Abstract
Repeated exposure of rabbits and other animals to ticks results in acquired resistance or immunity to subsequent tick bites and is partially elicited by antibodies directed against tick antigens. In this study we demonstrate the utility of a yeast surface display approach to identify tick salivary antigens that react with tick-immune serum. We constructed an Ixodes scapularis nymphal salivary gland yeast surface display library and screened the library with nymph-immune rabbit sera and identified five salivary antigens. Four of these proteins, designated P8, P19, P23 and P32, had a predicted signal sequence. We generated recombinant (r) P8, P19 and P23 in a Drosophila expression system for functional and immunization studies. rP8 showed anti-complement activity and rP23 demonstrated anti-coagulant activity. Ixodes scapularis feeding was significantly impaired when nymphs were fed on rabbits immunized with a cocktail of rP8, rP19 and rP23, a hall mark of tick-immunity. These studies also suggest that these antigens may serve as potential vaccine candidates to thwart tick feeding.
Introduction
Ixodes scapularis and Ixodes ricinus ticks transmit pathogens such as Borrelia, Babesia, Anaplasma and selected flaviviruses [1]. In order to acquire a successful blood meal, these ticks engorge for several days on a mammalian host and counter the haemostatic system and immune responses of the host by spitting tick saliva into the skin [2]. Tick saliva contains proteins that inhibit T-cells [3], B-cells [4], the complement system [5], [6], [7], [8], dendritic cells [9] and the coagulation system [10], [11], [12], [13]. Even though ticks modulate and dampen host immune responses to ensure successful feeding, upon repeated tick infestations some animals develop an immune response resulting in tick rejection. This phenomenon, referred to as ‘tick immunity’, was first described by William Trager in 1939, when he observed that Dermacentor variabilis ticks were not able to efficiently engorge on guinea pigs that had previously been exposed to several tick infestations [14]. Parameters of tick-immunity include decreased numbers of ticks feeding on the host, delayed time of engorgement, a reduction in tick weight, the inability to molt and decreased fecundity. Mast cells, basophils, eosinophils [15], and antibodies [16] against exposed and concealed [17] tick proteins play a role in tick-immunity.
In contrast to animals such as guinea pigs and rabbits, mice, do not develop the hall marks of tick-immunity upon repeated infestations with Ixodes scapularis ticks [18]. The mechanism underlying this difference remains to be understood. However, immune responses directed against tick proteins was shown to reduce Borrelia transmission when infected ticks fed on mice that were repeatedly infested with ticks [18]. Borrelia transmission in mice passively administered serum from tick-immune rabbits was also reduced when challenged with Borrelia burgdorferi-infected I. scapularis nymphs [19]. These observations uncouple tick feeding from pathogen transmission and suggest that while the tick-immune serum is unable to thwart tick feeding in mice, tick-immune serum contains antibodies directed against tick salivary proteins critical for Borrelia transmission to mice. Repeated exposure to tick bites is also associated with fewer episodes of Lyme disease in residents living in areas where B. burgdorferi infection is endemic [20]. Therefore, identification of tick salivary antigens that react with tick-immune serum would provide the preamble for a molecular understanding of tick feeding as well as pathogen transmission and also provide novel vaccine targets both to block tick feeding and pathogen transmission [21].
Immunoscreening of cDNA expression libraries using a phage display approach has identified several tick salivary proteins that react with tick-immune serum [22], [23]. A limitation with phage-displayed proteins is that they lack eukaryotic post-translational modifications that might contribute to critical epitopes, and preclude the identification of such antigens by phage display screening. Therefore, additional screening efforts that exploit novel high-throughput approaches would be essential to generate a comprehensive array of salivary antigens that react with tick-immune sera. Such a detailed catalog would help develop and distill a critical subset of tick salivary antigens that might serve as vaccines to block tick feeding and impair pathogen transmission. Towards this goal, we adapted the Yeast Surface Display (YSD) approach [24], that allows eukaryotic proteins to be displayed in a near-native form [25]. While YSD has been traditionally applied to study protein-protein interactions, we have in this report utilized the YSD approach to identify a subset of salivary proteins from I. scapularis nymphal stage that react with nymph-immune rabbit sera.
Results
Identification of antigenic I. scapularis salivary proteins from the nymphal stage
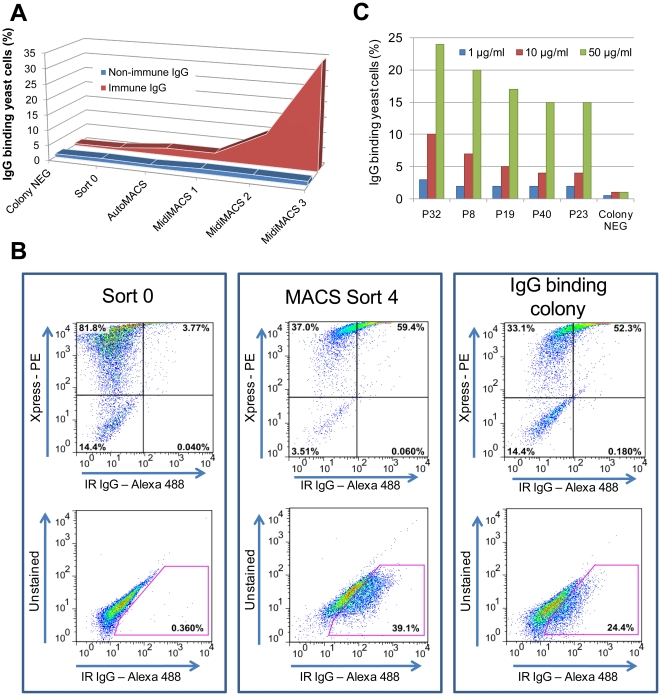
A YSD expression library of I. scapularis salivary gland cDNAs was probed with purified IgG from pooled sera from nymph-immune rabbits. After 4 rounds of magnetic-activated cell sorting (MACS) screen, a ∼110-fold enrichment of yeast cells expressing salivary proteins recognized by rabbit nymph-immune serum ( Fig. 1 A,B ) was obtained. The library sorted with IgG purified from non-immune serum did not show binding and did not provide enrichment of yeast cells ( Fig. 1 A ). Two hundred fifty colonies from the 4th sort were individually tested for their ability to bind to rabbit nymph-immune antiserum by fluorescence-activated cell sorting (FACS) analysis. Recombinant plasmids were isolated from 98 positive colonies and insert sizes compared by restriction digestion analysis. Clones with similar insert sizes were grouped and representative clones sequenced. Five unique clones encoding tick salivary proteins were identified and provided a unique identifier based on their putative molecular mass as shown in Table 1 . YSD was also useful in comparing the specificity and avidity of the antigen-antibody interaction between different clones using varying amounts of IgG from immune rabbits ( Fig. 1 C ). In silico analysis of P23 and P32 protein sequences revealed homology with putative secreted salivary gland proteins of I. scapularis, P19 was found to share homology with proteins from Rhipicephalus annulatus and Heamaphysalis qinghaiensis [26], [27]. P8 showed similarities with the I. scapularis anticoagulant protein, Salp14, and the putative anticoagulant Salp9pac. P40 was identical to I. scapularis Transducing (beta)-like 2 protein ( Table 1 ).
Figure 1. Enrichment and selection of yeast cells expressing immunogenic I. scapularis salivary proteins.
FACS analysis of yeast cells using nymph-immune rabbit IgG (red) and IgG derived from normal rabbit serum (blue) of transformed yeast cells (sort 0); autoMACS sort (AutoMACS); MidiMACS sort 1, 2 and 3 (MidiMACS1, 2 and 3). (B) Selection for IgG binding clones using 50 µg/ml Alexa-488 conjugated nymph immune rabbit IgG. Sort 0 was used as a negative control and MACS sort 4 as a positive control. Upper panel: mouse anti-Xpress antibody binding to induced yeast cells (PE). Lower panel: Cells binding nymph immune rabbit IgG (IR IgG) shown within the pink gate (Alexa-488). (C) Titration of binding of each unique using 50 µg/ml (green bars), 10 µg/ml (red bars) or 1 µg/ml (blue bars) of immune rabbit IgG. The percentage IgG binding yeast cells were determined by FACS analysis as was shown in the right lower panel of Fig. 1B.
Table 1. Antigenic Ixodes scapularis salivary proteins identified by immunoscreening a nymphal Ixodes scapularis yeast display library using nymph-immune rabbit serum.
| Positive colonies | GenbankAccession Number | Match to NR protein database# | ORF | MW* (kDa) | pI* | Domains | Paralogues† | Signal sequence‡ |
| P8 | HQ605983 | I. scapularis, Salp14GenBank: AAK97824.1E value 2e−43 I. scapularis, Salp9pacGenBank: AAN03859.1E value 5e−48 | 276-bp | 7.9 | 4.3 | NO | YES | YES |
| P19 | XM_002399589.1 | Rhipicephalus annulatus, Ba05GenBank: ABV53333.1E value 6e−78 Haemaphysalis qinghaiensis,Hq05GenBank AAX37829.1E value 1e−69 | 564-bp | 18.7 | 5.8 | NO | NO | YES |
| P23 | HQ605984 | I. scapularis, putative secreted SG peptide, GenBank: XP_002405271.1E value 4e−37 | 669-bp | 22.7 | 9.5 | NO | YES | YES |
| P32 | HM802761.1 | I. scapularis, putative secreted SG peptide, GenBank: AAV80775.1E value 3e−40 | 912-bp | 31.7 | 5.6 | NO | NO | YES |
| P40 | HM802762.1 | I. scapularis Transducing (beta)-like 2 (Tbl2) protein,GenBank: XM_002416416.1E value 9e−140 | 1089-bp | 40.1 | 7.2 | WD40(NCBI CDD:cl02567) | NO | NO |
#Homology search performed using BLAST (www.ncbi.nlm.nih.gov/BLAST).
*Theoretical molecular weight (MW) and isoelectric point (pI) using ExPASy proteomics server (http://www.expasy.ch/tools/pi_tool.html).
Proteins ≤40% identity using the database of VectorBASE (http://iscapularis.vectorbase.org/) and the GenBank database (www.ncbi.nlm.nih.gov/blast/Blast.cgi).
Secretory signal sequence as assessed by the SignalP 3.0 signal prediction server (www.cbs.dtu.dk/services/SignalP/).
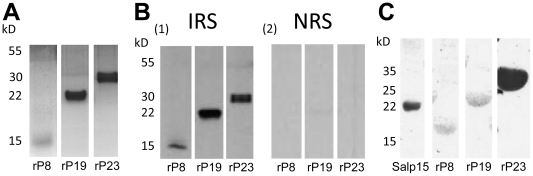
With the exception of P40, all other antigens identified contained a canonical secretory signal sequence. With an initial focus on secreted salivary proteins, we chose to exclude P40 in this study. P8, P19 and P23 were produced as recombinant proteins in a Drosophila expression system and purified using Ni-NTA Superflow chromatography ( Fig. 2 A ). Escherichia coli DH5α cells transformed with the pMT/Bip/V5-HisA plasmid containing p32 were not viable, which precluded plasmid generation for transfection of S2 cells and rP32 protein production. The three recombinant salivary proteins were recognized avidly by antibodies in nymph-immune rabbit serum ( Fig. 2 B , panel 1), and did not react with normal rabbit serum ( Fig. 2 B , panel 2). Analysis of the amino acid sequences of the proteins (www.cbs.dtu.dk/services/NetNGlyc/) revealed that P8 and P19 have 1 predicted N-glycosylation site and P23 has 3 predicted N-glycosylation sites. Indeed, consistent with the in silico glycosylation predictions, glycoprotein staining with periodic acid-Schiff (PAS) indicated that rP8 and rP19 were glycosylated, albeit to a lesser extent compared to rP23 ( Fig. 2 C ).
Figure 2. Purified recombinant Ixodes scapularis salivary proteins.
(A) Coomassie blue staining of purified recombinant I. scapularis salivary proteins rP8, rP19 and rP23 electrophoresed on SDS 12% polyacrylamide gel. (B) Western blot analysis of the recombinant proteins probed with nymph-immune rabbit serum (IRS) and normal rabbit serum (NRS). (C) PAS staining of rP8, rP19, rP23 and Salp15 electrophoresed on SDS 12% polyacrylamide gel.
Expression of the p19, p23, p32 and p8 genes in different I. scapularis stages
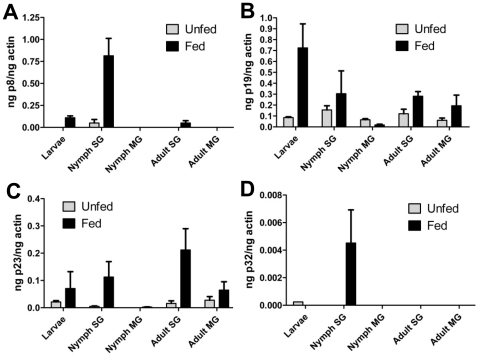
p19 and p23 were expressed in larval, nymphal and adult ticks, while p32 and p8 were primarily expressed in nymphs ( Fig. 3 ). As expected all 4 genes were expressed in tick salivary glands and significantly induced upon feeding. In addition, p19 and p23 showed additional expression in the tick gut in selected developmental stages and p8 and p32 were preferentially expressed in the nymphal salivary glands.
Figure 3. Expression of the genes coding for the four salivary proteins during several life stages of I. scapularis.
Quantitative reverse-transcription polymerase chain reaction performed on RNA isolated from whole larvae, and from salivary glands and midguts I. scapularis from nymphs and adults. Expression profile of: A. p8; B. p19; C. p23; and D. p32 in unfed (grey bars) and fed ticks (black bars). SG; salivary glands, MG; midguts.
rP8 protects Borrelia from complement-mediated killing
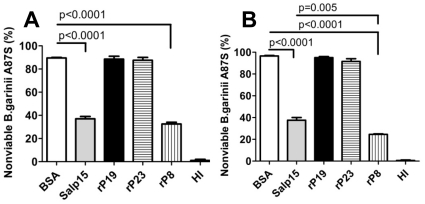
Since no functional domains were found by in silico analysis, we examined each of the three recombinant proteins in assays to test for predominant biochemical activities represented in tick saliva, i.e. anticomplement [6], [8], [28] and anticoagulant activity [10], [11], [12], [13], [29], [30]. Unlike, B. burgdorferi sensu stricto, B. garinii is a human complement sensitive strain [31]. Therefore, we utilized a B. garinii killing assay [8] to investigate whether rP8, rP19 and rP23 were able to protect spirochetes from or inhibit the human complement system. rP8 significantly reduced complement-mediated killing of B. garinii A87S ( Fig. 4 ) after 1.5 hours ( Fig. 4 A ; 90±0.9% for BSA versus 31±1.2% for rP8, p<0.0001) and 4.5 hours ( Fig. 4 B ; 97±0.6% for BSA versus 25±0.9% for rP8, p<0.0001), in a dose dependent manner. B. garinii spirochetes incubated with heat-inactivated NHS remained viable at all time points examined ( Fig. 4 ). None of the other recombinant proteins provided protection against complement-mediated killing ( Fig. 4 ). The I. scapularis salivary protein Salp15 was used as a positive control [8].
Figure 4. Influence of recombinant salivary proteins on human complement system.
Serum sensitive strain Borrelia garinii A87S was incubated with 12.5% NHS in the presence of BSA, Salp15, rP19, rP23 or rP8 for (A) 1.5 hours; or (B) 4.5 hours and the percentage of immotile spirochetes were determined. Control spirochetes were incubated with heat-inactivated NHS (HI). Two hundred spirochetes were counted. Results represent mean ± SEM of values from a representative of 3 replicate experiments.
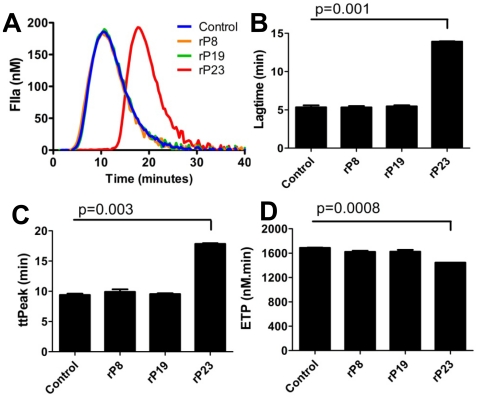
rP23 inhibits coagulation of human plasma
Calibrated automated thrombography (CAT) was used to assess the effect of the recombinant I. scapularis salivary proteins on tissue factor initiated thrombin generation. In normal human pooled plasma, recombinant rP23 delayed thrombin generation ( Fig. 5 A ), with significant prolongation of lag time and time to peak ( Fig. 5 B,C ) in a dose dependent manner, suggesting that rP23 may influence the initiation phase of coagulation. In addition, determination of the total amount of thrombin formed (Endogenous Thrombin Potential, ETP) showed that rP23 significantly reduced thrombin generation by 18% compared to ETP in the absence of rP23 ( Fig. 5 D ). rP8 and rP19 at similar concentrations did not affect thrombin generation ( Fig. 5 ).
Figure 5. Influence of recombinant salivary proteins on human coagulation system.
(A) Thrombin generation was initiated in human pooled normal plasma with 1 pM tissue factor (TF) in the presence of rP8 (orange), rP19 (green) or rP23 (red) and thrombin generation was measured using a fluorogenic substrate. (B) Lagtime, (C) time to peak (ttpeak) and (D) Endogenous Thrombin Potential (ETP) were measured. Unpaired t-test was used to determine statistical significance. Representatives of three experiments are shown. Results described represent the mean ± SEM.
Immunization with recombinant P8, P19 and P23 impairs nymphal tick feeding on rabbits
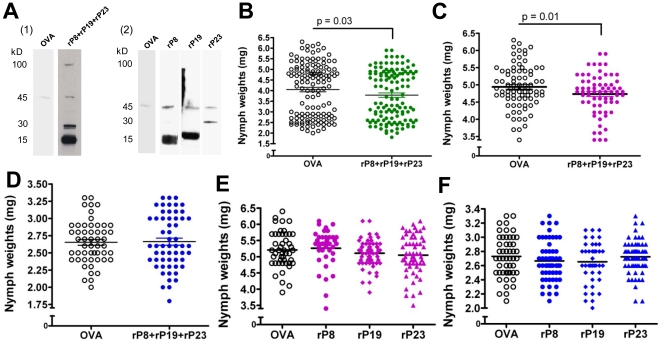
Three rabbits were immunized with a cocktail of rP8, rP19 and rP23. Nymphal salivary gland extract (SGE) probed with immune sera from each of the rabbits recognized the native proteins ( Fig. 6 A , panel 1). A 45 kDa band appeared in all blots most likely due to binding of anti-rabbit IgG to host globulins present in the fed SGE [32]. Immune sera from 3 control rabbits immunized with OVA did not react with proteins in the nymphal SGE ( Fig. 6 A , panel 1). Upon challenge of the immunized rabbits with I. scapularis nymphs, comparable numbers of nymphs fed to repletion on the control and experimental animals (127 nymphs on the immunized group versus 137 on the control group). Nymphs feeding on rabbits immunized with the cocktail of rP8, rP19 and rP23 were significantly lighter than nymphs feeding on the OVA control rabbits ( Fig. 6 B ; 3.7±0.1 mg and 4.0±0.1 mg, respectively, p = 0.03). An independent control experiment showed that, after feeding on normal rabbits, nymphs with a weight of 3.3 mg and below consistently molted into males, while the heavy group of nymphs (3.4 mg and above) molted into female adult ticks ( Table 2 ). Therefore, engorged ticks were also divided into subgroups (light and heavy) ( Fig. 6 C–D ), Analysis of the subgroups showed a significant reduction in engorgement weights in the heavy group of nymphs fed on the rP8, rP19 and rP23 cocktail immunized rabbits compared to the heavy group of nymphs fed on control rabbits (4.7±0.1 mg and 4.9±0.1 mg, p = 0.01) ( Fig. 6 C ). There was no significant difference between the light group of nymphs feeding on immunized rabbits versus controls ( Fig. 6 D ). Finally, when three rabbits per group were immunized with single proteins, nymphal SGE probed with immune sera from each of the rabbits recognized the native proteins ( Fig. 6 A , panel 2), but there was no difference in nymph weights, compared to the control OVA-immunized animals in both heavy ( Fig. 6 E ) and light groups ( Fig. 6 F ).
Figure 6. Nymph feeding after rabbit immunization with recombinant salivary proteins.
(A) Nymph salivary gland extract probed with rP8/rP19/rP23 immune rabbit serum (panel 1), rP8 or rP19 or rP23 immune rabbit serum (panel 2), and with serum from the control (OVA) rabbit. (B) Nymph weights recovered from the control and the rP8/rP19/rP23 immunized rabbits. Tick weights of the heavy group of nymphs (C) and light group of nymphs (D) fed on rP8/rP19/rP23 immunized rabbits compared to the control rabbits. Weights of the heavy group of nymphs (E) and light group (F) of nymphs fed on rabbits immunized singly with OVA, rP19, rP8 or rP23 respectively. The horizontal bars represent the means of the respective groups. Unpaired t-test was used to determine statistical significance.
Table 2. Relationship between post-engorgement weights and resultant sexes in Ixodes scapularis nymphs.
| Nymphal Engorgement Weights | Males | Females |
| 2.0–3.3 mg | 92 | 0 |
| 3.4–6.2 mg | 0 | 110 |
Discussion
Ixodes ticks transmit numerous pathogens, including bacteria, protozoa and selected flaviviruses [33]. Anti-tick vaccines could potentially prevent transmission of Borrelia, as well as other pathogens, from the tick to the host. Acquired resistance to tick feeding can also impair pathogen transmission [34], [35]. Several studies have shown a critical role for the humoral response in tick immunity [16], [36] and provided the impetus to identify antigens that elicit tick-immunity by exploiting approaches including immunoscreening of cDNA expression libraries generated in routine prokaryotic expression systems [22], [23]. However, the prokaryotic systems are not capable of displaying the antigens with eukaryotic post-translational modifications. When these modifications are critical determinants of antigenic epitopes, prokaryotic expression systems preclude identification of such antigens. It is therefore critical to exploit additional strategies to circumvent these limitations. It is also important to note that the proteome of the developmental stages of ticks are likely different [37] and antigens critical for one stage might not necessarily be critical for all stages. Therefore, it is important to develop viable and high-throughput strategies to screen for salivary antigens that react with stage-specific immune sera. This would help build a comprehensive catalog of salivary antigens that react with tick-immune serum against different developmental stages of Ixodes scapularis, and facilitate the development of functional vaccines targeting critical developmental stages. Since, I. scapularis nymphs are critical for disease transmission to humans [38], in this study we advance our understanding of nymphal salivary proteins that react with nymph-immune rabbit sera. It is expected that these antigens might play a role in tick feeding and immunity against these antigens might impair tick feeding. Such a phenotype would therefore also affect the success of nymphal molting to adults and would be conductive to control tick populations. Decreased feeding might also lead to decreased pathogen transmission. Additionally, these antigens might provide functions critical for pathogen transmission [6], [8], [39]. Therefore, we used the YSD approach, to identify salivary antigens that react with nymph-immune serum and examine their role in the context of nymphal feeding success.
We demonstrate that YSD technology is a robust tool for the identification of antigenic salivary gland proteins. The major advantage of YSD over other expression systems such as phage display, is that yeast cells have eukaryotic machinery allowing the display of eukaryotic proteins with post-translational modifications like glycosylation, phosphorylation and correct disulphide bond formation [40] and hence, utilized for several applications including protein affinity maturation, epitope mapping, and cell adhesion molecule engineering [25], [41]. To date, there are only a few reports describing the utility of YSD for cDNA library screening and have addressed human cDNA libraries [42], [43]. This study extends the utility of YSD to perform high throughput immunoscreening of a YSD library of nymphal I. scapularis salivary gland cDNAs to enrich for yeast cells expressing antigenic tick proteins ( Fig. 1 ).
The YSD library of I. scapularis salivary gland cDNA was prepared from RNA isolated from replete nymphs. We are cognizant that the salivary gland proteome is dynamic, and the protein profiles change during feeding. The current screening effort is therefore not likely to identify antigens expressed specifically at early time points of feeding. Five novel antigenic tick salivary proteins were identified using the YSD approach ( Table 1 ). While P23 and P32 only showed homology with putative secreted salivary gland proteins, with no known functions. P8 showed homology with the putative anticoagulant Salp9pac and anticoagulant Salp14 family of proteins. Salp14 was identified earlier by Das et al [22] by immunoscreening a phage expression library of I.scapularis nymphal cDNAs using tick-immune sera [10]. P19 was homologous to a larval immunogenic protein, Ba05 from Rhipicephalus annulatus [27] and the protein Hq05 from Haemaphysalis qinghaiensis [26] (93 and 82% identity respectively). Expression analysis of Hq05 showed expression specifically in salivary glands of nymphs and adults, but not in eggs and larvae of H. qinghaiensis [26]. P40 showed similarities with a putative G-protein from I. scapularis (XP_002416461.1) and with Transducin beta-like 2 proteins (NP_001008084) from several species including mammals. P40 contains 7 copies of the WD40 domain, which is found in several eukaryotic proteins that are involved in diverse functions including pre-mRNA processing, signal transduction, cytoskeleton assembly and cell cycle control [44], [45], [46]. P40 lacked a canonical secretory signal sequence and possibly not secreted in tick saliva. However, based on the reactivity of P40 with tick-immune sera ( Table 1 ), we speculate that P40 might be secreted in tick saliva by other/novel secretory mechanisms and warrants detailed verification and therefore not included in this study. Four of the five identified proteins, i.e. P8, P19, P23 and P32, had a predicted secretory signal sequence as assessed by the SignalP 3.0 signal prediction server (www.cbs.dtu.dk/services/SignalP/) and were therefore selected for further analysis. As expected, the identified genes p8, p19, p23 and p32 encoding for the antigenic tick proteins were all induced upon nymphal feeding ( Fig. 3 ). While p19 and p23 were expressed both during larval and adult stages, p8 and p32 were preferentially expressed in the nymphal stage, indicating that some salivary proteins might play a stage-specific role. Next, recombinant P8, P19 and P23 were made in S2 cells using a Drosophila expression system ( Fig. 2 ). Attempts to generate stable S2 cell lines expressing rP32 were unsuccessful. All three recombinant proteins showed glycosylations as seen by a positive reaction using the Periodic Acid Schiff method ( Fig. 2 C ), suggesting that these proteins might also be glycosylated in I. scapularis in vivo and might account for the slightly increased molecular masses of the native P8 and P23 ( Fig. 6 A ). In silico analysis of the proteins for potential glycosylation sites using the NetNGlyc server (www.cbs.dtu.dk/services/NetNGlyc/) also suggested one and 3 potential N-glycosylation sites on P8 and P23, respectively.
Despite the homology between P8 and the anticoagulant Salp14 [10], [47], rP8 did not have any effect on thrombin formation in human plasma. We have previously shown that Salp9pac, also homologous to Salp14, did not have anticoagulant properties [10]. The anticoagulant Salp14 has a positively charged stretch of 20 amino acids at the C-terminal tail compared to rP8 and Salp9pac, which is most likely important for interaction with the coagulant proteins [10]. rP23 demonstrated anticoagulant activity and significantly delayed thrombin generation in human plasma ( Fig. 5 ). Interestingly, P23 does not contain any known anti-coagulant domains and might represent a novel type of anticoagulant. The mechanism of anticoagulation by rP23 remains to be determined.
To investigate the three proteins for anti-complement activity we used a B. garinii killing assay, since B. garinii is a serum sensitive strain compared to B. burgdorferi sensu stricto [8], [31]. We have previously demonstrated that the serum-sensitive B. garinii A87S strain is killed by the human complement system after formation of the C5b-9 membrane attack complex and that Ixodes Salp15 provided protection against this complement-mediated killing [8]. When Borrelia was incubated with normal human serum, rP8 was also able to protect Borrelia against complement-mediated killing ( Fig. 4 ). Whether, rP8 provides this protection by virtue of an anticomplement activity or by physically binding to spirochetes and shielding the spirochetes from complement attack remains to be examined.
We next examined if immunity against these recombinant salivary proteins would recapitulate tick immunity in rabbits. When rabbits were immunized with a cocktail of rP8, rP19 and rP23 and challenged with pathogen-free nymphs, feeding efficiency was modestly, but significantly, decreased compared to nymphs that fed on OVA-immunized rabbits, as determined by engorgement weights ( Fig. 6 B ). Previous experiments in our laboratory, consistent with observations by Hu et al [48], have shown that the heavy group of nymphs (3.4 mg and higher) consistently molted into adult females, while the light group of nymphs (3.3 mg and lighter) molted into adult males ( Table 2 ). The impaired feeding phenotype was significant in the to-be-female group ( Fig. 6 C ), since when fed nymphs were separated into heavy (to-be-female) and light (to-be-male) groups of nymphs, no difference in post-engorgement weights was found in the to-be male group of nymphs fed on the rP8, rP19, rP23 immunized rabbits compared to controls ( Fig. 6 D ). In addition, two weight populations were seen in the to-be male group of nymphs fed on the immunized animals ( Fig. 6 D ), possibly due to to-be female ticks that showed impaired feeding due to immunization with the recombinant tick proteins controls. It is possible that the to-be male group of ticks differentially express salivary proteins while feeding on the host compared to the to-be female group of nymphs. P8, P19 and/or P23 might play a redundant role in feeding in the to-be male nymphs. Future studies will determine whether the cocktail vaccine (rP8, rP19 and rP23) might also impair pathogen transmission from the tick to the host.
In conclusion, these data show that cocktail immunization targeting multiple (functional) salivary proteins resulted in impaired tick feeding, while immunizations with individual proteins did not have an effect. It is conceivable that targeting 3 antigens simultaneously provided neutralization of antigens important for suppressing several arms of the host defense (in this case, host coagulation and complement) and resulted in impaired tick feeding. Further, the past decade has demonstrated that the tick transcriptome elaborates an array of functional paralogs [49]. Several anticomplement [6], [8], [28] and anticoagulant proteins [10], [11], [12], [13], [29], [30] have been described and characterized in Ixodes ticks. This functional redundancy is central to the tick's ability to feed successfully. To efficiently block tick feeding, it might be important to immunize animals with cocktails of several anticomplement or anticoagulant proteins to circumvent fall-back strategies of the tick. Since tick-immunity effectively disables the ability of the tick to feed, presumably, tick-immune serum targets the critical subsets of functional paralogs. Thus, intensive screening using the YSD approach would provide a comprehensive list of antigens that react with tick-immune serum. This would enable us to group subsets of functional paralogs critical for feeding and facilitate the development of an effective cocktail vaccine to block tick feeding.
Materials and Methods
Ticks and animals
I. scapularis adults, nymphs and larvae were obtained from a tick colony at the Connecticut Agricultural Experiment Station in New Haven CT, USA. Ticks were maintained at 23°C and 85% relative humidity under a 14 hour light, 10 hour dark photoperiod. For the immunization studies, 6 week old inbred New Zealand white rabbits (Charles River Laboratories) were used. The work reported in this study is fully compliant with and approved by institutional policies pertinent to biosafety and animal care protocols. The protocol for the use of mice and rabbits was reviewed and approved by the Yale Animal Care and Use Committee (protocol number 2008-07941, approval date is 03/31/10 to 3/31/11).
Construction of an I. scapularis salivary gland cDNA library
RNA was purified from the salivary glands from 1000 I. scapularis nymphs fed to repletion (repletion achieved between 72 and 96 h), and cDNAs directionally cloned into the EcoRI and NotI sites of the yeast expression vector pYD1 (Invitrogen, CA) to generate a salivary gland expression library wherein that tick salivary proteins were expressed as Aga2 fusion proteins on the yeast surface (Invitrogen, CA). PstI digestion of plasmids purified from 24 clones of the pYD1-salivary gland library, showed an average insert size of 2.1 kb and 100% of the clones contained inserts. The unamplified library titre was 0.5×106 cfu/ml. Growth of transformant yeast cells and induction of recombinant protein production was done essentially as detailed by Chao et al. [50]. Briefly, fresh Saccharomyces cerevisiae EBY100 cells (Invitrogen, CA) with 5 µg of DNA were electroporated and subsequently grown in SDCAA medium (2% dextrose, 0.67% yeast nitrogen base, 0.5% bacto amino acids, 30 mM NaHPO4, 62 mM NaH2PO4). Induction of surface protein expression was done as described below.
Purification and conjugation of IgG from tick-immune rabbit sera
Two I. scapularis nymph-immune rabbits were generated as described earlier [19] and sera tested by western blot analysis to confirm reactivity with nymph SGE. IgG was purified from the nymph-immune rabbit sera using the Melon Gel IgG purification kit (Thermo Fisher Scientific inc, Rockford, IL). IgG concentration was measured using the BCA protein assay kit (Thermo Fisher Scientific inc., Rockford, IL). Rabbit IgG was labeled with Alexa-488 using the Alexa Fluor® 488 Protein Labeling Kit (Invitrogen, CA) according to the manufacturer's protocol.
Selection of yeast cells expressing immunogenic I. scapularis nymph salivary proteins
To induce surface protein expression, approximately, 1×1010 transformed yeast cells were grown for 24 hours at 28°C and induced with galactose and selected by 4 rounds of MACS sorting, as described earlier [50]. To induce surface protein expression, approximately, 1×1010 transformed yeast cells were grown for 24 hours at 28°C in 100 ml of SGCAA medium (2% galactose, 0.67% yeast nitrogen base, 0.5% bacto amino acids, 30 mM NaHPO4, 62 mM NaH2PO4). After induction with galactose, surface expression was demonstrated by indirect immunostaining with an antibody against the Xpress-epitope located on the N-terminal part of the expressed salivary protein on the yeast cell surface [50]. Ten thousand cells were examined on a FACSCalibur flow cytometer (Beckton Dickinson, Franklin Lakes, NJ) and data analyzed using the FlowJo software (Tree Star, Ashland, OR). The first round of selection was done using AutoMACSTM (Miltenyi Biotec, Auburn, CA). 2×1010 transformed yeast cells were washed 3 times with cold MACS (0.5% BSA, 2 mM EDTA) buffer and pelleted at 600 xg for 10 minutes. Next, cells were resuspended in cold MACS buffer and incubated with 30 µg/ml of purified nymph-immune rabbit IgG and incubated with gentle rotation for 30 minutes at 4°C. Subsequently, cells were washed 2 times and resuspended in 15 ml MACS buffer. 1 ml of goat-anti rabbit microbeads (Miltenyi Biotec, Auburn, CA) was added and incubated for 15 minutes at 4°C. Cells were washed 3 times, resuspended in 150 ml of MACS buffer and subjected to magnetic separation. The sorted cells were grown in SDCAA medium with Pen/Strep for 24 hours at 30°C. Subsequently, the cells were further selected by 3 rounds of MidiMACS sorting under the same conditions as described above. For screening of individual clones, 1×107 induced yeast cells were incubated on a shaking incubator with 33 µg/µl Alexa-488 conjugated nymph-immune rabbit IgG in MACS buffer for 45 minutes at room temperature. The cells were washed and resuspended in MACS buffer and analyzed on a FACSCalibur flow cytometer as described above. Plasmid DNA was isolated from individual positive clones using the ZymoprepTM II Yeast Plasmid Miniprep kit (Zymo research, Orange, CA), transformed into E. coli DH5α (Invitrogen, CA) and plated on LB plates containing 100 µg/ml ampicillin. Plasmid DNA was then isolated from bacterial colonies using the Plasmid Miniprep kit (Qiagen, CA), digested with XhoI and BamHI (New England Biolabs, MA) to assess insert sizes and unique clones sent for sequencing (Keck Facility, Yale University).
Production of recombinant salivary proteins
p8, p19 and p23 cDNAs were cloned in frame into the pMT/Bip/V5-HisA plasmid containing a His tag, V5 epitope, and a blasticidin resistance gene (Invitrogen, CA), and validated by sequencing. Drosophila melanogaster S2 cells were transfected with the plasmids containing p8, p19 or p23 and the blasticidin selection vector pCOBlast using the Calcium Phosphate Transfection Kit (Invitrogen, CA) to generate stable transfectants and protein expression induced in 500 ml cultures with copper sulfate as described by the manufacturer (Invitrogen, CA). The supernatant was filtered using a 0.22-µm filter (Millipore, MA). rP8, rP19 and rP23 were purified from the supernatant by means of the Ni-NTA Superflow column chromatography (Qiagen, CA) and eluted with 250 mM imidazole. The eluted fractions were filtered through a 0.22-µm filter and concentrated with a 5-kDa concentrator (Sigma-Aldrich, MO) by centrifugation at 4°C, washed and dialyzed against PBS. The purity of rP8, rP19 and rP23 was checked by Coomassie blue staining after electrophoreses on SDS 12% polyacrylamide gel and the concentration was determined by BCA protein assay kit (Thermo Fisher Scientific inc., IL).
Serological analysis by immunoblotting
Equal amounts of purified recombinant salivary proteins (100 ng), were electrophoresed on a SDS 12% polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were blocked with PBS containing 5% milk powder and the immunoblots were probed with a 1∶250 dilution of serum. To demonstrate that sera from immunized rabbits recognize tick salivary proteins, nymphal SGE (2 µg) prepared as described earlier [19] was electrophoresed and blotted as positive control. Immunoreactive bands were visualized using horseradish peroxidase conjugated goat anti-rabbit secondary antibodies (Sigma-Aldrich, MO) and the enhanced chemiluminescence Western Blotting Detection System (GE Healthcare, NJ).
Detection of glycosylation modifications on recombinant proteins by Periodic acid-Schiffs (PAS) staining
Periodic acid-Schiff (PAS) staining of glycoproteins after electrophoreses of the 3 proteins (25 µg) on SDS 12% polyacrylamide gel [32] was performed with Salp15 as a positive control [51] and BSA as a negative control according to the manufacturer's specifications (Sigma-Aldrich, MO).
Tick RNA isolation and quantitative RT-PCR
Ticks were fed to repletion on experimental and control animals. Larval ticks were pooled (5 ticks), nymphs and adults were dissected and salivary glands and midguts were pooled (3 ticks), homogenized and RNA was extracted using the RNeasy minikit (Qiagen, CA). The same procedure was done with unfed ticks. cDNA was synthesized using the iScript RT-PCR kit (Biorad, CA) and analyzed by quantitative PCR for the expression of tick actin, p8, p19, p23 and p32 and genes using the primers listed in Table 3 . Quantitative real-time PCR was performed for all tick genes using the iQ Syber Green Supermix (Biorad, CA) on a MJ cycler (MJ Research, CA).
Table 3. Primers used.
| Target | Forward | Reverse |
| Tick actin | GGCGACGTAGCAG | GGTATCGTGCTCGACTC |
| p8 | TTACTGCTGGAACGCTGAGA | TGGCATTCTCCATTTTGACA |
| p19 | GAACGAGAGGCAACAGAAGG | GCGAGCTTCTTGTTCAGGAT |
| p23 | TCAACGCTACTTTCGACACG | ACACGGTCAGAACCTTGTCC |
| p32 | TTAGCATACGCCCCCTACAC | ACGTTTGAACCCTTTGTTGC |
Assay for detection of complement-mediated killing of Borrelia spirochetes
The serum-sensitive Borrelia garinii strain A87S was used (107 spirochetes ml−1) to determine complement-mediated killing as described earlier [8]. Spirochetes (2.5×105) were pre-incubated with bovine serum albumin (BSA), Salp15, rP19, rP23 or rP8 (0.24 µg/µl) respectively for 30 min at 33°C. They were then incubated with 12.5% normal human pooled serum (NHS) or heat-inactivated NHS and examined after 1.5 h and 4.5 h. Serum samples were checked for the absence of Borrelia-specific antibodies by western blot analysis. Heat inactivation of NHS was performed by incubation at 56°C for 30 minutes. Borreliacidal effect was recorded by screening for immobilization and bleb formation of the spirochetes. Immotile spirochetes were considered dead, as described previously [31]. The percentages of non-viable spirochetes from 200 spirochetes per well were assessed.
Thrombin generation
Thrombin generation was initiated by recalcification of human pooled normal plasma in the presence of 1 pM recombinant human tissue factor (Innovin, Siemens Healthcare Diagnostics, Germany), 4 µM phospholipids, 417 µM thrombin substrate (z-Gly-Gly-Arg-AMC) and Salp15, rP23, rP8 or rP19 respectively. A calibrated automated thrombogram was used to assay the generation of thrombin in clotting plasma using a Fluoroskan Ascent microtiter plate reading fluorometer (Thermo Fisher Scientific, MA) and Thrombinoscope software (Thrombinoscope BV, Netherlands) according to the manufacturer's instructions. Thrombin formation was followed for 40 min and measurements were taken at 20 second intervals. The endogenous thrombin potential (ETP), lag time and time-to-peak were calculated using the Thrombinoscope software. Experiments were performed in duplicate and repeated three times.
Immunization of rabbits with recombinant nymphal I. scapularis proteins
Rabbits were immunized subcutaneously with 3 doses containing 50 µg of each purified recombinant protein separate, or with a cocktail composed of 30 µg of each recombinant protein, emulsified with Complete Freund's Adjuvant (first dose) and two subsequent booster injections emulsified in Incomplete Freund's Adjuvant at 3-week intervals. Control rabbits were inoculated with adjuvant and OVA (50 µg and 90 µg for the single and cocktail immunizations, respectively). Two weeks after the last immunization, rabbits were infested with 50 I. scapularis nymphs on the ear of each rabbit and ticks kept in place using socks over each ear. Nymphs that had fed to repletion and detached were weighed. In an independent control experiment, nymphal ticks were weighed and allowed to molt individually at 23°C and 85% relative humidity under a 14 hour light, 10 hour dark photoperiod.
Statistical analysis
The significance of the difference between the mean values of the groups was analyzed using the Student t test with Prism 5.0 software (GraphPad Software, USA). p≤0.05 was considered statistically significant.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants 41440, 49200 and 32947 from the National Institutes of Health (E.F.). E.F. is an Investigator of the Howard Hughes Medical Institute. T.J.S. is supported by a grant from the OLVG (Onze Lieve Vrouwe Gasthuis, Amsterdam) research fund, and S.N. is supported by an Exploratory Research grant from the National Institute of Allergy and Infectious Diseases/NIH. S.D. is supported by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Estrada-Pena A, Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715. doi: 10.1023/a:1006241108739. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM, Valenzuela JG. Tick saliva: from pharmacology and biochemistry to transcriptome analysis and functional genomics, in Ticks – Biology, Disease and Control. Bowman and Nuttall (Cambridge University Press) 2008:92–107. [Google Scholar]
- 3.Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, et al. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 4.Hannier S, Liversidge J, Sternberg JM, Bowman AS. Characterization of the B-cell inhibitory protein factor in Ixodes ricinus tick saliva: a potential role in enhanced Borrelia burgdorferi transmission. Immunology. 2004;113:401–408. doi: 10.1111/j.1365-2567.2004.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A. The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect. 2007;9:247–250. doi: 10.1016/j.micinf.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Tyson K, Elkins C, Patterson H, Fikrig E, de Silva A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol. 2007;16:469–479. doi: 10.1111/j.1365-2583.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- 8.Schuijt TJ, Hovius JW, van Burgel ND, Ramamoorthi N, Fikrig E, et al. The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect Immun. 2008;76:2888–2894. doi: 10.1128/IAI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovius JW, de Jong MA, den Dunnen J, Litjens M, Fikrig E, et al. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog. 2008;4:e31. doi: 10.1371/journal.ppat.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narasimhan S, Koski RA, Beaulieu B, Anderson JF, Ramamoorthi N, et al. A novel family of anticoagulants from the saliva of Ixodes scapularis. Insect Mol Biol. 2002;11:641–650. doi: 10.1046/j.1365-2583.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- 11.Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, et al. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J Biol Chem. 2006;281:26361–26369. doi: 10.1074/jbc.M604197200. [DOI] [PubMed] [Google Scholar]
- 12.Decrem Y, Mariller M, Lahaye K, Blasioli V, Beaufays J, et al. The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. Int J Parasitol. 2008;38:549–560. doi: 10.1016/j.ijpara.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann A, Walsmann P, Riesener G, Paintz M, Markwardt F. Isolation and characterization of a thrombin inhibitor from the tick ixodes ricinus. Pharmazie. 1991;46:209–212. [PubMed] [Google Scholar]
- 14.Trager W. Acquired immunity to ticks. J Parasitology. 1939;25:57–81. [Google Scholar]
- 15.Brossard M, Fivaz V. Ixodes ricinus L.: mast cells, basophils and eosinophils in the sequence of cellular events in the skin of infested or re-infested rabbits. Parasitology. 1982;85(Pt 3):583–592. doi: 10.1017/s0031182000056365. [DOI] [PubMed] [Google Scholar]
- 16.Brossard M, Girardin P. Passive transfer of resistance in rabbits infested with adult Ixodes ricinus L: humoral factors influence feeding and egg laying. Experientia. 1979;35:1395–1397. doi: 10.1007/BF01964030. [DOI] [PubMed] [Google Scholar]
- 17.Nuttall PA, Trimnell AR, Kazimirova M, Labuda M. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 2006;28:155–163. doi: 10.1111/j.1365-3024.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 18.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, et al. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS ONE. 2007;2:e451. doi: 10.1371/journal.pone.0000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke G, Wikel SK, Spielman A, Telford SR, McKay K, et al. Hypersensitivity to ticks and Lyme disease risk. Emerg Infect Dis. 2005;11:36–41. doi: 10.3201/eid1101.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuijt TJ, Hovius JW, van der Poll T, van Dam AP, Fikrig E. Trends Parasitol; 2010. Lyme borreliosis vaccination: the facts, the challenge and the future. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, et al. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J Infect Dis. 2001;184:1056–1064. doi: 10.1086/323351. [DOI] [PubMed] [Google Scholar]
- 23.You M, Xuan X, Tsuji N, Kamio T, Igarashi I, et al. Molecular characterization of a troponin I-like protein from the hard tick Haemaphysalis longicornis. Insect Biochem Mol Biol. 2001;32:67–73. doi: 10.1016/s0965-1748(01)00081-9. [DOI] [PubMed] [Google Scholar]
- 24.Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol. 1997;15:553–557. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- 25.Pepper LR, Cho YK, Boder ET, Shusta EV. A decade of yeast surface display technology: where are we now? Comb Chem High Throughput Screen. 2008;11:127–134. doi: 10.2174/138620708783744516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Luo J, Fan R, Schulte-Spechtel UC, Fingerle V, et al. Characterization of a concealed antigen Hq05 from the hard tick Haemaphysalis qinghaiensis and its effect as a vaccine against tick infestation in sheep. Vaccine. 2009;27:483–490. doi: 10.1016/j.vaccine.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro SZ, Buscher G, Dobbelaere DA. Acquired resistance to Rhipicephalus appendiculatus (Acari: Ixodidae): identification of an antigen eliciting resistance in rabbits. J Med Entomol. 1987;24:147–154. doi: 10.1093/jmedent/24.2.147. [DOI] [PubMed] [Google Scholar]
- 28.Daix V, Schroeder H, Praet N, Georgin JP, Chiappino I, et al. Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Mol Biol. 2007;16:155–166. doi: 10.1111/j.1365-2583.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 29.Francischetti IM, Valenzuela JG, Andersen JF, Mather TN, Ribeiro JM. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood. 2002;99:3602–3612. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- 30.Decrem Y, Rath G, Blasioli V, Cauchie P, Robert S, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206:2381–2395. doi: 10.1084/jem.20091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dam AP, Oei A, Jaspars R, Fijen C, Wilske B, et al. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Nuttall PA. Excretion of host immunoglobulin in tick saliva and detection of IgG-binding proteins in tick haemolymph and salivary glands. Parasitology. 1994;109(Pt 4):525–530. doi: 10.1017/s0031182000080781. [DOI] [PubMed] [Google Scholar]
- 33.Goodman JL, Dennis DT, Sonenshine DE, editors. Washington DC: ASM Press; 2005. Tick-borne diseases of Humans; [Google Scholar]
- 34.Bell JF, Stewart SJ, Wikel SK. Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity. Am J Trop Med Hyg. 1979;28:876–880. [PubMed] [Google Scholar]
- 35.Nazario S, Das S, de Silva AM, Deponte K, Marcantonio N, et al. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am J Trop Med Hyg. 1998;58:780–785. doi: 10.4269/ajtmh.1998.58.780. [DOI] [PubMed] [Google Scholar]
- 36.Askenase PW, Bagnall BG, Worms MJ. Cutaneous basophil-associated resistance to ectoparasites (ticks). I. Transfer with immune serum or immune cells. Immunology. 1982;45:501–511. [PMC free article] [PubMed] [Google Scholar]
- 37.Vancova I, Hajnicka V, Slovak M, Nuttall PA. Anti-chemokine activities of ixodid ticks depend on tick species, developmental stage, and duration of feeding. Vet Parasitol. 2010;167:274–278. doi: 10.1016/j.vetpar.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 38.Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 39.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh CT. Publishers; 2006. Posttranslational Modification of Proteins: Expanding Nature's Inventory: Roberts and Co. [Google Scholar]
- 41.Walker LM, Bowley DR, Burton DR. Efficient recovery of high-affinity antibodies from a single-chain Fab yeast display library. J Mol Biol. 2009;389:365–375. doi: 10.1016/j.jmb.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wadle A, Mischo A, Imig J, Wullner B, Hensel D, et al. Serological identification of breast cancer-related antigens from a Saccharomyces cerevisiae surface display library. Int J Cancer. 2005;117:104–113. doi: 10.1002/ijc.21147. [DOI] [PubMed] [Google Scholar]
- 43.Bidlingmaier S, Liu B. Construction and application of a yeast surface-displayed human cDNA library to identify post-translational modification-dependent protein-protein interactions. Mol Cell Proteomics. 2006;5:533–540. doi: 10.1074/mcp.M500309-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Paroush Z, Finley RL, Jr, Kidd T, Wainwright SM, Ingham PW, et al. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 45.Komachi K, Johnson AD. Residues in the WD repeats of Tup1 required for interaction with alpha2. Mol Cell Biol. 1997;17:6023–6028. doi: 10.1128/mcb.17.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyers M, Jorgensen P. Proteolysis and the cell cycle: with this RING I do thee destroy. Curr Opin Genet Dev. 2000;10:54–64. doi: 10.1016/s0959-437x(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 47.Narasimhan S, Montgomery RR, DePonte K, Tschudi C, Marcantonio N, et al. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci U S A. 2004;101:1141–1146. doi: 10.1073/pnas.0307669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu R, Rowley WA. Relationship between weights of the engorged nymphal stage and resultant sexes in Ixodes scapularis and Dermacentor variabilis (Acari: Ixodidae) ticks. J Med Entomol. 2000;37:198–200. doi: 10.1603/0022-2585-37.1.198. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, et al. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, et al. Isolating and engineering human antibodies using yeast surface display. Nat Protoc. 2006;1:755–768. doi: 10.1038/nprot.2006.94. [DOI] [PubMed] [Google Scholar]
- 51.Hovius JW, Ramamoorthi N, Van't Veer C, de Groot KA, Nijhof AM, et al. Identification of Salp15 homologues in Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 2007;7:296–303. doi: 10.1089/vbz.2006.0624. [DOI] [PubMed] [Google Scholar]