Abstract
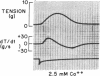
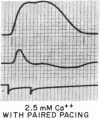
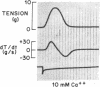
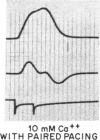
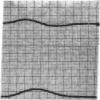
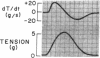
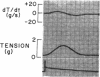
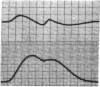
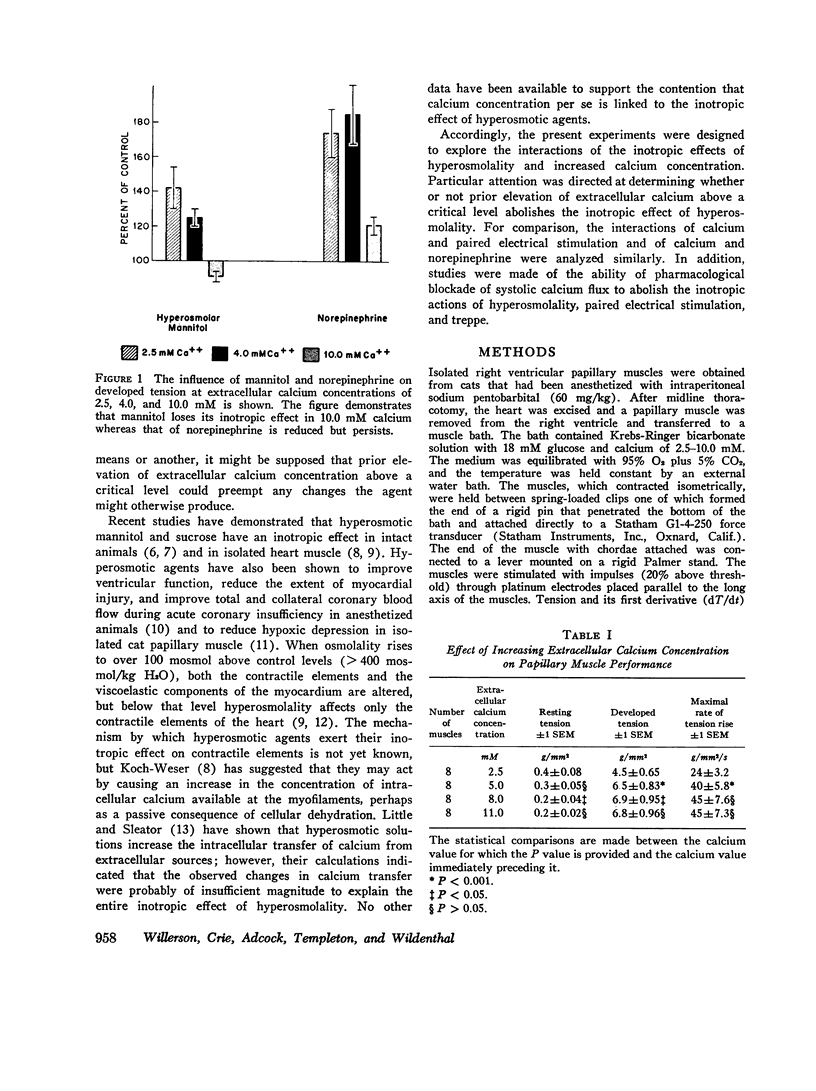
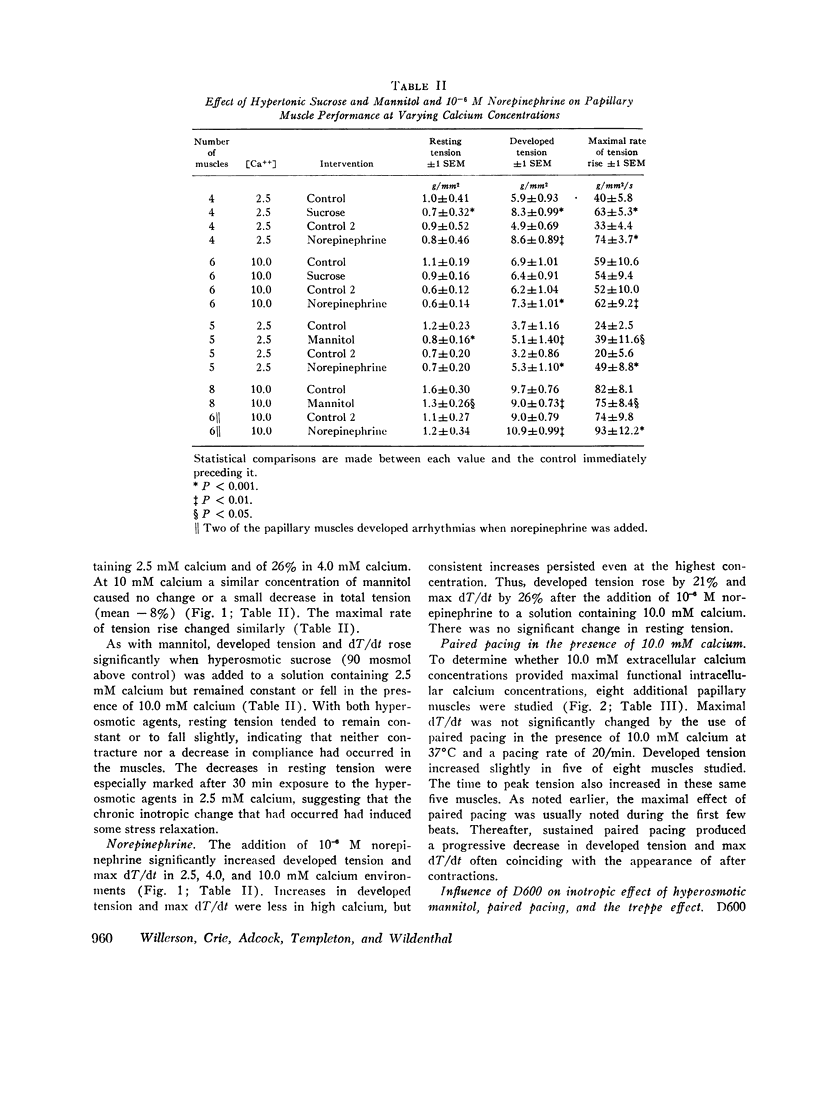
To analyze the interaction of calcium ion concentration with hypertonic agents and with other inotropic interventions, isolated right ventricular cat papillary muscles were studied under isometric conditions in Krebs-Ringer bicarbonate solution. Extracellular calcium concentrations were varied between 2.5 and 11.0 mM. Maximal inotropic effects occurred between 5 and 8.0 mM calcium and further elevation to 11.0 mM was without additional influence. The effect of hyperosmotic sucrose and mannitol on papillary muscle performance was compared with that of 10-6 M norepinephrine at calcium concentrations of 2.5 and 10.0 mM and with paired electrical stimulation in 10.0 mM calcium. Both norepinephrine and the hyperosmotic agents produced significant increases in developed tension and in the maximal rate of tension rise (dT/dt) in Krebs-Ringer in 2.5 and 4.0 mM calcium. In 10 mM calcium norepinephrine increased developed tension and dT/dt, but sucrose and mannitol caused no change or small reductions in both. Paired electrical stimulation, like hyperosmolality, caused no increase in dT/dt in 10 mM calcium.
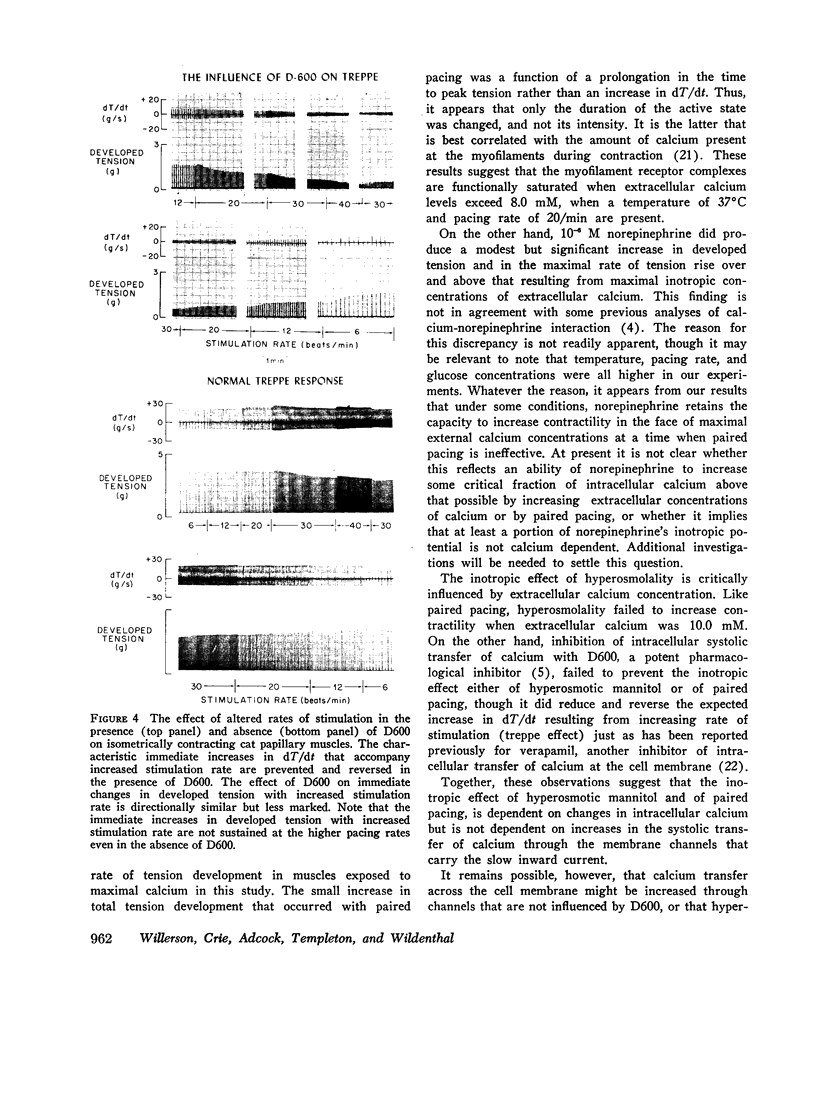
The presence of a potent pharmacological inhibitor of systolic calcium transfer across the cell membrane (D600, 10-6 M) reduced developed tension and dT/dt by 76±2.7 and 74±2.0%, respectively, and prevented and in fact reversed the expected increase in dT/dt associated with an increase in rate of stimulation (treppe). However, hypertonic mannitol and paired pacing persisted in causing marked increases in developed tension and dT/dt even in the presence of D600, suggesting that their inotropic effects are not dependent on increased intracellular transfer of calcium during systole through cell membrane channels in which D600 acts as a competitive inhibitor.
The results of these studies suggest that apparent functional saturation of intracellular calcium receptor sites eliminates any additional inotropic effect of hyperosmolality or paired pacing. The data are compatible with the hypothesis that the inotropic effects of hyperosmolality and of paired pacing result from an increase in calcium concentration at the myofilaments during contraction. The increase induced by hyperosmolality might occur because of an increase in the total amount of calcium released into the cytosol with each action potential and/or as a passive consequence of cellular dehydration. Norepinephrine has the capacity to increase contractility even when intracellular calcium receptor sites appear to be functionally saturated, suggesting that it may act at least in part by a mechanism that is independent of changes in net intracellular calcium concentration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. M., Wildenthal K., Horwitz L. D. Cardiovascular responses to hyperosmotic mannitol in anesthetized and conscious dogs. Am J Physiol. 1973 Jul;225(1):132–137. doi: 10.1152/ajplegacy.1973.225.1.132. [DOI] [PubMed] [Google Scholar]
- Buccino R. A., Sonnenblick E. H., Spann J. F., Jr, Friedman W. F., Braunwald E. Interactions between changes in the intensity and duration of the active state in the characterization of inotropic stimuli on heart muscle. Circ Res. 1967 Dec;21(6):857–867. doi: 10.1161/01.res.21.6.857. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Feigl E. O. Effects of stimulation frequency on myocardial extensibility. Circ Res. 1967 Apr;20(4):447–458. doi: 10.1161/01.res.20.4.447. [DOI] [PubMed] [Google Scholar]
- Friedman W. F., Buccino R. A., Sonnenblick E. H., Braunwald E. Effects of frequency of contraction and ionic environment on the responses of heart muscle to acetylcholine. Circ Res. 1967 Nov;21(5):573–582. doi: 10.1161/01.res.21.5.573. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- KOCH-WESER J. Influence of osmolarity of perfusate on contractility of mammalian myocardium. Am J Physiol. 1963 Jun;204:957–962. doi: 10.1152/ajplegacy.1963.204.6.957. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The intrinsic control of myocardial contraction--ionic factors. N Engl J Med. 1971 Nov 4;285(19):1065–1071. doi: 10.1056/NEJM197111042851910. [DOI] [PubMed] [Google Scholar]
- Little G. R., Sleator W. W. Calcium exchange and contraction strength of guinea pig atrium in normal and hypertonic media. J Gen Physiol. 1969 Oct;54(4):494–511. doi: 10.1085/jgp.54.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAYLER W. G. Influence of hypertonic solutions on ventricular contractile activity. Am J Physiol. 1961 Oct;201:682–686. doi: 10.1152/ajplegacy.1961.201.4.682. [DOI] [PubMed] [Google Scholar]
- NAYLER W. G. The importance of calcium in poststimulation potentiation. J Gen Physiol. 1961 Jul;44:1059–1072. doi: 10.1085/jgp.44.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenblick E. H., Parmley W. W., Buccino R. A., Spann J. F., Jr Maximum force development in cardiac muscle. Nature. 1968 Sep 7;219(5158):1056–1058. doi: 10.1038/2191056a0. [DOI] [PubMed] [Google Scholar]
- Sonnenblick E. H., Ross J., Jr, Covell J. W., Braunwald E. Alterations in resting length-tension relations of cardiac muscle induced by changes in contractile force. Circ Res. 1966 Nov;19(5):980–988. doi: 10.1161/01.res.19.5.980. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Rubio R. Ultrastructural changes produced by hypertonicity in cat cardiac muscle. J Mol Cell Cardiol. 1971 Oct;3(2):139–156. doi: 10.1016/0022-2828(71)90012-5. [DOI] [PubMed] [Google Scholar]
- Suko J., Ueba Y., Chidsey C. A. Intracellular calcium and myocardial contractility. II. Effects of postextrasystolic potentiation in the isolated rabbit heart. Circ Res. 1970 Aug;27(2):227–234. doi: 10.1161/01.res.27.2.227. [DOI] [PubMed] [Google Scholar]
- Templeton G. H., Mitchell J. H., Wildenthal K. Influence of hyperosmolality on left ventricular stiffness. Am J Physiol. 1972 Jun;222(6):1406–1411. doi: 10.1152/ajplegacy.1972.222.6.1406. [DOI] [PubMed] [Google Scholar]
- Ueba Y., Ito Y., Chidsey C. A., 3rd Intracellular calcium and myocardial contractility. I. Influence of extracellular calcium. Am J Physiol. 1971 May;220(5):1553–1557. doi: 10.1152/ajplegacy.1971.220.5.1553. [DOI] [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K., Mierzwiak D. S., Mitchell J. H. Acute effects of increased serum osmolality on left ventricular performance. Am J Physiol. 1969 Apr;216(4):898–904. doi: 10.1152/ajplegacy.1969.216.4.898. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Skelton C. L., Coleman H. N., 3rd Cardiac muscle mechanics in hyperosmotic solutions. Am J Physiol. 1969 Jul;217(1):302–306. doi: 10.1152/ajplegacy.1969.217.1.302. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Powell W. J., Jr, Guiney T. E., Stark J. J., Sanders C. A., Leaf A. Improvement in myocardial function and coronary blood flow in ischemic myocardium after mannitol. J Clin Invest. 1972 Dec;51(12):2989–2998. doi: 10.1172/JCI107126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson J. T., Weisfeldt M. L., Sanders C. A., Powell W. J., Jr Influence of hyperosmolar agents on hypoxic cat papillary muscle function. Cardiovasc Res. 1974 Jan;8(1):8–17. doi: 10.1093/cvr/8.1.8. [DOI] [PubMed] [Google Scholar]