Abstract
Purpose
The number of patients waiting for kidney transplantation is incessantly increasing, but the number of cadaveric kidney transplantations or ABO-compatible donors is so insufficient that ABO-incompatible kidney transplantation is being performed as an alternative. There are overseas studies and research showing that the 5-year survival rate and 5-year graft survival rate of ABO-incompatible kidney transplantation are not much different from those of ABO-compatible kidney transplantation. However, domestic research on the subject is rare. Therefore, we report the results of 22 ABO-incompatible kidney transplantation cases performed in our hospital.
Materials and Methods
This research was from 22 patients in our hospital who underwent ABO-incompatible kidney transplantation from 15 February 2007 to 20 May 2010.
Results
As yet, there have been no donor graft losses and no deaths after transplantation. The results of the two groups were analyzed by analysis of covariance of the creatinine value of the recipients at 6 months after the operation, corrected for the preoperative value in order to statistically identify whether there were differences in renal function after the operation between ABO-compatible and ABO-incompatible kidney transplantation. The results of the analysis of covariance showed no statistical difference in renal function after the operation between the two groups.
Conclusions
Even though there were not many cases, our initial results for ABO-incompatible kidney transplantation were positive. Considering the increasing number of patients waiting for kidney transplantation, longer-term domestic research studies of ABO-incompatible kidney transplantation are necessary.
Keywords: ABO blood-group system, Kidney transplantation, Living donors
INTRODUCTION
The number of patients with chronic renal failure is increasing along with the aging of the population, and renal transplantation is considered to be the most effective treatment for those patients [1]. On a broad scale, renal transplantation is divided into living kidney transplantation and cadaveric kidney transplantation. Living kidney transplantation in general shows a lower rejection rate, and it has the merit of restoring kidney function both in the short term and in the long term. Thus, living kidney transplantation is widely performed around the world [2]. In addition, owing to advanced kidney transplantation pretreatment and proper usage of immunosuppression, 1-year and 5-year survival rates are increasing [3,4].
Even though the number of patients waiting for kidney transplantation is incessantly increasing, the number of cadaveric renal transplantations or living donors is insufficient [5], and there are frequent transplantations using donors after cardiac death or marginal donors who are brain-dead. But the number of these is also limited [6,7].
Accordingly, ABO-incompatible kidney transplantation is being performed as an alternative. Because ABO-incompatible kidney transplantation may result in hyperacute rejection by ABO antibody, it has been avoided in general. But there have been successful results in Japan with antibody removal by plasmapheresis and injection of gammaglobulin and strong immunosuppressants. This method is now being performed instead of using brain-dead donors for many patients in Japan [8-11]. Studies in other countries, including the research from Tokyo Women's University and Johns Hopkins University, have reported that the 5-year survival rate and 5-year graft survival rate of ABO-incompatible kidney transplantation performed since 2000 are little different from those of ABO-compatible kidney transplantation [3,12]. In Korea, however, such results are rare. In fact, ABO-incompatible kidney transplantation is hardly conducted for various reasons.
Here we report the results of 22 ABO-incompatible kidney transplantations conducted during the latest 3-year and 3-month period in our hospital.
MATERIALS AND METHODS
Of the 83 cases of kidney transplantation in our hospital, 22 cases of ABO-incompatible kidney transplantation were chosen for study, excluding cases of ABO-compatible kidney transplantation. The recipients' and donors' gender, age range, and operation year; their relation; the cause of chronic renal failure; complications; and the survival rate of the graft and patients were recorded.
This study was based on the comparison of ABO-compatible and ABO-incompatible kidney transplantation. In order to identify whether there were statistically significant differences in postoperative renal function between the two groups, we used analysis of covariance (ANCOVA), in which the dependent variable was the creatinine value of the recipients at 6 months after the operation, and the main independent variable was ABO-compatibility (the two groups). We controlled for the preoperative creatinine level. Statistical analysis was conducted by using STATA ver. 11.0 (STATA Corp., College Station, TX, US).
To discern acute rejection, patients who showed certain abnormal symptoms from blood tests or clinical experiments were further examined by ultrasound-guided kidney biopsy. Graft failure was defined as the condition that the patient could not survive without dialysis and needed re-dialysis owing to the recurrence of renal failure or nephrectomy after transplantation. Each result was compared with that of 61 ABO-compatible kidney transplantations. ABO-incompatible kidney transplantation is, as its literal meaning indicates, the transplantation of kidney between a donor and a recipient with different blood types.
The inclusion criteria of our hospital for ABO-incompatible renal donors were the following: systolic blood pressure below 140 mmHg; diastolic blood pressure below 90 mmHg; quantitative proteinuria test result below 300 mg/24 h; no specific findings on the physical examination, general urine analysis, urine culture, excretory urography, and CT angiography; and results for general blood, serum electrolyte, and general biochemistry tests within the normal range. The exclusion criteria of our hospital for ABO-incompatible renal donors were drug addicts; incurable psychotics; patients with malignancy, abnormal urinary tract systems such as vesicoureteral reflex, or systemic disease such as severe infection and vascular diseases; and HIV-, HBV-, and HCV-positive respondents [13]. In addition, donors who had severe obesity, hypertension at the age of 40 years or younger, or were 60 years of age or older were also excluded. Meanwhile, even though they had a different blood type, kidney transplantations from O-type donors to other recipients, and from A- or B-type donors to AB-type recipients, were not classified as ABO-incompatible cases.
As kidney transplant pretreatment, rituximab was administered once a month intravenously before the operation without splenectomy. Before September 2008, 375 mg/m2 of rituximab was administered; after that date, 187 mg/m2 was administered. Plasmapheresis was started 7 to 14 days before kidney transplantation and plasma volume was replaced with 5% albumin. For plasmapheresis just before the operation or before and after kidney biopsy, equal amounts of albumin and fresh frozen plasma were used as the replacement in order to supplement the coagulation factors. In addition, 100 mg/kg IVIG was administered intravenously right after each plasmapheresis. Before transplantation, plasmapheresis was performed every day or every other day until the IgG or IgM ABO antibody titer was below 8. After transplantation, it was performed selectively only for the following cases for 2 weeks. First, plasmapheresis was performed when the IgG or IgM ABO antibody titer measurement was over 256 before rituximab treatment. Second, it was performed when the ABO antibody increased three phases more than the lowest measurement after the operation for 2 weeks. Third, when serum creatinine increased more than 15% to 20% after the operation for 2 weeks, plasmapheresis was performed empirically for 2 to 3 days until the target level was reached.
Tacrolimus, mycophenolate mofetil (MMF), and prednisolone were administered from the first plasmapheresis stage. Patients were treated with tacrolimus for 1 month until their blood concentration reached 8 to 12 ng/ml and after that until it reached 4 to 8 ng/ml. Mycophenolate was administered during the early 3 months at a dosage of 750 mg bid and after that at 500 mg bid. Steroids were administered orally at a dose of 0.3 mg/kg/day before the operation and then at 5 mg/kg bid intravenously on the day of the operation, at 4 mg/kg qd intravenously on the next day, at 2 mg/kg qd intravenously on the third day, and at 0.3 mg/kg/day on the fourth day and then gradually reduced by the third month after the operation but kept at 5 mg/day after that time.
Acute rejection was monitored both by the organizational diagnosis for kidney biopsy rejection with increased serum creatinine and by impressive diagnosis for cases not showing other reasons for disorder of kidney function clinically but showing symptoms of acute rejection. Because the first 3 weeks are a highly dangerous period for antibody-mediated rejection, kidney biopsy was performed for all cases when serum creatinine increased more than 15% to 20%.
RESULTS
The results of 22 ABO-incompatible kidney transplantations performed in our hospital over the past 3 years and 3 months were compared with those of 61 ABO-compatible kidney transplantations performed during the same period.
1. ABO-incompatible kidney transplantation by year
The first ABO-incompatible kidney transplantation was performed on February 15, 2007, and there were a total of 22 cases through May 20, 2010, with 2 cases in 2007, 11 in 2008, 5 in 2009, and 4 in 2010.
A total of 61 cases of ABO-compatible kidney transplantation were performed through May 20, 2010, with 12 cases in 2007, 19 in 2008, 23 in 2009, and 7 in 2010.
2. Characteristics of kidney donors and recipients
1) Range of age and gender
The ABO-incompatible donors' age range was 19-58 years with an average of 39 years. Of the 22 donors, 7 were males and 15 were females. The ABO-incompatible recipients' age range was 33-61 years and the average age was 45 years. Fourteen of the recipients were males, and the remaining 8 were females. The ABO-compatible donors' age range was 19-59 years with an average age of 41 years. Thirty of the compatible case donors were males and 31 were females. The ABO-compatible recipients' ages ranged from 22 to 64 years, and the average age was 4 years. The gender composition was 45 males and 16 females.
2) Cause of renal disease and dialysis before transplantation
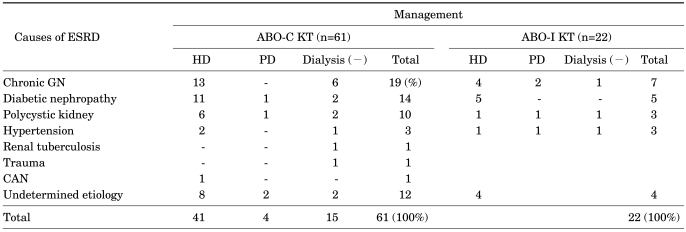
The cause of the recipients' end-stage renal disease and the status of dialysis before transplantation were as shown in Table 1.
TABLE 1.
Underlying renal disease and treatment before renal transplantation
ESRD: end stage renal disease, ABO-C KT: ABO-compatible kidney transplantation, ABO-I KT: ABO-incompatible kidney transplantation, HD: hemodialysis, PD: peritoneal dialysis, GN: Glomerulonephritis, CAN: chronic allograft nephropathy
3) Relation between donors and recipients and blood analysis
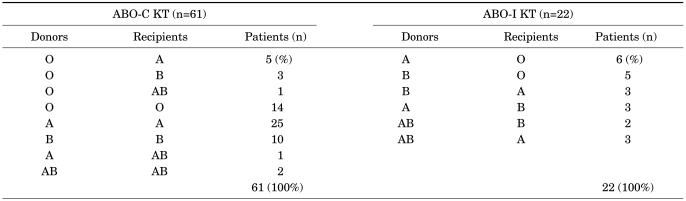
The ABO-incompatible donors' relations were husband (8), sibling (7), wife (2), parents (2), and others (3). The ABO-compatible donors' relations were husband (15), sibling (14), wife (6), parents (10), and others (16). The results of the blood analyses were as shown in Table 2.
TABLE 2.
Donor and recipient ABO type
ABO-C KT: ABO-compatible kidney transplantation, ABO-I KT: ABO-incompatible kidney transplantation
4) Operation method and changes in creatinine level
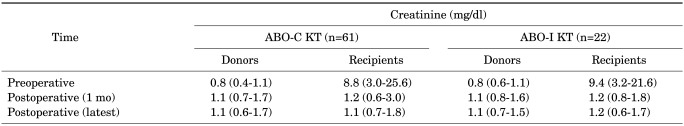
There were 17 left donor nephrectomies and 5 right donor nephrectomies. With respect to the operation method, there were 13 open donor nephrectomy (ODN) procedures and 9 hand-assisted laparoscopic donor nephrectomy (HALDN) procedures. Creatinine levels were as shown in Table 3. The creatinine level of the donors and the recipients was checked before and 1 month after the operation. There was an attempt to compare the creatinine level of both recipients and donors after 6 months or 1 year. However, even though the recipients' follow-up (F/U) was available for 6 months and 1 year, the donors' follow-up was not available on the scheduled date. Therefore, the latest creatinine figures for recipients and donors were checked.
TABLE 3.
Creatinine of donors and recipients
ABO-C KT: ABO-compatible kidney transplantation, ABO-I KT: ABO-incompatible kidney transplantation
5) Comparison of renal function after the operation between ABO-compatible and ABO-incompatible kidney transplantation
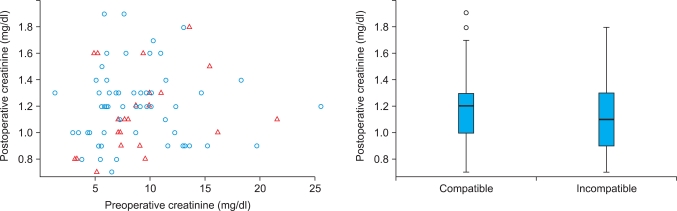
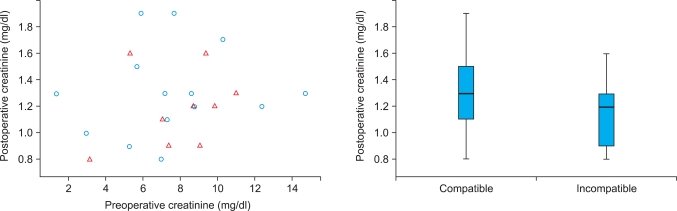
To compare the ABO-incompatible and ABO-compatible groups, we used analysis of covariance in which we controlled for the initial level of creatinine. In the ANCOVA, the null hypothesis was that the two groups were not different; the alternative hypothesis was that they differed. Because the p-value for the variable 'ABO compatibility' was 0.51, it was not statistically significant. Fig. 1 further assures such a conclusion. Fig. 1 displays the decrease in the creatinine level after the operation in each group. We can see that the postoperative result was slightly better on average for cases of ABO-incompatible transplants. However, the difference was not statistically different. In sum, the statistical analysis (ANCOVA), as well as the descriptive statistics, showed no statistical difference in renal function after the operation between the two groups.
FIG. 1.
Comparison of renal function after the operation between ABO-compatible and ABO-incompatible kidney transplantation; n=83 cases. Circles are compatible cases, and triangles are incompatible ones.
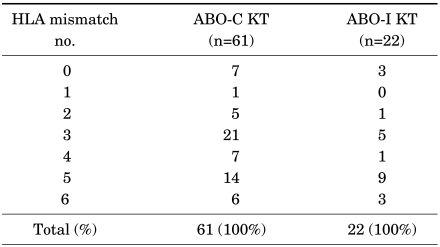
6) Comparison of human leukocyte antigen (HLA) antigen mismatched number
We compared the HLA mismatched number among 6 antigens, consisting of 1 pair of HLA-A, -B, and -DR. The HLA antigen mismatched results for ABO-compatible and ABO-incompatible kidney transplantation were as shown in Table 4. Because the number of cases of ABO-incompatible kidney transplantation was too small for drawing inferences in other groups, we compared the postoperative creatinine level between the ABO-compatible and -incompatible groups by using HLA mismatch no. 5, which had the largest number of ABO-I KT cases. The average for 9 ABO-incompatible kidney transplantation cases 1 month after operation was 1.2 (0.8-1.8), and the more recent average was 1.1 (0.8-1.8). On the other hand, the average for 14 ABO-compatible kidney transplantation cases 1 month after the operation was 1.4 (0.8-3.0), and the recent average was 1.3 (0.8-1.7). There were no graft losses in either case.
TABLE 4.
HLA mismatch number of ABO-I KT and ABO-C KT
ABO-I KT: ABO-incompatible kidney transplantation, ABO-C KT: ABO-compatible kidney transplantation, HLA: human leukocyte antigen
With the mismatch no. 5 cases, we conducted analysis of covariance to discern the effect of ABO-compatibility on the postoperative creatinine level. The numbers of cases were 14 and 9 for ABO-compatible and ABO-incompatible kidney transformation, respectively. As for the previous results with a greater number of cases, there was no statistically significant difference between the two groups, with the p-value associated with the variable ABO-incompatibility being 0.32 from the ANCOVA (Fig. 2). Hence, on the basis of these results, we can conclude that there was no difference in renal function after the operation between the two groups. ABO-incompatible kidney transplantation did not show any worse result than ABO-compatible kidney transplantation, even under the consideration of HLA mismatch.
FIG. 2.
Comparison of renal function after the operation between ABO-compatible and ABO-incompatible kidney transplantation: human leukocyte antigen (HLA) mismatch no. 5; n=23 cases.
3. Operative complications
The median time of follow-up was 12 months (range, 1-40 months), and no particular complications occurred for either donors or recipients, except for one recipient whose symptoms were not related to ABO-incompatible transplantation.
In one case, the recipient had delayed graft failure from exsanguination during the operation, received hemodialysis for 2 weeks, and was then treated with reduced tacrolimus. He showed acute cellular rejection IA in the kidney biopsy on the 14th day after the operation, but his ABO antibody titer showed the law 2 and then recovered shortly after receiving treatment including steroid pulse therapy.
Even though there was an antibody-mediated rejection related to ABO-incompatible transplantation, the patient recovered after plasmapheresis and is in good condition at the present. There was 1 post-transplantation case of diabetes mellitus, but it has been controlled well by diabetes medicine, and 4 cases of urinary tract infection, which were cured by antibiotics.
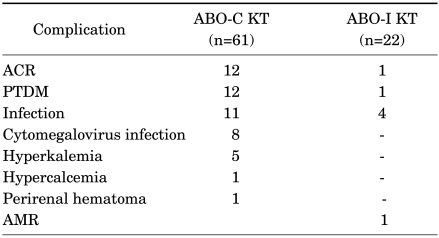
There were no other complications such as cytomegalovirus infection or carcinoma. On the other hand, there were complications of ABO-compatible kidney transplantation as shown in Table 5. All complications including acute cellular rejection have been controlled well with proper treatment until now.
TABLE 5.
Complications of recipients
ABO-C KT: ABO-compatible kidney transplantation, ABO-I KT: ABO-incompatible kidney transplantation, ACR: antibody cellular rejection, PTDM: posttransplantation diabetes mellitus, AMR: antibody-mediated rejection
4. Survival rate of grafts and patients
During the follow-up period of 1 to 40 months, there was no graft loss, and to date, no patients have died after the operation in either the ABO-incompatible or the ABO-compatible group.
DISCUSSION
The number of patients who are waiting for kidney transplantation is increasing more and more. In the current domestic situation in which cadaveric transplantation is not activated, the number of cadaveric grafts is insufficient. Organ donation is also not sufficient, and the number of living kidney transplantations is not increasing either [5]. According to the Korean Network for Organ Sharing (KONOS), in 2009, the number of approved living donor cases was 800 and the number of marginal donors from brain-dead persons was 495, for a combined 1295 cases of kidney transplantation. On the other hand, the number of patients waiting for kidney transplantation was 2,309 in 2000, 3,878 in 2003, 5,672 in 2006, and 8,488 in 2009 [5]. With the increasing number of patients in need of kidney transplantation, domestic hospitals began considering ABO-incompatible kidney transplantation. In fact, it has been only 10 years since the results for ABO-incompatible kidney transplantation became similar to those for ABO-compatible kidney transplantation. Although Hume tried to perform kidney transplantation between a B-type cadaveric donor and an O-type recipient for the first time in 1955, he reported that the transplanted kidney did not function [14]. Alexandre et al reported the results of 23 cases of ABO-incompatible kidney transplantation in 1987, but the success rates were lower than for ABO-compatible kidney transplantation, showing a 1-year graft survival rate of 79% and a 1-year patient survival rate of 88% [15,16].
Until the late 1990s, studies in Japan also reported lower records of success of ABO-incompatible transplantation than for ABO-compatible kidney transplantation. Since 2000, however, thanks to advanced immunosuppression, there have been reports that the results of ABO-incompatible kidney transplantation show little difference from those of ABO-compatible kidney transplantation in the aspect of 1-year and 5-year survival rates [3,4]. For example, Ishida et al reported obvious advancement in ABO-incompatible kidney transplantation by showing that the 1-year and 5-year graft survival rates of 117 patients using tacrolimus and mycophenolate mofetil during 2000 to 2004 were 94% and 90%, respectively, whereas those of 105 patients using cyclosporine and zazthioprine performed in a single center during 1989 to 1999 were 78% and 73%, respectively [3]. Tydén et al also reported advanced results for ABO-incompatible kidney transplantation since 2001 by showing 60 successful cases by use of a protocol before and after the operation that excluded blood antibody by immune absorption and used rituximab instead of splenectomy. They reported no antibody-mediated rejection during the follow-up period, which averaged 17 months, and that all of the grafts were living except in one case who stopped taking immunosuppression and in one case who died [17].
Acute antibody-mediated rejection by blood antibodies takes place mainly in the critical period of the early 1 to 3 weeks just after the operation. Takahashi et al reported that all 20 acute antibody-mediated rejections (AMRs) out of 441 ABO-incompatible patients occurred within the early 3 weeks: 17 (85%) were within 1 week and 3 were within 2 to 3 weeks [18]. This means that the danger of graft loss by ABO-incompatible transplantation is higher during the early days after the transplantation. Actually, the data on Japan's ABO-incompatible kidney transplantation show that the graft loss occurs intensively right after the operation, and its rate of loss after that period is no different from that of ABO-compatible cases [19]. Therefore, the success of ABO-incompatible transplantation may be dependent on keeping blood antibodies low at a safe level for the early 3 weeks. When antibody-mediated rejection is prevented by proper treatment during the early weeks after the operation, the longer term results of ABO-incompatible transplantation can be just as good as for ABO-compatible kidney transplantation.
As such, there have been studies from other countries providing good results owing to advanced pretreatment and immunosuppression. But domestic reports on ABO-incompatible kidney transplantation have been very rare, except for a few cases.
We have performed 22 cases of ABO-incompatible kidney transplantation since February 2007. In all 22 cases, rituximab was used instead of splenectomy and plasmapheresis was performed before and after the operation as mentioned above. In addition, tacrolimus, mycophenolate mofetil, and prednisolone were administered in proper doses for immunosuppression.
Until now, there has not been any graft or patient loss during the average follow-up period of 12 months (range, 1-40 months), except for 1 antibody-mediated rejection. Furthermore, there was no statistical difference in renal function after the operation between ABO-compatible and ABO-incompatible kidney transplantation.
CONCLUSIONS
Since 2000, there have been reports that the results of ABO-incompatible kidney transplantation show little difference from those of ABO-compatible kidney transplantation in the aspect of 1-year and 5-year survival rates owing to the proper usage of immunosuppression. The follow-up survey conducted by our hospital also showed the same results concerning patient and transplanted kidney survival rates. In addition, the comparison of renal function according to creatinine after the operation between the two groups also showed no statistical difference.
However, our study was conducted with only 22 cases, and the follow-up period was at most 38 months. Thus, further research will be necessary with a longer term follow-up period and many more subjects.
Footnotes
The authors have nothing to disclose.
References
- 1.Park CG, Lee JJ, Park HY. Donor factors influencing the graft survival of kidney transplants. Korean J Urol. 1999;40:1355–1359. [Google Scholar]
- 2.Wolf JS, Jr, Merion RM, Leichtman AB, Campbell DA, Jr, Magee JC, Punch JD, et al. Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation. 2001;72:284–290. doi: 10.1097/00007890-200107270-00021. [DOI] [PubMed] [Google Scholar]
- 3.Ishida H, Miyamoto N, Shirakawa H, Shimizu T, Tokumoto T, Ishikawa N, et al. Evaluation of immunosuppressive regimens in ABO-incompatible living kidney transplantation-single analysis. Am J Transplant. 2007;7:825–831. doi: 10.1111/j.1600-6143.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 4.Ichimaru N, Takahara S. Japan's experience with living-donor kidney transplantation across ABO barriers. Nat Clin Pract Nephrol. 2008;4:682–692. doi: 10.1038/ncpneph0967. [DOI] [PubMed] [Google Scholar]
- 5.Korean Network for Organ Sharring (KONOS) Seoul: KONOS; 2009. KONOS annual report, 2009 [internet] Available from: http://www.konos.go.kr. [Google Scholar]
- 6.Cortesini R, Pretagostini R, Bruzzone P, Alfani D. Living unrelated kidney transplantation. World J Surg. 2002;26:238–242. doi: 10.1007/s00268-001-0211-4. [DOI] [PubMed] [Google Scholar]
- 7.Audard V, Matignon M, Dahan K, Lang P, Grimbert P. Renal transplantation from extended criteria cadaveric donors: problems and perspectives overview. Transpl Int. 2008;21:11–17. doi: 10.1111/j.1432-2277.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Satio K. Present status of ABO-incompatible kidney transplantation in Japan. Xenotransplantation. 2006;13:118–122. doi: 10.1111/j.1399-3089.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe K, Takahashi K, Sonda K, Tokumoto T, Ishikawa N, Kawai T, et al. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation. 1998;65:224–228. doi: 10.1097/00007890-199801270-00014. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K. Accommodation in ABO-incompatible kidney transplantation: why do kidney grafts survive? Transplant Proc. 2004;36(2 Suppl):193S–196S. doi: 10.1016/j.transproceed.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Takahara S, Uchida K, Oshima S, Toma H, Sonoda T. Successful results after 3 years tacrolimus immunosuppression in ABO-incompatible kidney transplantation recipients in Japan. Transplant Proc. 2002;34:1604–1605. doi: 10.1016/s0041-1345(02)03039-7. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, et al. Plasmaphresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4:1315–1322. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang KH, Lee JO, Han BH. The renal function and the preoperative predictive factors influencing renal function after living donor nephrectomy. Korean J Urol. 2004;45:149–157. [Google Scholar]
- 14.Hume DM, Merrill JP, Miller BF, Thorn GW. Experiences with renal homotransplantation in the human: report of nine cases. J Clin Invest. 1955;34:327–382. doi: 10.1172/JCI103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandre GP, Squifflet JP, De Bruyere M, Latinne D, Moriau M, Ikabu N. Splenectomy as a prerequisite for successful human ABO-incompatible renal transplantation. Transplant Proc. 1985;17:138–143. [Google Scholar]
- 16.Alexandre GP, Latinne D, Carlier M, Moriau M, Pirson Y, Gianello P, et al. ABO-incompatibility and organ transplantation. Transplant Rev. 1991;4:230–241. [Google Scholar]
- 17.Tydén G, Donauer J, Wadström J, Kumlien G, Wilpert J, Nilsson T, et al. Implementation of a protocol for ABO-incompatible kidney transplantation--a three-center experience with 60 consecutive transplantations. Transplantation. 2007;83:1153–1155. doi: 10.1097/01.tp.0000262570.18117.55. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K. Recent findings in ABO-incompatible kidney transplantation: classification and therapeutic strategy for acute antibody-mediated rejection due to ABO-blood-group-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007;11:128–141. doi: 10.1007/s10157-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, et al. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4:1089–1096. doi: 10.1111/j.1600-6143.2004.00464.x. [DOI] [PubMed] [Google Scholar]