Abstract
Objective
A novel rodent model and a recent randomized trial of hyperuricemic adolescents with hypertension suggest a pathogenetic role of uric acid in hypertension, but it remains unknown whether these findings would be applicable to adult populations where the larger disease burden exists. We conducted a systematic review and meta-analysis to determine if hyperuricemia was associated with incident hypertension, particularly in various demographic subgroups.
Methods
We searched major electronic databases using Medical Subject Headings and keywords without language restrictions (through April 2010). We included prospective cohort studies with data on incident hypertension related to serum uric acid levels. Data abstraction was conducted in duplicate. We analyzed age, gender, and race subgroups.
Results
A total of 18 prospective cohort studies representing data from 55,607 participants were included. Hyperuricemia was associated with an increased risk for incident hypertension (adjusted risk ratio [RR], 1.41; 95% CI, 1.23–1.58). For 1 mg/dl increase in uric acid level, the pooled RR for incident hypertension after adjusting for potential confounding was 1.13 (95% CI, 1.06–1.20). These effects were significantly larger in younger study populations (p=0.02) and tended to be larger in women (p=0.059). Two studies suggested that the effect may also be larger among black individuals. Furthermore, later publication year and US-based studies were significantly associated with a lower effect estimate (p-values <0.02).
Conclusion
Hyperuricemia is associated with an increased risk for incident hypertension, independent of traditional hypertension risk factors. This risk appears more pronounced in younger individuals and women.
Keywords: hyperuricemia, hypertension, meta-analysis
INTRODUCTION
Hypertension affects approximately one-third of Americans(1) and is a leading cause of morbidity and mortality.(2) While the etiology of hypertension is unclear in many patients,(3) uric acid has been hypothesized to activate the renin-angiotensin system,(4) which can lead to injury to pre-renal blood vessels.(5) Recently, a novel rodent model of arteriolopathy and hypertension induced by mild hyperuricemia has brought renewed interest into this hypothesis.(5–8) Furthermore, a cross-over randomized trial of 30 hyperuricemic adolescents with hypertension demonstrated that lowering uric acid levels with allopurinol led to lowering blood pressure over a 4-week period.(9) These data support the pathogenetic role of uric acid in the development of hypertension in this specific demographic context, but it remains unknown whether these findings would be applicable to adult populations where the much larger share of hypertension disease burden exists.
To objectively summarize the published data about the relation between hyperuricemia and the risk for incident hypertension, particularly in various demographic subgroups, we performed a meta-analysis of prospective studies on the topic. We hypothesized that in addition to serving as an independent risk factor for incident hypertension in the general population, hyperuricemia may have important differential effects in age, gender, and racial subgroups.
MATERIALS AND METHODS
Literature Search
We searched three major electronic databases — MEDLINE (through April 2010), EMBASE (1980- April 2010), and the Cochrane Library (through April 2010) — using the following heading MeSH terms and keywords: [uric acid OR hyperuricemia OR urate OR hyperuric$] AND [hypertension OR hypertension, renal OR hypertension, renovascular OR hypertension, malignant OR blood pressure]. The wildcard character “$” insures that any word that has “hyperuric” at its beginning is included in the search results. We also hand searched bibliographies of identified reports and review articles for additional references.
Study Eligibility and Selection
To be eligible for inclusion, we only considered 1) prospective cohort studies without age restrictions, 2) with longer than one year of follow-up, 3) with a sample size of at least 100 subjects, and 4) an inception cohort free of hypertension. No geographic or language restrictions were applied.
Two authors (PCG and SYK) independently reviewed the studies for final determination of inclusion or exclusion. When multiple articles were published from the same cohort, we selected the reports that contained the most complete and relevant data on the association between hyperuricemia and hypertension.
Data Abstraction and Quality Assessment
All data were independently abstracted in duplicate by two authors (PCG and SYK) using a data abstraction form to retrieve information on study characteristics, participant information, definition of hyperuricemia and hypertension, outcome results, analyses, and adjustment. Two authors (PCG and SYK) assessed the quality of studies using the Newcastle–Ottawa Scale.(10) This quality score was calculated on the basis of three major components of cohort studies: selection of study groups (0–4 points), comparability of study groups (0–2 points), and ascertainment of the outcome of interest (0–3 points).(10) A higher score represented better methodological quality (Table 1). Areas of disagreement or uncertainty were resolved by discussion.
Table 1.
Study Characteristics
| Study name, Publication year |
Country, Cohort | Study population (% men) |
Age (mean) |
Follow- up (yr) |
Hyperuricemia definition (mg/dl) |
Hypertension definition |
Variables controlled (No.) |
Quality assessment score§ |
|---|---|---|---|---|---|---|---|---|
| Forman 2009(2) | USA, NHS | 1496 (0) | 43 | 8 | 4.6 | Self report | activity, age, BMI, chol, etoh, FH, GFR, homocysteine, insulin, sICAM,smoke, TG (12) | 4/2/2 |
| Zhang 2009(30) | China, Quinadao Port Health Study | 7220 (73.8) | 37 | 4 | 5.7 (M) 4.8 (F) |
>/= 140/90, meds | activity, age, BMI, chol, circumference, DBP, etoh, FH, glc, HDL, salt, SBP, smoke, TG (14) | 4/2/3 |
| Forman 2007(25) | USA, HPFS | 1454 (100) | 61 | 8 | 6.8 | Self report | activity, age, BMI, DBP, etoh, FH, GFR, race, SBP, smoke, weight change (11) | 4/2/2 |
| Krishnan 2007(26) | USA, MRFIT | 3073 (100) | 44 | 6 | 7.0 | >/= 140/90, meds | age, BMI, creatinine, chol, DBP, etoh, proteinuria, SBP, smoke (9) | 3/2/3 |
| Mellen 2006(22) | USA, ARIC | 9104 (45.5) | 53.3 | 9 | 7.0 | >/= 140/90, meds | age, BMI, DBP, DM, GFR, location, SBP, smoke (8) | 4/2/2 |
| Perlstein 2006(23) | USA, NAS | 2062 (100) | 41.7 | 21.5 | 7.0 | >/= 160/95, meds | age, chol, circumference, DBP, etoh, glc, SBP, smoke, TG (9) | 4/2/3 |
| Shankar 2006(24) | USA, Beaver Dam Eye Study | 2520 (43.7) | 58.3 | 10 | 6.6 | >/= 140/90, meds | A1C, activity, age, BMI, chol, DBP, DM, education, etoh, GFR pulse, SBP, sex, smoke (14) | 4/2/3 |
| Sundstrom 2005(21) | USA, Framingham | 3329 (44.4) | 48.7 | 4 | 6.4 | >/= 140/90, meds | age, BMI, cardiac meds, creatinine, DBP, DM, etoh, GFR, proteinuria, SBP, sex, smoke, weight change (13) | 4/2/3 |
| Nagahama 2004(29) | Japan, OGHMA | 4489 (65.2) | 46.9 | 3 | 7.0 (M) 6.5 (F) |
>/= 140/90 | age, chol, DM, etoh, FH, HDL, obesity, smoke, TG (9) | 4/2/3 |
| Nakanishi 2003(28) | Japan, Japanese Office Workers | 2310 (100) | 46.2 | 6 | 6.7 | >/= 140/90, meds | activity, age, BMI, chol, etoh, fasting glc, FH, HDL, MBP, smoke, TG (11) | 4/2/3 |
| Imazu 2001(33) | USA/Japan, Hawaii – LA – Hiroshima Study | 159 (35.7) | 54.8 | 15 | 6.0 | >/= 160/95, meds | age, BMI, change BMI, chol, fasting glc, insulin, SBP, sex, TG (9) | 3/2/2 |
| Taniguchi 2001(27) | Japan, Osaka Work Site | 6356 (100) | 41.6 | 9.7 | 6.2 | >/= 160/95 | activity, age, BMI, etoh, duration of walk to work, fasting glc, smoke (7) | 4/2/3 |
| Dyer 1999(20) | USA, CARDIA | 4747 (44.9) | 24.7 | 10 | Continuous | >/= 140/90, meds | activity, age, circum-ference, etoh, education, HDL, insulin, pulse, SBP, smoke, TG (11) | 4/2/3 |
| Jossa 1994(32) | Italy, Olivetti Heart Study | 505 (100) | 36.2 | 12 | Continuous | >/= 140/90, meds | age, BMI, chol, TG's (4) | 4/2/3 |
| Hunt 1991(19) | USA, Utah Cardiovascular Genetics Clinic | 1482 (97.3) | 34.4 | 7 | Continuous | Initiation of anti-hypertensive meds in clinic | age, BMI, SBP sex (4) | 3/2/2 |
| Selby 1990(18) | USA, Kaiser Permanente | 2062 (39.3) | 40.4 | 6 | Quintile 5 | >/= 160/95, meds | age, BMI, DBP, etoh, FH, salt, SBP (7) | 4/2/3 |
| Fessel 1973(17) | USA, Target Population and Screening Program | 335 (55) | 37 | 5 | 2 SD from matched mean | >/= 150/95 | Unadjusted (0) | 3/0/3 |
| Kahn 1972(31) | Israel, Israeli Heart Study | 2904 (100) | 40–60+ | 5 | 5.0 | >/= 160/95 | age, area of birth (2) | 3/0/3 |
Abbreviations - NHS: Nurses’ Health Study, HPFS: Health Professionals Follow-up Study, MRFIT: Multiple Risk factor Intervention Trial, ARIC: Atherosclerosis Risk in Communities, NAS: Normative Aging Study, OGHMA: Okinawa General Health Maintenance Association, CARDIA: Coronary Artery Risk Development in Young Adults, A1C: hemoglobin A1C, ACTIVITY: physical activity level, BMI: body mass index, CHOL: cholesterol, CIRCUMFERENCE: waist circumference, DBP: diastolic blood pressure, DM: diabetes mellitus,ETOH: alcohol use, FH: family history, GFR: glomerular filtration rate, GLC: glucose, HDL: high-density lipoprotein, INSULIN: fasting insulin, MBP: mean blood pressure, SALT: dietary salt intake, SBP: systolic blood pressure, sICAM: soluble intracellular adhesion molecule, SMOKE: tobacco use,. TG: triglycerides.
Derived from the Newcastle-Ottawa Scale for Cohort Studies(10).
Statistical Analysis
We converted serum uric acid levels in the studies that used the International system (SI) of units (µmol per liter) into the conventional units (milligram per deciliter), using a conversion rate of 16.81 (1 mg/dL = 59.48 µmol/L).(11)
Pooled estimates of both unadjusted and adjusted risk ratios (RRs) for incident hypertension were calculated using the DerSimonian and Laird random-effects model.(12) This statistical technique weights individual studies by sample size and variance (both within- and between-study variance) and yields a pooled point estimate and a 95% confidence interval (CI). The DerSimonian and Laird technique was considered appropriate because of the relative heterogeneity of the source population in each study. We analyzed hyperuricemia as both a categorical and continuous variable. For studies reporting hyperuricemia as categorical variable, we chose the category nearest to 6.8 mg/dL to define the hyperuricemic group.(13) We preferentially analyzed reported RRs but used raw data to create contingency tables and calculate RRs when not directly reported or when derived from linear trend across category rather than category of interest versus referent category. We added a value of 1 to all counts for those studies that contained a zero count.
We assessed for potential publication bias using the Begg’s and Egger’s tests and constructed funnel plots to visualize possible asymmetry.(14) To assess for heterogeneity, we calculated the I2-value, which quantifies the percentage of variability attributable to between-study difference.(15) We performed meta-regression analysis on pooled adjusted and unadjusted RRs to see if any detected heterogeneity could be due to differences in study characteristics using the maximum likelihood method of estimating the additive between-study variance. We evaluated the following study-level characteristics: gender, mean age of study population, length of follow up, publication year, study country, definition of hypertension, and number of adjusted variables (categorized as 0–5; 6–10; >10).
All statistical analyses were done using STATA 11 (Stata Corp, College Station, TX) or SAS 9.1 (SAS Institute, Cary, NC). We followed the Meta-analyses of Observational Studies in Epidemiology guidelines(16) in the report of this meta-analysis.
RESULTS
Study Characteristics
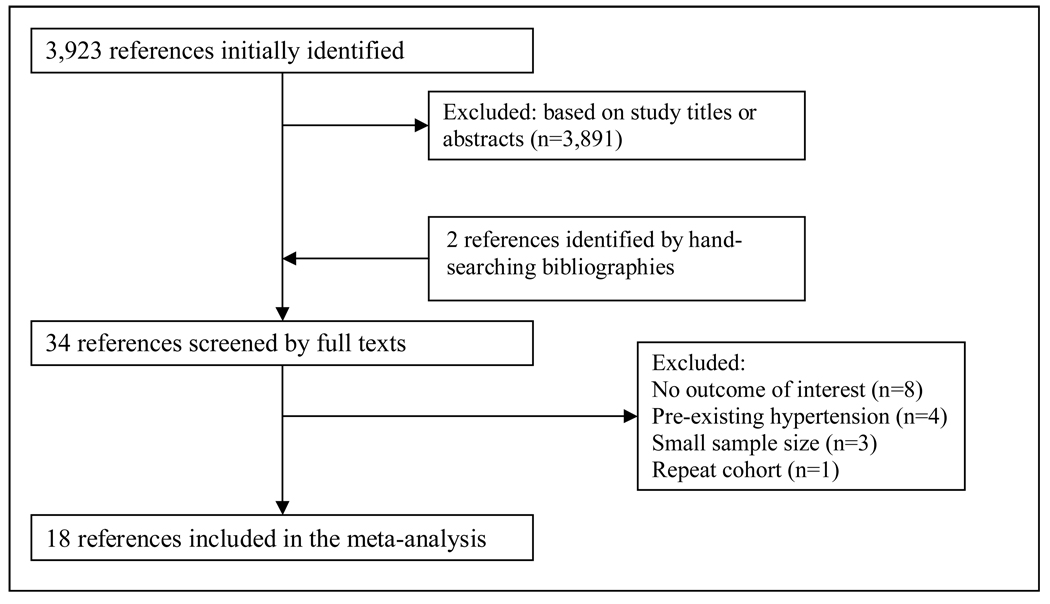
The electronic database search identified 3,923 references. We excluded 3,891 articles based on title or abstract resulting in 34 references for full text review. Review of references of articles selected for full text review and of relevant review articles yielded 2 additional references. A total of 18 prospective cohort studies representing data from 55,607 participants were included in our final review. There were no disagreements between the two reviewers regarding study inclusion (Figure 1).
Figure 1.
Study selection flow diagram.
Table 1 provides characteristics for the 18 included studies. All studies were written in English. Eleven studies were conducted in North America,(2, 17–26) four in Asia,(27–30) two in Europe,(31–32) and one in both North America and Asia.(33) Publication dates ranged from 1972 to 2009, whereas lengths of follow-up ranged from 3 to 21 years (average, 8 years). Hyperuricemia was defined on average at a level of 6.2 mg/dL (range, 4.6 – 7.0 mg/dL). Hypertension was typically defined as blood pressure ≥ 140/90 or ≥ 160/95, by self-report, or by use of anti-hypertensive medications.
Hyperuricemia and Incident Hypertension
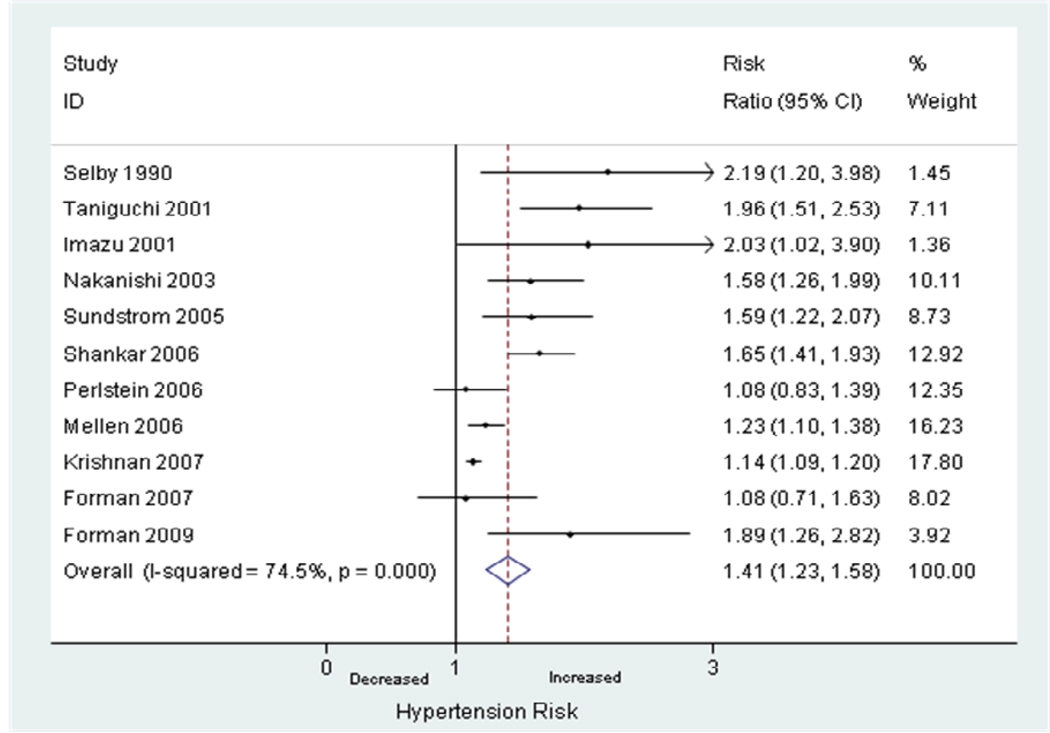
Twelve studies reported unadjusted RRs of hyperuricemia and incident hypertension.(2, 17–18, 21, 23–29, 31, 34) The pooled unadjusted RR was 1.81 (95% CI, 1.55–2.07) using the random effects model. There was significant heterogeneity between the studies reporting unadjusted RRs (I2 = 73.4%, p<0.0001). Eleven studies reported adjusted RRs of hyperuricemia and incident hypertension.(2, 18, 21–28, 33) The pooled adjusted RR was 1.41 (95% CI, 1.23–1.58). There was significant heterogeneity between the studies reporting adjusted RRs (I2 = 74.5%, p<0.0001) (Figure 2). In all studies that defined urate levels as a categorical variable, the adjusted RR for incident hypertension increased with each successive category of increasing baseline serum uric acid level.
Figure 2.
Random-effects analysis of adjusted risk ratios of hyperuricemia associated with incident hypertension.
Eleven studies defined urate levels as a continuous variable.(2, 19, 21–22, 24–28, 30, 32) Six studies defined hyperuricemia per 1mg/dl incremental increase,(2, 22, 24, 26–27, 32) and pooled adjusted RR per 1mg/dl increase was 1.13 (95% CI, 1.06–1.20). Eight studies defined hyperuricemia per 1 standard deviation (SD) incremental increase,(19, 21–22, 24–26, 28, 30) which ranged from 1.0–1.3 mg/dL. Pooled adjusted RR per 1 SD increase in uric acid level was 1.16 (95% CI, 1.07–1.26).
Analyses for Age, Gender, and Race
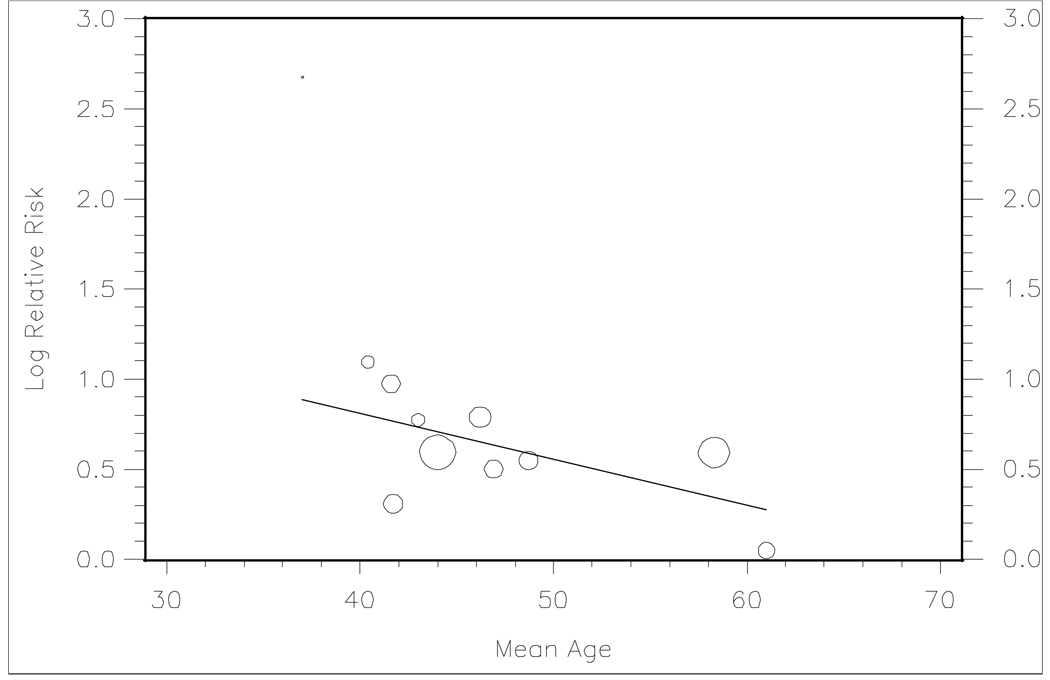
We found an inverse relationship between increasing mean study age and log-transformed unadjusted RRs among the eleven studies where this data was available (Figure 3).(2, 17–18, 21, 23–29) Similarly, meta-regression analysis showed that higher mean study age (coefficient −0.03, p=0.02) was significantly associated with a lower effect estimate for the risk of incident hypertension.
Figure 3.
Random effects determined meta-regression line (β1= −0.026, p=0.02) of reported unadjusted risk ratios versus mean study age in 11 studies. Bubble size represents sample size.
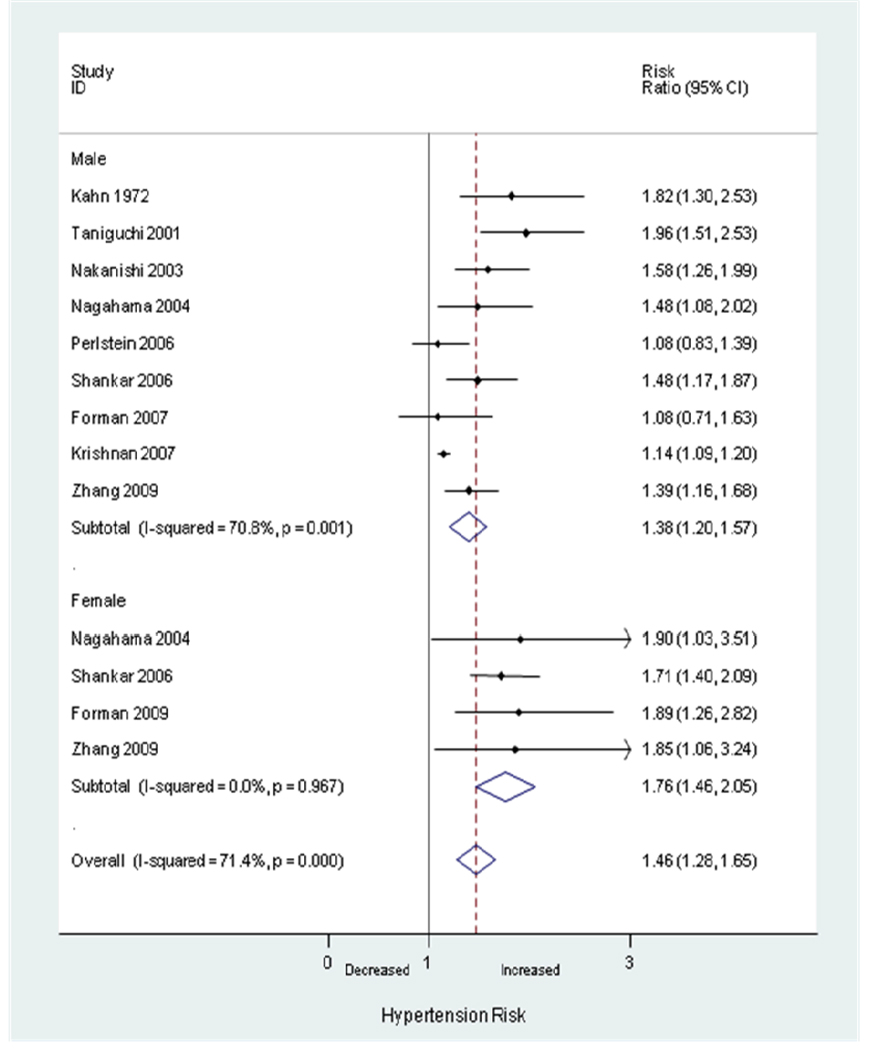
Gender-specific RRs were reported for men in nine studies(23–31) including seven limited to men(23–28, 31) and for women in four studies(2, 24, 29–30) including one limited to women.(2) The mean definition of hyperuricemia was 6.44 mg/dL for men and 5.63 mg/dL for women. The summary adjusted RRs of incident hypertension were 1.38 (95% CI, 1.20–1.57) in men and 1.76 (95% CI, 1.46–2.05) in women (Figure 4). Our meta-regression analysis suggested that female gender is associated with a stronger effect on incident hypertension (coefficient 0.25, p=0.059).
Figure 4.
Random-effects analysis of gender-specific adjusted risk ratios of hyperuricemia associated with incident hypertension.
Only two studies provided data regarding race as a potential effect modifier of the association between hyperuricemia and incident hypertension which precluded further analysis. Both studies reported the highest adjusted RR in black individuals. One study defined hyperuricemia as a continuous variable (per 1 SD increments),(20) and the other study defined hyperuricemia as a categorical variable.(22)
Other Potential Predictors of Effect Estimates
We performed meta-regression analysis on log-transformed adjusted RRs to investigate heterogeneity of effects across studies. Later publication year (coefficient −0.04, p=0.02) was associated with a significantly lower effect estimate for incident hypertension, while studies conducted outside the United States (coefficient: 0.32, p=0.02) were associated with a significantly higher effect estimate for incident hypertension. Unlike in studies reporting unadjusted RRs, mean study age and gender showed no significant effect on outcome in studies reporting adjusted RRs. Notably, all of these studies adjusted for age and gender within the respective analysis. Length of follow up, definition of hypertension, and number of adjusted variables were not statistically significant predictors of effect estimates in our analyses.
Publication Bias Assessment
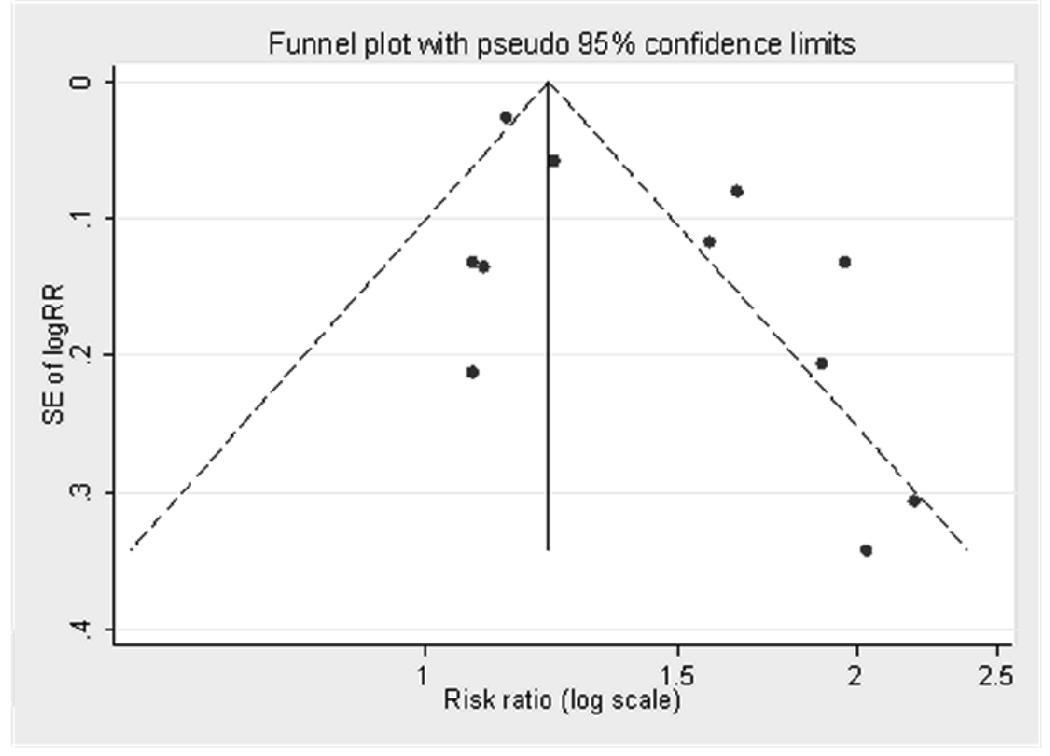
Evidence of publication bias for studies reporting adjusted RRs was noted in the funnel plot (Figure 5) and Egger’s test (p=0.03), but not in the Begg’s test (p=0.94).
Figure 5.
Funnel plot for publication bias in 11 studies reporting adjusted risk ratios of hyperuricemia associated with incident hypertension. Dashed lines indicate 95% confidence intervals. SE: standard error, RR: risk ratio.
DISCUSSION
In this systematic review and meta-analysis of published prospective studies, we found a modest but significantly increased RR for incident hypertension in subjects with hyperuricemia, independent of traditional risk factors for hypertension. The overall risk for incident hypertension increased by 13% per 1mg/dL increase in serum uric acid level and the risk was more pronounced in younger individuals and in women. Although serum uric acid level of 6.8 mg/dL was used to define hyperuricemia in this analysis, all original studies that employed categorical serum urate levels have reported that the risk of incident hypertension increases with increasing levels of serum uric acid. These data suggest that the relation between serum uric acid levels and hypertension is likely to be linear, rather than being dependent on a specific cut-point or threshold. Overall, these findings provide prospective evidence that individuals with higher serum uric acid, particularly younger adults and women, are at an increased risk for incident hypertension independent of other known risk factors.
Potential mechanisms behind the link between hyperuricemia and development of hypertension have included nitric oxide and renin-angiotensin pathways.(5, 35) Uric acid could lead to endothelial cell dysfunction via nitric oxide synthetase(36–38) and stimulate vascular smooth muscle cell proliferation.(7, 39) Furthermore, uric acid may also directly stimulate the renin-angiotensin system.(4, 40) A recent rodent model of induced hyperuricemia showed that uric acid could cause renal afferent arteriolopathy and tubulointerstitial disease, leading to hypertension.(6) The renal lesions and hypertension were prevented by lowering uric acid levels with allopurinol or benziodarone (a uricosuric agent) and reversed by angiotensin-converting enzyme inhibition.(6)
The stronger association observed among younger individuals is consistent with previous cross-sectional epidemiologic studies that showed a continuous relation of serum uric acid with blood pressure that was stronger in younger individuals and diminished over time.(41–42) Low prevalence of cardiovascular co-morbidities including renal disease in younger subjects may also explain the higher relative risk of hyperuricemia for incident hypertension in this group compared to older subjects. Additionally, children with primary hypertension often have elevated uric acid levels(43) and a recent randomized trial has demonstrated significant reduction of blood pressure and plasma renin activity with urate-lowering therapy in obese adolescents.(9) Experimental animal studies have demonstrated that once significant renal damage occurs, rats develop salt-sensitive hypertension regardless of the uric acid levels.(5) Thus, it is conceivable that hyperuricemia-related pathogenetic mechanisms may be more dominant in earlier stages of hypertension than later stages when salt-sensitivity becomes apparent. At this point, correction of the elevated uric acid level is no longer protective in the aforementioned rodent model of induced hyperuricemia.(44)
Hyperuricemia appears to have a stronger impact among women than among men. This trend would be consistent with gender-specific data from other cardiovascular outcomes. For example, a recent meta-analysis of prospective studies using the outcome of coronary artery disease has found that hyperuricemia is more strongly associated with the risk of coronary heart disease outcome in women.(45) Furthermore, a population based study reported that the association between gout and acute myocardial infarction was stronger in women than in men.(46) Serum urate levels in men are about 1 mg/dl higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the relative physiologic impact of having gout or a certain level of hyperuricemia may be stronger among women than men.
Similarly, race may be an important effect modifier for the link between hyperuricemia and incident hypertension. Although there were few studies addressing the issue of the race, hyperuricemic black individuals have been suspected of having the highest relative risk for incident hypertension.(20, 22) It would be valuable to investigate the impact of race in future studies.
Although we have found an association between hyperuricemia and incident hypertension, this association weakens in recently conducted studies. The forest plot of adjusted RRs (Figure 3) demonstrates that effect estimates approach the null in more recent studies. Additionally, meta-regression shows that later publication year predicts lower risk for incident hypertension. While one could suggest that later studies may have been of better quality, our quality scores of both earlier and later studies were consistently high. It is also conceivable that later studies tended to adjust for more covariates, and some of these covariates might have been at the causal pathway of interest (e.g. biomarker correlates). Thus, some of these adjustments might have led the effect estimates towards the null.
Interestingly, studies conducted outside of the US were associated with a higher effect estimate for incident hypertension than those done in the US. Although we attempted to minimize bias by imposing no geographic or language restrictions to our search strategy, all articles identified for inclusion were written in English. While the difference may represent a type of publication bias based on country-of-origin,(47) the difference may also reflect a biological divergence from different populations.
It remains unclear whether uric acid has a causal role in correlated metabolic conditions, such as insulin resistance and obesity(48). Nevertheless, all studies included in our meta-analysis that reported adjusted RRs controlled for adiposity (i.e. BMI or abdominal circumference) and most of the included studies adjusted for some measure of insulin resistance. If obesity or insulin resistance lies on a causal pathway between hyperuricemia and hypertension, then adjusting for these variables could bias the effect estimates towards the null. Given the increasing worldwide prevalence of obesity and metabolic syndrome in recent decades(49) and the high burden of these conditions in the US(50), adjusting for adiposity and insulin resistance might explain why there were lower effects estimates in studies conducted more recently and based on US populations. Interestingly, among studies reporting unadjusted RRs, publication year and study country showed no significant effect.
Several potential limitations inherent to meta-analysis of observational studies should be noted. First, even with our comprehensive search strategy, Egger’s test and the funnel plot suggested potential publication bias. While the rank-based Begg test did not find evidence of publication bias, this test tends to have lower power to detect publication bias. These test results do not allow us to gauge the degree of exaggeration in the pooled value. However, given that the largest studies(22, 26) indicate statistically significant increases in risk due to hyperuricemia, the increase in risk is likely real, although its magnitude may be overestimated. Second, statistical methods and degree of adjustment differed slightly in each study. Consequently, we performed separate analyses for hyperuricemia as both a continuous and categorical variable, we evaluated both unadjusted and adjusted RRs, and we selected the best adjusted RR per individual study. Third, in observational studies there is potential for bias from unmeasured confounding. Finally, although we focused on prospective studies, we cannot eliminate the potential for reverse causation (i.e. changes from pre-clinical hypertension leading to hyperuricemia).
Our study has several important strengths. We selected large-scale, prospective studies with inception cohorts free of disease, which helped precision of our estimates while minimizing heterogeneity. Furthermore, we evaluated the quality of observational studies with the Newcastle-Ottawa scale,(16) which scores the three most important domains of prospective cohort studies: selection of study participants, measurement of exposures and outcomes, and control of confounding. We also sought gender-specific analyses of the studies while adjusting for traditional hypertension risk factors. Finally, we performed meta-regression analysis to evaluate several potential sources of heterogeneity between studies.
In conclusion, our meta-analysis of published prospective studies indicates that hyperuricemia is associated with an increased future risk of incident hypertension, independent of other risk factors. This risk appears more pronounced in younger individuals and women. These data expand on well-established, cross-sectional associations between hyperuricemia and hypertension, and extend the link to the future risk of hypertension. Whether this link is causal remains to be clarified further by future studies. In particular, randomized trial data on the effect of urate-lowering medications on the prevention or treatment of hypertension, with particular attention paid to gender, age, and racial subgroups, would be valuable to clarify this question.
Acknowledgments
Financial supports or conflicts disclosure:
P Grayson - NIH T32 (AR 007598) Training Program in Rheumatic Disease
S Kim - NIH T32 (AR07442) Training Program in Rheumatic Disease
M LaValley - NIAMS P60 (AR 047785)
HK Choi - Served on the advisory boards for and received honorarium (less than $10,000) from Takeda Pharmaceuticals
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Peter C. Grayson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Grayson, Kim, Choi
Acquisition of data: Grayson, Kim, Choi
Analysis and interpretation of data: Grayson, Kim, LaValley, Choi
REFERENCES
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Archives of Internal Medicine. 2009;169(2):155–162. doi: 10.1001/archinternmed.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of Hypertension. Annals of Internal Medicine. 2003;139(9):761–776. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 4.Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, et al. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney International. 2004;66(4):1465–1470. doi: 10.1111/j.1523-1755.2004.00909.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 6.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 7.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. American Journal of Physiology - Renal Physiology. 2002;282(6 51-6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Lozada LG, Tapia E, Avila-Casado C, Soto V, Franco M, Santamaria J, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. American Journal of Physiology - Renal Physiology. 2002;283(5):F1105–F1110. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- 9.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells GSA, O'Connel D, Peterson J, Welch J, Loso M, et al. The NewCastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.html.
- 11.Young DS. SI units for clinical laboratory data. JAMA. 1978;240(15):1618–1621. [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox WR, Khalaf A, Weinberger A, Kippen I, Klinenberg JR. Solubility of uric acid and monosodium urate. Med Biol Eng. 1972;10(4):522–531. doi: 10.1007/BF02474201. [DOI] [PubMed] [Google Scholar]
- 14.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Fessel WJ, Siegelaub AB, Johnson ES. Correlates and consequences of asymptomatic hyperuricemia. Archives of Internal Medicine. 1973;132(1):44–54. [PubMed] [Google Scholar]
- 18.Selby JV, Friedman GD, Quesenberry CP., Jr Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. American Journal of Epidemiology. 1990;131(6):1017–1027. doi: 10.1093/oxfordjournals.aje.a115593. [see comment][erratum appears in Am J Epidemiol 1990 Sep;132(3):589] [DOI] [PubMed] [Google Scholar]
- 19.Hunt SC, Stephenson SH, Hopkins PN, Williams RR. Predictors of an increased risk of future hypertension in Utah. A screening analysis. Hypertension. 1991;17(6 Pt 2):969–976. doi: 10.1161/01.hyp.17.6.969. [DOI] [PubMed] [Google Scholar]
- 20.Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr, Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13(1):13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- 21.Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45(1):28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [see comment] [DOI] [PubMed] [Google Scholar]
- 22.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48(6):1037–1042. doi: 10.1161/01.HYP.0000249768.26560.66. [see comment] [DOI] [PubMed] [Google Scholar]
- 23.Perlstein TS, Gumieniak O, Williams GH, Sparrow D, Vokonas PS, Gaziano M, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031–1036. doi: 10.1161/01.HYP.0000248752.08807.4c. [see comment] [DOI] [PubMed] [Google Scholar]
- 24.Shankar A, Klein R, Klein BEK, Nieto FJ. The association between serum uric acid level and long-term incidence of hypertension: Population-based cohort study. Journal of Human Hypertension. 2006;20(12):937–945. doi: 10.1038/sj.jhh.1002095. [DOI] [PubMed] [Google Scholar]
- 25.Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. Journal of the American Society of Nephrology. 2007;18(1):287–292. doi: 10.1681/ASN.2006080865. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49(2):298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi Y, Hayashi T, Tsumura K, Endo G, Fujii S, Okada K. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: The Osaka Health Survey. Journal of Hypertension. 2001;19(7):1209–1215. doi: 10.1097/00004872-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi N, Li W, Fukuda H, Takatorige T, Suzuki K, Tatara K. Multiple Risk Factor Clustering and Risk of Hypertension in Japanese Male Office Workers. Industrial Health. 2003;41(4):327–331. doi: 10.2486/indhealth.41.327. [DOI] [PubMed] [Google Scholar]
- 29.Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertension Research - Clinical & Experimental. 2004;27(11):835–841. doi: 10.1291/hypres.27.835. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Sun K, Yang Y, Zhang H, Hu FB, Hui R. Plasma uric acid and hypertension in a Chinese community: prospective study and metaanalysis. Clin Chem. 2009;55(11):2026–2034. doi: 10.1373/clinchem.2009.124891. [DOI] [PubMed] [Google Scholar]
- 31.Kahn HA, Medalie JH, Neufeld HN, Riss E, Goldbourt U. The incidence of hypertension and associated factors: the Israel ischemic heart disease study. American Heart Journal. 1972;84(2):171–182. doi: 10.1016/0002-8703(72)90331-6. [DOI] [PubMed] [Google Scholar]
- 32.Jossa F, Farinaro E, Panico S, Krogh V, Celentano E, Galasso R, et al. Serum uric acid and hypertension: the Olivetti heart study. Journal of Human Hypertension. 1994;8(9):677–681. [PubMed] [Google Scholar]
- 33.Imazu M, Yamamoto H, Toyofuku M, Sumii K, Okubo M, Egusa G, et al. Hyperinsulinemia for the development of hypertension: data from the Hawaii-Los Angeles-Hiroshima Study. Hypertension Research - Clinical & Experimental. 2001;24(5):531–536. doi: 10.1291/hypres.24.531. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi N, Li W, Fukuda H, Takatorige T, Suzuki K, Tatara K. Multiple risk factor clustering and risk of hypertension in Japanese male office workers. Industrial Health. 2003;41(4):327–331. doi: 10.2486/indhealth.41.327. [DOI] [PubMed] [Google Scholar]
- 35.Mene P, Punzo G. Uric acid: bystander or culprit in hypertension and progressive renal disease? Journal of Hypertension. 2008;26(11):2085–2092. doi: 10.1097/HJH.0b013e32830e4945. [DOI] [PubMed] [Google Scholar]
- 36.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney International. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 37.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106(2):221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 38.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 39.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266(13):8604–8608. [PubMed] [Google Scholar]
- 40.Toma I, Kan J, Meer E, Pet-Peterdi J. Uric acid triggers renin release via a macula densa-dependent pathway. Presented at: American Society of Nephrology Annual Meeting; San Francisco, CA. 2007. F-P0240. [Google Scholar]
- 41.Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationship to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Archives of Internal Medicine. 1973;132(3):401–410. doi: 10.1001/archinte.132.3.401. [DOI] [PubMed] [Google Scholar]
- 42.Brand FN, McGee DL, Kannel WB, Stokes J, 3rd, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol. 1985;121(1):11–18. doi: 10.1093/oxfordjournals.aje.a113972. [DOI] [PubMed] [Google Scholar]
- 43.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 62(2):170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis. 2010 doi: 10.1136/ard.2009.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316(7124):61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keenan RT, Pillinger MH. Hyperuricemia, gout, and cardiovascular disease--an important "muddle". Bull NYU Hosp Jt Dis. 2009;67(3):285–290. [PubMed] [Google Scholar]
- 49.Seidell JC. Obesity, insulin resistance and diabetes--a worldwide epidemic. Br J Nutr. 2000;83 Suppl 1:S5–S8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 50.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]