Abstract
Objective
Behavioral and personality characteristics associated with excessive inhibition and disinhibition are observed in patients with eating disorders, but neural correlates of inhibitory control have not been examined in adolescents with these disorders.
Methods
Thirteen adolescents with binge eating and purging, i.e., bulimia nervosa or anorexia nervosa, binge-purge subtype ,14 with anorexia nervosa, restricting subtype, and 13 healthy controls performed a rapid jittered event related Go-NoGo task. FMRI images were collected using a 3T GE scanner and a spiral pulse sequence. A whole-brain 3-group ANOVA in SPM5 was used to identify significant activation associated with the Main Effect of Group, for the comparison of correct NoGo versus Go trials. The mean activation in these clusters was extracted for further comparisons in SPSS.
Results
The binge-purge group showed significantly greater activation than the healthy control group in the bilateral precentral gyri, anterior cingulate cortex, and middle and superior temporal gyri, and greater activation compared to both controls and anorexia nervosa restricting type group in the hypothalamus and right dorsolateral prefrontal cortex. Within-group analysis found that only the anorexia nervosa, restricting group showed a positive correlation between percent correct on NoGo trials and activation in posterior visual and inferior parietal cortex regions.
Conclusions
The current study provides preliminary evidence that during adolescence, eating disorder subtypes may be distinguishable in terms of neural correlates of inhibitory control. This distinction is consistent with differences in behavioral impulsivity in these patient groups.
Keywords: Eating Disorders - AJP0011, Biological Markers - AJP0082, Child Psychiatry - AJP0102, Adolescents - AJP0100, Cognitive Neuroscience - AJP0069
Introduction
Behavior and personality characteristics differ among patients with eating disorders depending on subtype. Patients with binge eating or purging behaviors, such as the anorexia nervosa binge-purge subtype and/or bulimia nervosa, often display impulsive and disinhibited personality characteristics. In contrast, those with anorexia nervosa, restrictive subtype (1–3) often show a restrictive and overly controlled behavioral style.
Disinhibition and impulsivity related to eating behaviors are hallmarks of bulimia nervosa (4, 5). In recognition of this core feature of the disorder, the DSM-IV diagnostic description of bulimia nervosa incorporates the requirement that binge eating episodes include this disinhibited (“out of control”) characteristic (6). Impulsivity may extend into other areas of life in addition to eating or purging (2). For example, individuals with bulimia nervosa often report alcohol and drug abuse, self-harm, sexual disinhibition, and shoplifting (5). Some data suggest that the basis of cognitive and behavioral disinhibition in bulimia nervosa may be related to serotonin dysregulation (7), while neuropsychological studies have found evidence of disinhibition at a neurocognitive level in affected individuals. For example, relative to healthy controls, subjects with bulimia nervosa who use laxatives were observed to make more errors of commission on a Go-NoGo task and endorse higher ratings for impulsive behaviors on a self-report assessment (4). Similarly, cognitive research into inhibitory processing using a motor stop signal paradigm and a motor Stroop task found that anorexia nervosa, restricting patients displayed superior response inhibition overall with fewer impulsive errors than patients with anorexia nervosa, binge-purge subtype (8). Anorexia binge-purge patients also made more response errors in a modified version of the Hayling sentence completion task compared to those with anorexia restricting subtype (8, 9). Thus, because of the evidence suggesting similarities in impulsive cognitive styles between anorexia nervosa, binge-purge type and bulimia nervosa, we group them together as a single group of binge-purge type eating disorders to compare it to anorexia nervosa, restricting type and healthy control groups in this study of cognitive inhibitory control.
Neural differences in executive functioning related to inhibitory control may be associated with the cognitive and clinical symptoms in bulimia nervosa (10–12). However, only limited functional imaging studies have examined inhibition and disinhibition in bulimia nervosa to date. A recent study by Marsh et al (2009) examined response inhibition in adult patients with bulimia nervosa (11). These authors found that adult subjects with bulimia nervosa responded more impulsively and made more errors on a response inhibition task (Simon Task) (13) compared to healthy controls, and patients with the most severe symptoms made the most errors. During correct responding on incongruent trials, patients failed to activate frontostriatal circuits to the same degree as healthy controls, including the bilateral inferior frontal gyrus, lenticular and caudate nuclei, and the anterior cingulate cortex. These authors conclude that diminished activity in these regions may contribute to the loss of control in the eating behavior of patients with bulimia nervosa. In contrast to patients with bulimia nervosa, clinical reports document perseverative, obsessive, and rigid thinking styles in patients with anorexia nervosa. Patients with anorexia nervosa are frequently perfectionistic, report obsessive-compulsive personality traits and obsessive-compulsive disorder in childhood (1). Studies also find that those recovered from anorexia nervosa continue to have anxiety, perfectionism, inflexible thinking, and over-concern with symmetry, exactness and order (14, 15). Neuropsychological research provides additional evidence of reduced cognitive inflexibility (set shifting) and an excessively detailed information processing style (weak central coherence), with a neglect of the overall picture (gestalt) in adults with anorexia nervosa (9, 12, 16, 17). Limited neuroimaging data in adults with anorexia nervosa support this idea as well. For example, Zastrow and colleagues (2009) found decreased activation in anterior cingulate and striatum associated with impaired cognitive-behavioral flexibility in patients with anorexia nervosa (12).
Adolescents with eating disorders have rarely been examined in structural or functional neuroimaging studies (18). It is well known that brain development undergoes significant alteration in adolescence (19) and development of executive functioning skills is a particularly dynamic process during this period (20), associated with increasing abilities pertaining to decision making, social processing, and inhibitory control. These refinements lead to what has been called the “collaborative brain” (21) wherein improved connections allow the prefrontal cortex to modulate critical interconnected subcortical structures (e.g. basal ganglia and amygdala). The lateral prefrontal cortex has a distinct architectonic trend within the frontal lobe (22). The lateral aspect develops later than other regions, both in ontogeny and phylogeny, and is important for making inhibitory processes more efficient.
In sum, phenomenological, clinical, neuropsychological, neuroimaging, and neurodevelopmental findings support the likely importance of examining inhibition/disinhibition in adolescents with eating disorders. Further, evidence suggests that aberrant functioning of the inferior frontal gyrus and the anterior cingulate gyrus are likely to be associated with impulsivity and disinhibition in patients that binge and purge, but not in those with anorexia nervosa, restricting subtype (11). Examination of response inhibition using fMRI in adolescents with eating disorders, specifically comparing those with anorexia nervosa, restricting subtype to those with bulimia nervosa or anorexia nervosa, binge-purge subtype, provides an opportunity to explore these processes in the developing brain and to distinguish these subtypes from each other on a neural basis, as well as from healthy controls. Examining neural correlates of inhibitory control in an adolescent population who are not severely emaciated and are not chronically ill may help to distinguish these features associated with the onset of the disorder as opposed to secondary effects associated with starvation and prolonged disease.
This preliminary study is the first to examine brain activation associated with response inhibition in adolescents with eating disorders, and, further, to compare patients with a binge-purge subtype to a restrictive subtype and a healthy control group. We hypothesized that brain activation associated with inhibitory control during a Go-NoGo task would differ in adolescents with eating disorders compared to healthy controls. We predicted that those with binge-purge behaviors would have abnormal activation in frontostriatal regions typically associated with response inhibition compared to healthy controls and those with anorexia nervosa, restricting type. We also predicted we would find evidence of excessive inhibition in the anorexia nervosa, restricting group compared to both healthy controls and those with binge-purge behaviors.
Method
This study was approved by the Stanford University internal review board and all participants signed informed consent forms (by parents if under the age of 18 years) and/ or assent (if participant was under the age of 18 years) before participation. Eating disordered subjects were current outpatients recruited from the Stanford University Child and Adolescent Psychiatry Clinic. Diagnoses of eating disorders and co-morbid psychiatric disorders were made by clinicians with expertise in eating disorders in children and adolescents and eating disorder diagnoses were confirmed by the Eating Disorder Examination (23) administered independently by trained interviewers. Participants with eating disorders were required to meet full DSM-IV criteria for their respective diagnosis within 3 months of study participation. Eating disordered subjects also completed other diagnostic and clinical assessments (see Table 1). Participants included 15 female subjects with anorexia nervosa, restricting type, 16 with binge-purge behaviors (including 12 with bulimia nervosa and 4 with anorexia nervosa, binge-purge subtype), and 15 healthy control subjects. None of the subjects with bulimia nervosa had a history of anorexia nervosa and none of the anorexia nervosa restricting type subjects had a history of bulimia nervosa. Healthy controls were recruited through advertisements in local papers. For all groups, eligible participants had no contraindications for MRI and no co-existing major neurological problems (e.g., seizure disorder, traumatic brain injury with loss of consciousness, multiple sclerosis) or psychotic disorders.
Table 1.
Clinical and Demographic Characteristics:
| Variable | Anorexia Nervosa Restrictor (R, n = 14) |
Binge/Purge (BP, n = 13) |
Healthy Controls (HC, n = 13) |
Statistical Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | ||
| Age (years) | 15.02 | 1.74 | 5.75 | 17.26 | 1.23 | 4.26 | 15.93 | 1.39 | 4.66 | B-P>HC, p= .016 |
| B-P>R, p= .002 | ||||||||||
| Duration of illness (months) | 32.07 | 19.91 | 61 | 28.92 | 18.03 | 56 | N/A | N/A | N/A | Ns |
| # Binging episodes 28 Days | 0 | 0 | 0 | 16.61 | 19.11 | 28 | 0.23 | 0.83 | 3 | BP>HC, p= .005 |
| BP>R, p= .003 | ||||||||||
| # Purging episodes 28 Days | 0.21 | 0.80 | 3 | 16.15μ | 23.02 | 63 | 0 | 0 | 0 | BP>HC, p= .018 |
| BP>R, p= .016 | ||||||||||
| EDE Global Score | 1.19 | 1.47 | 3.08 | 1.09 | 0.29 | 0.34 | B-P>HC, p= .000 | |||
| B-P>R, p= .001 | ||||||||||
| R>HC, p= .042 | ||||||||||
| MASC total score | 45.07 | 11.63 | 40 | 47.54 | 9.78 | 28 | 39.62 | 8.56 | 24 | B-P>HC, p= .038 |
| BDI total score | 14.07 | 12.75 | 41 | 23.62 | 13.42 | 43 | 4.92 | 5.35 | 19 | B-P>HC, p= .000 |
| R>HC, p= .024 | ||||||||||
| BIS 11 total | 53.66 | 6.27 | 26.25 | 68.84 | 9.46 | 33.75 | 57.02 | 8.14 | 33.75 | B-P>HC, p= .002 |
| B-P>R, p= .000 | ||||||||||
| Mean | SD | Min/Max | Mean | SD | Min/Max | Mean | SD | Min/Max | ||
| Weight | 92.4 | 11.85 | 66.5/107.6 | 126.12 | 29.24 | 84.4/136.4 | 127.63 | 17.3 | 97/162.25 | HC>R, p= .000 |
| BP>R, p= .001 | ||||||||||
| % Ideal Body Wt | 88.3* | 9.76 | 73.71/99.9 | 100.96 | 20.07 | 79.8/145.2 | 105.67 | 13.72 | 88.7/120.85 | HC>R, p= .001 |
| BP>R, p= .045 | ||||||||||
| Percent | Percent | Percent | ||||||||
| Weight below 86% | 42.86 | 15.38 | 0 | χ2 = 7.99, p = .018 | ||||||
| Current Psychotropic Meds‡ | 7 | 8 | 0 | Ns | ||||||
| Comorbid Dx† | 21 | 23 | N/A | Ns | ||||||
| Regular Menstruation 3 mth | 7.14 | 61.54 | 100 | BP>R, p= .000 | ||||||
| HC>R, p= .000 | ||||||||||
| B-P>HC, p= .020 | ||||||||||
| Caucasian | Asian | Mixed | Caucasian | Asian | Mixed | Caucasian | Asian | Mixed | ||
| Ethnicity Percent | 85.7 | 7.1 | 7.1 | 69.23 | 15.4 | 15.4 | 76.9 | 15.4 | 7.7 | |
| Handedness- Right | 100 | 84.62 | 84.62 | Ns | ||||||
Patients had to meet criteria within prior three months of scan- therefore, some had higher than 85% Ideal Body Weight (diagnostic criteria for anorexia).
Menstrual cycle information also collected for prior three months. One anorexia nervosa, restricting subtype participant met full Anorexia Nervosa criteria within past 3 months, though reported currently menstruating at 78 % Ideal Body Weight.
Current medications included one Binge-Purge subject on Prozac and one anorexia nervosa, restricting subtype subject on Lexapro
One patient omitted from calculation due to innumerable purging (specifically, vomiting) episodes.
Comorbid diagnoses included one Binge-Purge subject with Major Depressive Disorder & General Anxiety Disorder, one with General Anxiety Disorder, and one with Obsessive Compulsive Disorder; one anorexia nervosa, restricting subtype with Obsessive Compulsive Disorder, two with Major Depressive Disorder
MRI Acquisition
Imaging data were acquired on a 3.0T GE Signa Excite magnet housed in the Lucas Imaging Center of Stanford University, using a custom-built whole head coil that provides a 35% advantage in signal to noise ratio over that of the standard GE coil. Following a 3-plane localizer scan, fMRI data were collected using a spiral-in/out sequence which provides optimal signal to noise while minimizing susceptibility effects (24). Thirty axial slices (3 mm thick, 1 mm skip) parallel to the AC-PC line and covering the whole brain were imaged (TR = 2000 msec, TE = 30msec, flip angle = 90°, 1 interleave, field of view will be 22 cm2, 64×64 matrix, in-plane spatial resolution of 3.125mm). The task was presented using E-Prime software (Psychology Software Tools, Pittsburgh, PA), which also triggered the initiation of the scan. Visual stimuli were projected from the foot of the scanner onto a screen attached to the head coil, and viewed via a mirror.
Subjects performed a rapid jittered event related Go-NoGo task featuring a series of letters. Subjects pushed a button in response to all letters, except for the infrequent letter X. This task is a classic test of executive function, requiring effortful inhibition of a prepotent response (25). The task lasted 16 minutes, and presented 300 Go stimuli and 75 NoGo stimuli (1:4 ratio). The intertrial interval was jittered from 2 – 12 seconds.
Data Analysis
Functional data were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). FMRI images were corrected for slice timing, spatially realigned, motion repaired using the ArtRepair toolbox (http://cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm) normalized into an age-appropriate stereotactic template from the CCHMC (https://irc.cchmc.org/software/pedbrain.php), smoothed with a 7 mm Gaussian filter, and high-pass filtered. A fixed effects model compared correctly inhibited NoGo trials to correct Go trials for each subject.
Within groups t-tests were performed using random effects analyses and a threshold of p=.01 height and p=.01 extent, corrected for multiple comparisons. For all SPM5 analyses, the location of significant clusters and the coordinates of peak voxels in Talairach space were determined using the mni2tal function (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
The 3 subject groups were compared using a 2 step approach. First, a whole-brain ANOVA was performed in SPM5, including all 3 subject groups, for the contrast of correct NoGo to Go trials. Significant clusters of activation associated with the Main Effect of Group were identified using a threshold of p=.01 height and extent = 80 voxels, uncorrected. Although this threshold is uncorrected, it is more stringent than that used in many fMRI studies, and is appropriate for the initial step of this analysis that identifies clusters of interest. In the second part of the analysis, the mean activation in each of the identified clusters was extracted using the MARSBAR toolbox (http://marsbar.sourceforge.net/), and transferred to SPSS for follow-up t-tests. The t-tests conducted in SPSS included age as a covariate (because of group differences in age), and used a corrected threshold of p=.0083. This threshold was determined as p=.05 divided by the number of clusters identified in the SPM5 whole brain analysis (see results below). Limiting the follow-up t-tests to regions of interest (ROIs) identified by the 3-group ANOVA reduces the probability of Type I error. Extracting these ROIs into SPSS allows correlation analyses with clinical and behavioral variables and examination of distributions associated with each group. Behavioral and demographic data were also analyzed in SPSS using ANOVA and follow-up t-tests when appropriate.
Whole brain correlations with task accuracy were conducted in SPM5 using multiple regression. A threshold of p=.01 height and p=.01 extent, corrected for multiple comparisons was used to determine significance.
Correlations between clinical and behavioral measures and brain activation in the extracted ROIs were performed in SPSS using a Pearson’s correlation analysis. The most relevant clinical measures were chosen for analysis, including the total Eating Disorder Examination score, the Beck Depression Inventory, the Behavioral Inhibition Scale, and the Multidimensional Anxiety Scale for Children. A corrected alpha of p=.05/4 clinical measures = .0125 was chosen as a threshold.
Results
Two subjects from the anorexia nervosa, restricting group, 2 from the binge-purge group, and 2 from the healthy control group were removed from the data analyses, either for excessive motion in the scan, or for behavioral data with less than 50% correct responses, which suggested noncompliance with, or misunderstanding of task instructions. The remaining subjects included 14 in the anorexia nervosa, restricting group, 13 in the binge-purge group, and 13 in the healthy control group. Table 1 shows the demographic and clinical characteristics of each group. Handedness did not differ between groups (see Table 1). Age, Eating Disorder Examination scores, and behavioral symptom reports are consistent with the clinical presentation of these disorders in adolescents; therefore, participants with bulimia nervosa and anorexia nervosa, binge-purge subtype were at higher weights and were slightly older than participants with anorexia nervosa, restricting group (26). There was a significant difference in age across the groups, primarily associated with low variance in age within each group (F(2,37)=7.03, p=.003). The binge-purge group was older than the anorexia nervosa, restricting group and the healthy control groups (binge-purge mean age=17.26, SD=1.18; healthy control mean age = 15.93, SD=1.33; anorexia nervosa restricting group mean age=15.02, SD=1.74). Accordingly, we performed between-group comparisons of functional activation in SPSS with and without age as a covariate (see below). Further analyses of the effects of age on brain activation are included below.
Behavioral Performance
All participants performed with high accuracy on the Go trials, and a similar rate of false alarms on the NoGo trials. The means and standard deviations are as follows: Accuracy (percent correct) for Go trials: healthy controls 94.64 (9.96); binge-purge: 97.38 (2.65); anorexia nervosa, restricting group: 97.71 (3.31). Response time for Go trials (in milliseconds): healthy controls: 386.49 (55.88); binge-purge group: 403.46 (45.05); anorexia nervosa restricting group: 356.54 (90.68). Accuracy for NoGo trials: healthy controls: 73.74 (14.54); binge-purge group: 77.23 (10.77); and anorexia nervosa, restricting group: 70.86 (12.11). Response time for false alarms: healthy controls: 350.07 (43.74); binge-purge group: 333.09 (29.23); anorexia nervosa, restricting group 341.62 (25.13). There were no group differences in task accuracy for Go trials (F<1) or NoGo trials (F<1). Also, there were no group differences in response time for Go trials (F(2, 37)=1.68, p=.20).
Correlations between age and task performance were not significant across groups. Within groups, only 1 correlation was marginally significant: within the anorexia nervosa restricting group, age was correlated with response time for false alarms, r=.54, p=.047. Therefore, although older subjects took longer to make a false alarm on a NoGo trial, response time for false alarms was not significantly different between groups.
Neuroimaging Findings
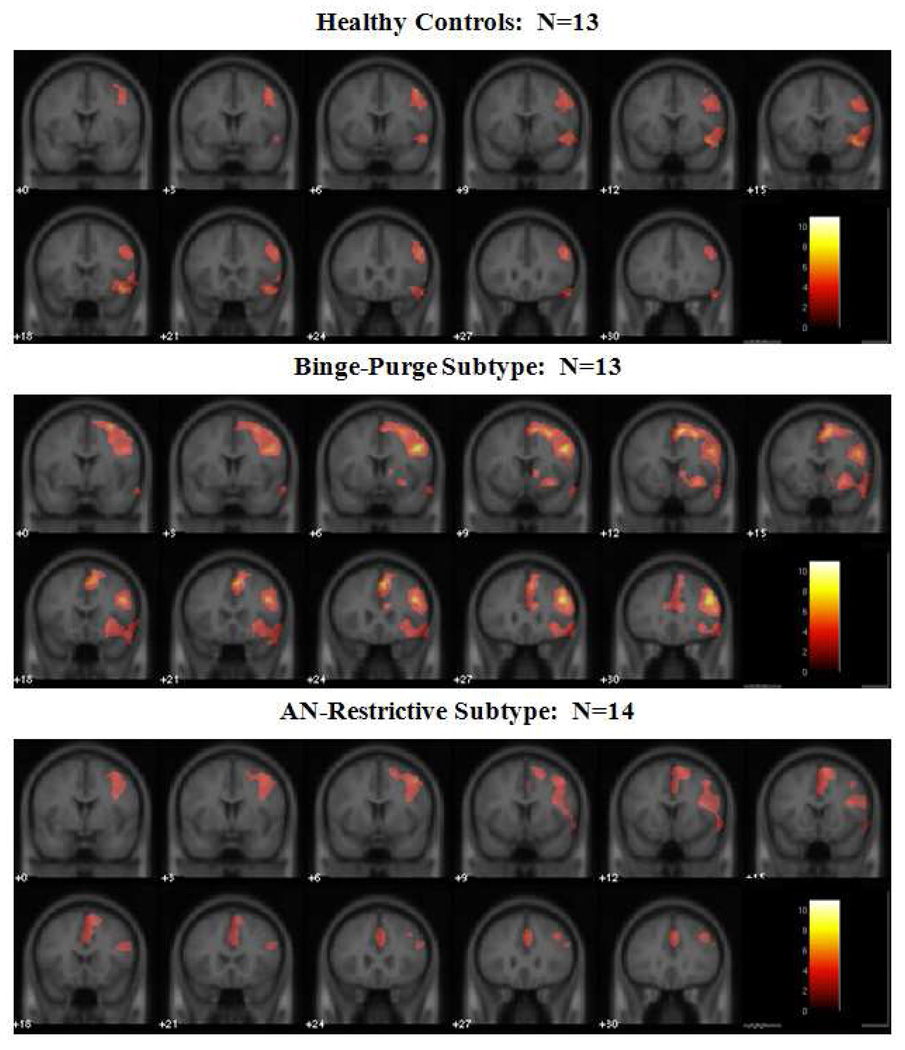
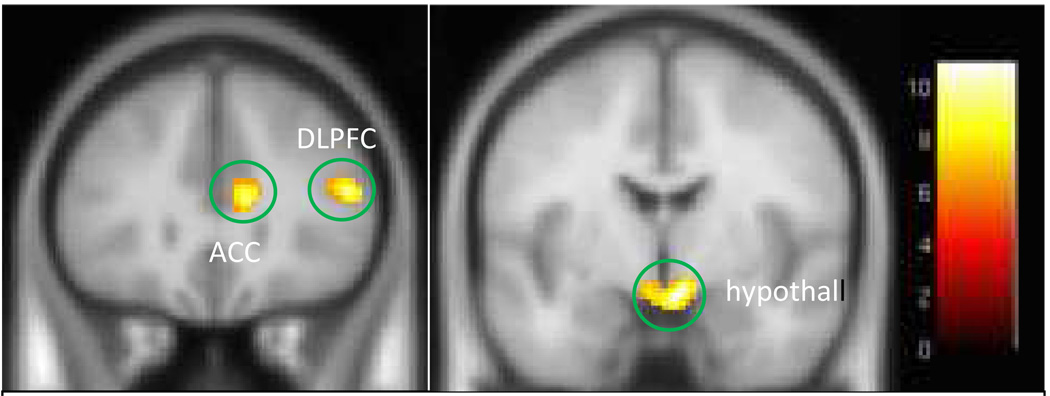
FMRI results showed that, within each group, right inferior and middle frontal gyri were activated during successful response inhibition trials (i.e., don’t press when observing the letter “X”) compared to successful response trials (NoGo-Go), see Figure 1 and Table 2. Also, the binge-purge group showed right dorsal caudate and anterior cingulate activation, and widespread frontal activation. The anorexia nervosa, restricting type group also showed anterior cingulate activation, but not caudate activation. A 3-group ANOVA showed a significant main effect of group in the bilateral hypothalamus, right dorsolateral prefrontal cortex, right anterior cingulate cortex, right middle temporal gyrus, and bilateral precentral gyri (see Figure 2 and Table 3). Follow-up between-group comparisons performed in SPSS (corrected p=.0083) showed that all of the group differences were attributed to increased activation in the binge-purge group. For left precentral gyrus, binge-purge>health control, p=.001, binge-purge> anorexia nervosa, restricting type, p=.011 (trend), for right precentral gyrus, binge-purge>health control, p=.0001, binge-purge>anorexia nervosa, restricting group, p=.006; for right anterior cingulate cortex, binge-purge>health control, p=0001; binge-purge>anorexia nervosa, restricting type, p=.013 (trend) ; for right middle/superior temporal gyrus, binge-purge>healthy control, p=.0001, binge-purge>anorexia nervosa, restricting group, p=.005; for hypothalamus, binge-purge>health control, p=.001; binge-purge>anorexia nervosa, restricting group, p=.001; for right dorsal lateral prefrontal cortex, binge-purge>health control, p=.001; binge-purge>anorexia nervosa, restricting group, p=.001. When the analysis was repeated using age as a covariate, all of the group differences between binge-purge and healthy control groups remained. Greater activation in the binge-purge group compared to the anorexia nervosa, restricting group remained only in the hypothalamus and the dosolateral prefrontal cortex (Table 3). As mentioned above, a corrected alpha of p=.0083 was used for significance (p=.05 / 6 regions = .0083).
Figure 1.
Significant Activation to correct NoGo-Go trials for each Group
Table 2.
Locations of Significantly Activated Regions Within each Group
| Region | Brodmann Area | # voxels | T/Z at peak | Location of Peak: Tal: X, Y, Z |
|---|---|---|---|---|
| Healthy Controls | ||||
| R inferior frontal gyrus | 45/47 | 967 | 7.36/4.45 | 42, 15, −6 |
| R Insula | 13 | 6.53/4.19 | 42, 15, −2 | |
| R superior temporal gyrus | 38 | 5.63/3.87 | 48, 13, −7 | |
| R middle temporal gyrus | 22/21 | 1639 | 6.44/4.16 | 67, −41, 4 |
| R inferior temporal gyrus | 37 | 6.41/4.15 | 66, −38, −4 | |
| R superior frontal gyrus | 9/8/10 | 3053 | 7.21/4.4 | 24, 48, 36 |
| R middle frontal gyrus | 6/9/10/46 | 5.61/3.86 | 42, 49, 12 | |
| Binge-Purge Subtype | ||||
| R middle frontal gyrus | 9/46 | 10820 | 11.01/5.29 | 46, 10, 36 |
| R inferior frontal gyrus | 45/47 | 4.75/3.5 | 34, 26, 2 | |
| R insula | 13 | 4.5/3.39 | 24, 14, −8 | |
| R putamen | 2.89/2.32 | 16, 12, 0 | ||
| R anterior cingulate gyrus | 32/24 | 4.85/3.54 | 4, 36, 26 | |
| R superior frontal gyrus | 8 | 8.58/4.77 | 4. 24. 49 | |
| R inferior parietal lobe | 40 | 7754 | 8.83/4.83 | 46, −56, 45 |
| R middle/inferior temporal | 21/22/37 | 6.96/4.33 | 56, −34, −4 | |
| R precuneus | 7 | 8.15/4.66 | 8, −71, 48 | |
| R superior parietal lobe | 7 | 7.44/4.47 | 44, −58, 49 | |
| AN-Restrictive Subtype | ||||
| R supramarginal gyrus | 40 | 3515 | 8.3/4.81 | 61, −51, 34 |
| R inferior parietal lobe | 40 | 6.23/4.17 | 48, −60, 47 | |
| R superior parietal lobe | 7 | 7.02/4.44 | 50, −64, 50 | |
| R middle frontal gyrus | 10/46/6 | 4936 | 6.04/4.1 | 44, 50, 20 |
| R superior frontal gyrus | 10 | 5.47/3.87 | 40, 51, 16 | |
| R inferior frontal gyrus | 44 | 3.13/2.54 | 48, 10, 18 | |
| R anterior cingulate gyrus | 32 | 3.75/5.05 | 0, 18, 42 |
Significance threshold was set at p=.01 height and p=.01 cluster extent, corrected; R=right
Figure 2.
Clusters of significant activation from the 3-group ANOVA: Main Effect of Group. From left to right, the clusters circled in green include the right ACC, right DLPFC, and bilateral hypothalamus. Talairach y=27 (left image) and 0 (right image). The colorbar represents values of F.
Table 3.
Significant clusters determined by ANOVA: Main Effect of Group
| Region | Brodmann Area |
# of voxels |
F/Z at peak | Location of Peak: Tal: X, Y, Z |
Group Difference in SPSS P=.0083, corrected |
|---|---|---|---|---|---|
| L precentral gyrus | 6/4 | 88 | 8.30/3.08 | −10, −18, 64 | B/P > HC p=.001 |
| R precentral gyrus | 6 | 150 | 9.61/3.33 | 10, −18, 64 | BP > HC p=.001 |
| R anterior cingulate gyrus | 24 | 132 | 9.24/3.26 | 8, 28, 15 | B/P > HC p=.001 |
| R middle/superior temporal gyrus | 37 | 242 | 8.61/3.14 | 50, −55, −2 | B/P > HC p=/0001 |
| R/L hypothalamus | N/A | 222 | 11.22/3.61 | 4, 4, −7 | B/P > HC p=.001; B/P > ANR p=.003 |
| R inferior/middle frontal gyri* | 46 | 83 | 9.78/3.36 | 50, 30, 15 | B/P > HC p=.003; B/P > ANR p=.006 |
A significance threshold of p=.01, extent=80 was used for the ANOVA: main effect of group;
Dorsolateral prefrontal cortex
Although the analyses were performed while co-varying for the group difference in age, we further tested whether age had an effect on activation in any of the regions of interest. We conducted Pearson’s correlations between activation in each brain region and age, both within each group and across all groups. None of the correlations were significant (at a threshold of p=.05), suggesting that age did not significantly influence our results.
The low rate of false alarms greatly reduces our ability to detect brain activation associated with false alarm trials. Typically about 30 trials are needed to detect activation in an event-related study, corresponding to a false alarm rate of 40% (of a possible 75 NoGo trials). Only 3 control subjects and 4 from the anorexia nervosa, restricting group, and none of the binge-purge group subjects made 40% or more false alarms. Instead, we performed a whole-brain correlation with percent of correctly inhibited trials within each group, using a threshold of p=.01 height and p=.01 cluster extent, corrected for multiple comparisons. Therefore, this analysis suggests group-specific neural strategies that are successful (positive correlation) or unsuccessful (negative correlation). We found that only the anorexia-nervosa, restricting type group showed a significant positive correlation with percent correctly inhibited trials in a single large cluster (cluster size=3657 voxels, peak Z=3.72, location: x=18, y=−80, z=28). The cluster included regions of the inferior parietal cortex (BA 7) and precuneus (BA 19/31) and posterior cingulate gyrus (BA 31). This suggests that, within the anorexia nervosa restricting group, successful inhibition is associated with greater recruitment of brain regions underlying visual attention (27) and visual working memory (27) (e.g., precuneus and inferior parietal cortex). Attention to detail is a cognitive feature associated with anorexia nervosa (17).
Association with Clinical Measures
Pearson’s correlation analysis was used to find associations between activation in the extracted ROIs and relevant clinical measures, including the Eating Disorder Examination score, Beck Depression Inventory, Behavioral Inhibition Scale, and Multidimensional Anxiety Scale for Children. A threshold of p=.05 / 4 = .0125 was required for significance. Across all groups combined, total Eating Disorder Examination was significantly correlated with activation in the bilateral precentral gyrus, right anterior cingulate cortex, right superior temporal gyrus, and hypothalamus. However, these correlations were attributable to group differences in total Eating Disorder Examination score. The correlations were repeated within each group separately and were not significant. In addition, there were no significant correlations with subscales on the Eating Disorder Examination or number of bulimic episodes. Similarly, for Beck Depression Inventory measures, significant correlations across all groups were found with the bilateral precentral gyrus and the right superior temporal gyrus, but these were due to group differences in Beck Depression Inventory scores. Correlations between Beck Depression Inventory and ROI values were not significant within each group separately. Scores on the Behavioral Inhibition Scale were not correlated with activation in any of the ROIs. The Multidimensional Anxiety Scale for Children was significantly correlated with right superior temporal gyrus activation across all groups (r=.48, p=.002). This was found to be attributable primarily to a significant correlation between Multidimensional Anxiety Scale for Children scores and right superior temporal gyrus activation within the binge-purge group (r=.70, p=.007), but was not significant within the other groups.
Possible effects of weight restoration on outcome were examined. In the binge-purge group, only 2 of the subjects weighed under 86% IBW; however, to check if this variable affected our results, we excluded the 2 low weight binge-purge subjects and re-ran the comparisons with the healthy control group. The results did not change for any of the 6 brain regions, even while covarying for age. Therefore, low weight did not appear to influence the comparisons between binge-purge and healthy control subjects. The comparisons with the anorexia-nervosa, restricting group are not possible, as 6 of the 14 subjects were below 86% IBW, though none were extremely emaciated (e.g. < 79% IBW) as all were outpatients. Because hormonal function is related to nutritional status, we conducted an analysis excluding the single participant with anorexia nervosa, restricting group who reported regular menstruation at the time of scanning and found no difference in activation patterns.
As scores on the Beck Depression Inventory were significantly higher for the binge-purge group, we examined the possible influence of major depression on our findings by removing from the analysis the 3 subjects who had a diagnosis of major depression. This included 2 subjects in the binge-purge group, and 1 subject in the anorexia nervosa, restricting group. None of the results changed. For all of the ROIs, activation in the binge-purge group remained significantly greater than in the healthy control group (p ranged from .002 to .0080, including covariance for age).
Discussion
This preliminary study supports the hypothesis that differences in neural function can be identified between anorexia nervosa, restricting type and binge-purge eating disorder subtypes, as well as between binge-purge and healthy control groups, during a task requiring inhibitory control. The binge-purge subtype showed increased activation in the right dorsolateral prefrontal cortex, an executive control region, suggesting inefficient or possibly compensatory activation (i.e., recruitment of additional brain regions and/or discrepant brain activation patterns leading to improved cognitive ability) (28). The finding of increased hypothalamic activation further suggests aberrant responses in a region associated with emotional function (29, 30). The finding of increased hypothalamic activation might also indicate that the binge-purge group is experiencing greater stress during response inhibition (31), possibly related to the additional effort needed to successfully complete the task. Binge-purge subjects use greater anterior cingulate resources as well, suggesting increased activity related to monitoring response conflict (32). This finding differs from that identified by Marsh et al (11) and Uher et al (33) who found decreased activation in the frontostriatal region in adults with bulimia nervosa. Also, Marsh found that the adult patients with bulimia nervosa had impaired task performance, while our study did not. These differences could result from task differences (Simon Task (13)-a response inhibition task to incongruent spatial stimuli that may be more challenging than the Go-NoGo Task (34, 35)), developmental differences (age, cognitive maturity), clinical severity or chronicity (duration of illness, binge purge behavior frequency), diagnosis (binge-purge behavior versus bulimia nervosa) and co-morbidity. The fact that our younger age group activated several additional brain regions not observed in the March et al. study (e.g. hypothalamus, dorsal lateral prefrontal cortex) might also reflect the influence of brain maturation on cognitive processing.
In a whole-brain correlation with percent correct on NoGo trials within each group, only the anorexia-nervosa, restricting group showed a significant positive correlation with activation in a cluster that included the inferior parietal cortex, precuneus, and posterior cingulate gyrus. The other groups showed no significant correlations with percent correct on NoGo trials.
Clinically, adolescents with anorexia nervosa, restricting type display characteristics of greater inhibitory control than healthy controls and those with bulimia nervosa or anorexia nervosa, binge-purge subtype, while those with bulimia nervosa or anorexia nervosa, binge-purge subtype display decreased inhibitory control compared to healthy controls and restricting subjects. Findings presented here provide putative neural correlates of decreased inhibitory control in adolescents that binge eat and purge subjects, but we did not find evidence of comparable correlates of increased inhibitory control in the anorexia nervosa, restricting group. The neurofunctional correlates of these cognitive characteristics in binge-purge subjects are consistent with expected locations of activation related to executive function (18, 30, 33). However, there were no correlations between Eating Disorder Examination subscales or binge-purge episodes, but this may be due to poor reliability of the Eating Disorder Examination subscales and low variability of rates of binge eating and purging in this small sample. This could also imply that severity of behavioral symptoms (e.g. binge-purge rates) do not predict activation within this group.
Our preliminary findings suggest that adolescent subjects with binge-purge behaviors and anorexia nervosa restricting type likely differ from each other on a neural level, and therefore risks and effective interventions may differ between these two groups. These findings in an adolescent group who are not severely malnourished and with relatively short duration of eating disorders symptoms suggest that these neural processes occur prior to or early in the evolution of the disorder, and may not be the result of chronic disease or state dependent starvation. Longitudinal studies are needed to help distinguish primary and secondary neural processes. Other studies might also follow individuals with early symptoms of an eating disorder to track development of behaviors and corresponding neural correlates. In addition, future studies might also examine the effects of cognitive therapies on these neural processes in anorexia nervosa restricting type and binge-purge subjects (36).
There are a number of limitations to these findings. The sample is small and therefore other group differences may well have not been detected. The participants were females, and although most eating disorder patients are females, 10% are male and these finding may not generalize to them. The Go-NoGo task does not capture all aspects of inhibitory control. Additional studies are needed to replicate and expand upon these preliminary findings. It is also important to note that depression symptoms are associated with some of the regions of interest examined in this study (37, 38). Depression is a common co-morbid condition in bulimia nervosa and Beck Depression Inventory scores were elevated in the binge-purge sample. However, there was no correlation found between Beck Depression Inventory score and any of the regions of interest within the binge-purge group in our study (results not shown).
An ongoing debate in the diagnostic literature is the potential cross-over between anorexia nervosa, restricting type, anorexia nervosa, binge-purge subtype, and bulimia nervosa, and particularly whether eating disorders are best considered a single transdiagnostic disorder or separate clinical entities (39). The current study provides preliminary evidence that, at least during adolescence, eating disorder subtypes may be distinguishable in terms of neural correlates of inhibitory control. This distinction is also consistent with the clinical reports of the later onset of binge eating and purging in general and in the anorexia nervosa, binge-purge subtype in particular (26). At the same time, the fronto-striatal circuit is known to be involved in a variety of psychiatric disorders (e.g., Tourette syndrome, bipolar disorder, obsessive-compulsive disorder, Attention Deficit Hyperactivity Disorder, and depression), thus these findings should be considered in this larger context (18). Addressing inhibitory control as an aspect of treatment in adolescents with eating disorders is another important consideration. Strategies to address response inhibition, as well as cognitive flexibility and perseverative thinking, using cognitive remediation therapy, have been preliminarily studied in adults with chronic anorexia nervosa, and may be a promising adjunctive treatment to standard interventions aimed at weight restoration and eating disorder related cognitions (36).
Acknowledgments
Funding for this research was provided from an unrestricted fund for pediatric research from Lucile Packard Children’s Hospital at Stanford.
Footnotes
Previous Presentation: Some data from this paper were presented as a poster at the American Academy of Child and Adolescent Psychiatry Annual Meeting, Honolulu, HI, October 27–31, 2009.
Disclosures: The authors have no disclosures of competing interests.
Contributor Information
James Lock, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305, Fax: 650-723-5531, Tel: 650-723-5473, jimlock@stanford.edu.
Amy Garrett, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine.
Judy Beenhakker, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine.
Allan Reiss, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine.
REFERENCES
- 1.Anderluch M, Tchanturia K, Rabe-Hesketh S, Treasure JL. Childhood obsessive compulsive personality traits in adult women with eating disorders: Defining a broader eating disorder phenotype. Am J Psychiatry. 2003;160:242–247. doi: 10.1176/appi.ajp.160.2.242. [DOI] [PubMed] [Google Scholar]
- 2.Wagner A, Barbarich-Marstellar N, GK F, Bailer U, Wonderlich S, Crosby R, Henry S, Vogel V, Plotnicov K, C M, WH K. Personality traits after recovery from eating disorders: do subtypes differ. Int J Eat Disord. 2006;39:276–284. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- 3.Strober M. The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: evidence from a family study with discussion of nosological and neurodevelopmental implications. Int J Eat Disord. 2007;40:S46–S51. doi: 10.1002/eat.20429. [DOI] [PubMed] [Google Scholar]
- 4.Bruce K, Koerner N, Steiger H, Young S. Laxative misuse and behavioral disinhibition in bulimia nervosa. Int J Eat Disord. 2003;33:92–97. doi: 10.1002/eat.10116. [DOI] [PubMed] [Google Scholar]
- 5.Rosval L, Steiger H, Bruce K, Israel M, Richardson I, Aubut M. Impulsivity in women with eating disorders: Problem of response inhibition, planning or attention? Int J Eat Disord. 2006;39:590–593. doi: 10.1002/eat.20296. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe B, Metzger E, Levine J, Finkelstein D, Cooper T, Jimerson D. Serontonin function following remission from bulimia nervosa. Neuropsychopharmacology. 1999;22:257–263. doi: 10.1016/S0893-133X(99)00117-7. [DOI] [PubMed] [Google Scholar]
- 7.Jimerson D, Wolfe B, Metzger E, Finkelstein D, Cooper T, Levine J. Decreased serotonin function in bulimia nervosa. Archives of General Psychiatry. 1997;55:529–534. doi: 10.1001/archpsyc.1997.01830180043005. [DOI] [PubMed] [Google Scholar]
- 8.Southgate L. Institute of Psychiatry, Maudsley Hospital. London: University of London; 2005. Response inhibition in anorexia nervosa and bulimia nervosa: An exploration of neuropsychological functions and their association with personality traits and behaviors. [Google Scholar]
- 9.Roberts M, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychol Med. 2005;37:1075–1084. doi: 10.1017/S0033291707009877. [DOI] [PubMed] [Google Scholar]
- 10.Gordon C, Dougherty D, Fishman A. Neural substrates of anorexia nervosa: a behavioral challenge study with positron emission tomography. J Pediatr. 2001;139:51–57. doi: 10.1067/mpd.2001.114768. [DOI] [PubMed] [Google Scholar]
- 11.Marsh R, Steinglass J, Gerber A, O-Leary G, Wang Z, Murphy D, Walsh BT, Peterson B. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Arch Gen Psychiatry. 2009;66:51–63. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, Belger A, Weisbrod M, Treasure J, Friederich H. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166:608–616. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- 13.Peterson B, Kane M, Alexander G, Lacadie C, Skidlarski P, Leong H, May J, Gore J. An event-related functinal MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 14.Kaye WH, Greeno C, Moss H, Fernstrom J, Fernstrom M, Lilenfeld L, Weltzin T, Mann J. Alterations in serotonin activity and psychiatric symptoms after recovery from bulimia nervosa. Arch Gen Psychiatry. 1998;(55):927–935. doi: 10.1001/archpsyc.55.10.927. [DOI] [PubMed] [Google Scholar]
- 15.Godart N, Flament M, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: A review. Int J Eat Disord. 2002;32 doi: 10.1002/eat.10096. [DOI] [PubMed] [Google Scholar]
- 16.Holliday J, Tchanturia K, Landau S, Collier D. Is Impaired Set-Shifting an Endophenotype of Anorexia Nervosa? Am J Psychiatry. 2005;162:2269–2275. doi: 10.1176/appi.ajp.162.12.2269. [DOI] [PubMed] [Google Scholar]
- 17.Southgate L, Tchanturia K, Treasure J. Neuropsychology in Eating Disorders. In: Wood S, Allen N, Pantelis C, editors. Handbook of Neuropsychology of Mental Illness. Cambridge: Cambridge University Press; 2009. pp. 316–325. [Google Scholar]
- 18.Marsh R, Maia T, Peterson B. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keverne B. Brain development and well-being. In: Hubbert F, Baylis N, Keverne B, editors. The science of well-being: integrating neurobiology, psychology, and social science. London: Philosophical Transactions of the Royal Society; 2004. pp. 1349–1358. [Google Scholar]
- 20.Nelson E, Leibenluft E, McClure E, Pine D. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 21.Luna B, Sweeney J. The emergence of collaborative brain function. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 22.Panya D, Barnes C. The frontal lobe revisited. In: Perecman E, editor. The frontal lobes revisited. New York: IRBN Press; 1987. [Google Scholar]
- 23.Cooper Z, Fairburn CG. The Eating Disorder Examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. International Journal of Eating Disorders. 1987;6:1–8. [Google Scholar]
- 24.Glover G, Lai S. Self-Navigated Spiral fMRI: Interleaved versus Single-shot. Mag. Resn. Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 25.Casey B, Trainor R, Orendi J, et al. A developmental functional MRI sutyd of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 26.Peebles R, Wilson J, Lock J. How do children and adolescents with eating disorders differ at presentation, Journal of Adolescent Health. Journal of Adolecent Health. 2006;39:800–805. doi: 10.1016/j.jadohealth.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Mayer J, Bittner R, Nikolić D, Bledowski C, Goebel R, Linden D. Common neural substrates for visual working memory and attention. Neuroimage. 2007;36:441–453. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Han SD, Bangen K, Bondi M. Functional magnetic resonance imaging of compensatory neural recruitment in aging and risk for Altzheimer's disease: Review and recommendations. Dement Geriatr Cogn Disord. 2009;27 doi: 10.1159/000182420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips K, Drevets W, Rauch S, Lane R. Neurobiology of emotion perception 1: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 30.Wagner A, Aizenstein H, Venkatraman V, Bischoff-Grethe A, Fudge J, May J, GK F, Bailer U, Fischer B, Putnam K, WH K. Altered striatal response to reward in bulimia nervosa after recovery. Int J Eat Disord On line publication. 2009 doi: 10.1002/eat.20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahs F, Furmark T, Michelgard A, Langstom B, Appel L, Wolf O, Kirschbaum C, Fredrikson M. Hypothalamic blood flow correlates positively with stress-induced cortisol levels in subjects with social anxiety disorder. Psychosomatic Medicine. 2006;68:859–862. doi: 10.1097/01.psy.0000242120.91030.d8. [DOI] [PubMed] [Google Scholar]
- 32.Yeung N, Nieuwenhuis S. Dissociating response conflict and error likelihood in anterior cingulate cortex. Journal of neuroscience. 2009;29:14506–14510. doi: 10.1523/JNEUROSCI.3615-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uher R, Murphy T, Brammer M, Dalgleish T, Philllips M, Ng V, Andrews C, Williams S, Campbell I, Treasure JL. Medial prefrontal cortex activit associated with symptom provocation in eating disorders. American Journal of Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 34.Aron A, Fletcher P, Bullmore E, Sahakian B, Robbins T. Stop signal inhibition disrupted by right inferior frontal gyrus in humans. National Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 35.Aron A, Robbins T, Poldrack R. Inhibition and the right inferior frontal cortex. Trends Cogn Science. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Tchanturia K, Davies H, Campbell I. Cognitive Remediation for patients with Anorexia Nervosa: preliminary findings. Annals of General Psychiatry. 2007;14:1–6. doi: 10.1186/1744-859X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halari R, Simic M, Pariante C, Papadopoulos A, Cleare A, Brammer M, Fombonne E, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication naieve adolescents with depression compared to controls. Journal of Child Psychology and Psychiatry. 2009;50:307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- 38.Killgore W, Gruber S, Yurgulun-Todd D. Depressed mood and lateralization prefrontal activity during a Stroop tast in adolescent children. Neuroscience Letters. 2007;416:43–48. doi: 10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fairburn C, Bohn K. Eating disorder NOS (EDNOS): An example of the troublesome eating disorder not otherwise specified(NOS) category in DSM-IV. Behav Res Ther. 2005;43:691–701. doi: 10.1016/j.brat.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]