Abstract
Background
Numerous epidemiological studies have reported a positive association between major depression (MD) and regular tobacco use (RU) or nicotine dependence (ND). However, few have used a genetically informative design to assess whether these traits share a common genetic and/or environmental liability.
Method
We assessed MD, RU and ND in same-sex twins from the population-based Swedish Twin Registry. In males, we examined both cigarette use and snus (smokeless tobacco) use. We used structural equation modeling to examine the relationship between MD, RU, and ND given RU.
Results
The results suggest modest correlations between MD and RU, and between MD and ND. In males, the liability shared between MD and RU is solely genetic for both cigarettes and snus, while MD and ND share both genetic and unique environmental influences. The continuation to ND given RU differed considerably between cigarette and snus users. In females, both MD–RU and MD–ND relationships are partially attributable to genetic and unique environmental correlations.
Conclusions
The relationship among MD, RU and ND is at least partially attributable to shared genetic and environmental risk factors. The genetic and environmental correlations between traits are modest. The nature of the shared liability differs by sex, and in males, by the type of tobacco product used. Differences between previous reports and results presented in the current study are suggestive of population differences in how MD and tobacco use inter-relate.
Keywords: Genetic risk, shared liability, twin modeling
Introduction
Many studies have demonstrated a positive association between tobacco use and depressive disorders (e.g. Breslau et al. 1991; Klungsoyr et al. 2006; Sihvola et al. 2008). Others have examined the relationship between nicotine dependence (ND) and major depression (MD) or depressive symptoms, with results suggesting that the risk of MD is higher with increasing levels of ND (Breslau & Johnson, 2000; Kessler et al. 2007; Manley et al. 2009). The causes underlying these associations remain unclear. Tobacco use could cause depressive symptoms, and/or depression could lead to tobacco use. Alternatively, the association could be non-causal, i.e. tobacco use and depression could share a common liability, in turn the result of genetic and/or environmental influences. These possibilities are not mutually exclusive.
Genetic epidemiological studies have been undertaken to address the possibility that MD and tobacco use traits (initiation of tobacco use, progression to regular use, and ND) share a common liability influenced by genetic and/or environmental factors. Kendler et al. (1993) tested a causal model, in which smoking causes MD or vice versa, and a non-causal model, in which the association between these traits is due to shared genetic and/or environmental liability. Their results supported a non-causal model in which shared liability was due predominantly to genetic factors. Other twin studies have also identified modest to moderate levels of genetic and/or environmental correlation between regular tobacco use or ND and depression or depressive symptoms, in both adolescents (McCaffery et al. 2008) and adults (Fu et al. 2002; Korhonen et al. 2007; Lyons et al. 2008). Kendler et al. (1993) reported a substantial genetic correlation between MD and regular smoking in women (ra = 0.56). While a similar estimate has been reported among adolescent females (ra = 0.62, McCaffery et al. 2008), lower estimates have also been reported (ra = 0.17–0.25, Korhonen et al. 2007; Lyons et al. 2008). McCaffery et al. (2008) found that the genetic correlation between these traits was not significant among adolescent males.
In the current report, we examine a large population-based sample of Swedish twins to assess whether MD, regular tobacco use and ND are influenced by shared genetic and/or environmental factors. We employ a modified version of the causal–contingent–common pathway (CCC) model (Kendler et al. 1999) to account for the contingency of ND on regular tobacco use. We fit models to cigarette use and cigarette-related ND in both males and females; snus (smokeless tobacco) use was sufficiently prevalent among males to also fit models addressing the relationship between MD, regular snus use and snus-related ND.
We address several questions: (i) is there evidence of a shared liability, or non-causal explanation, for the association between MD, tobacco use and ND?; (ii) does the structure of genetic and environmental influences on these traits differ between males and females?; (iii) in males, are there differences between genetic and environmental influences on cigarette use versus snus use and corresponding measures of ND?
Method
Sample
Data were collected for the Swedish Screening Across the Lifespan Twin (SALT) study, which is based on the Swedish Twin Registry (Lichtenstein et al. 2002; Pedersen et al. 2002) and has been described elsewhere (Kendler et al. 2006) (see also Supplementary Material, available online). The present analyses are based on same-sex twins of known zygosity (n = 27993) (Table 1). Zygosity was assigned as described elsewhere (Lichtenstein et al. 2002). Informed consent was verbally obtained prior to interviews and confirmed in a post-interview letter. The Swedish Data Inspection Authority, the Ethics Committee of the Karolinska Institute and the Institutional Review Board of the University of Southern California approved this project.
Table 1.
Prevalence of MD, tobacco use and levels of nicotine dependence in same-sex twin pairs of the SALT sample, and odds ratios between MD and tobacco use traits
| Trait | Prevalence, % |
Odds ratios (95% CI) |
||
|---|---|---|---|---|
| Males (maximum n = 12744) | Females (maximum n = 15249) | Males | Females | |
| MD** | 13.0 | 24.8 | N.A. | N.A. |
| Regular cigarette use | 66.3 | 66.7 | 1.21 (1.07–1.36)† | 1.52 (1.38–1.67)†† |
| Regular snus use** | 28.5 | 2.3 | 1.28 (1.14–1.45)†† | 2.01 (1.52–2.66)†† |
| Nicotine dependence – cigarettes** | ||||
| Very lowa | 46.0 | 53.1 | 1.00 (0.86–1.15) | 1.21 (1.09–1.36)†† |
| Low | 31.7 | 27.3 | 1.02 (0.87–1.20) | 1.51 (1.32–1.72)†† |
| Medium | 10.8 | 10.0 | 1.82 (1.48–2.24)†† | 2.32 (1.95–2.77)†† |
| High | 9.5 | 8.7 | 2.04 (1.65–2.52)†† | 2.95 (2.46–3.55)†† |
| Very high | 1.9 | 1.0 | 2.80 (1.89–4.16)†† | 4.34 (2.69–7.02)†† |
| Nicotine dependence – snus* | ||||
| Very lowa | 19.0 | 29.2 | 1.24 (0.98–1.57) | 2.45 (1.53–3.95)†† |
| Low | 36.1 | 33.9 | 1.09 (0.90–1.31) | 1.00 (0.99–1.00) |
| Medium | 34.2 | 28.3 | 1.39 (1.17–1.66)†† | 1.82 (0.90–3.69) |
| High | 10.7 | 8.6 | 1.71 (1.29–2.27)†† | 0.93 (0.31–2.77) |
MD, Major depression; SALT, Swedish Screening Across the Lifespan Twin; CI, confidence interval; N.A., not applicable.
Non-smokers/non-snusers served as the reference category for very low nicotine dependence.
Significant sex differences in prevalence:
p<0.001,
p<0.0001.
Significant odds ratios:
p<0.01,
p<0.001.
Measures
The measure of MD used for this sample has been described previously (Kendler et al. 2006). Briefly, the computerized Composite International Diagnostic Interview Short-Form was adapted to assess lifetime prevalence of depression; a small number (n = 205 of the current sample) volunteered that they were taking anti-depressants, were considered positive for a history of MD and included in the analysis, but were not assessed for depressive symptoms.
These analyses use data collected on cigarette use and snus use. Interview questions relevant to these analyses include: whether the participant had ever smoked or used snus; daily cigarette consumption; weekly snus consumption; and the frequency with which they smoked/used snus. We considered an individual as a ‘regular smoker’ or a ‘ regular snus user’ if at least one of the following was true: (i) they reported ever regularly smoking/snusing; (ii) they reported at least 1 year of smoking/snusing; (iii) they reported weekly use of cigarettes/snus. We will refer to regular use (RU) of cigarettes as RUC, and regular use of snus as RUS.
ND was assessed using items from the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al. 1991). FTND score was further categorized into five levels of dependence: scores of 0–2 are grouped as ‘very low’; 3–4 as ‘low’; 5 as ‘medium’; 6–7 as ‘high’; and 8–10 as ‘very high’. A subset of items from the FTND was also asked of snus users: (i) latency to first snus; (ii) which snus would you most hate to give up?; (iii) quantity snused; and (iv) do you snus more in the morning? To maintain consistence with the cigarette-oriented FTND, we converted snus consumption (originally reported in terms of snus boxes per week) to the comparable amount of cigarettes per day using Fagerstrom (2005) as a guide. Due to having fewer questions on this modified FTND scale, we grouped users into only four categories of dependence. We will refer to measures of ND derived from questions regarding cigarette use as NDC, and those derived from questions regarding snus use as NDS.
Due to the structure of our model (described below), we applied a number of contingencies to the coding of the data. Participants were scored for regular tobacco use only if they positively endorsed the question regarding initiation, and set to missing otherwise. Dependence scores were assigned only if the participant was scored positively as a regular tobacco user, and scores were assigned separately for each product (i.e. an individual using both cigarettes and snus regularly was assigned a separate dependence score for each method of administration).
Statistical analyses
We prepared data for use as raw ordinal data in the statistical modeling package Mx (Neale et al. 2003). This approach assumes that the ordinal categories are representative of an underlying normal distribution of liability, with thresholds in liability discriminating between categories. Measures of MD, RUC and RUS were coded 0 or 1; NDC was grouped into five categories coded from 0 to 4; NDS was grouped into four categories coded from 0 to 3.
In twin modeling, liability to traits such as depression or ND can be attributed to several latent sources of variance: additive genetic factors (A); shared environment (C); and unique environment (E). Estimates of each of these variance components are calculated by comparing the phenotypic correlation between monozygotic twins, who share all their genes, with dizygotic twins, who share half of their genes on average identically by descent.
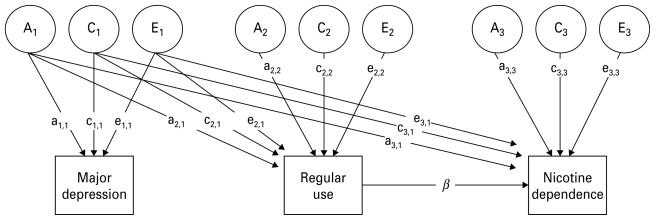
The saturated model used in these analyses is depicted in Fig. 1. This is a modification of the CCC model (Kendler et al. 1999), and takes into consideration the contingency of ND on regular tobacco use. Thus, liability to dependence is a function of both risk shared with regular tobacco use (depicted by the β pathway in Fig. 1) and risk factors independent of regular tobacco use. If all liability to dependence can be accounted for by liability to regular use, the β common pathway coefficient will approach 1, and the path coefficients a3,3, c3,3 and e3,3 will approach 0. Our model also includes MD, which is not contingent on regular tobacco use or ND, and allows for shared genetic and environmental liability between MD and both tobacco-related variables. This model allows us to assess quantitative sex effects by obtaining parameter estimates for both sexes and testing whether these parameters can be constrained to equality without a loss in model fit. Because of the complex model design, we opted not to test for qualitative sex effects (wherein the same trait is at least partially influenced by different genes in males versus females); thus, opposite-sex twin pairs are excluded from our analyses.
Fig. 1.
Saturated trivariate model depicting relationship between major depression, regular tobacco use and nicotine dependence. In this modified version of the causal–contingent–common pathway (CCC) model, the regression path (β) demonstrates that a score on the nicotine dependence scale is contingent on regular tobacco use. A, C and E represent latent additive genetic, common environmental and unique environmental sources of variance influencing the observed traits.
The fit of nested models was assessed as a function of the change in the value of twice the log likelihood of the data, which is distributed as a χ2 statistic with degrees of freedom (df) equal to the difference in the number of parameters estimated between models. A significant Δχ2 indicates a significant deterioration in model fit, which would result in rejection of the nested model. We also used Akaike’s Information Criterion (AIC; Akaike, 1987) to select models. A lower AIC value indicates a better balance between the explanatory power of a model and parsimony.
Equal environments assumption
We examined the similarity of childhood environment (number of years living together in home of origin) and adult environment (current frequency of contact or personal meetings with the co-twin) to determine whether monozygotic twins may have had more similar environmental experiences than dizygotic twins (Kendler, 1983). Using logistic regression while controlling for zygosity, we tested, separately by sex, whether the mean score on environmental measures predicted pair concordance for all phenotypes.
Results
Descriptive statistics
The prevalence of lifetime MD in the SALT sample has been described elsewhere (Kendler et al. 2006). Prevalence of MD, regular tobacco use and ND levels, along with measures of sex differences where appropriate, are provided in Table 1. Mean age at onset of MD was 39.2 years. Mean age of onset of tobacco use was 18.0 years. Additional details are provided in Supplementary Table 1 and Materials.
The relationship between regular tobacco use and MD is positive and statistically significant (Table 1) for both sexes, and for both types of tobacco use in males. Odds ratios for MD generally increased with increasing levels of ND, although in some cases the 95% confidence intervals (CIs) span unity. Tetrachoric and polychoric correlations between twins for MD and tobacco use variables are presented in Table 2. Monozygotic twins exhibit higher levels of correlation than do dizygotic twins, suggesting that the covariance between twins is at least partially attributable to genetic factors.
Table 2.
Tetrachoric and polychoric correlations between twins for variables of interest
| Sex and zygosity | Number of ‘complete’ pairs | Total number of individuals | Tetrachoric/polychoric correlation (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| Major depression | RUC | RUS | NDC | NDS | |||
| Female MZ twins | 2457 | 6081 | 0.46 (0.41–0.52) | 0.74 (0.69–0.79) | N.A. | 0.56 (0.50–0.62) | N.A. |
| Female DZ twins | 3413 | 9168 | 0.19 (0.13–0.25) | 0.44 (0.44–0.51) | N.A. | 0.30 (0.23–0.37) | N.A. |
| Male MZ twins | 1855 | 4883 | 0.33 (0.28–0.43) | 0.67 (0.61–0.72) | 0.64 (0.57–0.70) | 0.44 (0.37–0.51) | 0.53 (0.41–0.62) |
| Male DZ twins | 2727 | 7861 | 0.13 (0.11–0.22) | 0.42 (0.36–0.48) | 0.38 (0.32–0.45) | 0.24 (0.17–0.31) | 0.20 (0.06–0.32) |
| Opposite-sex DZ twinsa | 6426 | 16118 | 0.14 (0.14–0.15) | 0.23 (0.23–0.30) | N.A. | 0.19 (0.18–0.21) | N.A. |
CI, Confidence interval; RUC, regular cigarette use; RUS, regular snus use; NDC, nicotine dependence derived from cigarette use; NDS, nicotine dependence derived from snus use; N.A., not applicable; MZ, monozygotic; DZ, dizygotic.
Values for opposite-sex dizygotic twins are included for reference; these individuals were excluded from all other analyses.
Equal environments assumption
Zygosity was significantly (p < 0.0001) associated with both childhood and adult environmental measures for both sexes, controlling for age. The measure of childhood environment significantly predicted twin concordance for cigarette-based ND in males (p = 0.0299) and females (p < 0.0001). However, similarity in this environmental measure accounted for little of the total variance in twin concordance [Δr2 (rescaled) = 0.0032 in males and 0.0106 in females]. Neither measure of adult environment predicted concordance for any phenotype.
Trivariate analysis of MD, RUC and NDC
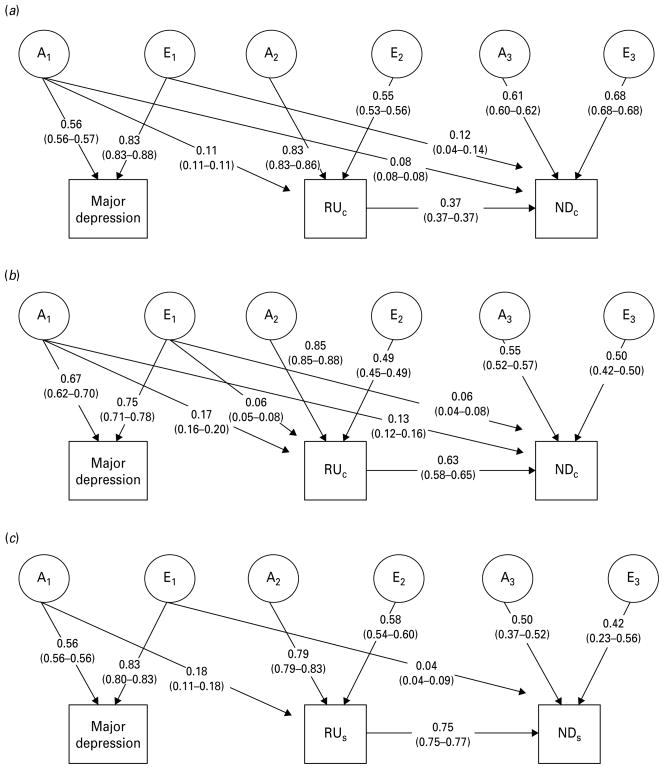
We first tested whether variance components could be constrained to be equal across the sexes in the saturated model. This resulted in a significant deterioration in fit (Δ χ2 = 42.58, Δdf = 16, AIC = + 10.58) (Table 3) so subsequent models did not incorporate this constraint. We next tested an AE model, in which shared environment does not contribute to variance, and a CE model, in which genetic factors do not contribute to variance. The AE model fit well (Δχ2 = 9.63, Δdf = 10, p = 0.473, AIC = −10.37); however, the CE model fit significantly worse (Δχ2 = 219.66, Δdf = 10, p < 0.001, AIC = + 199.66) and was rejected. We subsequently tested a series of models nested within the full AE model, e.g. to assess whether the genetic and environmental correlations between traits could be removed without a deterioration in fit. Ultimately, only the environmental correlation path between MD and regular cigarette use (path e2,1) could be removed, and only in males. Parameter estimates of the best-fitting models for these analyses are depicted in Figs. 2a and b.
Table 3.
Model fitting results for MD, regular tobacco use and ND
| Model comparison | Δdf | Δχ2 | p | ΔAIC | |
|---|---|---|---|---|---|
| MD, regular cigarette use and FTND | |||||
| 1. Full CCC modela | – | – | – | – | – |
| 2. Constrain sources of variance across the sexes | 2 v. 1 | 16 | 42.577 | <0.0001 | +10.577 |
| 3. AE model | 3 v. 1 | 10 | 9.634 | 0.473 | −10.366 |
| 4. CE model | 4 v. 1 | 10 | 219.662 | <0.0001 | +199.662 |
| 5. Set all genetic cross-trait correlations to 0 for both sexes | 5 v. 3 | 4 | 60.273 | <0.0001 | +52.273 |
| 6. Set all environmental cross-trait correlations to 0 for both sexes | 6 v. 3 | 4 | 23.543 | <0.0001 | +15.543 |
| 7. Set genetic and environmental correlation between MD and FTND to 0 for both sexes | 7 v. 3 | 4 | 67.44 | <0.0001 | +59.440 |
| 8. Set genetic and environmental correlation between MD and regular use to 0 for both sexes | 8 v. 3 | 4 | 105.583 | <0.0001 | +97.583 |
| 9. Set environmental correlation between MD and regular use to 0 in malesb | 9 v. 3 | 1 | 0.001 | 0.973 | −1.999 |
| 10. Test for quantitative genetic effects across the sexes | 10 v. 9 | 5 | 13.67 | 0.018 | +3.670 |
| MD, regular snus use and ND | |||||
| 1. Full CCC modelc | – | – | – | – | – |
| 2. AE model | 2 v. 1 | 5 | 3.779 | 0.582 | −6.221 |
| 3. CE model | 3 v. 1 | 5 | 106.859 | <0.0001 | +96.859 |
| 4. Set all genetic cross-trait correlations to 0 | 4 v. 2 | 2 | 19.525 | <0.0001 | +15.525 |
| 5. Set all unique environmental correlations to 0 | 5 v. 2 | 2 | 4.308 | 0.116 | +0.308 |
| 6. Set genetic correlation between MD and ND to 0 | 6 v. 2 | 1 | 0.77 | 0.38 | −1.23 |
| 7. Set unique environmental correlation between MD and ND to 0 | 7 v. 6 | 1 | 3.454 | 0.063 | +1.454 |
| 8. Set genetic correlation between MD and regular snus use to 0 | 8 v. 6 | 1 | 18.75 | <0.0001 | +16.750 |
| 9. Set unique environmental correlation between MD and regular snus use to 0d | 9 v. 6 | 1 | 1.63 | 0.202 | −0.370 |
MD, Major depression; ND, nicotine dependence; FTND, Fagerstrom Test for Nicotine Dependence; df, degrees of freedom; AIC, Akaike’s Information Criterion; CCC, causal–contingent–common pathway; A, additive genetic factors; E, unique environmental factors; C, shared environmental factors; LL, log likelihood.
−2LL = 83374.749; df = 60926; AIC = −38477.251.
Best fit model: −2LL = 83384.384; df = 60937; AIC = −38489.616.
−2LL = 29188.486; df = 25714; AIC = −22239.514.
Best fit model: −2LL = 29194.666; df = 25721; AIC = −22247.334.
Fig. 2.
Best-fitting models for major depression, regular use of cigarettes (RUC) and cigarette-based nicotine dependence (NDC) in males (a) and females (b) and for major depression, regular snus use (RUS) and nicotine dependence derived from snus use (NDS) in males (c). A and E represent latent additive genetic and unique environmental sources of variance influencing the observed traits.
The sample used for the present analyses differs slightly from that which was previously reported on (Kendler et al. 2006); consequently, our precise estimates differ slightly. Heritability estimates and 95% CIs for all traits are given in Table 4. Heritability estimates for RUC were similar in the two sexes (a2 = 0.70 in males and 0.76 females). NDC was less heritable than RUC, with estimates of a2 = 0.48 in males and a2 = 0.62 in females. The remainder of the variance is attributable to non-shared environmental factors.
Table 4.
Variance components from best-fit model
| Variance component | MD | RUC | NDC | RUS | NDS |
|---|---|---|---|---|---|
| a2m | 0.31 (0.22–0.40) | 0.70 (0.64–0.74) | 0.48 (0.41–0.54) | 0.66 (0.61–0.68) | 0.62 (0.56–0.66) |
| e2m | 0.69 (0.66–0.74) | 0.30 (0.30–0.36) | 0.51 (0.45–0.58) | 0.34 (0.31–0.34) | 0.38 (0.37–0.39) |
| a2f | 0.44 (0.39–0.49) | 0.76 (0.76–0.76) | 0.62 (0.62–0.63) | N.A. | N.A. |
| e2f | 0.56 (0.50–0.59) | 0.24 (0.20–0.26) | 0.35 (0.31–0.39) | N.A. | N.A. |
Values are heritability estimates and 95% confidence intervals.
MD, Major depression; RUC, regular cigarette use; NDC, nicotine dependence derived from cigarette use; RUS, regular snus use; NDS, nicotine dependence derived from snus use; a2m, heritability in males; e2m, environmental variance in males; a2f, heritability in females; N.A., not applicable; e2f, environmental variance in females.
In both sexes, the genetic correlation between MD and RUC was modest (ra = 0.13 in males and 0.19 in females) but could not be set to zero. The correlation between unique environmental influences on MD and RUC could be removed from the model for males; in females, the environmental correlation was modest (re = 0.12), but significant.
The genetic correlation between MD and NDC was 0.18 for males and 0.30 for females; the corresponding environmental correlations were lower (0.14 for males and 0.17 for females). The total genetic variance of NDC can be partitioned into its different sources: that which is specific to NDC (path a3,3); that which is shared only with RUC (a function of paths β and a2,2); and that which is shared with MD both independent of RUC (path a3,1) and through genetic factors shared between MD and RUC (a function of paths b and a2,1). Because the β path differs between the sexes (0.37 for males and 0.63 for females) the proportion of genetic and environmental variance specific to NDC varies considerably between the sexes: 79% of the genetic influences on NDC are specific to dependence in males, contrasted with 49% in females. Consequently, far less of the genetic variance affecting NDC is shared with regular smoking in males (20%) than in females (47%). Variance shared between NDC and MD accounts for very little of the total genetic variance influencing NDC (<5% for both sexes, Fig. 3), but cannot be removed from the model.
Fig. 3.
Contributions (%) to the total genetic variance of nicotine dependence (ND). NDC, ND based on cigarette use; NDS, ND based on snus use; ( ), ND-specific; (□), shared with regular use; (■), shared with major depression.
), ND-specific; (□), shared with regular use; (■), shared with major depression.
Trivariate analysis of MD, RUS and NDS
We tested, in males only, whether an ACE, AE or CE model most parsimoniously explained the data: fitting an AE model resulted in an improvement in AIC (Δχ2 = 3.779, Δdf = 5, p = 0.582, AIC = −6.221), while a CE model fit substantially worse (Δχ2 = 106.859, Δdf = 5, p < 0.001, AIC = + 96.859). Thus, we tested subsequent models nested within an AE model. We were able to further improve AIC by removing the direct genetic correlation between MD and NDS (path a3,1) and the unique environmental correlation between MD and RUS (path e2,1) (Table 3).
Our estimates of the contributions of genetic and unique environmental factors to MD are nearly identical to those obtained when modeling the relationship between MD, RUC and NDC (a2 = 0.31, e2 = 0.69). The heritability of RUS is quite similar to that of RUC (a2 = 0.66 and 0.70, respectively). The heritability of NDS is 0.62. The genetic correlation between MD and RUS (ra = 0.22) is slightly higher than that for cigarette use in males. As with MD and RUC, the unique environmental covariance between MD and RUS could be set to 0. The remaining variance in RUS and NDS is accounted for by unique environmental factors.
The genetic correlation between MD and NDS (ra = 0.17) is comparable with that of MD and NDC (ra = 0.18). The unique environmental correlation between MD and NDS is low (re = 0.07). However, the coefficient of the regression path (β) between RUS and NDS is higher than that found for cigarette use (0.75 v. 0.37). Accordingly, much of the variance in NDS can be accounted for by RUS rather than being ND-specific. Ultimately, liability to RUS accounts for 57% of the total heritability and 51% of the total unique environmental variance of NDS. NDS-specific variance accounts for much of the remainder (40% of the total genetic variance and 49% of the total environmental variance); only 3% of the total genetic variance influencing NDS is shared with MD (Fig. 3), and only 0.4% of the total environmental variance is shared with MD.
Discussion
We attempted to address three major questions in these analyses: (i) does shared genetic and/or environmental liability contribute to the correlation between MD and tobacco use?; (ii) does the structure of genetic and environmental influences differ across the sexes?; and (iii) among males, does this structure differ depending on the type of tobacco used?
Our results support the hypothesis that MD, regular tobacco use and ND share a common liability. In males, the nature of the non-causal relationship between MD and regular tobacco use – for both cigarettes and snus – can be accounted for entirely by genetic factors shared by these traits. In females, genetic and environmental influences contribute to this covariance. Estimates of the genetic correlation between MD and regular tobacco use or ND are modest, ranging from ra = 0.13 to 0.30, indicating that the genetic liability to these traits is largely specific to each. However, genetic correlations are higher than environmental correlations (re = 0–0.17).
A summary of previous reports of genetic correlations between MD and tobacco use traits is provided in Table 5. Our estimate of the genetic correlation between MD and regular smoking in females is substantially lower than has been previously reported in adult (Kendler et al. 1993) and adolescent (McCaffery et al. 2008) females. Our results for males are comparable with (but lower than) other reports of genetic correlations between MD and regular smoking. Our estimate of the genetic correlation between MD and cigarette-based ND is substantially lower than that reported by Lyons et al. (2008). This discrepancy may be partially attributable to their use of a dichotomous rating of ND, whereas the current report used five categories of dependence derived from the FTND; these scales might measure different aspects of ND (Hughes et al. 2004; Kandel et al. 2005).
Table 5.
Summary of previous reports of genetic correlations between depression and tobacco use
| Study | Sample population | Sample sex | Depression measure | Tobacco use measure | Genetic correlation |
|
|---|---|---|---|---|---|---|
| Women | Men | |||||
| Kendler et al. (1993) | Virginia, USA | Adult women | DSM-III-R | RUC | 0.56 | N.A. |
| Korhonen et al. (2007) | Finland | Adult men and women | BDI | RUC | Not modeled | 0.25 |
| Lyons et al. (2008)a | Vietnam era twins (USA) | Adult men | DSM-III-R | RUC and NDC | N.A. | RUC: 0.17 NDC: 0.71 |
| McCaffery et al. (2003) | USA | Adult men | CES-D | Current and lifetime RU | N.A. | Current RU: 0.05 Lifetime RU: 0.17 |
| McCaffery et al. (2008) | USA | Adolescent men and women | CES-D | Smoking frequency | 0.62 | 0 |
DSM-III-R, third revision of the Diagnostic and Statistical Manual of Mental Disorders, revised; RUC, regular cigarette use; N.A., not applicable; BDI, Beck Depression Inventory; NDC, cigarette-based nicotine dependence; CES-D, Center for Epidemiological Studies – Depression; RU, regular use.
Lyons et al. (2008) used the DSM-III-R diagnostic criteria to assess nicotine dependence.
Given the consistency in heritability estimates of MD across samples, and the likelihood that the genetic basis of ND – that is, which is specific to dependence rather than shared by initiation or regular use – is unlikely to be substantially different across populations, the high phenotypic correlations and modest genetic correlations between these traits suggest that social factors heavily influence smoking behavior. These social factors – and the genes influencing them – likely differ across countries, cultures and cohorts.
We detected significant sex differences for ND. Among cigarette smokers, ND was more highly heritable among women (a2 = 0.62) than men (a2 = 0.48). Furthermore, the proportion of genetic effects specific to ND – that is not shared with depression or regular use – was much greater in males (about 80%) than in females (49%). For both sexes, the remaining variance is shared mostly with regular cigarette use, and only a modest amount of the total genetic variance in ND is shared with depression. The difference between sexes in the proportion of ND-specific genetic effects is due mostly to the β path: for women, regular smoking and ND exhibit relatively high continuity, whereas additional, unidentified factors are relevant for men.
In general, our estimates for the heritability of regular smoking and cigarette-based ND are comparable with those from the Virginia Twin Registry (a2 = 0.80 for regular use and 0.67 for ND) (Maes et al. 2004), although in that sample variance could be constrained to be equal across the sexes. Heritability specific to dependence is also similar across the studies (a2 = 0.26 for the Virginia sample v. 0.37 for Swedish males and 0.30 for Swedish females).
A notable difference between these studies is in the continuity between regular smoking and subsequent ND. In contrast to our results, Maes et al. (2004) found that this parameter approached unity (β = 0.93). This difference is particularly striking among males in the current study (β = 0.37). Lyons et al. (2008) reported an intermediate result (β = 0.54) among men. An analysis of the pathway from smoking initiation to ND in female twins (Kendler et al. 1999) estimated the β path to be similar to that reported here for females (β = 0.77, v. 0.63 in the current study). Swedish males appear to be outliers in that the sources of variance influencing regular cigarette use are only modestly influential on ND. The causes underlying this deviation from previous results are unclear.
In our male sample, we sought to compare the genetic and environmental influences on cigarette use and snus use, as well as on ND as associated separately with these two different tobacco products. The heritabilities of regular snus use and regular cigarette use are similar; however, snus-based ND is more highly heritable, and the relationship between regular snus use and ND (i.e. the β path) is stronger than that between regular smoking and ND. Accordingly, relative to regular smoking and cigarette-based ND, far more of the total genetic variance of snus-based ND is shared with regular snus use (20% v. 57%, respectively). Furthermore, although the differences are modest, it is worth noting that the genetic correlation between MD and RUS (ra = 0.22) is higher than between MD and RUC (ra = 0.13), indicating that the genetic liability shared between MD and tobacco use is higher for this less common method of tobacco administration than for cigarette smoking.
Few studies have examined the relationship between MD and smokeless tobacco use. Sihvola et al. (2008) explored early (age 14 years) depressive disorders and later (age 17.5 years) cigarette use or smokeless tobacco use in a Finnish population, and found similar odds ratios for both types of tobacco use. Those findings are generally in agreement with our own. Schmitt et al. (2005) investigated cigarette and smokeless tobacco use in the male sample of the Virginia twin study and reported heritability estimates similar to those reported here for both regular cigarette use (a2 = 0.69 for males in the current study versus 0.64 by Schmitt et al.) and snus use (a2 = 0.66 v. 0.73).
Potentially relevant differences among samples include ethnicity, age and culture. Some evidence suggests that heritability increases in the context of permissive environments, such as increased acceptance of smoking among women in younger populations (Kendler et al. 2000), although this is not always the case (Kendler et al. 2004). We conducted post-hoc analyses to assess the extent to which heritability differed by cohort by dividing our sample into two using a median split by birth year. The resulting heritability estimates were quite similar in both groups (data not shown), though this does not preclude the possibility that cohort differences are contributing to the discrepant results across samples. Overall, our estimates of the heritabilities of these traits are comparable with previous reports.
Although shared genetic and environmental liabilities influence MD, regular tobacco use and ND, causal pathways might also contribute to the association among these traits. The details of causal hypotheses and how they might apply to our results are beyond the scope of this study. Briefly, evidence suggests that nicotine can have detrimental effects on neurotransmitter systems and neural integrity (e.g. white-matter lesions) (Ding et al. 2003; Malone et al. 2003), which could in turn have an impact on depressive symptoms (Balfour & Ridley; 2000; Tiemeier, 2003). Alternatively, individuals experiencing depressed mood might use nicotine as a form of self-medication (Parrott, 2006), in which case depressive symptoms could lead to tobacco use rather than vice versa.
To our knowledge, this is the only study that includes MD, regular tobacco use and ND, while accounting for the conditional nature of ND on tobacco use, in a single structural equation model. This design enables us to partition the genetic and environmental influences on ND into those that are ND-specific versus shared only with regular tobacco use, shared only with MD, or common to all three traits. Furthermore, we are able to compare these influences across the sexes, as well as between different types of tobacco use among males. Both the genetic and environmental components of this shared risk are modest, with genetic correlations slightly higher. These findings have potential clinical implications, in that individuals who regularly use cigarettes and/or snus might be at an increased risk for MD due to shared liability in addition to risk due to causal relationships among phenotypes. Furthermore, these results could be informative for gene-finding efforts, including genome-wide studies, since variants associated with a phenotype such as MD could be considered candidates for ND or regular tobacco use as well. Considered in the context of varied estimates of genetic correlation across sexes, cultures and cohorts, our results are consistent with the hypothesis that MD, tobacco use and ND share a statistically significant genetic and/or environmental liability, but do not preclude the existence of causal factors.
Limitations
These results should be considered in light of a number of limitations. First, our sample was limited to Swedish individuals, and the generalizability of these results to other populations is not known. Second, our measures of ND did not take into consideration the fact that many individuals use both cigarettes and snus, and could thus have higher levels of ND than they were assigned in these analyses. However, post-hoc analyses suggest that, among individuals who use both types of tobacco, few would be classified as having a different level of ND had we employed a more global assessment of dependence. Third, our data were cross-sectional and retrospective, and may be subject to errors in recall.
Fourth, our analyses assumed that the exposure to etiologically relevant environmental risk factors is equally correlated in monozygotic and dizygotic twin pairs. Concordance for cigarette-based ND was significantly predicted by our measure of childhood environment. How this environment might influence ND concordance is unclear, particularly given that it was not predictive of concordance for regular use of tobacco which we considered more likely to be influenced by social factors. The measure accounted for only a very small proportion of the total variance, and is within the CIs of our estimates. Therefore, we feel that it is unlikely to have introduced significant biases in our results. Finally, these analyses were not corrected for potentially confounding factors, such as other types of psychopathology, neuroticism or socio-economic status.
Supplementary Material
Acknowledgments
This study was supported in part by grants DA011287, DA018673, DA022989, DA024413 and MH020030 from the National Institutes of Health and grant AG 08724 from the Swedish Research Council, and the Swedish Ministry of Higher Education. The authors thank Dr Charles Gardner for assistance with data management and statistical input.
Footnotes
Note
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/psm).
Declaration of Interest
None.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction ? Pharmacology Biochemistry and Behavior. 2000;66:79–85. doi: 10.1016/s0091-3057(00)00205-7. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. American Journal of Public Health. 2000;90:1122–1127. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Archives of General Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Ding J, Nieto FJ, Beauchamp NJ, Longstreth WT, Jr, Manolio TA, Hetmanski JB, Fried LP. A prospective analysis of risk factors for white matter disease in the brain stem: the Cardiovascular Health Study. Neuroepidemiology. 2003;22:275–282. doi: 10.1159/000071190. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K. The nicotine market: an attempt to estimate the nicotine intake from various sources and the total nicotine consumption in some countries. Nicotine and Tobacco Research. 2005;7:343–350. doi: 10.1080/14622200500124875. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Archives of General Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Oliveto AH, Riggs R, Kenny M, Liguori A, Pillitteri JL, MacLaughlin MA. Concordance of different measures of nicotine dependence: two pilot studies. Addictive Behaviors. 2004;29:1527–1539. doi: 10.1016/j.addbeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Kandel D, Schaffran C, Griesler P, Samuolis J, Davies M, Galanti R. On the measurement of nicotine dependence in adolescence: comparisons of the mFTQ and a DSM-IV-based scale. Journal of Pediatric Psychology. 2005;30:319–332. doi: 10.1093/jpepsy/jsi027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Overview: a current perspective on twin studies of schizophrenia. American Journal of Psychiatry. 1983;140:1413–1425. doi: 10.1176/ajp.140.11.1413. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Prescott CA, Jacobson KC, Neale MC. Level of family dysfunction and genetic influences on smoking in women. Psychological Medicine. 2004;34:1263–1269. doi: 10.1017/s0033291704002417. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. American Journal of Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Archives of General Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in Swedish twins reared apart and reared together. Archives of General Psychiatry. 2000;57:886–892. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Borges G, Castilla-Puentes RC, Glantz MD, Jaeger SA, Merikangas KR, Nock MK, Russo LJ, Stang PE. Smoking and suicidal behaviors in the National Comorbidity Survey: Replication. Journal of Nervous and Mental Disease. 2007;195:369–377. doi: 10.1097/NMD.0b013e3180303eb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klungsoyr O, Nygard JF, Sorensen T, Sandanger I. Cigarette smoking and incidence of first depressive episode: an 11-year, population-based follow-up study. American Journal of Epidemiology. 2006;163:421–432. doi: 10.1093/aje/kwj058. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Broms U, Varjonen J, Romanov K, Koskenvuo M, Kinnunen T, Kaprio J. Smoking behaviour as a predictor of depression among Finnish men and women: a prospective cohort study of adult twins. Psychological Medicine. 2007;37:705–715. doi: 10.1017/S0033291706009639. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, Grant MD, Rende R, Eisen S, Eaves L, Tsuang MT. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine and Tobacco Research. 2008;10:97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychological Medicine. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Malone KM, Waternaux C, Haas GL, Cooper TB, Li S, Mann JJ. Cigarette smoking, suicidal behavior, and serotonin function in major psychiatric disorders. American Journal of Psychiatry. 2003;160:773–779. doi: 10.1176/appi.ajp.160.4.773. [DOI] [PubMed] [Google Scholar]
- Manley MJ, de Jonge P, Kershaw TS, Desai RA, Lin H, Kasl SV. Association of major depression with subtypes of nicotine dependence found among adult daily smokers: a latent class analysis. Drug and Alcohol Dependence. 2009;104:126–132. doi: 10.1016/j.drugalcdep.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Niaura R, Swan GE, Carmelli D. A study of depressive symptoms and smoking behavior in adult male twins from the NHLBI twin study. Nicotine and Tobacco Research. 2003;5:77–83. doi: 10.1080/14622200307259. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Papandonatos GD, Stanton C, Lloyd-Richardson EE, Niaura R. Depressive symptoms and cigarette smoking in twins from the National Longitudinal Study of Adolescent Health. Health Psychology. 2008;27:S207–S215. doi: 10.1037/0278-6133.27.3(suppl.).s207. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Virginia Institute for Psychiatric and Behavioral Genetics. Virginia Commonwealth University; Richmond, VA: 2003. Mx: Statistical Modeling. [Google Scholar]
- Parrott AC. Nicotine psychobiology: how chronic-dose prospective studies can illuminate some of the theoretical issues from acute-dose research. Psychopharmacology. 2006;184:567–576. doi: 10.1007/s00213-005-0294-y. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Lichtenstein P, Svedberg P. The Swedish Twin Registry in the third millennium. Twin Research. 2002;5:427–432. doi: 10.1375/136905202320906219. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Prescott CA, Gardner CO, Neale MC, Kendler KS. The differential heritability of regular tobacco use based on method of administration. Twin Research and Human Genetics. 2005;8:60–62. doi: 10.1375/1832427053435346. [DOI] [PubMed] [Google Scholar]
- Sihvola E, Rose RJ, Dick DM, Pulkkinen L, Marttunen M, Kaprio J. Early-onset depressive disorders predict the use of addictive substances in adolescence: a prospective study of adolescent Finnish twins. Addiction. 2008;103:2045–2053. doi: 10.1111/j.1360-0443.2008.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H. Biological risk factors for late life depression. European Journal of Epidemiology. 2003;18:745–750. doi: 10.1023/a:1025388203548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.