Abstract
The histaminergic system is exclusively localized within the posterior hypothalamus with projection to almost all the major regions of the central nervous system. Strong and consistent evidence exist to suggest that histamine, acting via H1 and/or H3 receptor has a pivotal role in the regulation of sleep-wakefulness. Administration of histamine or H1 receptor agonists induced wakefulness, whereas administration of H1 receptor antagonists promoted sleep. The H3 receptor functions as an auto-receptor and regulates the synthesis and release of histamine. Activation of H3 receptor decreased histamine release and promoted sleep. Conversely, blockade of H3 receptor promoted wakefulness. Histamine release in the hypothalamus and other target regions was highest during wakefulness. The histaminergic neurons displayed maximal activity during the state of vigilance, and cease their activity during NREM and REM sleep. The cerebrospinal levels of histamine were reduced in diseased states where hypersomnolence was a major symptom. The histamine deficient HDC KO mice displayed sleep fragmentation and increased REM sleep during the light period along with profound wakefulness deficit at dark onset, and in novel environment. Similar results were obtained when histamine neurons were lesioned. These studies strongly implicate the histaminergic neurons of the TMN to play a critical role in the maintenance of high vigilance state during wakefulness.
Keywords: Histamine, tuberomammillary nucleus, sleep, wakefulness, REM sleep, narcolepsy, orexins, H1 receptor, H3 receptor
INTRODUCTION
The state of wakefulness is an ensemble of multiple and coherent behaviors that allows the interaction with the external world. It is the manifestation of increased activity in the cortex (cortical activation or desynchronization). Increased activation of cortex is the result on concerted increase in the activity of multiple neuronal aggregates, localized in various brain regions, and utilizing multiple neurotransmitters. Each system is distinct and has a special role in the maintenance of wakefulness. For example, the glutamatergic neurons of brainstem reticular formation and the cholinergic neurons of the ponto-mesencephalic tegmentum and basal forebrain display maximal activity during wakefulness and rapid eye movement (REM) sleep and may promote cortical activation during wakefulness and REM sleep. In contrast, the monoaminergic systems namely, the norepinephrine containing locus coeruleus neurons, the serotonin containing neurons of the raphe nuclei and the histamine containing neurons of the tuberomammillary nucleus (TMN) are unique in the sense that these groups increase their discharge during wakefulness and completely cease firing during REM sleep. It is believed that the monoaminergic systems act in concert with other arousal systems to maintain wakefulness and inhibit REM sleep. We have focused this review on the histaminergic neurons of the TMN in the control of sleep-wakefulness. An interested reader is encouraged to consult more detailed reviews about other arousal systems and their role in sleep-wakefulness (1–5).
HISTORY OF HISTAMINE
It was in early 20th century when the sedative side effects of antihistamines were first discovered. This propelled histamine into the central nervous system (CNS) and histamine was termed as a “waking substance” (6). While research on other monoamines (norepinephrine and serotonin) in the CNS thrived during the first half of 20th century, research on histamine lagged behind, mainly because fluorescent histochemistry that revealed the presence of norepinephrinergic and serotonergic systems in the brain was unable to localize the histaminergic system in the CNS (7*), It was only after immunohistochemical studies revealed the presence of histaminergic system in the brain that histamine was awarded the coveted status of the “neurotransmitter” (8*;9*). Subsequent electrophysiological and biochemical studies demonstrated the presence of four G-protein coupled metabotropic receptors, H1, H2, H3 and H4 in the CNS (10; 11).
During the last decade, availability of numerous pharmacological and molecular tools to selectively manipulate histaminergic transmission has led to significant advancement into our understanding of the functional role of histamine in the CNS. It is now believed that histaminergic neurons plays a critical role in the regulation of various behavioral and physiological functions including arousal, stress, learning and memory, pain perception, fluid balance, thermoregulation and various neuroendocrine functions. This review is focused on the role of histamine in sleep-wakefulness. However, an interested reader is encouraged to read excellent reviews on role of histamine in vertebrates and invertebrates (12–15).
ANATOMICAL LOCALIZATION OF HISTMINE NEURONS
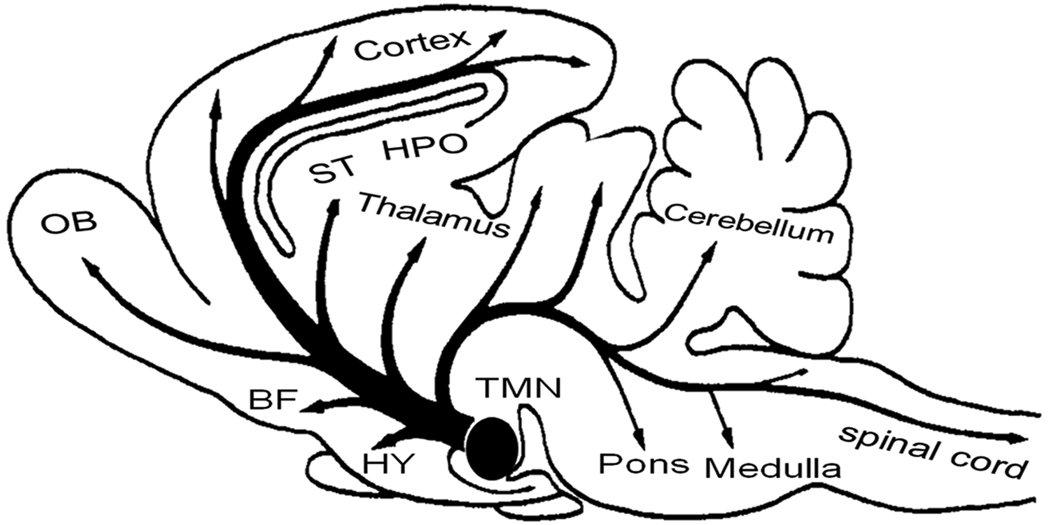
The histamine containing neurons (Fig 1) are mainly localized in the tuberomammillary nucleus [(TMN) derived from tuber cinerium meaning a pale swelling; see (16*)] and adjacent areas within the posterior hypothalamus. The TMN, (the name derived from tuber cinerium meaning a pale swelling) was named by Malone and consists of several dense clusters of large, characteristic neurons, as well as scattered neurons with the same morphology and staining properties in surrounding, more heterogeneous regions. It is located rostral to the mammillary bodies and caudal to optic chiasm forming the floor of the third ventricle in the posterior hypothalamus (16). In all mammalian species examined, the histaminergic neurons are mainly localized within the TMN and/or surrounding regions, although minor differences do exist. For examples, in humans, the histamine containing neurons are distributed in and around the TMN (7). Although majority of histaminergic neurons in the mouse brain are localized within the TMN, the TMN is less compact and contains fewer and smaller neurons (17). In contrast, the TMN in cat brain is compact and localized mainly in the ventrolateral posterior hypothalamus (18). In guinea pigs, the histaminergic neurons are more widely distributed than in the rat and the mouse (19).
Figure 1.
Schematic representation of the location and distribution of the histaminergic system in the brain. The histamine containing neurons are localized in the tuberomammillary nucleus (TMN) within the posterior hypothalamus and send projections throughout the brain. Abbreviations: BF = basal forebrain; HPO= hippocampus; HY = hypothalamus; OB = Olfactory bulb; ST= Striatum; TMN = tuberomammillary nucleus; Adapted from (118).
Ericson and his colleagues divided the TMN of rats into various subdivisions (20). The largest population of histaminergic neurons, termed as the ventral subgroup, was situated at the ventral surface of the brain, rostral and caudal to the mammillary bodies. The medial subgroup was situated on each side of the mammillary recess. The diffuse part of TMN consisted of a small group of neurons scattered within the posterior hypothalamus. There is no evidence to suggest that these different subgroups have different projections. The TMN is considered as a single functional unit of histamine containing neurons, although recent studies indicate functional heterogeneity (16; 21).
In addition to histamine and it synthesizing machinery, some TMN neurons also contains glutamate decarboxylase (GABA synthesizing enzyme), adenosine deaminase (enzyme involved in degradation of adenosine; only in rats but not in mice), galanin, substance P, and pro-enkephalin-derived peptides (16;22;23). The functional significance of these co-localized neuroactive substances is somewhat unclear.
The TMN neurons targets almost all major regions of the central nervous system, especially the wake-promoting basal forebrain and the orexinergic lateral hypothalamus and receives strong galanin and gamma amino butyric acid (GABA) inputs from the ventrolateral preoptic region (10;24)
SYNTHESIS AND METABOLISM OF HISTAMINE
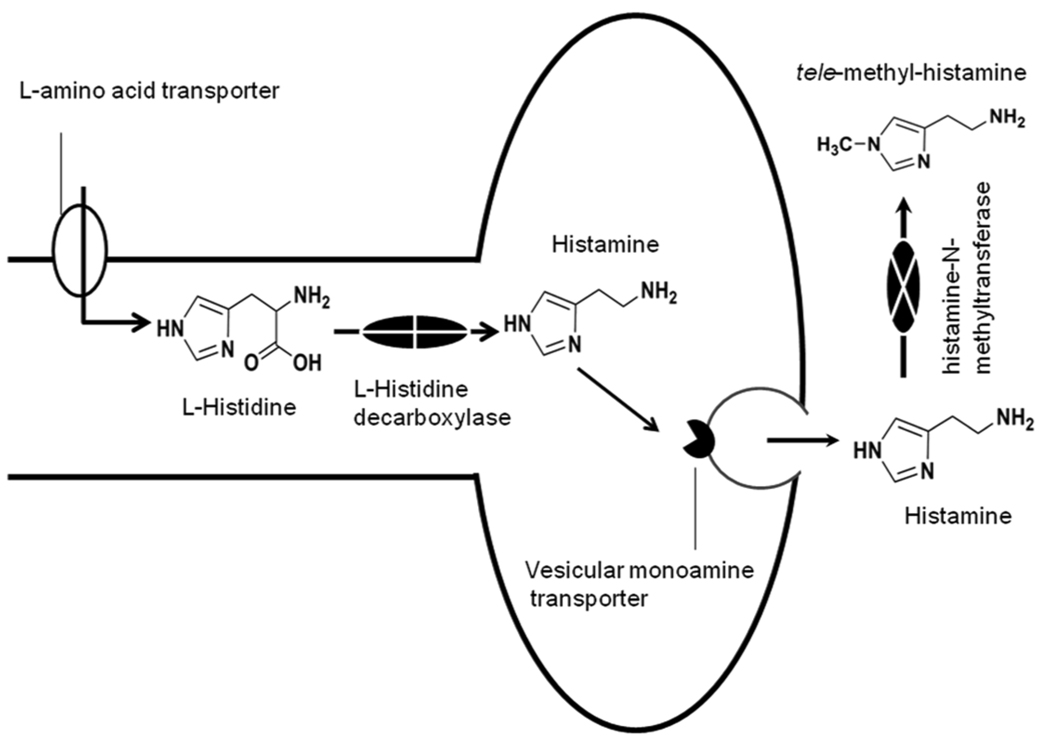
Two distinct pools of histamine exist in the brain: neuronal and the non-neuronal pool. All brain histaminergic actions are the result of histamine released by histamine neurons in the TMN. The histamine contribution from the non-neuronal pool (mainly by mast cells) is limited (10).The blood brain barrier is impermeable to histamine. Histamine in the brain is formed from the essential amino acid L-histidine (Fig 2). Histamine synthesis occurs in two steps: 1) neuronal uptake of L-histidine by L-amino acid transporter and, 2) subsequent decarboxylation of L-histidine by a specific enzyme L-histidine decarboxylase (HDC; E.C. 4.1.1.22). The rate limiting step for histamine synthesis is the availability of L-histidine. The enzyme HDC is selective for L-histidine. Once synthesized, histamine is taken up into the vesicles by vesicular monoamine transporter and stored until released. The histamine levels in the brain are lower as compared to other monoamines, however histamine has a fast turnover (half-life <30 min) that varies significantly with the functional state. Unlike other monoamines, there is no high affinity uptake system for histamine.
Figure 2.
Histamine synthesis and metabolism in neurons. The L-histidine is transported into neurons by L-amino acid transporter. Once inside the neuron, L-histidine is converted into histamine by a specific enzyme histidine decarboxylase. Subsequently histamine is taken up into vesicles by the vesicular monoamine-transporter and stored until released. In absence of high affinity uptake mechanism in the brain, released histamine is quickly degraded by histamine methyltransferase which is located post-synaptically and in glia to tele-methyl-histamine, a metabolite that does not show any histamine-like activity.
Histamine, once released, is inactivated by methylation almost exclusively by the enzyme histamine-N-methyltransferase (E.C. 2.1.1.8) in the CNS. The tele-methyl-histamine is subsequently degraded by monoamine oxidase-B (MAO-B) and aldehyde dehydrogenase to produce tele-methylimidazoleacetic acid (7; 18)
HISTAMINE IN SLEEP-WAKFULNESS
The first study examining the effects of antihistamines on sleep-wakefulness was performed in cats and reported increased NREM sleep coupled with reduced REM sleep (25). Similar results were also obtained in dogs and humans (26; 27). Intra-ventricular (icv) application of histamine in anesthetized rat caused a dose dependent decrease in narcosis duration, whereas in conscious animals, it produced classical signs of wakefulness including EEG desynchronization, increased grooming and exploratory behavior. While pre-treatment with H1 receptor antagonists blocked the effects of histamine, the H2 antagonists had no effect (28–30).
In rodents, histamine synthesis, release, and degradation peaked and troughed in a circadian fashion with the highest levels observed during the dark period, when wakefulness is a predominant state and lowest during the light period when sleep is the predominant state (31; 32). These initial findings suggested that histamine is responsible for the modulation of behavioral and cortical arousal via its action on histamine receptors in the brain.
Parallel research implicated the posterior hypothalamus (where the histaminergic neurons were later discovered) in the generation of arousal. It was Von Economo (1930), who first observed that lesion of the posterior hypothalamus produced sleep [cf: (33)]. Bilateral lesions in the area of the mammillary bodies induced somnolence in monkeys [Ranson, 1939, cf: (34*)]. Bilateral transections of the posterior hypothalamus reduced wakefulness in rats [Nauta, 1946, cf: (34)]. Localized and reversible inactivation by cooling the posterior hypothalamus induced behavioral sleep in rats (35). Bilateral electrolytic lesions of the posterior hypothalamus and sub-thalamic region produced a state of continuous sleep for more than one day in rats (36) and cats (37). These studies suggested that the posterior hypothalamus may contain the “wakefulness center”. These findings provided the initial impetus to pursue further research to unravel the role of histamine and posterior hypothalamus in sleep-wakefulness. The next cycle of research, substantiating the role of histamine in the sleep-wakefulness consisted mainly of pharmacological, electrophysiological, and biochemical/molecular and lesion studies.
PHARMACOLOGICAL STUDIES IN ANIMALS
The bulk of evidence supporting the role of histamine in control of wakefulness was derived from pharmacological studies in animal models. For easier reading these preclinical studies are divided into subgroups and described below:
Manipulation of histamine synthesis and degradation and its effects on sleep-wakefulness
The α-fluoro-methyl-histidine (α-FMH) is an irreversible inhibitor of HDC. A single systemic injection of α-FMH (10–50 mg/kg) can produce up to 90% inhibition of HDC activity within 60–120 min resulting in marked depression of histamine levels (34).
Systemic (intraperitoneal; ip) administration of α-FMH (50 and 100 mg/Kg) resulted in decreased wakefulness and increased NREM sleep in rats and in cats (38–40). The sleep inducing effect of α-FMH began after 8 hours and lasted for 24 hr (40). Bilateral administration of α-FMH (50 µg/1 µL) into the TMN induced sleep within 2 hours. The effects lasted for 9 hours (40). Bilateral administration of α-FMH into the preoptic region of cats produced similar results (41; 42).
Local TMN microinjections of SKF-91488 (50 µg/1 µL), a specific inhibitor of the catabolic enzyme histamine-N-methyltransferase, in cats immediately increased wakefulness and reduced sleep (both NREM and REM phases). The effects lasted for 6 hours (43).
These data suggests that reduction of endogenous histamine by pharmacological blockade of histamine synthesis reduced wakefulness. Conversely, increase in endogenous histamine by pharmacological blockade of histamine breakdown increased wakefulness.
Administration of histamine and its effect on sleep-wakefulness
Bilateral application of histamine into the TMN region increased arousal and latency to sleep coupled with reduction in NREM sleep in a site-specific, dose-dependent manner in cats. The highest dose (60 pg) produced maximal increase in wakefulness that lasted for 6 hours suggesting that histamine is a potent wakefulness promoting agent. Pretreatment with the histamine H1 receptor antagonist mepyramine completely blocked arousal inducing effects of histamine suggesting that the histamine mediates it wakefulness promoting effect via H1 receptors (40; 43). Similar effects were observed when histamine was bilaterally administered into the pontine tegmentum or preoptic region of cats. Pretreatment with H1 receptor antagonist mepyramine attenuated arousal inducing effects of histamine (41; 42).
Repeated low frequency stimulation of the midbrain reticular formation increased cortical EEG spectral power in low frequency bands (0–6 Hz). This effect was blocked by central (icv) administration of histamine and reversed by simultaneous administration of H1 receptors antagonist, but not H2 receptors antagonist (44; 45).
Bilateral reverse microdialysis administration of histamine into the cholinergic basal forebrain of rats produced a site specific, dose-dependent increase in wakefulness with a concomitant decrease in NREM sleep without affecting REM sleep implicating that histamine induced wakefulness may be mediated via the cholinergic basal forebrain (46).
Administration of H1 receptor agonists/antagonist and its effect on sleep-wakefulness
The H1 receptor is a typical G protein-coupled metabotropic receptor (~490 amino acids) with seven putative transmembrane domains (47). Encoded from the intronless region on human chromosome 3, the H1 receptor is coupled to phospholipase C through a pertussis toxin-insensitive (Gq/11) G protein (48). The H1 receptor is widely distributed throughout the brain with high to moderate levels found in sleep-wakefulness regulatory regions including the basal forebrain, locus coeruleus, raphe nuclei, mesopontine tegmentum and the thalamus (49).
Systemic (ip) administration of the first generation H1 receptor antagonists pyrilamine and diphenhydramine decreased W and increased NREM sleep in rats. Central administration of the H1-receptor agonist 2-thiazolylethylamine, dose-dependently, increased wakefulness and decreased both NREM and REM sleep. Furthermore, the H1 receptor antagonists pyrilamine blocked the wakefulness inducing effects of 2-thiazolylethylamine (50).
Bilateral administration of 2-thiazolylethylamine (50 µg/0.5 µL) into the pontine tegmentum of cats increased wakefulness and reduced NREM sleep during the first 3 hr post-injection. Conversely, bilateral application of mepyramine (5 µg/0.25 µL) into the pontine tegmentum of cats reduced wakefulness and increased NREM sleep suggesting that histamine may mediate wakefulness via activation of H1 receptors in wakefulness regulatory mesopontine tegmentum (42). Systemic injections of H1 receptor antagonist mepyramine (1 and 5 mg/Kg) dose-dependently increased NREM sleep and decreased wakefulness, REM sleep and latency to sleep within 1 hour after injections. However, local application of mepyramine (120 µg/µL) into the TMN reduced wakefulness and increased sleep (both NREM and REM phases) without affecting NREM sleep latency (40).
Systemic administration of H1-antagonists, promethazine, diphenhydramine, or chlorpheniramine had no effect on sleep-wakefulness in rats housed on sawdust. However, rats housed on grid above water displayed increased NREM sleep suggesting that the H1-antagonists had potent sedative effects only when the histaminergic system is activated (51).
Administration of H2 receptor agonists/antagonist and its effects on sleep-wakefulness
The H2 receptor is coupled to adenylyl cyclase via the GTP-binding Gs protein. Activation of H2 receptor causes an accumulation of cAMP and activation of protein kinase A (52). Medium to low densities of H2 receptor are observed in sleep-wakefulness regulatory centers including the thalamus, basal forebrain, posterior hypothalamus, locus coeruleus, raphe nuclei and TMN (53). However, central or systemic administration of H2-receptor agonists and/or antagonists had no effect on sleep-wakefulness in rats (50;54). Similar results were obtained after local application of H2 receptor agonist, impromidine (0.2µg/0.25 µL), in the pontine tegmentum. However, application of impromidine (1 µg/1 µL), into the preoptic region of the cat induced wakefulness (41; 42).
Administration of H3 receptor agonists/antagonist and its effects on sleep-wakefulness
Identified first in 1983, the H3 receptor functions as an auto-receptor and regulates histamine release (55). Coupled to pertussis toxin sensitive Gi/o protein, the H3 receptor has high constitutive signaling activity in vivo, a unique characteristic that is rarely observed in vivo (56; 57).
Oral administration of thioperamide, an H3 receptor antagonist, produced a dose dependent and protracted wakefulness. The highest dose (10 mg/Kg) produced maximal increase (>50 %) in wakefulness that lasted for 10 hours. The arousal effects of thioperamide were prevented by pretreatment with (R) α-methyl-histamine (H3 agonist; 20 mg/kg) or mepyramine (1mg/Kg). Conversely, oral administration of (R) α-methyl-histamine to cats enhanced NREM sleep in a dose dependent manner. The highest dose of (R) α-methyl-histamine (20 mg/Kg) increased NREM sleep (>50%) that lasted for 6 hr (58). These were among the first studies to demonstrate the role of H3 receptors in the regulation of wakefulness.
While systemic administration of thioperamide increased wakefulness and reduced sleep in a dose dependent fashion, systemic administration of (R) α-methyl-histamine had no effect on sleep in rats. In contrast, bilateral application of (R) α-methyl-histamine into the TMN region increased NREM sleep with a concomitant decrease in wakefulness and REM sleep suggesting that activation of H3 receptor in the TMN is likely to block histamine release resulting in reduced wakefulness. The NREM inducing effect of (R) α-methyl-histamine was attenuated if the rats were pretreated with thioperamide (59).
Oral administration of BP 2.94 (20–30 mg/Kg) produced a dose dependent increase in NREM sleep without affecting wakefulness or REM sleep in rats. Pretreatment with H3 receptor antagonist carboperamide (30 mg/Kg) prevented the sleep inducing effects of BP 2.94 in rats. Conversely, oral administration of carboperamide (20–30 mg/Kg) produced a dose dependent increase in wakefulness with a concomitant decrease in NREM and REM sleep (60). Similarly, oral administration of H3 receptor agonist BP 2.94 induced a dramatic increase in NREM sleep in cats (61*).
Oral administration (10 mg/kg) of H3 receptor agonist, Sch 50971, depressed locomotor activity and increased total sleep time in guinea pigs (62). In contrast, oral administration of ciproxifan (0.15–2 mg/kg), a H3 receptor antagonist, induced wakefulness in cats (63). However, ip injections of immepip (5 or 10 mg/kg; H3 receptor agonist) in rats did not produce major changes in sleep, although cortical histamine release was significantly reduced (64).
Systemic (subcutaneous; sc) administration of thioperamide (10 mg/kg), in H3 receptor (−/−) knockout (H3R-KO) mice had no effect on sleep. However, increased wakefulness coupled with decreased NREM sleep was observed during the first 2 h after administration (at lights-on) in wild type mice. REM sleep remained unaffected (65).
Systemic injections (1–10 mg/Kg; sc) of a highly selective and a novel non-imidazole H3 receptor antagonist, 1-[4-(3-piperidin-1-yl-propoxy)-benzyl]-piperidine (JNJ-5207852) increased wakefulness with a concomitant decrease in sleep (both NREM and REM phases) coupled with reduced total delta power in mice and rats (66).
Vanni-Mercier and his colleagues (67) investigated the effects of H3 receptor ligands on the discharge activity of TMN neurons in freely behaving cats. Systemic (intramuscular; im) injection of ciproxifan (1 mg/kg) increased wakefulness and rapidly (> 15 min) increased the activity of wake-active TMN neurons for 2 hours. Local TMN application of (R)-α-methyl-histamine blocked the effects of ciproxifan. Conversely, systemic injection of the H3 receptor agonist imetit (S-[2-4-(imidazolyl)ethyl]isothiourea), a (1 mg/kg, i.m.), reduced the activity of wake-active TMN neurons for 3 hour. Behaviorally the animals appeared drowsy and the EEG displayed increased slow wave and spindles. The effects of imetit were reversed by systemic injection of ciproxifan. Local administration of (R)-α-methyl-histamine into the TMN, dose dependently, decreased the activity of wake-active TMN neurons without affecting behavioral states. The highest dose (0.1 µg/0.2 µl) ceased the activity completely within 30 min post-injection (67).
Acute oral administration of GSK189254 (H3 receptor antagonist; 3 and 10 mg/kg) increased wakefulness and reduced sleep (NREM and REM phases) in wild type (orexin +/+) and orexin KO mice and reduced episodes of narcolepsy (sleep onset REM sleep) in orexin KO mice. However, after chronic administration of GSK189254 (10 mg/kg; twice daily for 8 days), the effect on wakefulness was significantly reduced in both wild types and orexin KO. In addition, significant increase in narcoleptic episodes was observed in orexin KO (68).
In another study, oral administration of tiprolisant (H3 antagonist; 20 mg/kg) in orexin KO, at dark onset, promoted wakefulness coupled with increased theta and gamma activity in the EEG and reduced episodes of narcolepsy (sleep onset REM sleep). However, there was no significant difference in cortical levels of tele-methyl histamine (histamine metabolite) between wild type (orexin (+/+) and orexin KO suggesting that histamine levels were not altered. Interestingly, co-administration of modafinil [currently used drug for the treatment of excessive daytime sleepiness in narcolepsy; 64g/kg; orally] with tiprolisant (orally, 20 mg/Kg) was strongly enhanced the effects of tiprolisant (69).
As described above, strong pharmacological evidence exists to suggest that administration of H3 receptor antagonist produced strong wakefulness. Interestingly, acute administration of H3 receptor antagonist reduced narcoleptic episodes and increased wakefulness, however, repeated dosing of H3 antagonist resulted in increased narcoleptic episodes coupled with reduced effects on wakefulness.
PHARMACOLOGICAL STUDIES IN HUMANS
As described above, the first generation anti-histamine can easily penetrate the blood brain barrier and cause drowsiness and sedation. Several of these anti-histamines including the non-selective HI receptor antagonists from the phenothiazine class and “over the counter” diphenhydramine, have been examined for their effects on daytime sleepiness as well as on subjective and objectives measures of nocturnal sleep in healthy human subjects sleep and are extensively reviewed elsewhere (70;71). We have sampled some studies and presented here.
Administration of promethazine at bed time, in healthy volunteers, induced a dose dependent reduction in REM sleep followed by a significant increase in REM sleep (REM rebound) on post-drug “withdrawal” night. Significant increases in Stage 2 NREM sleep (50 and 100 mg) and Stage III and IV (200 mg) were also observed (26). In the second part of the same study, administration of promethazine for 9 days (100 mg at bed time) induced a profound suppression of REM sleep that peaked on day 1 and returned to placebo values by day 9, followed by REM rebound on post-drug withdrawal day 10 (26). In contrast, Adam and Oswald (1986) reported increased stage II NREM after 20 and 40 mg dose of promethazine. Reduction in REM sleep was observed only with the 40 mg dose (72). Self assessment of sleep quality and sleep latency improvements substantiated by significant reduction in latency to NREM sleep (stage 1) was observed after bedtime administration of propiomazine (25 mg) for 5 days (73).
A single bed time dose (50 or 75 mg) of “over the counter”, antihistamine diphenhydramine, increased motor activity without affecting any subjective sleep parameter (74). Significant improvement in subjective sleep parameters were also observed after diphenhydramine (50 mg) administration in moderately insomniac (75).
The recent development of non-imidazole H3 receptor ligands with reduced risk of drug-drug interaction has provided many potential clinical candidates that can be used to correct sleep-wakefulness disorders including narcolepsy (76–78). In a recent sequential placebo-controlled, single-blind study, 21 narcoleptic patients were administered a fixed dose of 40 mg tiprolisant (H3 receptor antagonist) for 7 days and excessive daytime sleepiness (EDS) was examined by the subjective Epworth sleepiness scale. Significant improvements in EDS were observed with tiprolisant as compared to placebo (79). This study was among the first to demonstrate therapeutic relevance of H3 receptor antagonist as potential clinical candidates for the treatment of narcolepsy. Besides tiprolisant, many other H3 receptor antagonists including JNJ-17216498, GSK189254, BF2.649, APD916, and PF-03654746 are under investigation for the treatment of various sleep-wakefulness disorders including narcolepsy (76; 78; 80).
ELECTROPHYSIOLOGICAL STUDIES
IN VITRO STUDIES
The TMN neurons
The histamine containing TMN neurons are spontaneously active at resting potential (−50 mV) with broad shouldered spike (mid-amplitude duration ~2 ms), mainly due to fast Na+ and Ca+ conductions and a deep (~15 mV) and long lasting Ca+ independent after-hyperpolarization (~450 ms duration) that brings the membrane potential down to −80 mV (81). The onset of an action potential is the result of slow depolarizing potential mediated by voltage dependent Ca2+ current and a slow tetrodotoxin (TTX) sensitive Na+ current. The Ca2+ current is activated by a return to threshold following after-hyperpolarization, whereas the Na+ current appears to be persistent (82). The action potential is followed by after-hyperpolarization which is responsible for limiting the discharge activity. A fast transient K+ outward current (A-type) with two components may be critical for membrane re-polarization, and together with a hyperpolarization -activated current (Ih), it provides a strong inward- and outward rectifications similar to other monoaminergic neurons (10).
Post synaptic effects of histamine on other sleep-wakefulness regulatory sites Dorsal Raphe
Although, H2 receptor mediated depression of dorsal raphe firing has been reported (83), histamine activates the dorsal raphe neurons via H1 receptor mediated opening of a mixed cation channel of the transient receptor potential cation channel family (84;85).
Locus coeruleus
Majority of norepinephrine containing locus coeruleus neurons are excited by histamine via H1 receptor. The H2 receptor mediated excitation of norepinephrine containing locus coeruleus neurons has been observed. However, the H3 receptor mediated electrophysiological actions have not been reported (86).
Basal forebrain and the brainstem cholinergic neurons
The cholinergic neurons of the mesopontine tegmentum and basal forebrain are excited by histamine, mediated via the activation of H1 receptor (87; 88). Activation of H3 receptor results in the depression of cholinergic neurons coupled with reduction in cortical acetylcholine release (89; 90).
Ventrolateral and medial preoptic neurons
In vitro studies suggest that histamine does not have any effect on sleep-active neurons of the ventrolateral preoptic region (91). However, activation of H1 receptor causes excitation of GABA neurons in the medial preoptic region (92; 93).
Perifornical lateral hypothalamus
In vitro studies suggest that histamine has no direct effect on orexinergic neurons of the perifornical lateral hypothalamus, although strong anatomical connection are present (24; 94)
Thalamus
Histamine promotes depolarization of thalamic relay neurons via combined activation of H1 and H2 receptors (95).
IN VIVO STUDIES
Early studies examined extracellular single unit activity of presumed histaminergic neurons from the TMN in urethane-anesthetized and conscious rats, and conscious cats. These studies suggested that presumed histaminergic neurons displayed discharge pattern that was similar to other monoaminergic neurons; slow tonic discharge during wakefulness, reduced discharge during NREM sleep, complete cessation during REM sleep and resumption of firing in anticipation of wakefulness. These REM-off neurons displayed long duration action potentials and slow conduction velocity suggesting unmyelinated histaminergic axons (96–99)
Recently, extracellular activity was examined from identified histaminergic neurons from the TMN of non-anesthetized, head-restrained mice (100*). The histaminergic neurons displayed broad triphasic action potentials with a slow (<10 Hz) tonic, and repetitive firing pattern that strongly correlated with state of vigilance. The maximal activity was observed during attentive wakefulness that dramatically reduced during quite wakefulness. Complete cessation of activity was observed during the state of drowsiness, NREM and REM sleep state. A pronounced delay in the discharge activity was observed during the transition from sleep to wakefulness. The histaminergic neurons resumed their activity only after the animal was fully alert. Based on these results, the authors concluded that histamine neurons may play a pivotal role in the maintenance of an arousal state of high vigilance that is required for cognitive processes. Conversely, cessation of histaminergic activity may play a role in initiation and maintenance of sleep (100).
BIOCHEMICAL/MOLECULAR STUDIES
Measurement of histamine release and turnover
The histaminergic system appears to be under strong circadian control. Microdialysis measurement of histamine release from the anterior hypothalamic area of freely behaving rats, maintained under 12:12 h light:dark cycle, revealed that histamine release anticipated wakefulness and increased during the second half of the light period. The histamine release peaked during the dark period when the rats were fully awake and active (101). Similar results were obtained after push-pull cannula measurement of histamine release from the posterior hypothalamus in freely moving rats (102). Diurnal variations in the levels of histamine metabolites (tele-methylhistamine and tele-methylimidazoleacetic acid), with the peaks during active period (daytime) and troughs during inactive period, were observed in the cerebrospinal fluid (CSF) of rhesus monkey (103). Diurnal variations in CSF levels of telemethylhistamine (histamine metabolite) in human children has also been reported (104).
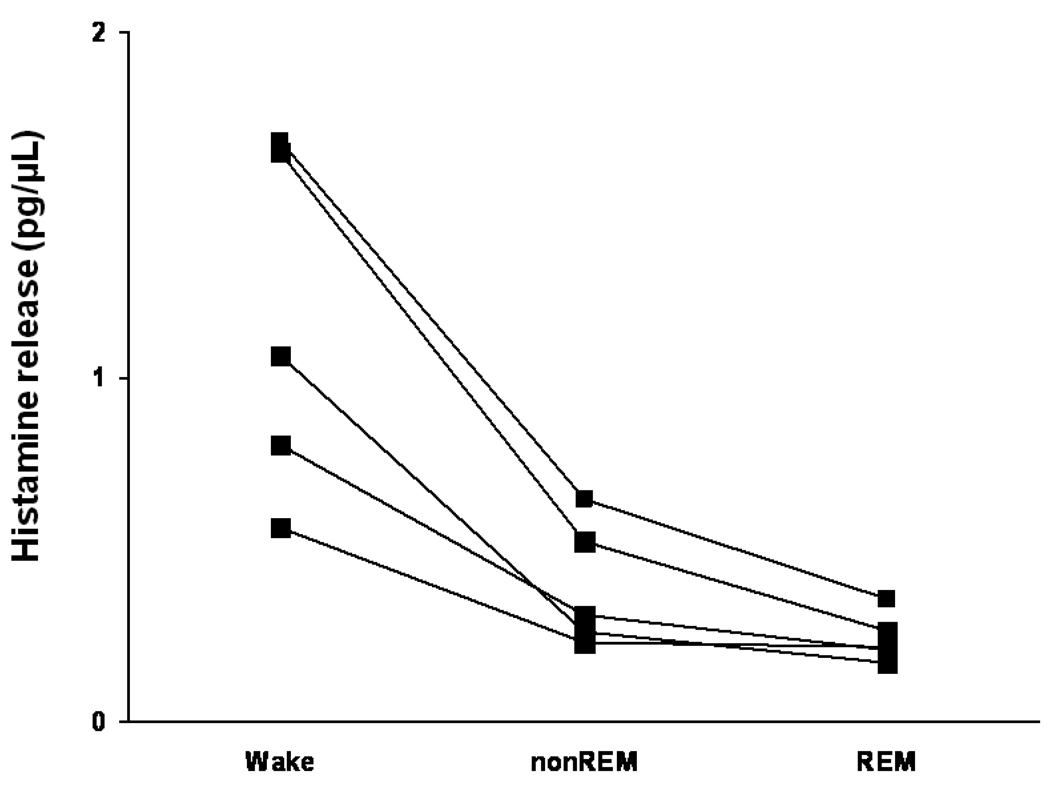
Microdialysis measurements of histamine, from pre-optic/anterior hypothalamus, across behavioral states in freely behaving cats suggested that histamine levels peaked during wakefulness and troughed during REM sleep (Fig.3). Interestingly, histamine levels did not increase during sleep deprivation suggesting that the histamine levels do not convey the “sleep drive” information to the sleep-promoting neurons of the preoptic hypothalamus (105).
Figure 3.
Histamine release measured from the preoptic/anterior hypothalamus of freely behaving cats across sleep-wakefulness. Histamine release was higher during wakefulness as compared non-REM and REM sleep values in each experiment (represent by each line) producing a highly significant group effect [N=5; for details see (105); Adapted from (105).
Measurement of c-Fos immunoreactivity as a marker of neuronal activation
Since its discovery in the 1980s, expression of immediate-early gene c-fos, and the subsequent accumulation of c-Fos protein has often been used as a maker of neuronal activation (106). This characteristic of c-Fos protein has been extensively used, in sleep research, to identify the neurochemical phenotype of activated neurons across sleep-wakefulness (107)
Systemic administration of a H3 receptor antagonist, ciproxifan (1 mg/kg in 0.2 ml, i.m), induced c-Fos expression in vast majority of histaminergic neurons in the TMN (67). Systemic administration of anesthetic agent pentobarbital (100 mg/Kg) and propofol (140 mg/Kg), GABAA receptor agonist muscimol (10 mg/Kg), and α2 receptor agonist dexmedetomidin (500 µg/Kg) produced significant reduction in c-Fos protein in the TMN (108;109). Infusion of prostaglandin D2 (200 pmol/min) or adenosine A2a receptor agonist CGS21680 (20 pmol/min) into the subarachnoid space, during the dark period, increased NREM sleep and reduced c-Fos expression in the TMN of rats. In contrast, infusion of adenosine A1 receptor agonist N6-cyclopentyl-adenosine (2 pmol/min) in the same area did not have any effect on sleep-wakefulness or c-Fos expression in the TMN (110; 111).
Rats maintained in free running condition (constant darkness) display increased wakefulness and c-Fos protein in the TMN during the subjective night irrespective of the lighting condition suggesting a strong co-relationship between c-Fos expression in the TMN and the amount of wakefulness independent of circadian cycle (112).
Measurement of sleep-wakefulness in knockout mice
Sleep-wakefulness in H1 receptor (−/−) KO
The H1 receptor KO (H1R-KO) displayed essentially normal sleep-wakefulness under normal baseline conditions (113). Minor deficits including loss of brief awakenings (<16 sec bouts) and increased NREM sleep duration were observed in H1R-KO as compared to wild type mice. Systemic injection of ciproxifan induced wakefulness in wild type mice. No such effect was observed in H1R-KO validating the loss of H1 receptor knockdown. However, ciproxifan induced histamine release in the frontal cortex was identical in both wild type and H1R-KO mice suggesting that the histamine release in not controlled by H1 receptor (113).
Sleep-wakefulness in H3R- KO
Similar to H1R-KO, the H3 receptor KO (−/−; H3R-KO) mice display essentially normal sleep-wakefulness under baseline condition with a small reduction in locomotor activity during the dark period (114). However, as described above, systemic administration of thioperamide was ineffective in H3R-KO, but increased wakefulness in wild type mice (H3 +/+) mice suggesting that the H3 receptors have an important role in mediating behavioral arousal (114).
Sleep-wakefulness in HDC (−/−) KO and it comparison with orexin KO
The recently discovered orexin neurons are exclusively localized in the perifornical hypothalamus, in close proximity to the histaminergic neurons, and like the histaminergic neurons send widespread projections to the entire brain including those involved in the regulation of sleep wakefulness. Like the histaminergic neurons, the orexin neurons are wake-active, reduce their activity during quite wakefulness and are completely silent during NREM and REM sleep. In addition, orexin neurons send strong projections to the histaminergic neurons and orexin receptors are localized on histaminergic neurons. A large body of evidence indicates that deficiency in orexins is the cause for the pathogenesis of human and animal narcolepsy (115–117). Reduced histamine has been observed in CSF of narcoleptics (discussed below). These similarities between orexins and histamine raised questions: Do orexin and histamine containing neurons have similar or distinct role in control of wakefulness? Are these two systems co-responsible for narcolepsy? Anaclet et al., (2009) compared the behavioral and sleep-wakefulness phenotypes of HDC (−/−) KO mice with those of orexin (prepro-orexin −/−) KO mice. The HDC KO mice displayed histamine deficiency due to complete absence of histamine synthesis (17; 118). The orexin KO displayed orexin deficiency. Both orexin and HDC KO mice displayed sleep fragmentation and increased REM sleep. However, there were several major differences: 1) The HDC KO displayed increased REM sleep during the light (inactive) period; the orexin KO displayed increased REM sleep during the dark (active) period. 2) The HDC KO displayed wakefulness deficit at dark onset and in novel environment; the orexin KO do not. 3) The orexin KO displayed impaired circadian distribution for both wakefulness and REM sleep; the HDC KO do not. 4) When faced with a motor challenge, the orexin KO displayed narcolepsy; the HDC KO do not. Based on these results, the authors concluded that the orexins and histamine neurons exert a distinct, but complementary and synergistic control in wakefulness maintenance (119*).
Role of orexins in the histaminergic TMN
Local reverse microdialysis administration of orexin A (5 and 25 pmol/min) into the TMN of rats increased histamine release and wakefulness with a concomitant reduction in sleep (both NREM and REM phases). Furthermore, central infusion of orexin A (1.5 pmol/min) increased wakefulness in wild-type mice, but not in H1 receptor (−/−) KO mice suggesting that the arousal effect of orexins are dependent on the activation of H1 receptor in the TMN (120).
Role of adenosine in the histaminergic TMN
A distinctive feature of many (but not all) histaminergic neurons in the TMN of rats (not the mouse) is the presence of high levels of adenosine deaminase, a key enzyme involved in deamination of adenosine (7). Adenosine is a mediator of homeostatic sleep regulation (121; 122). Recently, it has been demonstrated that adenosine A1 receptors are expressed on histaminergic neurons of the TMN. Activation of A1R or inhibiton of adenosine deaminase in the TMN decreased histamine release in the frontal cortex and increased non-rapid eye movement (NREM) sleep without affecting rapid eye movement (REM) sleep. Activation of A1 promoted sleep in wild type mice, but not in A1 receptor or H1 receptor knockout mice (123).
INACTIVATION/LESION STUDIES
Inactivation of the ventro-lateral posterior hypothalamus (in and around the TMN) by local application of muscimol (0.1–1.0 µg/0.5 µL), a potent agonist of GABA, induced long-lasting NREM sleep followed by a significant increase in REM sleep. Systemic administration of p-chlorophenylalanine, a potent serotonin synthesis inhibitor produces long lasting insomnia. Local administration of muscimol in these insomniac cats induced NREM and REM sleep with short latency (124)
Local administration of the neurotoxin saporin conjugated to orexin B (hypocretin 2, 50 ng in 0.25 µL) into the TMN, destroyed up to 83% of histaminergic neurons along with other neurons in the TMN. The sleep-wakefulness remained unaffected. However, compensatory effects following lesion cannot be ruled out because the neurons take several days to die following the administration of the neurotoxin (125).
In a subsequent study, sleep-wakefulness was examined following simultaneous lesions of three wake-promoting neuronal groups with three different saporin-conjugated neurotoxins. The basal forebrain cholinergic neurons were lesioned by 192-IgG-saporin (6 µg/3µ; icv). The noradrenergic neurons were lesioned by bilateral locus coeruleus injections of anti-dopamine-β-hydroxylase-saporin (0.25 µg/0.25 µL/side), and the histaminergic neurons were lesioned by bilateral and injections of orexin B-saporin conjugate (62.5 ng/0.25 µL/side) into the TMN. Simultaneous lesion of three wakefulness-promoting regions did not affect sleep-wakefulness, except that rats with triple lesions displayed wakefulness deficit at dark onset. It is important to note that wakefulness deficit at dark onset is also observed in HDC-KO. Thus, these data clearly suggest that the histamine neurons may have a major role in the maintenance of the wakefulness state (126).
HISTMINE IN DISEASED STATES
The monoaminergic LC and 5-HT neurons reduced or ceased their activity during cataplexy in narcoleptic dogs. However, the histaminergic neurons of the TMN remained active (127). Narcoleptic dogs displayed histamine deficit in the cortex and thalamus. In contrast, dopamine and NE levels were elevated in the same brain structures (128). Similarly, CSF levels of histamine were reduced in non-medicated human subjects with narcolepsy or idiopathic hypersomnia. However, similar reduction was not observed in human subjects with obstructive sleep apnea (*129; 130). The authors concluded that CSF histamine can be used as a biomarker reflecting the degree of hypersomnia of central origin (129). While these finding are interesting and novel, they were some limitations. For example, considerable overlap in histamine values was observed between controls and patients. In addition, substantial variability of histamine levels was observed within groups (131).
CONCLUSION
Does histaminergic system regulate wakefulness? The histaminergic system is localized within the TMN, which is a nucleus in the posterior hypothalamus. Since the early part of the 20th century, posterior hypothalamus has been implicated in the regulation of wakefulness. The histaminergic neurons send strong projections especially to the wakefulness promoting regions including the orexin rich perifornical hypothalamus and the cholinergic rich basal forebrain. The discharge activity of identified histaminergic neurons peaked during the state of high vigilance and ceased during NREM and REM sleep. Histamine release parallels histaminergic discharge, highest during wakefulness and lowest during sleep. Considerable pharmacological evidence suggests that the H1 and H3 receptor (but not the H2 receptors) are key mediators of histaminergic action on wakefulness. The HDC KO displayed wakefulness deficit at dark onset and in novel environment. Thus, there is plethora of evidence implicating histaminergic system to play a crucial role in the regulation of wakefulness. The challenges for the future are: 1) to identify the precise, cellular and the molecular mechanisms by which histamine acts to maintain the high state of vigilance, 2) to examine whether histamine or one of its metabolites can be used to diagnostic tool in diseased states and 3) to create selective and specific histamine H3 receptor ligands that can be used to treat sleep-disorders.
PRACTICE POINTS
-
1
The histaminergic neurons in the brain are exclusively localized in the tuberomammillary nucleus in the posterior hypothalamus.
-
2
The histaminergic neurons display increased discharge activity during the state of high vigilance and are completely silent during states of drowsiness, NREM and REM sleep.
-
3
Mice with constitutive deletion of L-histidine decarboxylase gene display histamine deficit coupled with sleep-wake disruptions.
RESEARCH AGENDA
-
4
To explore the possibility of using CSF histamine or one of its metabolites as biomarkers in diseases state with hypersomnolence of central origin.
-
5
To develop novel and selective histamine H3 receptor ligands that can be used to treat sleep disorders including narcolepsy.
-
6
To further understand the role of histamine in cognition and novelty induced arousal.
ABBREVIATIONS
- α-FMH
α-fluoro-methyl-histidine
- CSF
cerebrospinal fluid
- GABA
Gama amino butyric acid.
- HDC
L-histidine decarboxylase.
- H1R-KO
Histamine H1 receptor knockout
- H3R-KO
Histamine H3 receptor knockout
- icv
intraventricular
- ip
intraperitoneal
- im
intramuscular
- KO
constitutive knockout mice
- NREM
non-rapid eye movement sleep
- REM
Rapid Eye Movement
- sc
subcutaneous
- TMN
Tuberomammillary nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: Reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31(5):775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aston-Jones G. Brain structures and receptors involved in alertness. Sleep Med. 2005 Jun;6 Suppl 1:S3–S7. doi: 10.1016/s1389-9457(05)80002-4. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, McCarley RW. Brain Control of Sleep and Wakefulness. New York: Kluwer-Elsevier; 2005. [Google Scholar]
- 4.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005 Nov;26(11):578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Monti JM. The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010 Feb 11; doi: 10.1016/j.smrv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Monnier M, Fallert M, Battacharya IC. The waking action of histamine. Experientia. 1967 Jan 15;23(1):21–22. doi: 10.1007/BF02142244. [DOI] [PubMed] [Google Scholar]
- 7.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008 Jul;88(3):1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 8.Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano Y, et al. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295(1):13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- 10.Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003 Feb;4(2):121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 11.Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RL, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol. 2009 May;157(1):55–63. doi: 10.1111/j.1476-5381.2009.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart AE. From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron. 1999 Mar;22(3):431–433. doi: 10.1016/s0896-6273(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 13.Ohtsu H. Progress in allergy signal research on mast cells: the role of histamine in immunological and cardiovascular disease and the transporting system of histamine in the cell. J Pharmacol Sci. 2008 Mar;106(3):347–353. doi: 10.1254/jphs.fm0070294. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman H. Histamine receptors in the central nervous system. Pharm Weekbl Sci. 1989 Oct 20;11(5):146–150. doi: 10.1007/BF01959461. [DOI] [PubMed] [Google Scholar]
- 15.Thakkar MM, McCarley RW. Histamine in the control of sleep-wakefulness. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. Neurochemistry of sleep and wakefulness. New York: Cambridge University Press; 2008. pp. 144–178. [Google Scholar]
- 16.Kohler C, Swanson LW, Haglund L, Wu JY. The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience. 1985 Sep;16(1):85–110. doi: 10.1016/0306-4522(85)90049-1. [DOI] [PubMed] [Google Scholar]
- 17.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002 Sep 1;22(17):7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JS, Kitahama K, Fort P, Panula P, Denney RM, Jouvet M. Histaminergic system in the cat hypothalamus with reference to type B monoamine oxidase. J Comp Neurol. 1993 Apr 15;330(3):405–420. doi: 10.1002/cne.903300309. [DOI] [PubMed] [Google Scholar]
- 19.Smits RP, Steinbusch HW, Mulder AH. The localization of histidine decarboxylase-immunoreactive cell bodies in the guinea-pig brain. J Chem Neuroanat. 1990 Mar;3(2):85–100. [PubMed] [Google Scholar]
- 20.Ericson H, Watanabe T, Kohler C. Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against L-histidine decarboxylase as a marker. J Comp Neurol. 1987 Sep 1;263(1):1–24. doi: 10.1002/cne.902630102. [DOI] [PubMed] [Google Scholar]
- 21.Miklos IH, Kovacs KJ. Functional heterogeneity of the responses of histaminergic neuron subpopulations to various stress challenges. Eur J Neurosci. 2003 Dec;18(11):3069–3079. doi: 10.1111/j.1460-9568.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- 22.Kukko-Lukjanov TK, Panula P. Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro. J Chem Neuroanat. 2003 Jul;25(4):279–292. doi: 10.1016/s0891-0618(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 23.Patel BT, Tudball N, Wada H, Watanabe T. Adenosine deaminase and histidine decarboxylase coexist in certain neurons of the rat brain. Neurosci Lett. 1986 Jan 16;63(2):185–189. doi: 10.1016/0304-3940(86)90059-5. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001 Dec 1;21(23):9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jewett RE. Effects of promethazine on sleep stages in the cat. Exp Neurol. 1968 Jul;21(3):368–382. doi: 10.1016/0014-4886(68)90048-4. [DOI] [PubMed] [Google Scholar]
- 26.Risberg AM, Risberg J, Ingvar DH. Effects of promethazine on nocturnal sleep in normal man. Psychopharmacologia. 1975 Sep 17;43(3):279–284. doi: 10.1007/BF00429264. [DOI] [PubMed] [Google Scholar]
- 27.Wauquier A, Van den Broeck WA, Awouters F, Janssen PA. A comparison between astemizole and other antihistamines on sleep-wakefulness cycles in dogs. Neuropharmacology. 1981 Sep;20(9):853–859. doi: 10.1016/0028-3908(81)90078-2. [DOI] [PubMed] [Google Scholar]
- 28.Wolf P, Monnier M. Electroencephalographic, behavioural and visceral effects of intraventricular infusion of histamine in the rabbit. Agents Actions. 1973 Oct;3(3):196. doi: 10.1007/BF01965754. [DOI] [PubMed] [Google Scholar]
- 29.Kalivas PW. Histamine-induced arousal in the conscious and pentobarbital-pretreated rat. J Pharmacol Exp Ther. 1982 Jul;222(1):37–42. [PubMed] [Google Scholar]
- 30.Monnier M, Hatt AM. Afferent and central activating effects of histamine on the brain. Experientia. 1969 Dec 15;25(12):1297–1298. doi: 10.1007/BF01897510. [DOI] [PubMed] [Google Scholar]
- 31.Orr E, Quay WB. Hypothalamic 24-hour rhythms in histamine, histidine, decarboxylase and histamine-N-methyltransferase. Endocrinology. 1975 Apr;96(4):941–945. doi: 10.1210/endo-96-4-941. [DOI] [PubMed] [Google Scholar]
- 32.Friedman AH, Walker CA. Circadian rhythms in rat mid-brain and caudate nucleus biogenic amine levels. J Physiol. 1968 Jul;197(1):77–85. doi: 10.1113/jphysiol.1968.sp008547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001 Dec;24(12):726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 34.Monti JM. Involvement of histamine in the control of the waking state. Life Sci. 1993;53(17):1331–1338. doi: 10.1016/0024-3205(93)90592-q. [DOI] [PubMed] [Google Scholar]
- 35.Naquet R, Denavit M, be-Fessard D. Comparison between the role of the subthalamus and that of the different bulbomesencephalic structures in the maintenance of wakefulness. Electroencephalogr Clin Neurophysiol. 1966 Feb;20(2):149–164. doi: 10.1016/0013-4694(66)90159-3. [DOI] [PubMed] [Google Scholar]
- 36.McGinty DJ. Somnolence, recovery and hyposomnia following ventro-medial diencephalic lesions in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(1):70–79. doi: 10.1016/0013-4694(69)90035-2. [DOI] [PubMed] [Google Scholar]
- 37.Swett CP, Hobson JA. The effects of posterior hypothalamic lesions on behavioral and electrographic manifestations of sleep and waking in cats. Arch Ital Biol. 1968;106(3):283–293. [PubMed] [Google Scholar]
- 38.Monti JM, D'Angelo L, Jantos H, Pazos S. Effects of a-fluoromethylhistidine on sleep and wakefulness in the rat. Short note. J Neural Transm. 1988;72(2):141–145. doi: 10.1007/BF01250237. [DOI] [PubMed] [Google Scholar]
- 39.Kiyono S, Seo ML, Shibagaki M, Watanabe T, Maeyama K, Wada H. Effects of alpha-fluoromethylhistidine on sleep-waking parameters in rats. Physiol Behav. 1985 Apr;34(4):615–617. doi: 10.1016/0031-9384(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 40.Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27(2):111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 41.Lin JS, Sakai K, Jouvet M. Hypothalamo-preoptic histaminergic projections in sleep-wake control in the cat. Eur J Neurosci. 1994;6(4):618–625. doi: 10.1111/j.1460-9568.1994.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 42.Lin JS, Hou Y, Sakai K, Jouvet M. Histaminergic descending inputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat. J Neurosci. 1996 Feb 15;16(4):1523–1537. doi: 10.1523/JNEUROSCI.16-04-01523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin JS, Sakai K, Jouvet M. Role of hypothalamic histaminergic systems in the regulation of vigilance states in cats. C R Acad Sci III. 1986;303(11):469–474. [PubMed] [Google Scholar]
- 44.Tasaka K, Chung YH, Sawada K. Excitatory effect of histamine on EEGs of the cortex and thalamus in rats. Agents Actions. 1989 Apr;27(1–2):127–130. doi: 10.1007/BF02222218. [DOI] [PubMed] [Google Scholar]
- 45.Tasaka K, Chung YH, Mio M, Kamei C. The pathway responsible for EEG synchronization and effect of histamine on this system. Brain Res Bull. 1993;32(4):365–371. doi: 10.1016/0361-9230(93)90201-l. [DOI] [PubMed] [Google Scholar]
- 46.Ramesh V, Thakkar MM, Strecker RE, Basheer R, McCarley RW. Wakefulness-inducing effects of histamine in the basal forebrain of freely moving rats. Behav Brain Res. 2004 Jul 9;152(2):271–278. doi: 10.1016/j.bbr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita M, Ito S, Sugama K, Fukui H, Smith B, Nakanishi K, et al. Biochemical characterization of histamine H1 receptors in bovine adrenal medulla. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1233–1239. doi: 10.1016/0006-291x(91)90673-u. [DOI] [PubMed] [Google Scholar]
- 48.Leurs R, Church MK, Taglialatela M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002 Apr;32(4):489–498. doi: 10.1046/j.0954-7894.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 49.Palacios JM, Wamsley JK, Kuhar MJ. The distribution of histamine H1-receptors in the rat brain: an autoradiographic study. Neuroscience. 1981;6(1):15–37. doi: 10.1016/0306-4522(81)90240-2. [DOI] [PubMed] [Google Scholar]
- 50.Monti JM, Pellejero T, Jantos H. Effects of H1- and H2-histamine receptor agonists and antagonists on sleep and wakefulness in the rat. J Neural Transm. 1986;66(1):1–11. doi: 10.1007/BF01262953. [DOI] [PubMed] [Google Scholar]
- 51.Tokunaga S, Tsutsui R, Obara Y, Ishida T, Kamei C. Effects of histamine H1-antagonists on sleep-awake state in rats placed on a grid suspended over water or on sawdust. Biol Pharm Bull. 2009 Jan;32(1):51–54. doi: 10.1248/bpb.32.51. [DOI] [PubMed] [Google Scholar]
- 52.Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International union of pharmacology .13. Classification of histamine receptors. Pharmacol Rev. 1997 Sep;49(3):253–278. [PubMed] [Google Scholar]
- 53.Vizuete ML, Traiffort E, Bouthenet ML, Ruat M, Souil E, Tardivel-Lacombe J, et al. Detailed mapping of the histamine H2 receptor and its gene transcripts in guinea-pig brain. Neuroscience. 1997 Sep;80(2):321–343. doi: 10.1016/s0306-4522(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 54.Monti JM, Orellana C, Boussard M, Jantos H, Olivera S. Sleep variables are unaltered by zolantidine in rats: are histamine H2-receptors not involved in sleep regulation? Brain Res Bull. 1990 Aug;25(2):229–231. doi: 10.1016/0361-9230(90)90065-8. [DOI] [PubMed] [Google Scholar]
- 55.Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983 Apr 28;302(5911):832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 56.Rouleau A, Ligneau X, Tardivel-Lacombe J, Morisset S, Gbahou F, Schwartz JC, et al. Histamine H3-receptor-mediated [35S]GTP gamma[S] binding: evidence for constitutive activity of the recombinant and native rat and human H3 receptors. Br J Pharmacol. 2002 Jan;135(2):383–392. doi: 10.1038/sj.bjp.0704490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, et al. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000 Dec 14;408(6814):860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- 58.Lin JS, Sakai K, Vanni-Mercier G, Arrang JM, Garbarg M, Schwartz JC, et al. Involvement of histaminergic neurons in arousal mechanisms demonstrated with H3-receptor ligands in the cat. Brain Res. 1990;523(2):325–330. doi: 10.1016/0006-8993(90)91508-e. [DOI] [PubMed] [Google Scholar]
- 59.Monti JM, Jantos H, Boussard M, Altier H, Orellana C, Olivera S. Effects of selective activation or blockade of the histamine H3 receptor on sleep and wakefulness. Eur J Pharmacol. 1991 Dec 3;205(3):283–287. doi: 10.1016/0014-2999(91)90911-9. [DOI] [PubMed] [Google Scholar]
- 60.Monti JM, Jantos H, Ponzoni A, Monti D. Sleep and waking during acute histamine H3 agonist BP 2.94 or H3 antagonist carboperamide (MR 16155) administration in rats. Neuropsychopharmacol. 1996 Jul;15(1):31–35. doi: 10.1016/0893-133X(95)00151-3. [DOI] [PubMed] [Google Scholar]
- 61.Lin JS. Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Medicine Reviews. 2000 Oct;4(5):471–503. doi: 10.1053/smrv.2000.0116. [DOI] [PubMed] [Google Scholar]
- 62.McLeod RL, Aslanian R, del PM, Duffy R, Egan RW, Kreutner W, et al. Sch 50971, an orally active histamine H3 receptor agonist, inhibits central neurogenic vascular inflammation and produces sedation in the guinea pig. J Pharmacol Exp Ther. 1998 Oct;287(1):43–50. [PubMed] [Google Scholar]
- 63.Ligneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, et al. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. The Journal Of Pharmacology And Experimental Therapeutics. 1998 Nov;287(2):658–666. [PubMed] [Google Scholar]
- 64.Lamberty Y, Margineanu DG, Dassesse D, Klitgaard H. H3 agonist immepip markedly reduces cortical histamine release, but only weakly promotes sleep in the rat. Pharmacol Res. 2003 Aug;48(2):193–198. doi: 10.1016/s1043-6618(03)00094-x. [DOI] [PubMed] [Google Scholar]
- 65.Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, et al. Behavioral Characterization of Mice Lacking Histamine H3 Receptors. Molecular Pharmacology. 2002 Aug 1;62(2):389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- 66.Barbier AJ, Berridge C, Dugovic C, Laposky AD, Wilson SJ, Boggs J, et al. Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H3 antagonist. Br J Pharmacol. 2004 Nov;143(5):649–661. doi: 10.1038/sj.bjp.0705964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanni-Mercier G, Gigout S, Debilly G, Lin JS. Waking selective neurons in the posterior hypothalamus and their response to histamine H3-receptor ligands: an electrophysiological study in freely moving cats. Behav Brain Res. 2003 Sep 15;144(1–2):227–241. doi: 10.1016/s0166-4328(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 68.Guo RX, Anaclet C, Roberts JC, Parmentier R, Zhang M, Guidon G, et al. Differential effects of acute and repeat dosing with the H3 antagonist GSK189254 on the sleep-wake cycle and narcoleptic episodes in Ox−/− mice. Br J Pharmacol. 2009 May;157(1):104–117. doi: 10.1111/j.1476-5381.2009.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007 Feb 10;369(9560):499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 70.Barbier AJ, Bradbury MJ. Histaminergic control of sleep-wake cycles: recent therapeutic advances for sleep and wake disorders. CNS Neurol Disord Drug Targets. 2007 Feb;6(1):31–43. doi: 10.2174/187152707779940790. [DOI] [PubMed] [Google Scholar]
- 71.Welch MJ, Meltzer EO, Simons FE. H1-antihistamines and the central nervous system. Clin Allergy Immunol. 2002;17:337–388. [PubMed] [Google Scholar]
- 72.Adam K, Oswald I. The hypnotic effects of an antihistamine: promethazine. Br J Clin Pharmacol. 1986 Dec;22(6):715–717. doi: 10.1111/j.1365-2125.1986.tb02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Almqvist M, Liljenberg B, Hetta J, Rimon R, Hambert G, Roos BE. Effects of propiomazine on the EEG sleep of normal subjects. Pharmacol Toxicol. 1987 Nov;61(5):278–281. doi: 10.1111/j.1600-0773.1987.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 74.Borbely AA, Youmbi-Balderer G. Effect of diphenhydramine on subjective sleep parameters and on motor activity during bedtime. Int J Clin Pharmacol Ther Toxicol. 1988 Aug;26(8):392–396. [PubMed] [Google Scholar]
- 75.Rickels K, Morris RJ, Newman H, Rosenfeld H, Schiller H, Weinstock R. Diphenhydramine in insomniac family practice patients: a double-blind study. J Clin Pharmacol. 1983 May;23(5–6):234–242. doi: 10.1002/j.1552-4604.1983.tb02730.x. [DOI] [PubMed] [Google Scholar]
- 76.Celanire S, Wijtmans M, Talaga P, Leurs R, De EI. Keynote review: histamine H3 receptor antagonists reach out for the clinic. Drug Discov Today. 2005 Dec;10(23–24):1613–1627. doi: 10.1016/S1359-6446(05)03625-1. [DOI] [PubMed] [Google Scholar]
- 77.Passani MB, Lin JS, Hancock A, Crochet S, Blandina P. The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders. Trends Pharmacol Sci. 2004 Dec;25(12):618–625. doi: 10.1016/j.tips.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Sander K, Kottke T, Stark H. Histamine H3 receptor antagonists go to clinics. Biol Pharm Bull. 2008 Dec;31(12):2163–2181. doi: 10.1248/bpb.31.2163. [DOI] [PubMed] [Google Scholar]
- 79.Lin JS, Dauvilliers Y, Arnulf I, Bastuji H, Anaclet C, Parmentier R, et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin−/− mice and patients. Neurobiol Dis. 2008 Apr;30(1):74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Clinical Trials. 2010 http://clinicaltrials gov/
- 81.Haas HL, Reiner PB. Membrane properties of histaminergic tuberomammillary neurones of the rat hypothalamus in vitro. J Physiol. 1988 May;399:633–646. doi: 10.1113/jphysiol.1988.sp017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevens DR, Eriksson KS, Brown RE, Haas HL. The mechanism of spontaneous firing in histamine neurons. Behav Brain Res. 2001 Oct 15;124(2):105–112. doi: 10.1016/s0166-4328(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 83.Lakoski JM, Gallager DW, Aghajanian GK. Histamine-induced depression of serotoninergic dorsal raphe neurons: antagonism by cimetidine, a reevaluation. Eur J Pharmacol. 1984 Aug 3;103(1–2):153–156. doi: 10.1016/0014-2999(84)90202-4. [DOI] [PubMed] [Google Scholar]
- 84.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002 Oct 15;22(20):8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sergeeva OA, Korotkova TM, Scherer A, Brown RE, Haas HL. Co-expression of non-selective cation channels of the transient receptor potential canonical family in central aminergic neurones. J Neurochem. 2003 Jun;85(6):1547–1552. doi: 10.1046/j.1471-4159.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- 86.Korotkova TM, Sergeeva OA, Ponomarenko AA, Haas HL. Histamine excites noradrenergic neurons in locus coeruleus in rats. Neuropharmacology. 2005 Jul;49(1):129–134. doi: 10.1016/j.neuropharm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Khateb A, Serafin M, Muhlethaler M. Histamine excites pedunculopontine neurones in guinea pig brainstem slices. Neurosci Lett. 1990 May 4;112(2–3):257–262. doi: 10.1016/0304-3940(90)90213-s. [DOI] [PubMed] [Google Scholar]
- 88.Khateb A, Muhlethaler M, Alonso A, Serafin M, Mainville, Jones BE. Cholinergic nucleus basalis neurons display the capacity for rhythmic bursting activity mediated by low-threshold calcium spikes. Neuroscience. 1992 Dec;51(3):489–494. doi: 10.1016/0306-4522(92)90289-e. [DOI] [PubMed] [Google Scholar]
- 89.Doreulee N, Yanovsky Y, Flagmeyer I, Stevens DR, Haas HL, Brown RE. Histamine H(3) receptors depress synaptic transmission in the corticostriatal pathway. Neuropharmacology. 2001;40(1):106–113. doi: 10.1016/s0028-3908(00)00101-5. [DOI] [PubMed] [Google Scholar]
- 90.Blandina P, Giorgetti M, Bartolini L, Cecchi M, Timmerman H, Leurs R, et al. Inhibition of cortical acetylcholine release and cognitive performance by histamine H3 receptor activation in rats. Br J Pharmacol. 1996 Dec;119(8):1656–1664. doi: 10.1111/j.1476-5381.1996.tb16086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000 Apr 27;404(6781):992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 92.Szymusiak R, Gvilia I, McGinty D. Hypothalamic control of sleep. Sleep Med. 2007 Jun;8(4):291–301. doi: 10.1016/j.sleep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 93.Tsai CL, Matsumura K, Nakayama T, Itowi N, Yamatodani A, Wada H. Effects of histamine on thermosensitive neurons in rat preoptic slice preparations. Neurosci Lett. 1989 Jul 31;102(2–3):297–302. doi: 10.1016/0304-3940(89)90095-5. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Gao XB, Sakurai T, Van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002 Dec;36(6):1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 95.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol. 1992 Apr;9(2):212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- 96.Reiner PB, McGeer EG. Electrophysiological properties of cortically projecting histamine neurons of the rat hypothalamus. Neurosci Lett. 1987 Jan 2;73(1):43–47. doi: 10.1016/0304-3940(87)90028-0. [DOI] [PubMed] [Google Scholar]
- 97.Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999 Sep 4;840(1–2):138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- 98.Vanni-Mercier G, Sakai K, Jouvet M. [Specific neurons for wakefulness in the posterior hypothalamus in the cat]. [French] Comptes Rendus de l Academie des Sciences - Serie Iii, Sciences de la Vie. 1984;298(7):195–200. [PubMed] [Google Scholar]
- 99.Sakai K, el Mansari M, Lin JS, Zhang JG, Vanni-Mercier G. The Posterior Hypothalamus in the regulation of wakefulness and paradoxical sleep. In: Mancia M, Marini G, editors. The Diencephaon and Sleep. New York: Raven Press Ltd; 1990. pp. 171–198. [Google Scholar]
- 100.Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006 Oct 4;26(40):10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav. 1992 Feb;51(2):391–394. doi: 10.1016/0031-9384(92)90157-w. [DOI] [PubMed] [Google Scholar]
- 102.Prast H, Dietl H, Philippu A. Pulsatile release of histamine in the hypothalamus of conscious rats. J Auton Nerv Syst. 1992 Jun 15;39(2):105–110. doi: 10.1016/0165-1838(92)90050-q. [DOI] [PubMed] [Google Scholar]
- 103.Prell GD, Khandelwal JK, Burns RS, Green JP. Diurnal fluctuation in levels of histamine metabolites in cerebrospinal fluid of rhesus monkey. Agents Actions. 1989 Mar;26(3–4):279–286. doi: 10.1007/BF01967291. [DOI] [PubMed] [Google Scholar]
- 104.Kiviranta T, Tuomisto L, Airaksinen EM. Diurnal and age-related changes in cerebrospinal fluid tele-methylhistamine levels during infancy and childhood. Pharmacol Biochem Behav. 1994 Dec;49(4):997–1000. doi: 10.1016/0091-3057(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 105.Strecker RE, Nalwalk J, Dauphin LJ, Thakkar MM, Chen Y, Ramesh V, et al. Extracellular histamine levels in the feline preoptic/anterior hypothalamic area during natural sleep-wakefulness and prolonged wakefulness: An in vivo microdialysis study. Neuroscience. 2002 Sep 2;113(3):663–670. doi: 10.1016/s0306-4522(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 106.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988 Jun 3;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 107.Pompeiano M, Cirelli C, Arrighi P, Tononi G. c-Fos expression during wakefulness and sleep. Neurophysiol Clin. 1995;25(6):329–341. doi: 10.1016/0987-7053(96)84906-9. [DOI] [PubMed] [Google Scholar]
- 108.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003 Feb;98(2):428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 109.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002 Oct;5(10):979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 110.Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci U S A. 1998 Jun 23;95(13):7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107(4):653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 112.Ko EM, Estabrooke IV, McCarthy M, Scammell TE. Wake-related activity of tuberomammillary neurons in rats. Brain Res. 2003 Dec 5;992(2):220–226. doi: 10.1016/j.brainres.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 113.Huang ZL, Mochizuki T, Qu WM, Hong ZY, Watanabe T, Urade Y, et al. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4687–4692. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, et al. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol. 2002 Aug;62(2):389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- 115.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999 Aug 20;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 116.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene [see comments] Cell. 1999 Aug 6;98(3):365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 117.Thakkar MM, Ramesh V, Cape EG, Winston S, Strecker RE, McCarley RW. REM sleep enhancement and behavioral cataplexy following orexin (hypocretin)-II receptor antisense perfusion in the pontine reticular formation. Sleep Res Online. 1999;2(4):112–120. [PubMed] [Google Scholar]
- 118.Watanabe T, Yanai K. Studies on functional roles of the histaminergic neuron system by using pharmacological agents, knockout mice and positron emission tomography. Tohoku J Exp Med. 2001 Dec;195(4):197–217. doi: 10.1620/tjem.195.197. [DOI] [PubMed] [Google Scholar]
- 119.Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009 Nov 18;29(46):14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic Basal forebrain. J Neurosci. 2003 May 15;23(10):4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003;122(4):1107–1113. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 123.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989 Feb 13;479(2):225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 125.Gerashchenko D, Chou TC, Blanco-Centurion CA, Saper CB, Shiromani PJ. Effects of lesions of the histaminergic tuberomammillary nucleus on spontaneous sleep in rats. Sleep. 2004 Nov 1;27(7):1275–1281. doi: 10.1093/sleep/27.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007 Dec;19(51):14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004 May 27;42(4):619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nishino S, Fujiki N, Ripley B, Sakurai E, Kato M, Watanabe T, et al. Decreased brain histamine content in hypocretin/orexin receptor-2 mutated narcoleptic dogs. Neurosci Lett. 2001 Nov 9;313(3):125–128. doi: 10.1016/s0304-3940(01)02270-4. [DOI] [PubMed] [Google Scholar]
- 129.Kanbayashi T, Kodama T, Kondo H, Satoh S, Inoue Y, Chiba S, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009 Feb 1;32(2):181–187. doi: 10.1093/sleep/32.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nishino S, Sakurai E, Nevsimalova S, Yoshida Y, Watanabe T, Yanai K, et al. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep. 2009 Feb 1;32(2):175–180. doi: 10.1093/sleep/32.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scammell TE, Mochizuki T. Is low histamine a fundamental cause of sleepiness in narcolepsy and idiopathic hypersomnia? Sleep. 2009 Feb 1;32(2):133–134. doi: 10.1093/sleep/32.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]