Abstract
Purpose
To analyze the biochemical recurrence-free and cancer-specific survival after radical prostatectomy in a consecutive series of patients with prostate cancer.
Materials and Methods
We retrospectively reviewed data for 1,822 patients who underwent radical prostatectomy with pelvic lymph node dissection at our institution between 1990 and 2009. After excluding 498 patients who were treated with neoadjuvant androgen deprivation therapy or who were followed up for ≤6 months, we included 1324 patients (mean age, 64.4 years; mean prostate-specific antigen [PSA] level, 12.3 ng/ml). We assessed patient age at the time of surgery, preoperative PSA concentration, biopsy and pathologic Gleason scores, pathologic stage, surgical margin status, disease progression, and survival.
Results
The mean follow-up time was 40 months (range, 6-193 months). The 5- and 10-year biochemical recurrence-free survival rates were 73.2% and 66.2%, respectively, and the 10-year cancer-specific survival rate was 92.4%. The mean time from surgery to biochemical recurrence was 18 months. In the multivariate analysis, Gleason score (4+3 vs. 2-6, p=0.004; 8-10 vs. 2-6, p<0.001), pathologic stage (pT3a vs. pT2, p=0.001; pT3b-4 vs. pT2, p<0.001; pN1 vs. pT2, p<0.001), and resection margin status (p<0.001) were statistically significant predictors of biochemical recurrence, with only pathologic stage (pT3b-4 vs. pT2, p=0.006; pN1 vs. pT2, p=0.010) being a statistically significant predictor of cancer-specific survival.
Conclusions
Radical prostatectomy resulted in favorable cancer control in more than 70% of patients after 5 years and a low (<10%) cancer-specific mortality rate after 10 years. The factors predictive of biochemical recurrence were Gleason score, pathologic stage, and resection margin status.
Keywords: Prostatectomy, Prostatic neoplasms
INTRODUCTION
Prostate cancer is the most frequently detected cancer in males and is the second leading cause of cancer-related death among men in the United States [1]. The widespread use of prostate-specific antigen (PSA) as a diagnostic marker has enabled prostate cancer to be diagnosed in younger patients, in patients with lower PSA concentrations, and in a higher proportion of patients with organ-confined disease, thus reducing the death rate by 20% [2,3]. Large variations in the incidence of and mortality from prostate cancer have been observed among countries and racial/ethnic groups, with the lowest incidence seen among Asian men [4]. Similarly, various clinicopathologic characteristics of prostate cancer have been described in different ethnic groups. In Korea in 2007, prostate cancer was the fifth most common cancer among men, with an incidence of 21.5 per 100,000 men [5]. We found that a significant proportion of prostate cancers in Korean men are poorly differentiated, regardless of initial serum PSA level or clinical stage at presentation; such poorly differentiated tumors adversely affect prognosis, causing a greater rate of PSA failure [6]. Radical prostatectomy (RP) is the treatment of choice for men with localized prostate cancer [7,8]. In addition, surgeons can now anatomically dissect the prostate, thus reducing perioperative morbidity during and after RP [9].
In recent years, the number of RPs has increased dramatically in Korea, but the prognosis after RP has not been reported in Korean men. Here we update the follow-up data on our large series of patients who were treated by RP. We report the 20-year outcomes of 1,324 consecutive men who underwent RP for prostate cancer at our institution between 1990 and 2009.
MATERIALS AND METHODS
We retrospectively reviewed data on 1,822 patients who underwent RP with pelvic lymph node dissection at our institution between 1990 and 2009. After excluding 498 patients who received neoadjuvant androgen deprivation therapy or who were followed up for 6 months or less, we included 1,324 patients (mean age, 64.4 years; mean PSA concentration, 12.3 ng/ml). We assessed patient age at the time of surgery, preoperative PSA level, Gleason scores (of biopsy and pathological specimens), pathologic stage (2002 TNM stage), surgical margin status, disease progression (biochemical and clinical), and survival. Preoperative evaluation included magnetic resonance imaging (MRI) and bone scanning. PSA concentration was measured by immunoradiometry with 125I-IRMA kits (CIS Bio International, Gif-Sur-Yvette, France).
Biopsy results indicated that RP was the first choice of curative treatment, regardless of Gleason score (GS). Patients underwent either open retropubic or robot-assisted laparoscopic RP, as determined by the treating surgeon.
RP specimens were examined microscopically after histological sectioning at multiple levels when routine fixation was concluded. Briefly, each surgical specimen was weighed and the external surface covered in India ink before fixing in 10% formalin. The prostate gland and seminal vesicles were step-sectioned perpendicular to the apicalbasal axis. All lymph nodes were analyzed by routine paraffin sectioning, in addition to evaluation of previous frozen sections. Tumor differentiation was evaluated by surgical Gleason scoring according to WHO consensus conference recommendations [10]. Surgical margins were considered positive when tumor foci were found by microscopy.
All patients were seen 3 and 6 months after surgery, and at least once yearly thereafter. Each visit included a clinical examination and PSA measurement. Biochemical recurrence was defined as any increase in PSA concentration >0.2 ng/ml, determined more than 1 month postoperatively, and increasing on at least two subsequent occasions, or when adjuvant therapy was required for advanced disease.
Fisher's exact test was used to compare percentage frequencies, and Student's t-test was used to compare means. Cancer-specific survival (CSS) and biochemical recurrence-free survival (BCRFS) were analyzed by the Kaplan-Meier method and were compared by the log-rank test. Prognostic factors were identified by univariate analysis, and those that were significant were entered into multivariate analysis by using Cox's regression method. A p-value less than 0.05 was considered significant. All statistical analyses were performed by using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA).
RESULTS
The clinical and pathologic characteristics of the 1,324 included patients are summarized in Table 1. Of these, 342 (25.8%) were aged 60 years or younger, and 90 (6.8%) had serum PSA concentrations below 4.0 ng/ml. Moreover, 814 patients (61.5%) had localized prostate cancer and 80 (6.0%) had lymph node invasion. GSs were 8 or higher in more than 24% of patients and were 2 to 6 in fewer than 24%. Positive resection margins were observed in 402 patients (30.4%).
TABLE 1.
Patient clinical and demographic characteristics
PSA: prostate-specific antigen
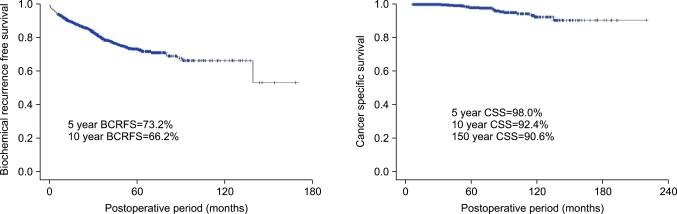
All patients were followed up for at least 6 months (mean, 40 months; range, 6-193 months). The 5- and 10-year BCRFS rates were 73.2% and 66.2%, respectively, and the 10-year CSS rate was 92.4%. The mean time from surgery to biochemical recurrence was 18 months (Fig. 1).
FIG. 1.
Biochemical recurrence-free and cancer-specific survival in patients after radical prostatectomy. BCRFS: biochemical recurrence-free survival, CSS: cancer-specific survival.
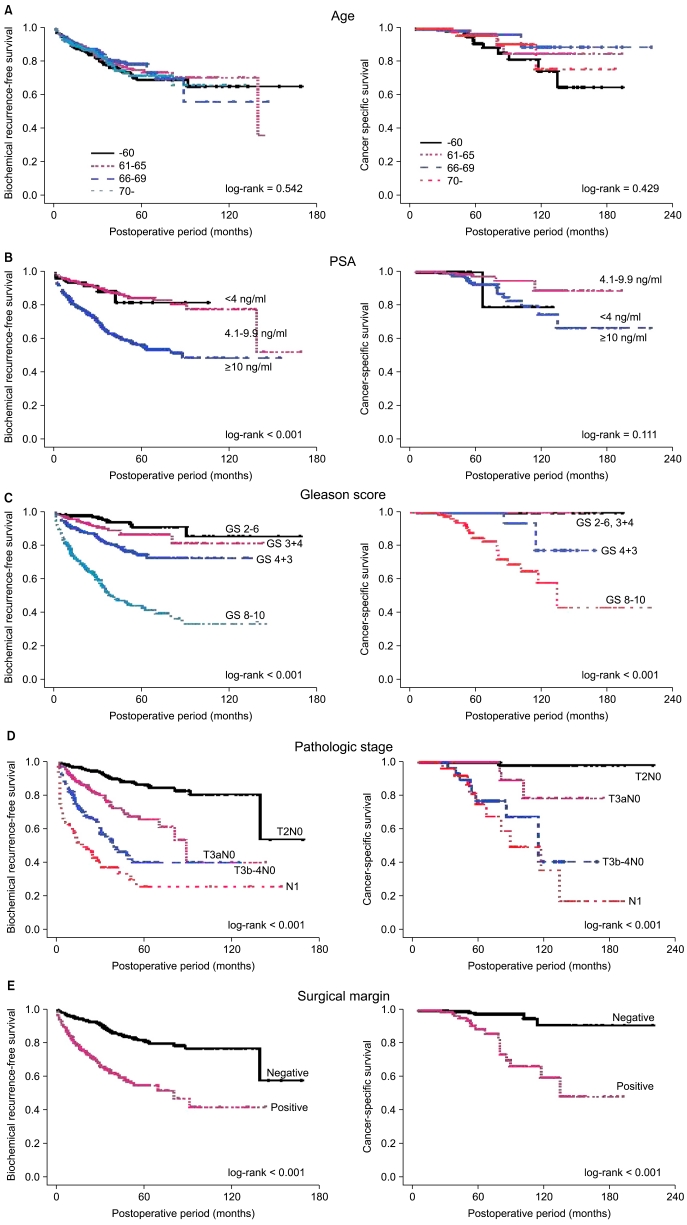
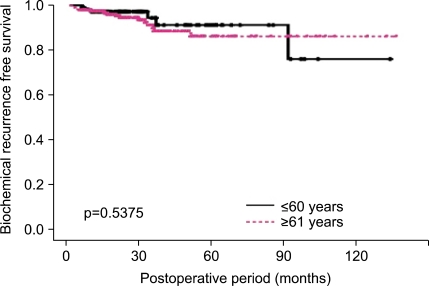
When the patients were divided by age into four groups, those aged ≤60 years, 61-65 years, 66-69 years, and ≥70 years, the 5- and 10-year BCRFS rates and the 10-year CSS rates did not differ significantly (Fig. 2A). A total of 316 patients who underwent RP had insignificant prostate cancer, as defined by Epstein et al [11] (clinical stage ≤T1c, PSA density <0.15 ng/ml/ml, biopsy GS ≤6, and number of positive cores ≤2). A total of 94 (29.4%) patients were younger than 60 years and 222 (70.3%) were older than 61 years. There was a trend for a higher rate of insignificant prostate cancer in the age group younger than 60 years, but the difference was not statistically significant (≤60 years vs. ≥61 years, 27.6% vs. 22.6%, p=0.066). When comparing these two age groups, they were not significantly different regarding pathologic GS, pathologic stage, or positive resection margin (p>0.05). There were no significant differences in patients with upgraded prostate cancer (pathologic GS ≥7; 42.6% vs. 51.4%), patients with advanced prostate cancer (pathologic stage ≥T3a; 17.0% vs. 22.1%), or BCRFS rates between the two age groups (p>0.05) (Fig. 3).
FIG. 2.
Biochemical recurrence-free and cancer-specific survival after radical prostatectomy by clinicopathological factors. PSA: prostate-specific antigen.
FIG. 3.
Biochemical recurrence-free survival in patients with insignificant prostate cancer according to age (≤60 years, ≥61 years).
However, poorly differentiated tumors were significantly more common in patients aged ≥70 years than in those aged ≤60 years (GS 8-10: 22.5% in ≤60 years and 28.9% in ≥70 years). Grouping of patients by preoperative PSA concentration showed that the 5-year BCRFS rate was significantly lower in patients with PSA levels ≥10 ng/ml than in those with PSA <10 ng/ml (56.7% vs. 84.8%, p<0.001), but the 10-year CSS rates did not differ significantly (89.8% vs. 95.5%, p=0.111) (Fig. 2B).
Grouping of patients by pathologic GSs showed that the 5-year BCRFS rates in patients with tumors classified as GS 2-6, 3+4, 4+3, and 8-10 were 91.3%, 86.8%, 74.8%, and 44.0%, respectively (p<0.001). None of the men with tumors classified as GS ≤3+4 died from prostate cancer, whereas those with a GS of 8-10 had a 10-year CSS rate of 83.2% (p<0.001) (Fig. 2C).
The 5-year BCRFS and 10-year CSS rates were 86.6% and 99.3%, respectively, in patients with tumors classified as pathologic stage pT2; 61.2% and 91.4%, respectively, in patients with tumors classified as pT3a; 40.0% and 76.1%, respectively, in patients with tumors classified as pT3b-4; and 25.5% and 74.0%, respectively, in patients with tumors classified as pN1 (p<0.001) (Fig. 2D). Although the 5-year BCRFS rates decreased gradually with pathologic stage, the 10-year CSS rates were considerably lower in patients with seminal vesicle or lymph node invasion than for other patients.
We found that the 5-year BCRFS (54.5% vs. 81.4%, p<0.001) and 10-year CSS (83.9% vs. 96.6%, p<0.001) rates were significantly lower in patients with positive than in those with negative surgical margins (Fig. 2E).
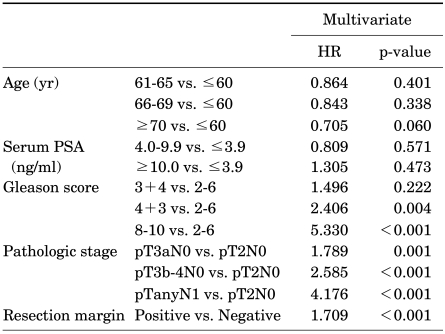
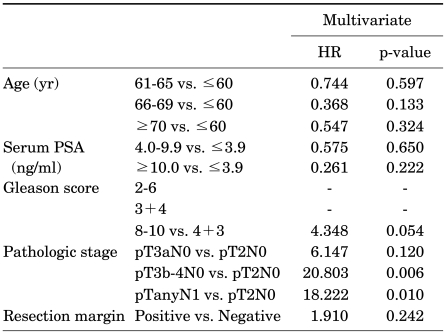
Table 2 and 3 show the multivariate Cox's regression analyses of factors predictive of biochemical recurrence and CSS. In the multivariate analysis, GS (4+3 vs. 2-6, p=0.004; 8-10 vs. 2-6, p<0.001), pathologic stage (pT3a vs. pT2, p=0.001; pT3b-4 vs. pT2, p<0.001; pN1 vs. pT2, p<0.001), and resection margin status (p<0.001) were statistically significant predictors of biochemical recurrence, whereas only pathologic stage (pT3b-4 vs. pT2, p=0.006; pN1 vs. pT2, p=0.010) was a statistically significant predictor of CSS.
TABLE 2.
Multivariate analysis of factors significantly predictive of biochemical recurrence
HR: hazard ratio, PSA: prostate-specific antigen
TABLE 3.
Multivariate analysis of factors significantly predictive of cancer-specific survival
HR: hazard ratio, PSA: prostate-specific antigen
DISCUSSION
We describe here the clinical characteristics and outcomes of a large cohort of men who underwent RP for prostate cancer. We found that 61.5% of our patients had organ-confined disease, 23.2% showed extracapsular extension, 9.3% had seminal vesicle invasion, and 6.0% had lymph node metastases, findings that are similar to previous results from our hospital [12] and also to those reported by others [13,14]. We found, however, that the percentage of men with a preoperative PSA concentration <4 ng/ml and a pathologic GS of 2-6 was lower (6.8% and 23.6%, respectively) than in earlier studies (18-28.1% and 46.1-69%, respectively) [13-17]. Although PSA range distribution and GS have varied from study to study, about 60% of Western men had GSs of 2-6 and more than 70% of those in a recent study had GSs ≥7. The GS distributions in studies of Asian patients were similar, with 46.7% of Chinese and 56.2% of Japanese patients having GSs ≥7 [18,19]. We have already reported on this discrepancy among ethnic groups [6]. The results presented here suggest that, stage-for-stage, prognosis may be similar in Westerners and Asians, except that Korean men may be at greater risk of biochemical relapse because more of their tumors are poorly differentiated.
We found that the overall 10-year BCRFS was 66.2% and that fewer than 10% of our patients died of prostate cancer within 10 years of RP. Although these results confirm that RP affords excellent long-term cancer control, the rates were lower than seen in previous studies of large numbers of patients [14,15,17]. Preoperative PSA concentration, pathologic stage, and GS have been found to be factors predictive of prognosis in patients with prostate cancer who underwent RP [15]. Thus, the presence of a greater proportion of patients with high-grade cancer in a study series may adversely affect surgical outcomes, especially after control for preoperative PSA and pathologic stage, as shown by the high BCRFS and CSS rates observed here.
We found no difference in prognosis according to age. However, an earlier study suggested that younger men present with more treatable disease [20]. We found that a higher percentage of younger men had GSs of 2-6, whereas a greater percentage of older men had GSs of 8-10, although there was no between-group difference in prognosis. Indeed, compared with previous studies, which found that 50% and 30% of patients had GSs of 2-6 and 7, respectively, fewer of our patients had GSs of 2-6 and more of our patients (>50%) had GSs of 7 [13-15,17]. We found, however, that the distribution of GSs did not differ with patient age.
PSA screening results in the detection of disease that is not clinically significant in many patients [21]. Recent studies have shown that there is a trend for a higher rate of preoperative insignificant prostate cancer in younger patients, but no difference in pathologic outcomes between the two age groups. We showed that age is not an important factor for disease-specific mortality in patients with insignificant prostate cancer. Furthermore, pathologic GS ≥7 and advanced prostate cancer was detected in more than 40% and 17%, respectively, of younger patients with insignificant prostate cancer, which indicates that patients with insignificant prostate cancer need definitive treatment.
Although a preoperative serum PSA concentration ≥10 ng/ml was significantly associated with the risk of recurrence, we found that, after controlling for tumor grade, stage, and margin status, PSA level was not predictive of recurrence. In contrast, previous studies found that pretreatment PSA concentration was one of the most predictive prognostic factors in patients undergoing RP [15]. Moreover, although biochemical progression-free survival rates were previously found to differ significantly between patients with PSA levels <4 ng/ml and ≥4 ng/ml [22], we did not observe such a difference in the current study. It may be relevant that, in the cited earlier report, 20% of patients had PSA <4 ng/ml, compared with 6.8% of our patients. Because PSA is highly associated with pathologic stage and GS, we suggest that PSA may not be a predictor of biochemical recurrence in multivariate analysis.
Patients with Gleason stage 8-10 tumors had a 5-year biochemical recurrence rate exceeding 50% and a 10-year cancer-specific death rate of about 20%, which was thus higher than the 10% rate in patients with GSs of 7. After controlling for preoperative PSA concentration, pathologic stage, and resection margin status, we found that the risk of biochemical failure was >5-fold higher for patients with GSs of 8-10 than for those with GSs of 6. The biochemical failure rate did not differ significantly in patients with GSs of 6 and 3+4, in contrast with previous findings, which reported a statistically significant difference in recurrence rate between these two groups of patients [23]. In the cited study, 62% of patients had GSs of 2-6, including 17% with GSs of 2-5, compared with 23.6% of our patients. Interestingly, none of the men with GSs of 2-6 or 3+4 died of prostate cancer.
The 5-year BCRFS rate was <90% in patients with organ-confined disease and approximately 60% in those with extracapsular extension disease, a 10% difference compared with previous studies with large numbers of patients [14,23]. In contrast, the 5-year BCRFS rates of patients with seminal vesicle invasion and lymph node metastases were 40% and 25%, respectively, which is similar to those of previous studies. The differences are likely attributable to variation in the distributions of GSs. In previous studies, about 59% to 69% of patients had GSs of 6 [15,17,23], compared with 23.6% of our patients, including 27.1% with organ-confined disease and extracapsular extension.
We found that 30.4% of our patients had positive surgical margins, higher than the 21.2% of patients in the Surveillance, Epidemiology, and End Results (SEER) program database [24]. However, the cited study included only patients with T2 and T3a prostate cancer, with 86.7% of patients being classified as T2. Pathologic stage has been associated with surgical margin status, with 17.7% of T2 patients and 43.8% of T3a patients in the SEER database having positive margins; the surgical margin positivity rate we observed was only slightly higher. Many studies have demonstrated an association between positive surgical margins and a higher rate of recurrence after radical prostatectomy [24-26]. In agreement with these results, we found that a positive surgical margin was associated with increased risks of biochemical recurrence and cancer-specific death on univariate analysis, but only with biochemical recurrence on multivariate analysis. Compared to patients with negative surgical margins, the risk of biochemical recurrence was 70% higher. Except for the SEER database study, no other report has found a significant association between positive surgical margins and cancer-specific mortality [24].
We found that positive surgical margin, pathologic stage, and GS were predictive of biochemical recurrence in patients with prostate cancer who underwent RP, whereas only pathologic stage was predictive of cancer-specific mortality. Because more than 60% of our patients underwent RP over the last 4 years, our follow-up period is currently too short to evaluate long-term, cancer-specific mortality after RP.
Our study had several important limitations. Our findings represent outcomes from a single institution, over 20 years, and describe the work of eight surgeons who, between them, performed 1,822 consecutive radical prostatectomies. Thus, patient selection and surgical technique may have differed over time, and cancer control rates may have varied somewhat. However, two surgeons (H. A. and C. S. K.) performed over 90% of the RP procedures delivered during our reporting period, thus reducing the probability of surgery-associated variation. In addition, the mean follow-up period of our patients was only 40 months. Further evaluation is needed to determine the long-term rates of CSS and metastatic progression.
CONCLUSIONS
The present study, which presented our intermediate results on work with a large number of Korean patients who underwent RP, shows that long-term PSA recurrence outcomes after RP remain suboptimal, because nearly 50% of men are at risk for PSA recurrence 15 years after surgery. However, our CSS outcomes were excellent, in that about 90% of men remained alive 5 years after surgery. Because favorable cancer control was associated with negative surgical margins, complete resection during prostatectomy for prostate cancer remains important.
Footnotes
The authors have nothing to disclose.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Lubeck DP, Mehta SS, Carroll PR. Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE) J Urol. 2003;170:S21–S25. doi: 10.1097/01.ju.0000095025.03331.c6. [DOI] [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 4.Whittemore AS. Prostate cancer. Cancer Surv. 1994;19-20:309–322. [PubMed] [Google Scholar]
- 5.The statistics report: the incidence of cancer on 2003-2005 and the survival rate on 1993-2005. National Cancer Center. 2009. [accessed Apr 1, 2009]. http://www.ncc.re.kr.
- 6.Song C, Ro JY, Lee MS, Hong SJ, Chung BH, Choi HY, et al. Prostate cancer in Korean men exhibits poor differentiation and is adversely related to prognosis after radical prostatectomy. Urology. 2006;68:820–824. doi: 10.1016/j.urology.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 8.Hammad FT. Radical prostatectomy. Ann N Y Acad Sci. 2008;1138:267–277. doi: 10.1196/annals.1414.032. [DOI] [PubMed] [Google Scholar]
- 9.Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorinis plexus during radical retropubic surgery. J Urol. 1979;121:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 10.Murphy GP, Busch C, Abrahamsson PA, Epstein JI, McNeal JE, Miller GJ, et al. Histopathology of localized prostate cancer. Consensus Conference on Diagnosis and Prognostic Parameters in Localized Prostate Cancer. Stockholm, Sweden, May 12-13, 1993. Scand J Urol Nephrol Suppl. 1994;162:7–42. [PubMed] [Google Scholar]
- 11.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 12.Song C, Kang T, Lee MS, Ro JY, Lee SE, Lee E, et al. Clinico-pathological characteristics of prostate cancer in Korean men and nomograms for the prediction of the pathological stage of the clinically localized prostate cancer: a multi-institutional update. Korean J Urol. 2007;48:125–130. [Google Scholar]
- 13.Xu DD, Sun SD, Wang F, Sun L, Stackhouse D, Polascik T, et al. Effect of age and pathologic Gleason score on PSA recurrence: analysis of 2911 patients undergoing radical prostatectomy. Urology. 2009;74:654–658. doi: 10.1016/j.urology.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 14.Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 15.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ, Jr, Yossepowitch O, Vickers AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter CR, Kodama K, Gibbons RP, Correa R, Jr, Chun FK, Perrotte P, et al. 25-year prostate cancer control and survival outcomes: a 40-year radical prostatectomy single institution series. J Urol. 2006;176:569–574. doi: 10.1016/j.juro.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 18.Egawa S, Suyama K, Arai Y, Tsukayama C, Matsumoto K, Kuwao S, et al. Treatment outcome by risk group after radical prostatectomy in Japanese men. Int J Urol. 2001;8:295–300. doi: 10.1046/j.1442-2042.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- 19.Hsu YS, Wang JS, Wu TT. E-cadherin expression in prostate adenocarcinomas in Chinese and its pathological correlates. Urol Int. 2004;73:36–40. doi: 10.1159/000078802. [DOI] [PubMed] [Google Scholar]
- 20.Khan MA, Han M, Partin AW, Epstein JI, Walsh PC. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology. 2003;62:86–91. doi: 10.1016/s0090-4295(03)00404-7. [DOI] [PubMed] [Google Scholar]
- 21.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr, Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 23.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 24.Wright JL, Dalkin BL, True LD, Ellis WJ, Stanford JL, Lange PH, et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. 2010;183:2213–2218. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245–1250. doi: 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 26.Pettus JA, Weight CJ, Thompson CJ, Middleton RG, Stephenson RA. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol. 2004;172:129–132. doi: 10.1097/01.ju.0000132160.68779.96. [DOI] [PubMed] [Google Scholar]