Abstract
Purpose
To investigate patients who had transrectal ultrasonography (TRUS)-guided prostate biopsy to define the role of the serum testosterone level in predicting prostate cancer risk and its association with a high Gleason score.
Materials and Methods
A total of 568 patients who underwent prostate biopsy were entered in this study. We divided the patients into two groups according to serum testosterone level (median level, 3.85 ng/ml): the high-testosterone group (n=285) and the low-testosterone group (n=283). Multivariate regression analysis was used to define the effect of age, prostate volume, serum prostate-specific antigen (PSA) level and PSA density, and serum testosterone level on the risk of prostate cancer and a high Gleason score.
Results
Baseline characteristics did not differ significantly between the two groups. Compared with the high-testosterone group, the low-testosterone group had a significantly higher prostate cancer incidence (38.9% vs. 29.5%, p=0.018). Factors associated with an increased risk of prostate cancer were increased age (odds ratio [OR]=1.08, 95% confidence interval [CI]=1.25-3.16, p=0.001), a high serum PSA level (OR=3.35, 95% CI=2.63-4.25, p=0.001), a low prostate volume (OR=0.183, 95% CI=0.11-0.30, p=0.001), and a low serum testosterone level (OR=1.99, 95% CI=1.25-3.16, p=0.001). Among these, only the serum PSA level was a strong predictor of high-grade prostate cancer (Gleason score ≥7) (OR=2.19, 95% CI=1.57-2.95, p=0.001).
Conclusions
Patients with lower levels of serum testosterone had a higher risk of prostate cancer than did patients with high serum testosterone. Even though a lower serum testosterone level was a predictor of prostate cancer risk, it was not associated with an increased risk of high-grade prostate cancer.
Keywords: Prostatic neoplasms, Risk factors, Testosterone
INTRODUCTION
The morbidity and mortality of prostate cancer is rapidly increasing in Korea. Despite some efforts to identify cancers with an unfavorable prognosis preoperatively, until now, no ideal marker has been recognized. The Gleason score of the specimen, pathological stage (TNM 1997), and prostate-specific antigen (PSA) are prognostic factors for prostate cancer aggressiveness. Although PSA is the leading marker for prostate cancer, it has a low sensitivity and does not have a clear cutoff value that can be used to recommend biopsy. The critical challenge in PSA testing is to discriminate the non-prostate cancer-induced elevation from the prostate cancer-related increase in serum PSA. Consequently, an additional screening test is needed that can further validate the PSA-weighted risk for prostate cancer in the "gray" (4.0-10 ng/ml) diagnostic area. Several parameters have been suggested as surrogate markers, e.g., PSA density, PSA velocity, and the free/total PSA ratio, and attempts are being made to develop new markers, such as prostate-specific membrane antigen and glutathione S-transferases [1-3]. Circulating androgens, particularly testosterone, are known to play fundamental roles in prostate growth. The underlying mechanism of action remains to be identified because androgens mediate complex interactions among many prostate growth factors, hormones, and cell lines. As early as 1941, Huggins and Hodges noted a close relationship between testosterone and prostate cancer [4]. They reported that a noticeable improvement occurred in the clinical status of patients with locally advanced or metastatic prostate cancer who underwent surgical castration.
In contrast, some investigators have reported that prostate cancer is often associated with low testosterone levels [5,6]. We know that testosterone concentrations drop with age and that the incidence of prostate cancer is highest in elderly patients. Despite this, the association of serum testosterone levels with prostate cancer development is not completely understood. In the present study, we investigated patients who underwent transrectal ultrasonography (TRUS)-guided prostate biopsy to define the role of the serum testosterone level in predicting prostate cancer risk and its association with a high Gleason score.
MATERIALS AND METHODS
1. Patients
Between January 2005 and May 2009, 568 patients who were selected as candidates for TRUS-guided prostate biopsy due to a serum PSA level >4 ng/ml or an abnormal result on a digital rectal examination were enrolled in this study. The patients were grouped according to their serum testosterone level (median value, 3.85 ng/ml). The patients whose serum testosterone level was 3.85 ng/ml or higher were classified as the high-testosterone group, and those with a level lower than 3.85 ng/ml were classified as the low-testosterone group, respectively. No patients had ever received testosterone replacement therapy.
2. Serum testosterone measurement and histopathological analysis
Blood samples for testosterone measurement were obtained in the morning before any intervention, when the testosterone concentration is relatively stable and is at its highest level. The testosterone level was measured by radioimmunoassay with the use of the DPC total testosterone kit (Nippon DPC Corp, Tokyo, Japan). Whereas other studies have chosen the hypogonadal range as a cutoff value [7,8], we applied the median value of serum testosterone as the cutoff value.
3. Statistical analysis
The age, PSA, PSA density, and prostate volume of patients in the low- and high-testosterone groups were compared by using Student's t-test. Rates of prostate cancer in the two groups were compared by using Pearson's chi-square test. Multivariate logistic regression analysis was performed to determine the correlation between prostate cancer or a high Gleason score (Gleason score≥7) and age, total PSA, prostate volume, low testosterone, and PSA density. p-values less than 0.05 were considered statistically significant. A statistical software package SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
RESULTS
1. Patient characteristics
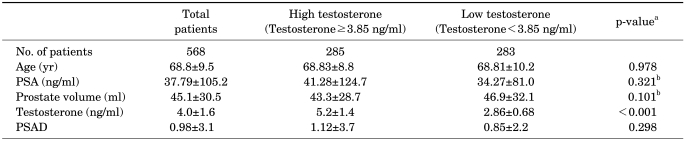
Table 1 shows the characteristics of the 568 patients and their mean testosterone levels. The number of patients in the high- and low-testosterone groups was 285 and 283, respectively. The mean age of all patients was 68.8±9.5 years, their mean PSA was 37.79±105.2, and their mean prostate volume was 45.1 g. The mean serum testosterone level of the high- and low-testosterone groups was 5.2±1.4 and 2.86±0.68 ng/ml, respectively. The mean PSA density of the total patients was 0.98±3.1.
TABLE 1.
Patient characteristics
PSA: prostate-specific antigen, PSAD: prostate-specific antigen density, a: Student's t-test, b: logarithmically adjusted
Table 1 also shows a comparison between the high- and low-testosterone groups. Baseline characteristics such as age, serum total PSA, prostate volume, and PSA density did not differ significantly between the 2 groups. Only the serum total testosterone level was significantly different (p<0.001).
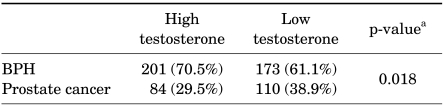
The number of prostate cancer patients in each group was 84 and 110, respectively. Patients with low testosterone had an increased risk of prostate cancer compared with patients with higher testosterone. The ratio of patients with a prostate cancer diagnosis was higher among those with serum testosterone ≤3.85 ng/ml (38.9 vs. 29.5%; p=0.018), as shown in Table 2.
TABLE 2.
Relation between prostate cancer and serum testosterone
BPH: benign prostatic hyperplasia, a: Pearson chi-square test
2. Correlation between prostate cancer and each variable
Factors associated with an increased risk of prostate cancer were increased age (odds ratio [OR]=1.08; 95% confidence interval [CI]=1.05-1.11, p=0.001), a high serum PSA level (OR=3.35, 95% CI=2.63-4.25, p=0.001), low prostate volume (OR=0.183, 95% CI=0.11-0.30, p=0.001), and a low serum testosterone level (OR=1.99, 95% CI=1.25-3.16, p=0.001). PSA density did not have an association with increased risk for prostate cancer (p>0.05) (Table 3).
TABLE 3.
Multivariate logistic regression analysis for estimating prostate cancer risk
PSA: prostate-specific antigen, PSAD: prostate-specific antigen density, a: logarithmically adjusted
3. Correlation between Gleason score and each variable
As shown in Table 4, age, prostate volume, serum testosterone level, and PSA density were not associated with the Gleason score. Only the serum total PSA level had validity for estimating high-grade prostate cancer (OR=2.19, 95% CI=1.59 to 3.00, p<0.001).
TABLE 4.
Multivariate logistic regression analysis for estimating a high Gleason score
PSA: prostate-specific antigen, PSAD: prostate-specific antigen density, a: logarithmically adjusted
DISCUSSION
In this study, patients with low testosterone had an increased risk of prostate cancer compared with patients with high testosterone. Recently, a growing number of studies has suggested that low testosterone is associated with worrisome features of prostate cancer. For instance, lower serum testosterone has been associated with high-grade cancer [8-13], more aggressive disease [14], and advanced pathological stage at the time of radical prostatectomy [15,16].
Hoffman et al indicated that lower serum-free testosterone might be a marker for more aggressive disease [8]. Yano et al reported that serum testosterone levels are an independent significant predictor of a positive prostate biopsy [13].
However, the relationship between serum testosterone and prostate cancer is not yet clear. Some reports showed that there was no relationship between prostate cancer and serum testosterone [17,18]. The cause of the association between high-risk prostate cancer and low serum testosterone is still under investigation. One explanation for the association between low testosterone and prostate cancer is the apparent suppression of testosterone by prostate cancer by the hypothalamic-pituitary axis. Miller et al revealed that prostate cancer inhibits testosterone production, apparently by local production of inhibin or other substances that initiate negative feedback on the hypothalamic-pituitary axis [19]. Indeed, after radical prostatectomy, testosterone concentrations are reported to rise sharply [19].
Also, Risbridger et al evaluated tumor expression of inhibin-α in 174 radical prostatectomy specimens and concluded that elevated expression of inhibin-α is related to a higher risk of PSA failure [20]. According to Lukkarinen et al, these endocrine changes are not seen after simple prostatectomy for benign prostatic hyperplasia (BPH) [21].
The correlation between low serum testosterone and high-risk prostate cancer could be also secondary to hormonal change in chronic disease [22].
Schatzl et al reported a statistically significant difference in mean Gleason score between patients with partial androgen deficiency (testosterone<300 ng/dl) and those without androgen deficiency (testosterone≥300 ng/dl) [12]. Zhang et al found lower testosterone in patients with high-grade tumors than in those with moderate grade tumors or in patients with prostate cancer [11]. They classified patients in the high grade, moderate, or negative group according to Gleason score after transrectal prostate biopsy. Whereas we classified high-grade cancer from a Gleason score of 7, they used a Gleason score of 8 as the cut-off value. The number in the high-grade group was small (n=18). In this study, serum total testosterone did not correlate with Gleason score. There is no clear reason for this discrepancy. Genetic factors and environmental factors might be responsible for the discordance, but further study would be required to resolve this question. As expected, the serum total PSA level was a significant factor for estimating the Gleason score (adjusted OR=2.19; 95% CI=0.34 to 1.34, p<0.001).
There are some differences between our study and others. Most other studies grouped patients according to biopsy results, whereas we divided the patients according to testosterone level. We consider the testosterone level to be a more relevant classification for identifying the relationship between testosterone level and prostate cancer. Also, in our study, we used the median value of testosterone as a cutoff value, whereas other studies applied the hypogonadal range to sort patients into the high- and low-testosterone groups. Moreover, there is the possibility of selection bias in other studies owing to age-matched grouping.
Besides testosterone, estrogen modulates prostate growth, differentiation, and function in several ways [23]. In addition, estrogen is considered to be one of the hormonal risk factors in the development of prostate cancer [24,25]. The principle source of estrogen in men is the aromatization of androgens to estrogens, which takes place predominantly in fatty tissue. Estradiol promotes prostate epithelial cell growth through binding of the estrogen receptor-α and inhibits growth by effects mediated through the estrogen receptor-β [23]. Signoretti and Loda demonstrated that the estrogen receptor-β also plays an important role in the initiation of prostate cancer [26]. Hsing et al concluded that estrogens may have anti-tumor action through their effects on the hypothalamic-pituitary axis, whereas at physiologic levels, estrogen, alone or in conjunction with androgens, may promote tumor growth [27].
Although several studies have revealed the relationship between estrogen and prostate cancer, its role is still controversial. Further prospective studies are essential to demonstrate the true relationship.
Our study had some limitations, including its retrospective nature and the absence of data for free testosterone. Actually, free testosterone is considered the more biologically active form and may be fundamental in predicting prognosis. Hoffman et al obtained results suggesting that low serum free testosterone is a marker of more aggressive disease [8]. Because our results depended on transrectal prostate biopsy, we did not include more precise data such as clinical stage, surgical margin, or cancer-specific survival. The true association between low testosterone and prostate cancer in the long term needs validation in large prospective studies.
CONCLUSIONS
Patients with lower levels of serum testosterone had a higher risk of prostate cancer than did those with high serum testosterone. Even though a lower serum testosterone level was a predictor of prostate cancer risk, it was not associated with an increased risk of high-grade prostate cancer. Further prospective studies are needed to explain the relation between a low serum testosterone level and a poor prognosis of prostate cancer.
Footnotes
The authors have nothing to disclose.
References
- 1.Vergho DC, Heine K, Wolff JM. The role of PSA in diagnosis of prostate cancer and its recurrence. Pathologe. 2005;26:473–478. doi: 10.1007/s00292-005-0789-7. [DOI] [PubMed] [Google Scholar]
- 2.Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res. 2004;10:3943–3953. doi: 10.1158/1078-0432.CCR-03-0200. [DOI] [PubMed] [Google Scholar]
- 3.Singh AS, Chau CH, Price DK, Figg WD. Mechanisms of disease: polymorphisms of androgen regulatory genes in the development of prostate cancer. Nat Clin Pract Urol. 2005;2:101–107. doi: 10.1038/ncpuro0091. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 5.Rannikko S, Adlercreutz H. Plasma estradiol, free testosterone, sex hormone binding globulin binding capacity, and prolactin in benign prostatic hyperplasia and prostatic cancer. Prostate. 1983;4:223–229. doi: 10.1002/pros.2990040302. [DOI] [PubMed] [Google Scholar]
- 6.Kumar VL, Wadhwa SN, Kumar V, Farooq A. Androgen, estrogen, and progesterone receptor contents and serum hormone profiles in patients with benign hypertrophy and carcinoma of the prostate. J Surg Oncol. 1990;44:122–128. doi: 10.1002/jso.2930440213. [DOI] [PubMed] [Google Scholar]
- 7.Morgentaler A, Bruning CO, 3rd, DeWolf WC. Occult prostate cancer in men with low serum testosterone levels. JAMA. 1996;276:1904–1906. [PubMed] [Google Scholar]
- 8.Hoffman MA, DeWolf WC, Morgentaler A. Is low serum free testosterone a marker for high grade prostate cancer? J Urol. 2000;163:824–827. [PubMed] [Google Scholar]
- 9.Nishiyama T, Ikarashi T, Hashimoto Y, Suzuki K, Takahashi K. Association between the dihydrotestosterone level in the prostate and prostate cancer aggressiveness using the Gleason score. J Urol. 2006;176:1387–1391. doi: 10.1016/j.juro.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 10.Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005;14:1262–1269. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 11.Zhang PL, Rosen S, Veerramachaneni R, Kao J, DeWolf WC, Bubley G. Association between prostate cancer and serum testosterone levels. Prostate. 2002;53:179–182. doi: 10.1002/pros.10140. [DOI] [PubMed] [Google Scholar]
- 12.Schatzl G, Madersbacher S, Thurridl T, Waldmüller J, Kramer G, Haitel A, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47:52–58. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 13.Yano M, Imamoto T, Suzuki H, Fukasawa S, Kojima S, Komiya A, et al. The clinical potential of pretreatment serum testosterone level to improve the efficiency of prostate cancer screening. Eur Urol. 2007;51:375–380. doi: 10.1016/j.eururo.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro M, Ruff P, Falkson G. Low serum testosterone and a younger age predict for a poor outcome in metastatic prostate cancer. Am J Clin Oncol. 1997;20:605–608. doi: 10.1097/00000421-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Imamoto T, Suzuki H, Fukasawa S, Shimbo M, Inahara M, Komiya A, et al. Pretreatment serum testosterone level as a predictive factor of pathological stage in localized prostate cancer patients treated with radical prostatectomy. Eur Urol. 2005;47:308–312. doi: 10.1016/j.eururo.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, Amling C, et al. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169:1670–1675. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]
- 17.Stattin P, Lumme S, Tenkanen L, Alfthan H, Jellum E, Hallmans G, et al. High levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective study. Int J Cancer. 2004;108:418–424. doi: 10.1002/ijc.11572. [DOI] [PubMed] [Google Scholar]
- 18.Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Hsing AW, et al. Endogenous sex hormones and the risk of prostate cancer: a prospective study. Int J Cancer. 2008;122:2345–2350. doi: 10.1002/ijc.23326. [DOI] [PubMed] [Google Scholar]
- 19.Miller LR, Partin AW, Chan DW, Bruzek DJ, Dobs AS, Epstein JI, et al. Influence of radical prostatectomy on serum hormone levels. J Urol. 1998;160:449–453. [PubMed] [Google Scholar]
- 20.Risbridger GP, Shibata A, Ferguson KL, Stamey TA, McNeal JE, Peehl DM. Elevated expression of inhibin alpha in prostate cancer. J Urol. 2004;171:192–196. doi: 10.1097/01.ju.0000100048.98807.b7. [DOI] [PubMed] [Google Scholar]
- 21.Lukkarinen O, Hammond GL, Kontturi M, Vihko R. Peripheral and prostatic vein steroid concentrations in benign prostatic hypertrophy patients before and after removal of the adenoma. Scand J Urol Nephrol. 1980;14:225–227. doi: 10.3109/00365598009179566. [DOI] [PubMed] [Google Scholar]
- 22.Iversen P, Rasmussen F, Christensen IJ. Serum testosterone as a prognostic factor in patients with advanced prostatic carcinoma. Scand J Urol Nephrol Suppl. 1994;157:41–47. [PubMed] [Google Scholar]
- 23.Härkönen PL, Mäkelä SI. Role of estrogens in development of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:297–305. doi: 10.1016/j.jsbmb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;27:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 25.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 26.Signoretti S, Loda M. Estrogen receptor beta in prostate cancer: brake pedal or accelator? Am J Pathol. 2001;159:13–16. doi: 10.1016/s0002-9440(10)61666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–235. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]