Abstract
Ocean acidification is a well recognised threat to marine ecosystems. High latitude regions are predicted to be particularly affected due to cold waters and naturally low carbonate saturation levels. This is of concern for organisms utilising calcium carbonate (CaCO3) to generate shells or skeletons. Studies of potential effects of future levels of pCO2 on high latitude calcifiers are at present limited, and there is little understanding of their potential to acclimate to these changes. We describe a laboratory experiment to compare physiological and metabolic responses of a key benthic bivalve, Laternula elliptica, at pCO2 levels of their natural environment (430 µatm, pH 7.99; based on field measurements) with those predicted for 2100 (735 µatm, pH 7.78) and glacial levels (187 µatm, pH 8.32). Adult L. elliptica basal metabolism (oxygen consumption rates) and heat shock protein HSP70 gene expression levels increased in response both to lowering and elevation of pH. Expression of chitin synthase (CHS), a key enzyme involved in synthesis of bivalve shells, was significantly up-regulated in individuals at pH 7.78, indicating L. elliptica were working harder to calcify in seawater undersaturated in aragonite (ΩAr = 0.71), the CaCO3 polymorph of which their shells are comprised. The different response variables were influenced by pH in differing ways, highlighting the importance of assessing a variety of factors to determine the likely impact of pH change. In combination, the results indicate a negative effect of ocean acidification on whole-organism functioning of L. elliptica over relatively short terms (weeks-months) that may be energetically difficult to maintain over longer time periods. Importantly, however, the observed changes in L. elliptica CHS gene expression provides evidence for biological control over the shell formation process, which may enable some degree of adaptation or acclimation to future ocean acidification scenarios.
Introduction
The ocean and the land are ‘sinks’ for the excess atmospheric CO2 produced by burning of fossil fuels, deforestation and land use changes. Twenty five to thirty percent of the total anthropogenic CO2 emissions produced since the industrial revolution have been absorbed by the oceans [1]. This excess CO2 dissolves in the surface ocean, causing increased hydrogen ion (H+) concentrations and decreased carbonate ion (CO3 2−) concentrations in seawater [2]. The result is a decline in ocean pH and calcium carbonate (CaCO3) saturation states. Through this process, known as ‘ocean acidification’, pH has already dropped by 0.1 pH units (an increase in H+ concentration of almost 30%) since the 1800s, and the rate of change is predicted to increase considerably, with a further decline of 0.2–0.5 pH units expected by 2100 [3], [4].
While the implication of ocean acidification to the chemistry of the open ocean is reasonably well understood, the ecological implications for marine fauna and flora are harder to predict [5], [6]. The taxonomic groups considered most susceptible to ocean acidification are calcifying organisms with CaCO3 skeletons and shells, such as corals, coralline algae, coccolithophores and molluscs. This is due to the predicted reduced availability of the CO3 2− ions they require for precipitation of CaCO3 [5], [7]. The degree of susceptibility of these calcifiers to ocean acidification is thought to be influenced by their mineralogy (but see [8], [9]). For example, skeletons made of the CaCO3 polymorph aragonite are generally more soluble than those comprised of calcite, but calcite skeletons containing high proportions of magnesium may be even more soluble than aragonite [10]. Organisms without CaCO3 skeletons or shells have also been shown to be affected through disruption of their acid-base balance and respiration (e.g., [11]–[13]). Experiments to date have shown that species’ responses are variable, due in part to differences in functional responses and environmental conditions (e.g., [14], [15]). Importantly too, not all species, nor all life stages of a particular species, have responded negatively to pCO2 conditions predicted for the future (e.g., [16], [17]).
Especially fast rates of change are expected in the Southern Ocean due to its cold water temperatures (and thus higher solubility of CaCO3, [18]) and CO3 2− saturation levels that already are lower than temperate regions. As early as 2030 during winter months, the Southern Ocean is predicted to become undersaturated in aragonite [19], [20]; a situation that has probably not occurred in at least the last 400,000 years [4]. Studies of potential effects of future levels of pCO2 on high latitude calcifying organisms are at present limited. Those conducted to date have shown reduced calcification and higher dissolution rates of shells of live pteropods [4], [21] and dissolution of bivalve, gastropod and brachiopod shells [9], and have documented a 30% decline in shell weights of foraminifera since the late 1800s [22]. In contrast, fertilisation, early embryogenesis and larval development of high Antarctic sea urchins were unaffected except at pH below 7.3 (i.e., pCO2 predicted for 2300; [23], [24]), and similar results were noted for fertilisation and early development of nemerteans [24].
Laternula elliptica is a large, infaunal, suspension-feeding bivalve with a circum-Antarctic distribution. It has a wide depth range (1–460 m), but is particularly common in shallow waters where it is routinely found at densities of 10's of individuals m−2 [25] and often much higher (>170 ind. m−2; [26], authors' unpubl. obs). In McMurdo Sound, Ross Sea, L. elliptica occurs in a variety of habitat types, ranging from homogeneous soft sediments to coarse pebbly sand deposits between rocks (authors' unpubl. obs). L. elliptica is a key species in Antarctic coastal benthic ecosystems through its influence on benthic-pelagic coupling [25] and has a shell comprised purely of aragonite [27]. In a laboratory study, [9] demonstrated significant dissolution of adult L. elliptica shells after 28 days exposure to pH 7.4. However, as their study was conducted on empty valves only, the biological response by the L. elliptica and their potential to compensate for this dissolution remains unknown.
Here we describe a laboratory experiment designed to assess the effect of a change in ocean pH on the functioning of adult Laternula elliptica from the Ross Sea, Antarctica. We compared L. elliptica's biological response at current Antarctic pH (7.99) to that predicted for the high Antarctic in the following decades (7.78; [4], [19]),and to a considerably elevated, ‘glacial’ pH (8.32). A suite of response variables, chosen to incorporate a range of molecular level (gene function, of stress and growth proteins) and whole organism responses (respiration and physiological condition) important in the functioning of this bivalve, were assessed. Specifically, we monitored effects on L. elliptica (i) shell, via measurement of chitin synthase (CHS) gene expression, a key enzyme involved in the shell formation process [28], and changes in shell weight; (ii) metabolism and growth, via measurement of oxygen consumption [29], physiological condition [30] and protein synthesis (i.e., total RNA, [31]); and (iii) stress, via measurement of heat shock proteins (HSP, specifically HSP70 gene expression, [32]). By examining this range of interlinked variables we were able to build a more comprehensive picture of the likely effect of ocean acidification on the functioning of this key Antarctic bivalve.
Methods
Laternula elliptica were collected from 20 m depth at Granite Harbour, Ross Sea, Antarctica by SCUBA divers on 15 November 2008. Their habitat consisted of loosely compacted coarse, sandy sediments, interspersed with pebbles and cobbles, and with very low CaCO3 content (<1%). Live L. elliptica (average 72.8 mm shell length (SL), range 54.2–87.9 mm) were transported to Wellington, New Zealand on 20 November 2008, where they were immediately transferred to a laboratory facility and held in running seawater. Seawater was sourced from the adjacent Wellington Harbour (WH) and was used in a single pass flow through system, filtered on a 0.1 µm filter and chilled to the experimental temperature of −1.76°C (± 0.0008 SE; range −1.93 to −1.60°C). WH seawater was of similar salinity to that at the Granite Harbour collection site (34.1 and 34.6, respectively).
pH/pCO2 manipulation
Three pH treatment levels were chosen for the experiment. These included an ‘Antarctic control’ (pH 7.99), a lowered pH (pH 7.78) which represents that predicted for 2100 by [4], and an elevated pH (pH 8.32) which last occurred in the Antarctic 20,000 years ago [33]. The Antarctic control pH treatment level was chosen based on measurements of water samples collected at the study site. The calculated pCO2 equivalents for the elevated, Antarctic control, and lowered pH treatments are 187, 430, and 735 µatm, respectively (see Table 1).
Table 1. Chemical characteristics of seawater from the experimental treatments, field measurements made at two McMurdo Sound locations, and relevant polar studies.
| ExperimentElevated pH pH 8.316±0.001 | ExperimentAntarctic control pH 7.993±0.002 | ExperimentLowered pH pH 7.775±0.002 | Field Granite Harbour pH 8.024 | Field New Harbour pH 7.996±0.007 | Cape Armitage pH 8.0 [23] | Arctic Fjord pH 8.12 [21] | Arctic Fjord pH 7.78 [21] | Artificial seawater pH 7.4 [9] | Artificial seawater pH 8.2 [9] | |
| pCO2 ppm (c) | 186.5+0.3 | 429.5+0.8 | 734.6+0.9 | 410 | 439.6±6.9 | nr | 320 | 765 | nr | nr |
| AT µmol kg−1 | 2253.2±3.9 | 2256.5±3.9 | 2258.3±2.8 | 2338.6 | 2329±2.8 | 2336 (c) | 2312 | 2295 | 2558 | 2678 |
| CT µmol kg−1 | 2043.7±3.7 (c) | 2169.4±3.9 (c) | 2235.0±2.8 (c) | 2238.2 | 2238.1±2.3 | nr | 2148 (c) | 2237 (c) | nr | nr |
| ΩAr (c) | 2.178±0.004 | 1.133±0.002 | 0.710±0.001 | 1.261 | 1.18±0.02 | 1.03 | 1.81 | 1.0 | 0.47 | 2.66 |
| [CO3 2−] µmol kg−1 (c) | 144.0±0.3 | 74.9±0.1 | 47.0±0.1 | 83.5 | 78.4±1.2 | nr | nr | nr | nr | nr |
| Temperature (°C) | −1.76±0.001 | −1.76±0.001 | −1.76±0.001 | −1.92 | −1.92 | −1.9 | 2.2 | 5.0 | 4.0 | 4.0 |
| Salinity (ppt) | 34.1 | 34.1 | 34.1 | 34.6 | 34.6 | 34.6 | 34.9 | 34.8 | 35 | 35 |
Values from this study are mean ± standard errors. AT = average total alkalinity, CT = total dissolved CO2, ΩAr = aragonite saturation index. (c) indicates calculated values, all other values are measured, nr = not reported. pH on the total hydrogen scale except for [23] ( = NBS scale) and [9] ( = not specified).
The pH of the chilled seawater was manipulated by bubbling food-grade 100% CO2 gas into a 60 litre tank of WH seawater to reduce the pH to 7.6 pH units. This low pH water was then mixed with WH seawater in additional 60 litre ‘header’ tanks to produce the Antarctic control and lowered pH treatments, respectively. The quantity of low pH water pumped to the header tanks was controlled via a Proportional Integrative Derivative feedback loop from industrial pH controllers (ATI Q 45P, Analytical Technology Inc. USA). The controllers measured the header tank pH through high quality glass pH electrodes (Hamilton, Switzerland) (Liq-Glass rated to -10°C to +100°C) and were temperature compensated using PT100 resistance thermometers (Servotech, New Zealand). The pH electrodes were calibrated regularly. The more accurate pH measurements derived from spectrophotometric readings (described below) were used to correct for any drift in the pH control system between calibrations. The elevated pH treatment was generated by chilling WH seawater to experimental temperature. Water from the header tanks was gravity-fed to replicate tanks (each 20 l) at a rate of 140 ml min−1. There were six replicates of each of the three experimental treatments.
Experimental setup
Laternula elliptica were individually numbered using permanent marker pen, their SL measured using electronic callipers, and 8 individuals were added to each replicate tank. Each L. elliptica was placed into tanks in a small mesh basket, which ensured they were held in a lifelike orientation (siphons uppermost). The tanks did not contain sediment.
Seawater pH in the replicate tanks was initially set at chilled WH levels (8.32). The pH was then gradually lowered to the target levels over the following 24 h, reaching these on 27 November 2008 (Day 0). L. elliptica were fed twice per week with a commercial algae mix (Shellfish diet, Reed Mariculture, US).
Chemical characteristics of seawater
Water samples for alkalinity (AT) were collected from the header tanks throughout the experiment, on December 4th and 18th 2008, and March 25th 2009 (n = 13, 14 and 13 for pH 8.32, 7.99 and 7.78, respectively), and preserved using HgCl2. AT was determined using a closed cell potentiometric titration method [34]. The accuracy of this method is estimated to be 1.5 µmol kg−1, based on the analyses of Certified Reference Material supplied by Andrew Dickson. pCO2, aragonite saturation (ΩAr), carbonate ion concentrations ([CO3 2−]) and dissolved inorganic carbon (CT) were calculated from the measured AT and pH at the average experimental water temperature and salinity using the refitted [35] equilibrium constants [36]. Salinity of WH seawater was measured daily as conductivity with Endress Hausser CLS30 - DIC4A probes.
For the first 38 days of the experiment (from 26/11/08 to 4/1/09), pH was measured manually twice daily using a pH electrode (WTW Multi 340i with sensorex glass probe). From 20/12/08 onwards, pH was measured spectrophotometrically using an automated system (see [37] for details) which sampled the header tanks hourly and the replicate tanks every 12 h. The dual measurements made during the 16 day overlapping time period allowed us to correct the electrode-based measurements using the more accurate spectrophotometric measurements, and thus attain a continuous record for the experiment. For spectrophotometric pH measurements, seawater from the tank being measured was drawn into a syringe pump (Kloehn model 55022, fitted with a 50 ml syringe), then mixed with an aliquot of thymol blue dye (mixing ratio 49:1). The dye-seawater mixture was pumped into a spectrometer (Ocean Optics USB4000) fitted with a 1 cm path length quartz cuvette. The spectral data were combined with reference data (no dye) to determine the absorbance at 435, 596 and 735 nm. The syringe pump and spectrophotometer assembly were located in a box which was temperature controlled at 4.2°C, and the temperature of the cuvette was recorded along with the absorbance data. The water sampling, pH measurement and data logging was automated, and controlled by a LabView programme. pH on the total hydrogen scale was calculated from the measured absorbances and the thymol blue dye parameters (pK2, e1, e2, and e3) at the cuvette temperature and a salinity of 34.1, using the algorithms of [38]. The pH at experimental water temperature was then calculated from the pH measured at 4.2°C using the AT and the [35] equilibrium constants as refitted by [36].
Laternula elliptica sampling
L. elliptica were sacrificed from each replicate tank on Days 0, 21 and 120 (27 November, 18 December 2008 and 28 March 2009, respectively) for determination of physiological and/or biochemical parameters. On Day 21, individuals were assessed for gene expression characteristics indicative of stress (i.e., heat shock protein, HSP70) and calcification activity (CHS). On Days 21 and 120, assessments of overall protein synthesis potential (i.e., total RNA content) were made. Also on Day 120, the individuals used for protein synthesis potential were first used to determine oxygen consumption rates as a proxy for energy use. On Days 0 and 120, physiological condition of the individuals was determined. Different individuals were assessed for physiological and biochemical parameters, respectively, on any one sampling date. Processing of L. elliptica for each of these measured is detailed below.
1. Molecular analyses
Mantle and adductor tissue were carefully dissected from each individual, snap frozen in liquid nitrogen and stored at −80°C prior to analysis for (a) HSP70 and CHS gene expression levels and (b) total RNA quantification, respectively, as described below.
A. Gene expression analysis
The messenger RNA (mRNA) levels in mantle tissue of L. elliptica target genes (CHS, HSP70) were measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR), a highly sensitive method which provides a measure of the relative expression levels of the target mRNA versus a control or reference gene (such as β-actin). Mantle tissue was chosen for analysis as: 1) this is the site of synthesis of calcification proteins, such as our target protein, CHS; and 2) highest expression of HSPs and constitutive expression of HSP70 occurs in this tissue in L. elliptica [39]. Expression analysis was achieved by first isolating total RNA, followed by the generation of cDNA, which was subsequently used in RT-qPCR. As the CHS gene sequence has not previously been determined from L. elliptica, this first necessitated PCR amplification of a partial CHS cDNA fragment using degenerate primers followed by cloning and sequencing prior to the development of a RT-qPCR assay for this gene (ii below).
Total RNA was extracted from 100 mg of L. elliptica mantle tissue using TRIzol reagent (Invitrogen Co, Grand Island, NY, USA) and resuspended in DEPC-treated water. The concentration of total RNA was determined by measuring ultraviolet absorbance at 260 nm. RNA purity was checked by determining the A260/A280 ratio, and RNA integrity was checked by agarose gel electrophoresis.
RNA samples were treated with Deoxyribonuclease I (Sigma-Aldrich) to remove any contaminating genomic DNA. Single-strand cDNA was reverse transcribed from 100 ng total RNA in a final volume of 10 µl using random primers and MMLV RT as per the SuperScript VILO cDNA synthesis kit (Invitrogen). Reactions were incubated at 25°C for 10 min, then at 42°C for 90 min, and terminated by heating at 85°C for 5 min. cDNA was stored at −20°C until required for cloning or PCR.
Degenerate primers for amplifying a circa 800 bp cDNA fragment of the L. elliptica CHS gene were designed on the basis of known molluscan CHS cDNA sequences (Table 2). PCR was performed using 10–100 ng of cDNA as a template in PCR buffer containing 3 mM MgCl2, 0.2 mM dNTPs, 0.4 µM of each primer and 1 unit of Platinum Taq Polymerase (Invitrogen) in a total volume of 10 µl. The thermal cycling parameters used were 95°C denaturation for 2 min, followed by 35 amplification cycles of 95°C for 30 sec, 55°C for 30 sec, 72°C for 2 min, and a final extension at 72°C for 10 min. The PCR products were gel-purified and sub-cloned into pCR4 TOPO TA vector (Invitrogen), and sequenced on an ABI Prism 3130 sequencer from both the 5′ and 3′ ends using the ABI PRISM dye terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, CA, USA). Complete clone insert sequences were identified using the blast or blastx search programs within the National Center for Biotechnology Information, in order to confirm their identity as CHS.
Table 2. Oligonucleotide primers used for cloning and qPCR of target genes from Laternula elliptica.
| Gene Target | Oligo | Primer DNA sequence (5′-3′) |
| Chitin Synthase | ||
| Cloning primer | CHS3F | TGyGCnACnATGTGGCAyGArAC |
| CHS3R | GCyTTyTGnArCCArTGnCC | |
| qPCR primer | LE qCHSF | TGTCCGCTCCTATCAAAACC |
| LE qCHSR | GGCCTTATCTCCTTCCTTGG | |
| HSP70 * | ||
| qPCR primer | HspF | AGATGAGGCTGTTGCATACG |
| HspR | GGTGACGTCAAGAAGAAGCA | |
| β-actin* | ||
| qPCR primer | ACTF | GGTCGTACCACAGGTATTGT |
| ACTR | CATCAGGTAGTCGGTCAAAT |
PCR amplifications of target (CHS, HSP70) and reference (β-actin) genes were performed in 25 µl reactions containing: cDNA generated from 200 pg of original RNA template (10 µl of 1∶500 diluted cDNA generated from 100 ng of original RNA template), 0.2 µM of each gene specific primer (GSP, Table 2), 10 nM fluorescein, 0.2 mM dNTPs, 3 mM MgCl2, 0.33x Sybr Green, 0.15% Triton X-100, 1 U of Platinum Taq polymerase (Invitrogen) and 1X PCR buffer (Invitrogen (20 mM Tris-HCl (pH 8.4), 50 mM KCl)). Note that primers have been previously developed for β-actin and HSP70 qRT-PCR [39]. Amplification was performed and monitored using the Icycler IQ real-time PCR detection system (Biorad, Hercules, CA, USA) with the following thermal cycle protocol: initial denaturation and enzyme activation at 95°C for 2 min, 40 amplification cycles of 94°C for 15 sec, 58°C for 15 sec and 72°C for 15 sec, followed by a melt curve analysis. Normalisation was performed by collecting dynamic well factors using the fluorescein background signal detected during the first few PCR cycles, in order to compensate for any system or pipetting non-uniformity and optimise fluorescent data quality and analysis.
Each template was analysed in triplicate. A four point standard curve for each primer pair being assayed, containing 10-fold dilutions of cDNA prepared from stock cDNA covering five orders of magnitude (1, 0.1, 0.001, 0.0001, and also encompassing the target nucleic acid interval), was included in triplicate on each 96-well PCR plate.
Data were collected as quantification cycle (Cq, formerly cycle threshold (Ct)) values using the iCycler iQ Optical System Software Version 3.1. The Cq values were normalised to sample quantities using the standard curve method for relative quantification. The primer concentrations (0.2 µM) were empirically determined based on lowest Cq values and highest efficiencies. The β-actin gene of L. elliptica was used as a reference gene. Data were normalised against the expression of β-actin (expressed as a ratio) to compensate for any differences in loading or reverse transcriptase efficiency. β-actin, a widely used reference gene in qPCR analysis, has previously been used for qPCR analyses in L. elliptica [40], and 1-way ANOVA demonstrated this gene did not vary across each time point in response to our pH treatments (data not shown).
B. Total RNA content
Total RNA on Days 21 and 120 was determined by pulverising freeze dried adductor tissue in a glass mortar and subsampling for RNA quantification. Total RNA was extracted using the TRIzol Reagent (Invitrogen # 15596-018; [41]), and quantified spectrophotometrically. Approximately 15 mg of tissue was used for the analyses and an additional ethanol wash was added to the manufacturer's protocol to ensure samples of satisfactory purity. Total RNA was quantified by reading the absorption against 0.5% SDS at 260 and 280 nm with a spectrophotometer using quartz cells (1.4 ml volume). In addition, absorption spectra were obtained (200–320 nm) as an indicator of sample purity. To standardise RNA content between individuals of different sizes, we normalised results to the average SL of the L. elliptica used in the experiment, 73 mm, using the following formula:
Total RNA ind Aadj = [RNA ind A (µg mg−1)/SL ind A (mm)]×73
Results are expressed as µg RNA mg−1 tissue dry weight.
2. Oxygen consumption
Respiration rates of L. elliptica were determined on Day 120. Individuals were placed in closed 600 ml chambers in their equivalent treatment water, with continuous, gentle propeller-driven mixing, after first brushing and rinsing to remove microflora. Chambers were placed in a water bath and temperatures maintained at −1.4°C (range = −1.3 to −1.6°C). After 50 min the chambers were sealed and oxygen consumption measurements were made over 184 min. Fifty min was considered to be an appropriate settling period to eliminate any effects of handling stress, based on trial experiments that we had conducted and other studies (e.g., [42], [43]). Measurements were made using hand held probes (YSI55, YSI550A and WTW Multi 340i with CellOx probe), which were first calibrated using fully saturated (100%) water and then sulfite (H2S) water (0%). Dissolved oxygen saturation in each chamber was not allowed to fall below 70%. O2 consumption was recorded every 2 min as % saturation and every 5 min as mg l−1. These values were later adjusted for the volume of the animal and for consumption rates recorded in chambers without animals.
To convert O2 consumption values to per unit ash free dry weight (AFDW; methods provided below), we derived the following relationship between L. elliptica AFDW and wet tissue weight:
AFDW = 0.37014 wet weight0.55134 ; R2 = 0.63
As there were no differences in this relationship between animals from different treatments (standard errors of all parameter estimates overlap for all treatments) we considered this to be a good estimator of AFDW for the O2 consumption animals.
3. Physiological condition
On Days 0 and 120, L. elliptica from each experimental replicate were dissected to remove flesh, the flesh wet weight determined, and the flesh and shell oven dried (60°C) and air dried, respectively, to constant weight (hereafter ‘FW’ and ‘SW’, respectively). The dry flesh was then ashed in a muffle furnace at 470°C for 24 h to determine AFDW (AFDW = FW- ashed weight). Two physiological condition indices were calculated: the ratio of FW to SL (CIFW:SL), and the ratio of SW to SL (CISW:SL). We also examined the change in each condition index over the 120 day experiment (Day 120 – Day 0).
Statistical analyses
A 2-way ANOVA with Day as a random factor and Treatment as a fixed factor was initially conducted to confirm that SL of the animals did not vary between treatments.
Where simple monotonic relationships between each response variable and pH were indicated, the significance of the relationship was tested by correlation analysis (either Pearson's or Spearman's) due to the well known insensitivity of ANOVA to monotonic gradients in responses [44]–[46]. For response variables exhibiting non-monotonic relationships, 1-way ANOVAs were used to test for differences between treatments, after determining whether normality and homogeneity of variances assumptions were met. When this was not the case and transformations did not help, a Kruskal-Wallis test was used.
Antarctic field conditions
Background information on the water chemistry in McMurdo Sound was determined from day time water samples and longer term instrument deployments. On 10 November 2008 water samples were collected for CT and AT analyses from 3 m below the surface at the Granite Harbour collection site and later analysed to determine the Antarctic control treatment pH level. Near bottom (18 m deep) water samples for CT and AT analyses were collected from New Harbour on seven occasions in the following year (6th and 8–14 November 2009). All samples were immediately preserved with HgCl2. AT was analysed as described above, and CT was determined using coulometric measurement of the CO2 evolved from an acidified sample [34]. In situ pH, pCO2, ΩAr and [CO3 2−] were calculated from the measured values using the refitted [35] equilibrium constants [36]. Information on temperature and salinity required for these calculations was obtained at both sites from Seabird Electronics SBE-37 microcat deployments.
Results
Experimental conditions
The chemical characteristics of our experimental treatments and Antarctic field sites are given in Table 1. Also provided for comparison are characteristics of water reported in other relevant polar studies from the literature. These values show good agreement with our experimental conditions. Of interest are the high pCO2 levels of our McMurdo Sound water samples (410 and 440 µatm at Granite Harbour and New Harbour, respectively).
Laternula elliptica response
There was no mortality over the 120 day experiment, and L. elliptica appeared to behave normally, extending siphons and feeding. There was also no measurable increase in SL of these adult L. elliptica over the experiment. Sizes of individuals did not differ significantly between treatments (p>0.05).
1. Molecular analyses
A. HSP70, heat shock protein gene expression
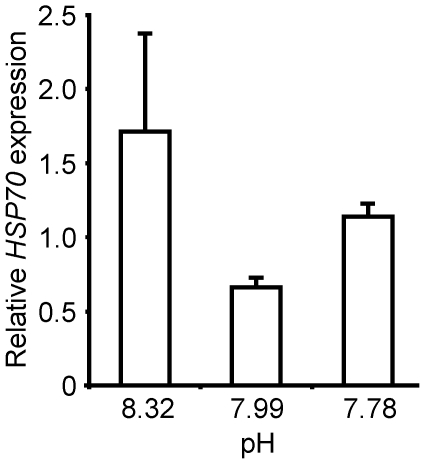
L. elliptica mRNA expression levels of HSP70 showed a curvilinear response to seawater pH, with lowest levels in the Antarctic control treatment after 21 days. There was considerably higher variability in HSP70 levels of individuals from the elevated pH treatment than in those of the other pH treatments (Figure 1). There was no significant correlation between pH and HSP70 gene expression relative to reference gene β-actin expression. However, HSP70 expression showed some indication of differences between treatments (Kruskal-Wallis Chi-Square = 5.3450, p = 0.0691), with the Antarctic control differing from both the elevated and lowered pH treatments (unadjusted p-values were 0.0451 and 0.0039, respectively).
Figure 1. mRNA expression of HSP70 in Laternula elliptica mantle tissue after 21 days at experimental pH.
Levels shown are means (+ SE) relative to β-actin (expressed as a ratio, fold induction).
B. CHS cloning, sequence determination and gene expression
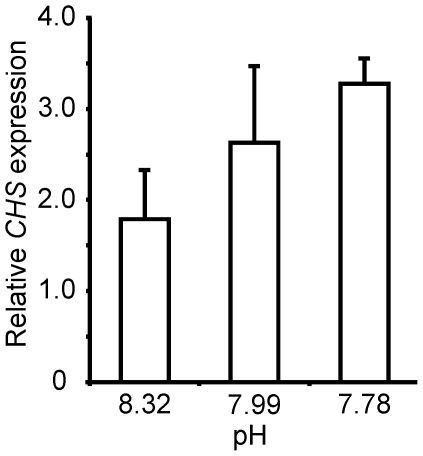
There was a significant negative correlation between mRNA expression levels of CHS relative to β-actin reference gene expression in L. elliptica and pH (log10 transformed) on Day 21 of the experiment (Pearson's R −0.61, p = 0.0060, n = 18), with an increase in CHS with decreasing pH (Figure 2).
Figure 2. mRNA expression of CHS in Laternula elliptica mantle tissue after 21 days at experimental pH.
Levels shown are means (+ SE) relative to β-actin (expressed as a ratio, fold induction).
The L. elliptica CHS partial cDNA sequence of 953 nucleotides (Genbank accession number HQ186262) is provided in Figure S1 overlaid with the deduced protein sequence of 317 amino acids. Highly conserved motif regions 1–4 found in many family 2 glycosyltransferases (GTF2) enzymes, including CHS, are indicated. BLAST analysis showed that the L. elliptica CHS nucleotide sequence gene shares homology with other known CHS genes, indicating that the cloned gene encodes CHS protein (Figure S2). A multiple sequence alignment of the deduced protein sequence of L. elliptica CHS with chitin synthases of other known bivalves is provided in Figure S2. L. elliptica CHS shows highest homology in its sequence to Atrina rigida (Japanese pearl oyster) and Pinctada fucata (stiff penshell oyster), with 72% identity at the nucleotide level and 82% and 80% identity over the deduced protein sequences, respectively (Table S1).
C. Total RNA
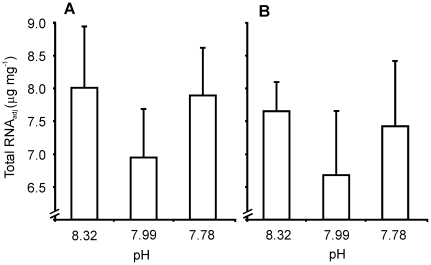
Total RNA levels were lowest in L. elliptica from the Antarctic control pH treatment after both 21 and 120 days. Levels in the elevated and lowered pH treatments were higher, and similar to each other (Figure 3). This curvilinear response, similar to that noted for HSP70, was observed on each Day, although neither was statistically significant (Day 21: F = 0.40, p = 0.6815; Day 120: F = 0.37, p = 0.6975).
Figure 3. Laternula elliptica adductor tissue Total RNAadj after A. 21 and B. 120 days at experimental pH.
2. Oxygen consumption
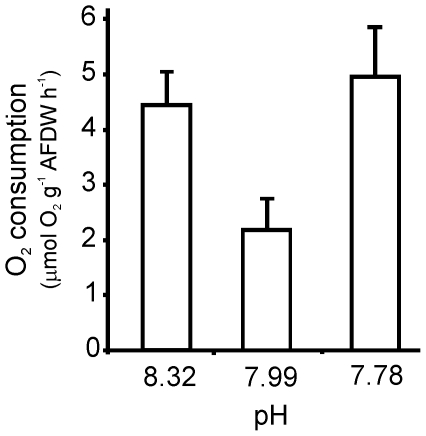
O2 consumption rates were significantly higher in individuals from the elevated and lowered pH treatments compared with those from the Antarctic control at the end of the experiment (F = 5.53, p = 0.0159; Figure 4). There was no significant relationship between O2 consumption and L. elliptica SL (F = 1.17, p = 0.295, R2 = 0.067).
Figure 4. O2 consumption (µmol O2 g−1 AFDW h−1) of Laternula elliptica after 120 days at experimental pH.
3. Physiological condition
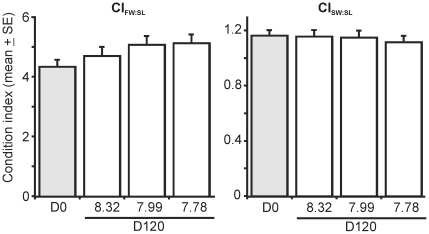
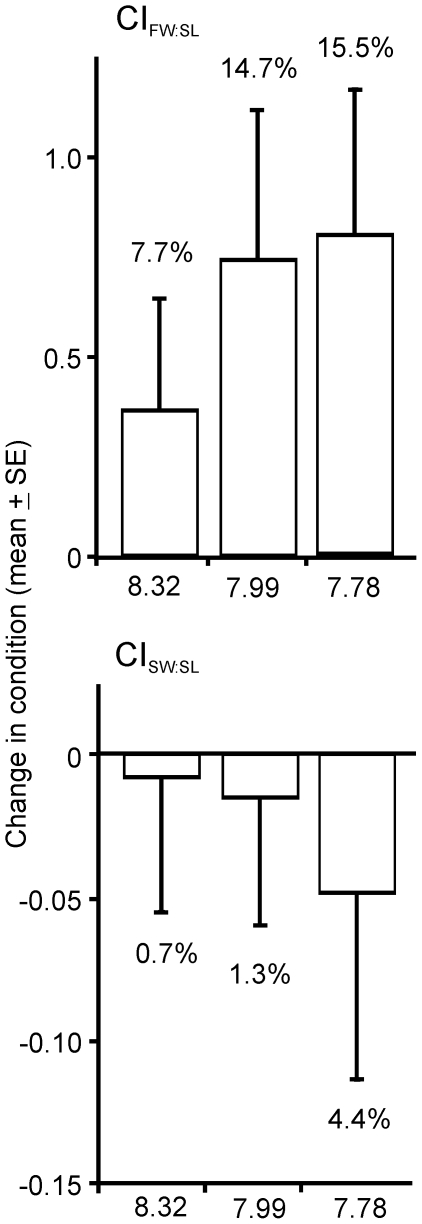
Physiological condition of L. elliptica as measured by CIFW:SL on Day 120 did not differ between treatments, but overall condition had increased relative to Day 0 in all treatments, indicating the experimental conditions were favourable for maintenance and survival of the L. elliptica (Figure 5; 2-way ANOVA, Treatment: P = 0.3245; Day: P = 0.0443; Treatment*Day: P = 0.9434). While no significant difference between treatments on Day 120 was detected, the magnitude of average change in condition between the beginning and end of the experiment did not appear to be random (Figure 6, Pearson's R = −0.48, p = 0.0450, n = 18). The average increase in CIFW:SL over the experiment was lowest at elevated pH (7.7%; Figure 6).
Figure 5. Physiological condition of Laternula elliptica in each experimental treatment on Day 120.
Condition of individuals on Day 0 is also shown.
Figure 6. The change in each physiological condition index between Days 0 and 120.
The % change that this represents is also given.
The CISW:SL index on Day 120 was not significantly different between treatments, nor was there any significant difference between Days 0 and 120 (Figure 5; Treatment: p = 0.9956; Day: p = 0.6332; Treatment*Day: p = 0.7730). Although Figure 6 suggests the magnitude of average change in CISW:SL between the beginning and end of the experiment may not be random, no significant correlation with pH was observed due to the high within-treatment variation, mainly due to the presence of a single high value in both the Antarctic control and low pH treatments (Figure 6, Pearson's R 0.29, p = 0.2655, n = 18). Preliminary examination of the outer surfaces of the L. elliptica shells did not reveal any obvious signs of degradation.
As there was no increase in shell length of the slow growing adult L. elliptica over the experiment, any change in CIFW:SL or CISW:SL can be attributed to a change in flesh weight or shell weight, respectively.
Discussion
Functioning of adult Laternula elliptica was affected by pH levels predicted for the Southern Ocean in coming decades (pH 7.78) after as little as 21 days exposure. Although no mortality occurred, L. elliptica in this lowered pH treatment were more stressed (Figure 1) and exhibited significantly higher basal metabolic rates (Figure 4) than L. elliptica from the Antarctic control (pH 7.99). Interestingly, this response was also noted for L. elliptica from the elevated pH treatment (pH 8.32), thus demonstrating a negative response by this bivalve to a change in pH in general. Most importantly, we noted a differential response of L. elliptica to lowered compared to elevated pH for CHS gene activity (as indicated by mRNA transcript abundance, Figure 2). CHS expression increased with decreasing pH, indicating an effect on the shell formation process of L. elliptica which appears specific to a decrease in pH. Total RNA content and HSP70 gene expression level did not show a statistically significant response to our experimental treatments, although the pattern of both responses mirrored that of the basal metabolic rates (cf. Figures 1, 3 and 4). The HSP70 response was close to being significant, with clear differences between individuals from the Antarctic control and both the elevated and lowered pH (Figure 1). The different response variables measured were influenced by pH in differing ways, indicating the importance of assessing a variety of different factors to determine the likely effect of pH change on organism functioning. While these effects on L. elliptica did not translate to statistically significant differences in either of the physiological condition indices we measured over the 120 day experiment (Figure 5), we suggest that the sustained cost of increased stress, metabolic and calcification rates may well affect L. elliptica condition (and thus growth and reproduction) in the longer term [47].
Metabolism and growth
Reduced metabolism is a recognised strategy in Antarctic invertebrate fauna to minimise energy expended during routine ‘maintenance’ and thus have more energy available to invest in reproduction and growth [48]. In this experiment we have used oxygen consumption rates as a proxy for basal metabolism (e.g., [29], [49]). In less than optimal environmental conditions, we may expect to see basal metabolic rates increase as the organism works harder to maintain the status quo, and this may in turn result in changes in overall physiological condition. In our experiment oxygen consumption rates of L. elliptica in the Antarctic control were 2.2 µmol g−1 AFDW h−1, 2.3 and 2.1 times lower than in the lowered and elevated pH treatments, respectively. These results illustrate that while L. elliptica can function at pH's different from those they currently experience in Antarctica it is energetically more stressful for them to do so, indicating they are optimally adapted to their present environment. Metabolic rates (also measured as O2 consumption) in the bivalve Yoldia eightsi increased under higher temperatures [50], and a similar pattern has been noted for a range of other Antarctic marine invertebrates [51], [52]. Seasonal increases of 3 and 3.7 times between winter and summer have been reported for Antarctic Peninsula L. elliptica [53], [54], a likely response to changes in temperature and food availability. As no field measurements of oxygen consumption by McMurdo Sound (or indeed, Ross Sea) L. elliptica have been made, we are unable to comment on the magnitude of the differences between our experimental treatments relative to seasonal or spatial differences in Ross Sea L. elliptica. However, we do note that the O2 consumption rates in our Antarctic control animals were considerably lower than those recorded for L. elliptica from the Antarctic Peninsula region. [55] measured average rates of 73.3 and 49.6 µmol O2 h−1 10.6 g−1 AFDW for L. elliptica from Rothera and Signy Island, respectively, at 0.0°C. When converted to enable direct comparison to our measurements, these rates are considerably higher than the average 2.2 µmol h−1 g−1 AFDW noted in the −1.8°C Antarctic control treatment of this experiment (i.e., Rothera 6.9, Signy 4.7 µmol h−1 g−1 AFDW), perhaps reflecting the influence of temperature on respiration. Furthermore, these differences in L. elliptica basal metabolism between Antarctic locations indicate that the functional response of this bivalve to ocean acidification may vary depending on their Antarctic environment.
Metabolic differences can also be reflected in short term growth rates as measured by total RNA (protein synthesis potential; [56]). Total RNA levels provide a measure of proteins synthesised during tissue growth as well as during physiological stress responses, and were relatively high in L. elliptica from the elevated and lowered pH treatments compared with the Antarctic control (Figure 3). Effects on total RNA levels were variable and, consequently, were not statistically significant on either Day 21 or Day 120 (Figure 3). We expect that they may have become significant given a longer experimental duration. Adductor muscle total RNA levels in our experiment were within the range of those found in L. elliptica collected at three other McMurdo Sound locations (i.e., Dunlop Island: 8.20±1.17 µg mg−1, n = 6; Spike Cape: 4.41±0.22 µg mg−1, n = 10; Cape Evans: 3.96±0.31 µg mg−1, n = 6; Joanna Norkko, unpublished data).
Stress
An increase in protein synthesis (including production of heat shock proteins, HSPs) has also been noted in L. elliptica in response to another stressor, temperature [39], [40], [57]. HSPs in the normal cell state assist in the folding of native polypeptides, and their induction under stress conditions prevents production of cytotoxins and stabilises denatured proteins (e.g., [32], [58], [59]). HSPs can be produced in response to a large variety of environmental stressors (e.g., freshwater input in the intertidal [60]; osmotic stress [61]; presence of oxygen radicals and toxicants [62]), and we may expect a similar response to pH change. We have demonstrated a substantial up-regulation (increased production) of HSP70 gene transcript levels in L. elliptica mantle tissue in response to pH levels that are both lowered and elevated relative to existing Antarctic conditions (Figure 1). This up-regulation is not unexpected as either pH directionality is likely to register as a stress to L. elliptica, stimulating induction of HSPs. HSP mRNA levels typically rapidly rise within hours following introduction to a stressor and generally reach their maxima following three hours of post-stress recovery [63], [64]. Our analysis shows that at 21 days following the initiation of the stress exposure (high or low pH), HSP70 levels in these bivalves remain elevated relative to individuals from the Antarctic control. The magnitude of the HSP70 up-regulation we observed is in line with experimental observations of the heat shock response (HSR [64]) in L. elliptica in response to the more classically studied stressor, temperature. L. elliptica constitutively express HSP70 [39], a common phenomenon for Antarctic species, and further sustained up-regulation in response to pH as a stressor may in fact lead to deleterious effects on other cellular and organismal processes (e.g., [65]). There is an energetic cost associated with induction of the HSR and the HSR system in L. elliptica is likely to have evolved under strong trade-off constraints [59]. Thus, in this experiment, the increased respiration rates we observed at pHs either elevated or decreased relative to the Antarctic control may relate in part to the extra energy required to mount and maintain the HSR over an extended period of time.
Shell (mineralogy and dissolution)
As L. elliptica shell is comprised purely of aragonite, one of the most soluble forms of CaCO3, it may be considered particularly susceptible to ocean acidification (e.g., [5], [66]). Aragonite was undersaturated in our lowered pH treatment (pH 7.78, ΩAr = 0.71) and the % change (loss) in CISW:SL after 120 days was the highest of all treatments (4.4%; Figure 6B, or 0.037% per day), although this correlation was not statistically significant. [9] reported a 2.767±0.607% loss in shell weight for L. elliptica shells held at 7.4 pH (ΩAr = 0.47) for 63 days, the equivalent of 0.044% per day. While a greater dissolution rate may be expected in the more undersaturated ΩAr conditions of the [9] experiment, we would urge caution in direct comparison of daily shell weight loss between experiments given that [9] studied empty shells and that dissolution may not be linear with time.
After just 21 days we found that lowering pH resulted in a significant increase in expression (up-regulation) of the CHS gene, which codes for a key enzyme involved in synthesis of bivalve shells [28], [67], thus indicating the animals were working harder to calcify. In contrast, elevated pH resulted in decreased CHS gene expression (down-regulation). Chitin is a major component of the bivalve shell [28], and forms the organic ‘framework’ within which CaCO3 minerals are subsequently deposited [67]. The enzymes involved in chitin synthesis, and chitin synthase in particular, are important not only in providing mechanical strength, but also in coordinating the shell formation and mineralisation process [67]. Inhibition of chitin synthesis has been clearly demonstrated to negatively affect survival and increase abnormal shell development rates in early Mytilus galloprovincialis larvae [67], [68]. In addition, larval shells formed under chitin synthesis-inhibited conditions were shown to be more soluble in distilled water [67]. Although CHS gene expression is not a direct measure of calcification, given that it encodes for an enzyme that is important in the shell mineralisation processes, effects on CHS gene expression could potentially affect the basic structure as well as the mineralisation, solubility and thus integrity of the shell. This is the first study examining changes in expression of CHS in response to ocean acidification (or other stressors) in any mollusc. The up- and down-regulation of CHS gene expression observed in our lowered and elevated pH treatments, respectively, is an important finding as it provides evidence for biological control over this process in response to changing environmental conditions, which may afford the organism a mechanism for some degree of acclimation or adaptation to future ocean acidification scenarios.
Experimental vs Antarctic conditions
The conditions of our laboratory experiment were as close as possible to those of the Antarctic situation. Our pH and ΩAr measurements from Granite Harbour and New Harbour, and the pH reported for Cape Armitage by [23] show high agreement with the experimental values of our Antarctic control treatment (Table 1). The AT of the seawater used in our experiment was naturally lower than that measured in the McMurdo Sound environment (by approximately 80 µmol kg−1; Table 1), although it was similar to that reported by [10] for cold, high latitude waters (2271±28.3). At -1.76°C, our experimental water temperatures were only slightly warmer than ambient spring water temperatures recorded for several locations in McMurdo Sound (−1.92°C; [69], [70]), and were very similar to those recorded in Terra Nova Bay where L. elliptica are also common (−1.8°C, authors' unpublished data). Salinities were also similar to those measured in McMurdo Sound in this study (Table 1), and by [70].
The experimental conditions were generally favourable for the maintenance and survival of L. elliptica, as shown by the increase in physiological condition CIFW:SL over the 120 day experiment in all treatments (Figure 5). Because there was no measurable increase in SL of the adult L. elliptica over the experiment, this change was purely due to an increase in flesh weight. There was no significant difference in absolute CIFW:SL between treatments on Day 120 (Figure 5), although the magnitude of this gain in condition between Day 0 and Day 120 increased significantly with decreasing pH (Figure 6). However, the significance of this was driven by the lower gain in condition of individuals in the elevated pH treatment, with little real difference between individuals from the lowered and Antarctic control pH treatments (Figure 6). For logistical reasons, L. elliptica were not held in sediments during these experiments, thus these experiments have not allowed for any buffering of the response to changing pH by burial. However, as pH within the sediment column is generally lower than the water column above [71], there will still be direct contact between the overlying seawater and the infaunal organism during feeding and respiration, and the CaCO3 content of the Granite Harbour sediments was very low, we do not anticipate that our results have overestimated the magnitude of the L. elliptica response.
In ocean acidification experiments, organisms are subjected to often-large pH changes which, of necessity, occur at a much faster rate than that predicted for their natural environments (i.e., years-decades). This has led to concern that these are in fact ‘shock-response’ experiments. It is also worth pointing out that in coastal and estuarine environments [72] and areas of oceanic upwelling [73], pH may change markedly over time scales more akin to those of experiments (i.e., hours to days). Of note are the high pCO2 levels of our McMurdo Sound water samples (410 and 440 µatm at Granite Harbour and New Harbour, respectively). The distance between these two coastal locations (ca. 90 km) and the fact that they were sampled in different years, indicates that the pCO2 levels of these high latitude coastal sites is already high in spring/early summer. Data are urgently needed to determine spatial and temporal variation in pCO2 and pH in Antarctic coastal regions, to put results of experiments into context of natural environmental conditions.
Concluding comments
L. elliptica are a long lived species: their life span is estimated at 36 years [74], and they take about 20 years to reach 100 mm SL [26], [75]. The duration of this experiment is just a very small portion of their life span, and the effect on functioning of L. elliptica at a lowered pH of 7.78 is of concern given the predicted changes in Southern Ocean pH and aragonite saturation for the coming decades [19]. While many studies have focussed their investigations on juveniles due to the increased susceptibility of early life stages to environmental perturbations (e.g., [76]), examining effects on adults is also important, particularly for long lived Antarctic species which will experience this change in ocean chemistry within their generation.
We have shown significant effects on some crucial functions of L. elliptica at a pH only 0.2 units below current levels. Importantly, the observed changes in L. elliptica CHS gene expression provides evidence for biological control over the shell formation process, which may provide a mechanism for adaptation or acclimation to future changes in seawater carbonate conditions. We anticipate, however, that the energetic costs of maintaining these responses may have more serious implications for the condition of this key Antarctic bivalve in the longer term. In addition, increases in temperature predicted for Antarctic waters over the next 100 years, and associated changes in food supply (e.g., [77]), are likely to modify any effect of pH reductions alone on the behaviour and functioning of key benthic invertebrates. Future investigations should study synergistic effects, and should incorporate a range of response variables to build a more comprehensive picture of the likely ecological impacts of impending environmental change.
Supporting Information
Designation of CHS gene family member status.
(DOC)
Nucleotide and deduced amino acid sequences of Laternula elliptica chitin synthase (CHS) Numbered boxes refer to highly conserved regions found in many family 2 glycosyltransferases (GTF2) enzymes, including CHS. Regions 1 and 2, UDP-binding; region 1 is similar to the Walker A/P-loop motif and to the R-β-GKR consensus sequence of GTF2; region 2 is similar to the Walker B motif and to the K-β-DDGS consensus sequence of GTF2. Regions 3 and 4, donor saccharide-binding; region 3 is similar to the DXD motif; region 4 is similar to the G(X)4(Y/F)R consensus sequence important for enzyme processivity. Positions of the primers used in RT‐qPCR are highlighted on the cDNA sequence.
(DOC)
Multiple alignment of deduced amino acid sequences of Laternula elliptica CHS with chitin synthases of other bivalves. The CHS abbreviations, species and the Genbank accession numbers are as follows: LECHS, Laternula elliptica, HQ186262; PFCHS, Pinctada fucata, AB290881; ARCHS, Atrina rigida, DQ081727; MGCHS, Mytilus galloprovincialis, EF535882. Bivalve sequences are numbered according to their complete Genbank entry whilst the amino acid sequence deduced from the LECHS mRNA fragment is numbered 1‐318.
(DOC)
Acknowledgments
We thank Malcolm Reid (University of Otago), and Jeremy Bulleid and Claire Coppard for advice on and construction of the spectrophotometer, respectively, Sarah Allen for her work in establishing the containment facility, and the K082 dive team (all NIWA) for Laternula elliptica collection. Antarctica New Zealand are thanked for their excellent logistical support on the ice, and we are particularly grateful to Paul Woodgate for his efforts in ensuring the safe importation of L. elliptica to NZ. This manuscript was improved by the helpful comments of two anonymous reviewers.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was funded by the New Zealand Foundation for Research, Science and Technology International Polar Year project COX01707, and the National Institute of Water and Atmospheric Research Capital Expenditure fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sabine CL, Feely RA, Gruber N, Key RM, Lee K, et al. The oceanic sink for anthropogenic CO2. Science. 2004;305:367–71. doi: 10.1126/science.1097403. [DOI] [PubMed] [Google Scholar]
- 2.Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, et al. Ocean acidification due to increasing atmospheric carbon dioxide. 2005. 57 Policy document 12/05. The Royal Society, London.
- 3.Caldeira K, Wickett ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res. 2005;110:C09S04. [Google Scholar]
- 4.Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 5.Fabry VJ, Seibel BA, Feely, RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci. 2008;65:414–432. [Google Scholar]
- 6.Widdicombe S, Spicer JI. Predicting the impact of ocean acidification on benthic biodiversity: what can physiology tell us? J Exp Mar Biol Ecol. 2008;366:187–197. [Google Scholar]
- 7.Kleypas JA, Feely RA, Fabry VJ, Langdon C, Sabine CL, et al. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research. 2006. 88 Report of a workshop held 18–20 April 2005, St. Petersburg, FL, sponsored by NSF, NOAA, and the U.S. Geological Survey.
- 8.Harper EM. Are calcitic layers an effective adaptation against shell dissolution in the Bivalvia? J Zool Lond. 2000;251:179–186. [Google Scholar]
- 9.McClintock JB, Robert A, Angus RA, Mcdonald MR, Amsler CD, et al. Rapid dissolution of shells of weakly calcified Antarctic benthic macroorganisms indicates high vulnerability to ocean acidification. Antarct Sci. 2009;21:449–456. [Google Scholar]
- 10.Andersson AJ, Mackenzie FT, Bates NR. Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar Ecol Prog Ser. 2008;373:265–273. [Google Scholar]
- 11.Langenbuch M, Pörtner HO. Energy budget of hepatocytes from Antarctic fish (Pachycara brachycephalum and Lepidonotothen kempi) as a function of ambient CO2: pH-dependent limitations of cellular protein biosynthesis? J Exp Biol. 2003;206:3895–3903. doi: 10.1242/jeb.00620. [DOI] [PubMed] [Google Scholar]
- 12.Ishimatsu A, Kikkawa T, Hayashi M, Lee KS, Kita J. Effects of CO2 on marine fish: larvae and adults. J Oceanogr. 2004;60:731–741. [Google Scholar]
- 13.Munday PL, Donelson JM, Dixson DL, Endo GGK. Effects of ocean acidification on the early life history of a tropical marine fish. Proc R Soc B. 2009;276:3275–3283. doi: 10.1098/rspb.2009.0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Findlay HS, Wood HL, Kendall MA, Spicer JI, Twitchett RJ, et al. Calcification, a physiological process to be considered in the context of the whole organism. Biogeosciences Discuss. 2009;6:2267–2284. [Google Scholar]
- 15.Miller AW, Reynolds AC, Sobrino C, Riedel GF. Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PLoS ONE. 2009;4e566 doi: 10.1371/journal.pone.0005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havenhand J, Schlegel P. Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. . Biogeosciences. 2009;6:3009–3015. [Google Scholar]
- 17.Ries J, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37:1131–1134. [Google Scholar]
- 18.Revelle RR, Fairbridge, RW Carbonates and carbon dioxide. In: Treatise on Marine Ecology and Paleoecology. Geol Soc Am Mem. 1957;67:239–296. [Google Scholar]
- 19.McNeil BI, Matear RJ. Southern Ocean acidification: a tipping point at 450-ppm atmospheric CO2. PNAS. 2008;105:18860–18864. doi: 10.1073/pnas.0806318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenton A, Codron F, Bopp L, Metzl N, Cadule P, et al. Stratospheric ozone depletion reduces ocean carbon uptake and enhances ocean acidification. Geophys Res Lett. 2009;36, L12606 doi: 10.1029/2009GL038227. [Google Scholar]
- 21.Comeau S, Gorsky G, Jeffree R, Teyssie J-L, Gattuso J-P. Impact of ocean acidification on a key Arctic pelagic mollusc (Limacina helicina). Biogeosciences. 2009;6:1877–1882. [Google Scholar]
- 22.Moy AD, Howard WR, Bray SG, Trull TW. Reduced calcification in modern Southern Ocean planktonic foraminifera. Nat Geosci Lett. 2009 DOI: 10.1038/NGEO460. [Google Scholar]
- 23.Clark D, Lamare M, Barker M. Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among a tropical, temperate, and a polar species. Mar Biol. 2008;156:1125–1137. [Google Scholar]
- 24.Ericson J, Lamare MD, Morely SA, Barker MF. The response of two ecologically important Antarctic invertebrates (Sterechinus neumayeri and Parborlasia corrugatus) to reduced seawater pH: effects on fertilisation and embryonic development. Mar Biol. 2010 DOI 10.1007/s00227-010-1529-y. [Google Scholar]
- 25.Ahn IY. Enhanced particle flux through the biodeposition by the Antarctic suspension-feeding bivalve Laternula elliptica in Marian Cove, King George Island. J Exp Mar Biol Ecol. 1993;171:75–90. [Google Scholar]
- 26.Ahn IY, Chung H, Choi KS. Some ecological and physiological features of the Antarctic clam, Laternula elliptica (King and Broderip) in a nearshore habitat on King George Island. Ocean Polar Res. 2001;23:419–424. [Google Scholar]
- 27.Harper EM, Dreyer H, Steiner G. Reconstructing the Anomalodesmata (Mollusca: Bivalvia): morphology and molecules. Zool J Linn Soc-Lond. 2006;148:395–420. [Google Scholar]
- 28.Weiss IM, Schönitzer V, Eichner N, Manfred S. The chitin synthase involved in marine bivalve mollusk shell formation contains a myosin domain. FEBS Lett. 2006;580:1846–1852. doi: 10.1016/j.febslet.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 29.Pörtner HO, Hardewig I, Peck LS. Mitochondrial function and critical temperature in the Antarctic bivalve, Laternula elliptica. Comp Biochem Physiol. 1999;124A:179–189. [Google Scholar]
- 30.Roper DS, Pridmore RD, Cummings VJ, Hewitt JE. Pollution related differences in the condition cycles of Pacific oysters Crassostrea gigas from Manukau Harbour, New Zealand. Mar Environ Res. 1991;31:197–214. [Google Scholar]
- 31.Norkko J, Norkko A, Thrush SF, Cummings VJ. Detecting growth under environmental extremes: spatial and temporal patterns in nucleic acid ratios in two Antarctic bivalves. J Exp Mar Biol Ecol. 2005;326:144–156. [Google Scholar]
- 32.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 33.Petit JR, Jouzel J, Raynaud D, Barkov NI, Barnola J-M, et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399:429–436. [Google Scholar]
- 34.Dickson AG, Sabine CL, Christian JR, editors. Guide to best practices for ocean CO2 measurements. PICES Special Publication 3. IOCCP Report. 2007;8:191. [Google Scholar]
- 35.Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;19:897–907. [Google Scholar]
- 36.Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 1987;34:1733–1743. [Google Scholar]
- 37.McGraw CM, Cornwall CE, Reid MR, Currie KI, Hepburn CD, et al. An automated pH-controlled culture system for laboratory-based ocean acidification experiments. Limnol Oceanogr-Meth [Google Scholar]
- 38.Zhang H, Byrne RH. Spectrophotometric pH measurements of surface seawater at in-situ conditions: absorbance and protonation behaviour of thymol blue. Mar Chem. 1996;52:17–25. [Google Scholar]
- 39.Clark M, Fraser K, Peck L. Antarctic marine molluscs do have an HSP70 heat shock response. Cell Stress Chaperon. 2008;13:39–49. doi: 10.1007/s12192-008-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park H, Ahn IY, Lee HE. Expression of heat shock protein 70 in the thermally stressed Antarctic clam Laternula elliptica. Cell Stress Chaperon. 2007;12:275–282. doi: 10.1379/CSC-271.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–536. [PubMed] [Google Scholar]
- 42.Hoegh-Guldberg O, Manahan D. Coulometric measurement of oxygen consumption during development of marine invertebrate embryos and larvae. J Exp Biol. 1995;198:19–30. doi: 10.1242/jeb.198.1.19. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AC, Davenport J, Allen JA. Anoxic survival, oxygen consumption and hemocyanin characteristics in the protobranch bivalve Nucula sulcata Bronn. Comp Biochem Physiol A. 1995;112:333–338. [Google Scholar]
- 44.Ellis JI, Schneider DC. Evaluation of a gradient sampling design for environmental impact assessment. Environmental Monitoring and Assessment. 1997;48:157–172. [Google Scholar]
- 45.Somerfield PJ, Clarke KR, Olsgard F. A comparison of the power of categorical and correlational tests applied to community ecology data from gradient studies. J Anim Ecol. 2002;71:581–593. [Google Scholar]
- 46.Cottingham KL, Lennon JT, Brown BL. Knowing when to draw the line: designing more informative ecological experiments. Front Ecol Environ. 2005;3:145–152. [Google Scholar]
- 47.Wood HL, Spicer JI, Widdicombe S. Ocean acidification may increase calcification rates, but at a cost. Proc R Soc Lond B Biol Sci. 2008;275:1767–1773. doi: 10.1098/rspb.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke A. Temperature, latitude and reproductive effort. Mar Ecol Prog Ser. 1987;38:89–99. [Google Scholar]
- 49.Heilmayer O, Brey T. Saving by freezing? Metabolic rates of Adamussium colbecki in a latitudinal context. Mar Biol. 2003;143:477–484. [Google Scholar]
- 50.Abele D, Tesch C, Wencke P, Pörtner HO. How oxidative stress parameters relate to thermal tolerance in the Antarctic bivalve Yoldia eightsi? Antarct Sci. 2001;13:111–118. [Google Scholar]
- 51.Peck LS, Webb KE, Bailey DM. Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct Ecol. 2004;18:625–630. [Google Scholar]
- 52.Peck LS. Prospects for survival in the Southern Ocean: vulnerability of benthic species to temperature change. Antarct Sci. 2005;17:497–507. [Google Scholar]
- 53.Brockington S. The seasonal energetics of the Antarctic bivalve Laternula elliptica (King and Broderip) at Rothera Point, Adelaide Island. Polar Biol. 2001;24:523–530. [Google Scholar]
- 54.Morley SA, Peck, LS, Miller AJ, Pörtner H-O. Hypoxia tolerance associated with activity reduction is a key adaptation for Laternula elliptica seasonal energetics. Oecologia. 2007;153:29–36. doi: 10.1007/s00442-007-0720-4. [DOI] [PubMed] [Google Scholar]
- 55.Morely SA, Hirse T, Pörtner H-O, Peck LS. Geographic variation in thermal tolerance within Southern Ocean marine ectotherms. Comp Biochem Physiol A. 2009;153:154–161. doi: 10.1016/j.cbpa.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Norkko J, Thrush SF, Wells RM. Indicators of short-term growth in bivalves: detecting environmental change across ecological scales. J Exp Mar Biol Ecol. 2006;337:38–48. [Google Scholar]
- 57.Clark MS, Fraser KPP, Peck LS. Lack of an HSP70 heat shock response in two Antarctic marine Invertebrates. Polar Biol. 2008;31:1059–1065. [Google Scholar]
- 58.Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: Implications for limits of thermotolerance and biogeography. J Exp Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- 59.Sorenson JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. [Google Scholar]
- 60.Clark MS, Peck LS. Triggers of the HSP70 stress response: environmental responses and laboratory manipulation in an Antarctic marine invertebrate (Nacella concinna). Cell Stress Chaperon. 2009;14:649–660. doi: 10.1007/s12192-009-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang ES. Stressed-out lobsters: crustacean hyperglycemic hormone and stress proteins. Integr Comp Biol. 2004;45:43–50. doi: 10.1093/icb/45.1.43. [DOI] [PubMed] [Google Scholar]
- 62.Lindquist S. The heat shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 63.Franzellitti S, Fabbri E. Differential HSP70 gene expression in the Mediterranean mussel exposed to various stressors. Biochem Biophys Res Co. 2005;336:1157–1163. doi: 10.1016/j.bbrc.2005.08.244. [DOI] [PubMed] [Google Scholar]
- 64.Fabbri E, Valbonesi P, Franzellitti S. HSP expression in bivalves. Invert Surviv J. 2008;5:135–161. [Google Scholar]
- 65.Hauton C, Tyrrell T, Williams JE. The subtle effects of sea water acidification on the amphipod Gammarus locusta. . Biogeosciences Discuss. 2009;6:919–946. [Google Scholar]
- 66.Guinotte JM, Fabry VJ. Ocean acidification and its potential effects on marine ecosystems. Ann NY Acad Sci. 2008;1134:320–342. doi: 10.1196/annals.1439.013. [DOI] [PubMed] [Google Scholar]
- 67.Schönitzer V, Weiss IM. The structure of mollusc larval shells formed in the presence of the chitin synthase inhibitor Nikkomycin Z. BMC Struct Biol. 2007;7:71. doi: 10.1186/1472-6807-7-71. doi: 10.1186/1472-6807-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiss IM, Schonitzer V. The distribution of chitin in larval shells of the bivalve mollusk Mytilus galloprovincialis. J Struct Biol. 2006;153:264–277. doi: 10.1016/j.jsb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Cummings VJ, Thrush SF, Norkko A, Andrew NL, Hewitt JE, et al. Accounting for local scale variability in benthos: implications for future assessments of latitudinal trends in the coastal Ross Sea. Antarct Sci. 2006;18:633–644. [Google Scholar]
- 70.Schwarz A-M, Hawes I, Andrew N, Norkko A, Cummings V, et al. Macroalgal photosynthesis near the southern global limit for growth; Cape Evans, Ross Sea, Antarctica. Polar Biol. 2003;26:789–799. [Google Scholar]
- 71.Green MA, Waldbusser GG, Reilly SL, Emerson, K, O'Donnell S. Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnol Oceanogr. 2009;54:1037–1047. [Google Scholar]
- 72.Waldbusser GG, Voigt EP, Bergschneider H, Green MA, Newell RIE. Biocalcification in the eastern oyster (Crassostrea virginica) in relation to long-term trends in Chesapeake Bay pH. Estuaries Coasts. 2010 DOI 10.1007/s12237-010-9307-0. [Google Scholar]
- 73.Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science. 2008;320:1490–1492. doi: 10.1126/science.1155676. [DOI] [PubMed] [Google Scholar]
- 74.Philipp E, Brey T, Pörtner HO, Abele D. Chronological and physiological ageing in a polar and a temperate mud clam. Mech Ageing Dev. 2005;126:589–609. doi: 10.1016/j.mad.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Urban H-J, Mercuri G. Population dynamics of the bivalve Laternula elliptica from Potter Cove, King George Island, South Shetland Islands. Antarct Sci. 1998;10:153–160. [Google Scholar]
- 76.Dupont S, Thorndyke MC. Impact of CO2-driven ocean acidification on invertebrates early life-history – what we know, what we need to know and what we can do. Biogeosciences Discuss. 2009;6:3109–3131. [Google Scholar]
- 77.Norkko A, Thrush SF, Cummings VJ, Gibbs MM, Andrew NL, et al. Trophic structure of coastal Antarctic food webs associated with changes in food supply and sea ice extent. Ecology. 2007;88:2810–2820. doi: 10.1890/06-1396.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Designation of CHS gene family member status.
(DOC)
Nucleotide and deduced amino acid sequences of Laternula elliptica chitin synthase (CHS) Numbered boxes refer to highly conserved regions found in many family 2 glycosyltransferases (GTF2) enzymes, including CHS. Regions 1 and 2, UDP-binding; region 1 is similar to the Walker A/P-loop motif and to the R-β-GKR consensus sequence of GTF2; region 2 is similar to the Walker B motif and to the K-β-DDGS consensus sequence of GTF2. Regions 3 and 4, donor saccharide-binding; region 3 is similar to the DXD motif; region 4 is similar to the G(X)4(Y/F)R consensus sequence important for enzyme processivity. Positions of the primers used in RT‐qPCR are highlighted on the cDNA sequence.
(DOC)
Multiple alignment of deduced amino acid sequences of Laternula elliptica CHS with chitin synthases of other bivalves. The CHS abbreviations, species and the Genbank accession numbers are as follows: LECHS, Laternula elliptica, HQ186262; PFCHS, Pinctada fucata, AB290881; ARCHS, Atrina rigida, DQ081727; MGCHS, Mytilus galloprovincialis, EF535882. Bivalve sequences are numbered according to their complete Genbank entry whilst the amino acid sequence deduced from the LECHS mRNA fragment is numbered 1‐318.
(DOC)