH3-23), 0.86 (3H, s, CH3-25), 0.95 (3H, s, CH3-27), 1.03 (3H, s, CH3-26), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.95 (3H, d, J = 1.2 Hz, AZT-CH3), 1.97 (2H, quint, J = 7.2 Hz, 3-O-glutaryl H2-3'), 2.32–2.51 (2H, m, AZT-H2-2'), 2.40 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-2'), 2.42, 2.49 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.43 (2H, t, J = 7.2 Hz, 3-O-glutaryl H2-4'), 2.48, 2.56 (each 1H, d, J = 15.2 Hz, 28-O-dimethylglutaryl H2-4"), 3.79, 4.27 (each 1H, d, J = 10.8 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.28 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.4 Hz, AZT-H-5a'), 4.44 (1H, dd, J = 4.4, 12.4 Hz, AZT-H-5b'), 4.49 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.14 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.30 (1H, d, J = 0.8 Hz, AZT-H-6), 9.18 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26, −25), 16.6 (C-24), 18.2 (C-6), 20.1 (3-O-glutaryl C-3'), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.7 (C-2), 26.8 (C-12), 26.9 (C-15), 27.9, 28.0 (28-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 32.9 (3-O-glutaryl C-4'), 33.7 (3-O-glutaryl C-2'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.6 (AZT-C-2'), 37.8 (C-4), 38.4 (C-1), 40.9 (C-8), 42.9 (C-14), 44.5 (28-O-dimethylglutaryl C-4" and C-19), 45.1 (28-O-dimethylglutaryl C-2"), 46.4 (C-17), 48.1 (C-18), 50.0 (C-9), 55.4 (C-5), 60.4 (AZT-C-3'), 62.7 (C-28 and AZT-C-5'), 81.1 (C-3), 81.9 (AZT-C-4'), 85.3 (AZT-C-1'), 111.3 (AZT-C-5), 135.4 (AZT-C-6), 150.1 (AZT-C-2), 163.8 (AZT-C-4), 171.2 (28-O-dimethylglutaryl 5"-COO-AZT), 172.3 (28-O-dimethylglutaryl 1"-COO−), 172.7 (3-O-glutaryl 1'-COO−), 177.3 (3-O-glutaryl 5'-COOH). HRESIMS (positive) m/z 950.5876 [M+H]+ (calcd for C52H80N5O11, 950.5854).
3'-Azido-3'-deoxythymidine-5'-yl 3-O-(3',3'-dimethylglutaryl)-lup-28-yl 3,3-dimethylglutarate (62)
Yield 76.4% (starting from 87.7 mg of 48); white solid. +0.64° (c 2.78, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 0.77, 0.84 (each 3H, d, J = 8.0 Hz, CH3-29, 30), 0.83 (3H, s, CH3-24), 0.85 (6H, s, CH3-23, CH3-25), 0.95 (3H, s, CH3-27), 1.03 (3H, s, CH3–26), 1.15 (6H, s, 3-O-dimethylglutaryl CH3), 1.12, 1.15 (each 3H, s, 28-O-dimethylglutaryl CH3), 1.95 (3H, br s, AZT-CH3), 2.32–2.51 (2H, m, AZT-H2-2'), 2.41, 2.47 (each 1H, d, J = 14.4 Hz, 3-O-dimethylglutaryl H2-2'), 2.42, 2.48 (each 1H, d, J = 14.4 Hz, 28-O-dimethylglutaryl H2-2"), 2.47 (2H, s, 3-O-dimethylglutaryl H2-4'), 2.48, 2.56 (each 1H, d, J = 15.2 Hz, 28-O-dimethylglutaryl H2-4"), 3.79, 4.27 (each 1H, d, J = 10.8 Hz, H2-28), 4.07 (1H, q-like, AZT-H-4'), 4.20–4.28 (1H, m, AZT-H-3'), 4.26 (1H, dd, J = 3.6, 12.0 Hz, AZT-H-5a'), 4.44 (1H, dd, J = 4.4, 12.0 Hz, AZT-H-5b'), 4.50 (1H, dd, J = 5.2, 10.8 Hz, H-3), 6.14 (1H, t-like, J = 6.4 Hz, AZT-H-1'), 7.29 (1H, d, J = 1.2 Hz, AZT-H-6), 9.19 (1H, br s, AZT-NH); 13C NMR (CDCl3, 100 MHz) δ 12.5 (AZT-CH3), 14.6 (C-27), 14.9 (C-29), 16.0 (C-26, −25), 16.6 (C-24), 18.2 (C-6), 20.8 (C-11), 21.6 (C-21), 22.9 (C-30), 23.8 (C-2), 26.8 (C-12), 26.9 (C-15), 27.9 (28-O-dimethylglutaryl CH3), 27.9, 28.0 (3-O-dimethylglutaryl CH3), 28.0 (C-23), 29.4 (C-20), 29.9 (C-16), 32.6 (28-O-dimethylglutaryl C-3"), 32.7 (3-O-dimethylglutaryl C-3'), 34.2 (C-7), 34.7 (C-22), 37.0 (C-10), 37.2 (C-13), 37.6 (AZT-C-2'), 37.7 (C-4), 38.4 (C-1), 40.9 (C-8), 42.9 (C-14), 44.6 (28-O-dimethylglutaryl C-4" and C-19), 45.1 (28-O-dimethylglutaryl C-2"), 45.2 (3-O -glutaryl C-4'), 45.7 (3-O-glutaryl C-2'), 46.4 (C-17), 48.1 (C-18), 50.0 (C-9), 55.4 (C-5), 60.4 (AZT-C-3'), 62.7 (C-28, and AZT-C-5'), 81.4 (C-3), 81.8 (AZT-C-4'), 85.3 (AZT-C-1'), 111.3 (AZT-C-5), 135.4 (AZT-C-6), 150.1 (AZT-C-2), 163.8 (AZT-C-4), 171.2 (28-O-dimethylglutaryl 5"-COO-AZT), 172.3 (28-O-dimethylglutaryl 1"-COO−, 3-O-dimethylglutaryl 1'-COO−), 175.6 (3-O-dimethylglutaryl 5'-COOH). HRESIMS (positive) m/z 1000.5991 [M+Na]+ (calcd for C54H83N5O11Na, 1000.5987).
HIV-1NL4-3 Inhibition Assay in MT-4 Lymphocytes
A previously described procedure was used in the experiments.21, 23
Supplementary Material
Acknowledgement
This investigation was supported by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (Y.K.) as well as a Grant AI-077417 from the National Institute of Allergy and Infectious Diseases (K.H.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Cichewicz RH, Kouzi SA. Med. Res. Rev. 2004;24:90. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- 2.Alakurtti S, Mäkelä T, Koskimies S, Yli-Kauhaluoma J. Eur. J. Pharm. Sci. 2006;29:1. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Yu D, Morris-Natschke SL, Lee KH. Med. Res. Rev. 2007;27:108. doi: 10.1002/med.20075. [DOI] [PubMed] [Google Scholar]
- 4.Gautam R, Jachak SM. Med. Res. Rev. 2009;29:767. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- 5.Mullauer FB, Kessler JH, Medema JP. Anticancer Drugs. 2010;21:215. doi: 10.1097/CAD.0b013e3283357c62. [DOI] [PubMed] [Google Scholar]
- 6.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. J. Med. Chem. 1996;39:1016. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 7.Martin DE, Blum R, Wilton J, Doto J, Galbraith H, Burgess GL, Smith PC, Ballow C. Antimicrob. Agents Chemother. 2007;51:3063. doi: 10.1128/AAC.01391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith PC, Ogundele A, Forrest A, Wilton J, Salzwedel K, Doto J, Allaway PA, Martin DE. Antimicrob. Agents Chemother. 2007;51:3574. doi: 10.1128/AAC.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. http://www.myriadpharma.com/product-pipeline/clinical/mpc-4326#4326-recent-presentations.
- 10.Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Marin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. Proc. Natl. Acad. Sci. USA. 2003;100:13555. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen IC, Shen JK, Wang HK, Cosentino LM, Lee KH. Bioorg. Med. Chem. Lett. 1998;8:1267. doi: 10.1016/s0960-894x(98)00202-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen IC, Wang HK, Kashiwada Y, Shen JK, Cosentino LM, Chen CH, Yang LM, Lee KH. J. Med. Chem. 1998;41:4648. doi: 10.1021/jm980391g. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwada Y, Chiyo J, Ikeshiro Y, Nagao T, Okabe H, Cosentino LM, Fowke K, Lee KH. Bioorg. Med. Chem. Lett. 2001;11:183. doi: 10.1016/s0960-894x(00)00635-1. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwada Y, Sekiya M, Ikeshiro Y, Fujioka T, Kilgore NR, Wild CT, Allaway GP, Lee KH. Bioorg. Med. Chem. Lett. 2004;14:5851. doi: 10.1016/j.bmcl.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Anti-HIV data for compounds 2, 4, 5, and 6 evaluated by the HIV-1IIIB infected H9 lymphocytes assay, 2: EC50 <0.00035 µM, TI >20,000, 4: EC50 2×10−5 µM, TI 1.12 ×106, 5: EC50 0.00066 µM, TI 21,515, 6: EC50 0.00087 µM, TI 42,400.
- 16.Zimmermann GR, Lehra J, Keith CT. Drug Discov. Today. 2007;12:34. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Jia J, Zhu F, Ma XH, Cao ZW, Li YX, Chen YZ. Nat. Rev. Drug Discov. 2009;8:111. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 18.Morphy R, Kay C, Rankovic Z. Drug Discov. Today. 2004;9:641. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 19.Mitsuya H, Weinhold KJ, Furman PA, St. Clair MH, Nusinoff Lehrman S, Gallo RC, Bolognesi D, Barry DW, Broder S. Proc. Natl. Acad. Sci. USA. 1985;82:7096. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto F, Kashiwada Y, Cosentino LM, Chen CH, Garrett PE, Lee KH. Bioorg. Med. Chem. 1997;5:2133. doi: 10.1016/s0968-0896(97)00158-2. [DOI] [PubMed] [Google Scholar]
- 21.Qian KD, Yu DL, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, Salzwedel K, Reddick M, Allaway GP, Lee KH. J. Med. Chem. 2009;52:3248. doi: 10.1021/jm900136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian KD, Kuo RY, Chen CH, Huang L, Morris-Natschke SL, Lee KH. J. Med. Chem. 2010;53:3133. doi: 10.1021/jm901782m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu DL, Sakurai Y, Chen CH, Chang FR, Huang L, Kashiwada Y, Lee KH. J. Med. Chem. 2006;49:5462. doi: 10.1021/jm0601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.