Abstract
The pathophysiology of dystonia has been best studied in patients with focal hand dystonia. A loss of inhibitory function has been demonstrated at spinal, brainstem and cortical levels. Many cortical circuits seem to be involved. One consequence of the loss of inhibition is a failure of surround inhibition, and this appears to directly lead to overflow and unwanted muscle spasms. There are mild sensory abnormalities and deficits in sensorimotor integration; these also might be explained by a loss of inhibition. Increasing inhibition may be therapeutic. A possible hypothesis is that there is a genetic loss of inhibitory interneurons in dystonia and that this deficit is a substrate on which other factors can act to produce dystonia.
Dystonia is a syndrome characterized primarily by unwanted muscle spasms giving rise to involuntary movements and abnormal postures. There are many types of dystonia, and while some aspects of the physiology may well be shared among them, this is not at all certain (Breakefield et al., 2008). Much of the physiology has been obtained in patients with focal hand dystonia, and this review will emphasize that entity. It is likely that the adult onset focal dystonias, the group that focal hand dystonia belongs to, are all related to each other (Defazio et al., 2007). Some patients have more than one of them, and the different entities can be seen in different members of the same family. Hence, the physiology of these disorders is likely to be shared.
Dystonia, like almost any other disorder, is a product of genetic factors and modifiers, including environmental modifiers. Some of the physiology should represent the endophenotype stemming from the genetic makeup of the person, while other physiology should correlate with the overt manifestations of the specific entity from which the person suffers. Which is endophenotypic and which is correlative is not always clear by our current understanding.
Focal hand dystonia commonly starts as an “occupational cramp”. This is a task specific disorder very much related to a repetitive action often done in the context of an occupation or hobby. The most common are writer's cramp and musician's cramp. It is clear that repetitive activity must be one of the environmental factors. Task specificity is a fascinating clinical problem, but its physiology is not well known (Hallett, 2006). Some patients do not progress beyond this stage. Others gradually worsen, with the dystonia involving other tasks, spreading proximally, or even having dystonia at rest. Dystonia can sometimes be produced in the dystonic hand by performing the relevant task with the contralateral, apparently normal hand; this is called mirror dystonia.
Analyzing the clinical features, the spasms are the most obvious. It is also clear that there is a problem with motor control, particularly, for example, in the control of individual fingers. Trying to move one finger will lead to multiple finger movement or even more proximal movement. This is called overflow. An additional feature of dystonia, seen most commonly in cervical dystonia, but occasionally in hand dystonia is the sensory trick, the geste antagoniste. A light touch can ameliorate the dystonia, but typically only for a short time.
Loss of selectivity and overflow
The excess movement in dystonia seems due to a loss of inhibition in motor control (Hallett, 2004). The nervous system is composed of excitatory and inhibitory circuits in balance with each other, and in dystonia it appears that inhibition is defective leading to loss of selectivity and overflow. In particular, it is likely that in making a movement, the motor control signal is both an excitatory command for the desired movement and an inhibitory command for undesired movements (Mink, 1996; Sohn and Hallett, 2004b). The inhibition in this combined command can be called surround inhibition or lateral inhibition. This organization is analogous to the well known center-surround organization of sensory systems, which acts to sharpen sensory perceptions. In the motor system, it should sharpen the motor command. In dystonia, a failure of surround inhibition would logically lead to the clinical features observed.
Loss of inhibition
Loss of inhibition in dystonia was first demonstrated in spinal and brainstem reflexes. Examples are the loss of reciprocal inhibition in the arm of patients with focal hand dystonia (Nakashima et al., 1989; Panizza et al., 1990) and abnormalities of blink reflex recovery in blepharospasm (Berardelli et al., 1985). Loss of reciprocal inhibition (Tisch et al., 2006b) and decreased inhibition in blink reflex recovery (Tisch et al., 2006a) can also be found in patients with generalized dystonia. Loss of reciprocal inhibition can be partly responsible for the co-contraction of antagonist muscles that characterizes voluntary movement in dystonia. Such abnormalities likely reflect abnormal supraspinal control signals. One exception to the loss of spinal inhibition is the cutaneous silent period. This is the pause in tonic EMG caused by stimulation of a cutaneous nerve, and is increased in patients (Espay et al., 2006).
Loss of inhibition can also be demonstrated for motor cortical function with a variety of techniques using transcranial magnetic stimulation (TMS) (Hallett, 2007). Each of these techniques evaluates an inhibitory circuit, most within the cortex itself. The inhibitory circuit includes at least one class of inhibitory interneurons, and it is possible that some of the methods might tap some of the same interneurons.
Short intracortical inhibition (SICI) is obtained with paired-pulse stimulation and reflects interneuron influences in the cortex (Ziemann et al., 1996). In such studies, an initial conditioning stimulus is given, sufficient to activate cortical neurons, but small enough that no descending influence on the spinal cord can be detected. A second test stimulus, at suprathreshold level, follows at short interval. Intracortical influences initiated by the conditioning stimulus modulate the amplitude of the motor evoked potential (MEP) produced by the test stimulus. At short intervals, less than 5 ms, there is inhibition that is likely largely a GABAergic effect, specifically mediated by GABA-A receptors (Di Lazzaro et al., 2000a). At intervals between 8 and 30 ms, there is facilitation, called intracortical facilitation (ICF). There is a loss of intracortical inhibition in patients with focal hand dystonia (Ridding et al., 1995). The loss of inhibition was found in both hemispheres of patients with focal hand dystonia indicating that this abnormality is more likely a substrate for dystonia (since the hemisphere opposite the normal hand also had a loss of inhibition). No abnormality was indentified for ICF, giving evidence to the idea that the fundamental problem is a loss of inhibition, not an increase in facilitation. A deficiency of SICI has been confirmed in some studies (Espay et al., 2006; Huang et al., 2010; McDonnell et al., 2007b) but not in others (Brighina et al., 2009; Stinear and Byblow, 2004a). In a single case, a decrease of the late I-wave volley in the corticospinal tract was identified correlating with the decrease of SICI in a patient with dystonia (Di Lazzaro et al., 2009). The SICI abnormality might be better evoked by looking at the threshold for producing inhibition (Stinear and Byblow, 2004a). Thus, SICI does seem abnormal, but the “conventional” method does not seem to be the most sensitive way to demonstrate it.
SICI can be demonstrated using TMS with the direction of induced current in the brain from posterior-to-anterior (PA), which is the most common experimental technique, or with the direction of current from anterior-to-posterior (AP). The corticospinal tract volley produced by TMS is composed of a series of waves, a direct wave (D wave) followed by a series of indirect waves (I waves) that are numbered in sequence. Different directions of TMS induce different patterns of these waves. In particular, PA current produces a prominent I1 wave, while AP current does not produce a prominent I1 wave, but does produce a prominent I3 wave (Di Lazzaro et al., 2001; Hanajima et al., 1998). PA current is ordinarily used since it tends to produce a larger MEP at shorter latency. Since the I3 wave is more sensitive to GABA modulation, the MEP produced by the AP current is likely more sensitive to influences on GABA interneurons. SICI was shown to be abnormal in focal hand dystonia with PA current, but not AP current suggesting that the MEP in dystonia arises less from I3 than from I1 (Hanajima et al., 2008). The origin of the I1 wave is not well known, but, as the authors suggest, might come more directly from basal ganglia influences.
The silent period (SP) is a pause in ongoing voluntary EMG activity produced by TMS. While the first part of the SP is due in part to spinal cord refractoriness, the latter part is entirely due to cortical inhibition (Fuhr et al., 1991). This type of inhibition is likely mediated by GABA-B receptors (Werhahn et al., 1999). SICI and the SP show different modulation in different circumstances and clearly reflect different aspects of cortical inhibition. The SP is shortened in focal dystonia (Chen et al., 1997; Espay et al., 2006; Kimberley et al., 2009), although this was not seen in all investigations (Stinear and Byblow, 2005). This abnormality may be restricted to the symptomatic hand (Chen et al., 1997). Another observation is that the abnormality of the SP was seen in a pincer grasp task, but not a power grip task in patients with writer's cramp (Tinazzi et al., 2005). This might indicate some task specificity for the abnormality.
Intracortical inhibition can also be assessed with paired suprathreshold TMS pulses at intervals from 50 to 200 ms (Valls-Solé et al., 1992). This is called long intracortical inhibition, or LICI, to differentiate it from SICI. LICI and SICI are clearly different phenomena as demonstrated by the facts that with increasing test pulse strength, LICI decreases but SICI tends to increase, and that there is no correlation between the degree of SICI and LICI in different individuals (Sanger et al., 2001). The mechanisms of LICI and the SP may be similar in that both seem to depend on GABA-B receptors. LICI in patients with writer's cramp is deficient also (Chen et al., 1997; Espay et al., 2006), but this might be only in the symptomatic hand and only with background contraction (Chen et al., 1997). This abnormality is particularly interesting since it is restricted to the symptomatic setting, and, therefore, might be a correlate of the development of the dystonia.
Another set of inhibitory circuits can be probed by delivering an electric shock to a peripheral nerve prior to motor cortex TMS. There are at least four types of this inhibition (Classen et al., 2000). Some effects are at short latency, about 20 ms, short afferent inhibition (SAI), while some effects are at long latency, about 200 ms, long afferent inhibition (LAI). Both SAI and LAI can be produced by stimulating a nerve closely related to a muscle, homotopic effects, or somewhat distant to the muscle, heterotopic effects. SAI is mediated by both cholinergic (Di Lazzaro et al., 2000b) and GABA-A influences (Di Lazzaro et al., 2007). Homotopic SAI at rest and during tonic movement was examined in patients with FHD by stimulating the median nerve and recording MEPs in the abductor pollicis brevis (APB) (Kessler et al., 2005). No abnormality was identified. In a single case, a decrease of the late I-wave volley in the corticospinal tract was identified correlating with the decrease of SAI in a patient with dystonia (Di Lazzaro et al., 2009). A similar study of homotopic LAI at rest showed that patients converted the inhibition into facilitation (Abbruzzese et al., 2001). This dramatic abnormality was not seen in patients with cervical dystonia in the same report suggesting that this abnormality might be specific to the type of focal dystonia. Using cutaneous stimuli rather than mixed nerve stimuli, in one study no abnormality of SAI was identified (Richardson et al., 2007). In another study, a deficiency of SAI was found in patients with focal hand dystonia (McDonnell et al., 2007b). Again with cutaneous stimuli rather than mixed nerve, there was only a trend seen for a loss of LAI in patients (Pirio Richardson et al., 2009).
One technique to evaluate central reciprocal inhibition also shows deficient inhibition in a mixed group of dystonia patients (including some with focal hand dystonia). The influence of a median nerve stimulus on the MEP of forearm extensor muscles can be evaluated at different intervals between the two stimuli. Inhibition is seen maximal between 15 and 18 ms, and this was less in the patients (Bertolasi et al., 2003). This observation was subsequently confirmed in a larger group of patients with focal hand dystonia, and extended by showing a similar loss of inhibition in forearm extensor muscles after stimulation of the radial nerve (Lourenco et al., 2007).
There are also premotor influences on the primary motor cortex (M1). The influence of the dorsal premotor cortex can be on the contralateral motor cortex cortex (transcallosal), and this has been investigated. There was less inhibition produced in patients with focal hand dystonia at a 10 ms interval with a high intensity stimulus, but a facilitatory effect at a different interstimulus interval (8 ms) with a less intense stimulus was normal (Koch et al., 2008). Moreover, it was possible to look at intracortical effects within the premotor cortex itself using a triple pulse technique. A double pulse was given to the premotor cortex prior to stimulating the contralateral primary motor cortex. The second pulse over the premotor cortex was given so that an inhibitory effect was anticipated (8 ms and “strong stimulus”). The first pulse over the premotor cortex was subthreshold and given at various intervals similar to the conditioning stimulus in the SICI paradigm. An increase in the inhibitory effect was seen at 4 and 8 ms; this was absent in the patients suggesting a lack of inhibition within the premotor cortex itself.
Stimulation over the cerebellum can also influence the MEP amplitude. A depression of the MEP is found at intervals from 5 to 7 ms (Ugawa et al., 1995). It is also the case that cerebellar stimulation will decrease SICI and increase ICF (Brighina et al., 2009). In dystonia, none of these three effects were seen (Brighina et al., 2009).
Curiously, patients with psychogenic dystonia appear to have many of the same abnormalities as to patients with focal hand dystonia (Espay et al., 2006). This includes SICI, LICI and SP. ICF was similarly normal. Peripheral reciprocal inhibition was similarly abnormal as well, and the patients with psychogenic dystonia had an even larger deficit of inhibition in the second phase. The cutaneous silent period was similarly increased in both patient groups. These findings suggest either that these abnormalities are a consequence of the dystonia rather than the cause, or that the patients with psychogenic dystonia have a similar predisposition to dystonic motor phenomena but with a different precipitating mechanism.
It should be noted that all these experiments with TMS are certainly valuable, but they remain at a phenomenological level. They indicate loss of inhibition, but their clinical correlate is not obvious. Additionally, they are not necessarily specific to dystonia, and some of these types of inhibition, such as SICI, have been found to be abnormal in other disorders.
One clinical correlate has been suggested by studies in non-human primates. Loss of cortical inhibition in monkey motor cortex can give rise to dystonic-like movements. Local application of bicuculline, a GABA-A antagonist, onto the motor cortex led to disordered movement and changed the movement pattern from reciprocal inhibition of antagonist muscles to co-contraction (Matsumura et al., 1991). In a second study, they showed that bicuculline caused cells to lose their crisp directionality, converted unidirectional cells to bidirectional cells, and increased firing rates of most cells including turning silent cells into active ones (Matsumura et al., 1992).
There is an interesting animal model for blepharospasm that supports the idea of a combination of genetics and environment, and, specifically, that the background for the development of dystonia could be a loss of inhibition (Schicatano et al., 1997). In this model, rats were lesioned to cause dopamine depletion thus reducing inhibition. Additionally, the orbicularis oculi muscle was weakened which leads to increased blink reflex drive in order to produce an adequate blink. Together, but not separately, these two interventions produced spasms of eyelid closure, similar to blepharospasm. Shortly after the animal model was presented, several patients with blepharospasm after a Bell's palsy were reported (Baker et al., 1997; Chuke et al., 1996). This could be a human analog of the animal experiments. The idea is that those patients who developed blepharospasm were in some way predisposed. Evidence for the predisposition is that inhibition in the blink reflex recovery curve is diminished in patients with Bell's palsy (Syed et al., 1999). A gold weight implanted into the weak lid of one patient, aiding lid closure, improved the condition suggesting that when the abnormal increase in reflex drive was removed, the dystonia could be ameliorated (Chuke et al., 1996). In another similar case, the blepharospasm was improved by subcutaneous apomorphine suggesting that the other part of the model might also be relevant in humans (Cattaneo et al., 2005).
There is also neuroimaging evidence consistent with a loss of inhibition; this topic will be discussed in detail in another article in this series. Dopamine D2 receptors are deficient in the putamen in focal dystonias (Perlmutter et al., 1997). There is also direct evidence for reduced GABA concentration both in basal ganglia and motor cortex utilizing magnetic resonance spectroscopy (Levy and Hallett, 2002). An attempt to reproduce the GABA abnormality showed only a trend for decrease in the basal ganglia (Herath et al., 2010).
Loss of surround inhibition
Surround inhibition can be demonstrated with TMS methods. The basic idea is to show that muscles not involved in a specific movement will show active inhibition during the movement. In one of the first demonstrations of this phenomenon, with movement of one finger there was simultaneous widespread inhibition of muscles in the contralateral limb (Sohn et al., 2003). Specifically, significant suppression of MEP amplitudes was observed when TMS was applied between 35 and 70 ms after EMG onset in the willed finger movement. There is also, and importantly, inhibition of muscles in the ipsilateral limb when those muscles are not involved in any way in the movement (Sohn and Hallett, 2004b). TMS was delivered to the left motor cortex from 3 ms to 1000 ms after EMG onset in the flexor digitorum superficialis muscle. MEPs from abductor digiti minimi were slightly suppressed during the movement of the index finger in the face of slightly increased background EMG level and increased F-wave amplitude and persistence. While F-wave data are not easy to interpret, together with the increase in background EMG activity, it could be considered surprising that MEPs are reduced. Further evidence for increased spinal excitability in surround muscles came from experiments using a reaction time movement of the index finger (first dorsal interosseus, FDI) evaluating excitability in the abductor pollicis brevis muscle (APB). The APB can have an H reflex in some subjects and this is a better indicator of spinal excitability than is the F wave. In this situation, it was demonstrated that the H reflex was already increased in the reaction time as well as during the willed movement of the FDI (Beck et al., 2008). The MEP was reduced at these two times, showing that the surround inhibition was present when spinal excitability was increased. It seems clear that surround inhibition must have a supraspinal origin.
Surround inhibition is a stronger effect in the dominant hemisphere of right handed subjects than in the non-dominant hemisphere (Shin et al., 2009). Surround inhibition is also stronger with a more complex task (Beck and Hallett, 2010). Additionally, surround inhibition has been studied when varying the force in the agonist muscle; inhibition peaks at 10% of maximal force and is lost at 40% maximal force (Beck et al., 2009a). These findings appear to indicate a role for surround inhibition in dexterity.
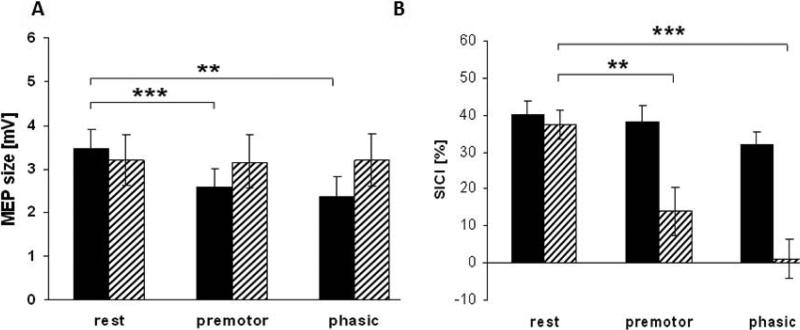
Surround inhibition was studied similarly in the abductor digiti minimi (ADM) muscle in patients with focal hand dystonia making index finger movements (Sohn and Hallett, 2004a). The MEPs were enhanced similarly in the flexor digitorum superficialis and ADM indicating a failure of surround inhibition. Using another experimental paradigm, looking for surround inhibition in the APB with movement of the index finger (FDI), there was a similar loss of surround inhibition (Beck et al., 2008; Stinear and Byblow, 2004d). In one of these experiments, the movement was a reaction time movement rather than a self-paced movement. This allowed looking at surround inhibition during the reaction time. Surround inhibition begins to appear prior to the reaction time movement, and this is also lacking in patients with focal hand dystonia (Figure 1A) (Beck et al., 2008).
Figure 1.
Study of surround inhibition in normal subjects (black bars) and patients with focal hand dystonia (hatched bars). Subjects made reaction time movements of their index fingers, and excitability was assessed in a thumb muscle (surround muscle). Part A shows motor evoked potential (MEP) size in mV in the abductor pollicis brevis (APB) muscle in the period before a reaction time stimulus (rest), during the reaction time (premotor), and at the time of the quick onset of the movement (phasic). Part B shows the short intracortical inhibition (SICI) at the same time points in the two groups. **p<0.01, ***p<0.005. Modified from (Beck et al., 2008) with permission.
As noted earlier, surround inhibition is a functional inhibition, presumably directly influencing motor performance. The question can be raised as to what is its mechanism. Many types of inhibition are known, as just reviewed. Perhaps one or more of them can be identified as contributing to surround inhibition, and perhaps some of them are abnormal in focal hand dystonia.
Although one experiment suggested that SICI might contribute to surround inhibition in normal subjects (Stinear and Byblow, 2004d), this was not confirmed in two other experiments, one using the same surround muscle (Beck et al., 2008; Sohn and Hallett, 2004a). Studies of LICI (Sohn and Hallett, 2004b), SAI (Richardson et al., 2007), LAI (Pirio Richardson et al., 2009), and IHI (Beck et al., 2009b) all show decreases of inhibition either just before movement or during movement. Hence, they make surround inhibition more difficult to produce. Another possibility would be that surround inhibition could be caused by a reduction in a facilitatory process. In this regard, ICF was studied and showed no change (Sohn and Hallett, 2004b). At the present time, no clear mechanism has been demonstrated that would produce surround inhibition.
On the other hand, abnormal mechanisms have been found in patients with focal hand dystonia that would seem to explain, at least in part, the abnormality that they show in surround inhibition. There is a dramatic loss of SICI in patients (Beck et al., 2008; Beck et al., 2009a; Stinear and Byblow, 2004d) and this includes during the reaction time period (Figure 1B) (Beck et al., 2008). Inhibition can also be produced by interhemispheric effects. Stimulation of one motor cortex will inhibit the other one at a 10 ms interval; this is called interhemispheric inhibition (IHI). There is a loss of IHI just in the subset of focal hand dystonia patients exhibiting mirror dystonia (Beck et al., 2009b); therefore, it might be relevant for that phenomenon rather than surround inhibition. There was no abnormality of LAI (Pirio Richardson et al., 2009), and (homotopic) SAI increased more than in normal subjects, suggesting that this mechanism might actually be compensatory in the patients (Richardson et al., 2007).
Loss of ability of patients with focal hand dystonia to focus movement selectively was also demonstrated with movement imagination. In normal subjects, thinking about a movement of the index finger led to an increase of the MEP only in the FDI muscle, but in patients the excitability spread to surrounding muscles (Quartarone et al., 2005).
Another result of loss of inhibition in patients with focal hand dystonia is an abnormality in their ability to inhibit a pre-planned response. Patients and normal subjects were cued at various times prior to a planned response at a specific time (Stinear and Byblow, 2004b). Patients had more difficulty in doing so and required a longer warning time.
Sensory abnormalities
While dystonia is clearly mainly a motor problem and sensory changes are not obvious, it turns out that there are mild sensory abnormalities. There are generally no sensory complaints, and the abnormalities are brought out only by special testing. One type of abnormality is spatial discrimination. This has been demonstrated both with single touch localization and with JVP domes (Bara-Jimenez et al., 2000a). The latter assesses the capability of the subject to detect the orientation of a linear grating with variable spacing when pressed on the skin. Another abnormality is in temporal discrimination (Bara-Jimenez et al., 2000b; Tinazzi et al., 2002). This is the ability to know that there are two stimuli rather than one when they are close together in time. Interestingly, these abnormalities are present in both hands of patients with unilateral focal hand dystonia and even in the hands of patients with cervical dystonia and blepharospasm (Molloy et al., 2003; Scontrini et al., 2009). The abnormalities are also present in the hands of some of the relatives of patients with dystonia suggesting that this abnormality could detect a non-manifesting carrier of a yet unknown gene with incomplete penetrance (O'Dwyer et al., 2005). The abnormality of temporal discrimination extends to two stimuli in different modalities (Fiorio et al., 2003).
Kinesthesia is also abnormal. The perceptual threshold for being able to identify the direction of a passive finger movement correctly was increased in patients with focal dystonia (Putzki et al., 2006). There are also abnormalities of the perception of vibratory tendon stimulation. Patients can match the movement of one elbow with the other one for passive movements, but not if the movement is produced by the tonic vibration reflex (Grunewald et al., 1997). Perception of the vibration-induced illusion of movement is abnormal for patients with different types of focal dystonia in multiple body joints (Yoneda et al., 2000). Similar to temporal discrimination abnormalities, a deficit of vibration-induced illusion was also identified in asymptomatic first degree relatives of patients (Frima et al., 2008).
To a certain extent, the search for abnormalities of sensation were stimulated by the observation in a primate model of dystonia that showed enlarged and overlapped receptive fields (Byl et al., 1996). This observation also stimulated a search for abnormal somatotopy in the somatosensory cortex. Abnormal somatotopy was demonstrated with somatosensory evoked potential mapping with EEG (Bara-Jimenez et al., 1998), MEG (Meunier et al., 2001) and fMRI (Butterworth et al., 2003; Nelson et al., 2009). Not only were finger representation locations closer together than normal, but sometimes the representations were not in the correct order. These abnormalities are present on the unaffected side of patients with unilateral hand dystonia, and might even be more abnormal on the unaffected side (Meunier et al., 2001).
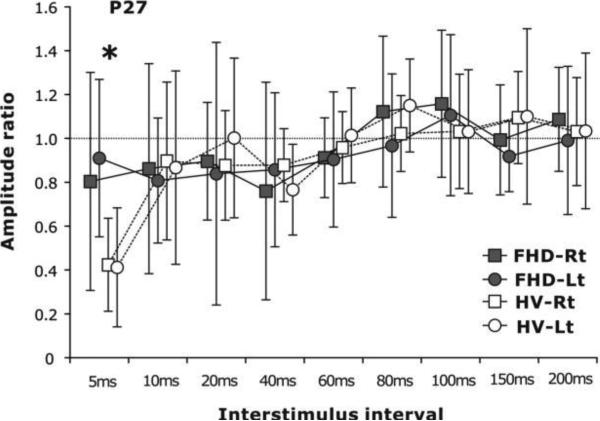
One possible pathophysiological mechanism for these abnormalities could be a loss of lateral inhibition. That lateral inhibition can be lacking has been demonstrated with a study of somatosensory evoked potentials (SEPs) from the median and ulnar nerves. If the median and ulnar SEPs are produced together the combined SEP should be less than the sum of the two individual ones because of mutual inhibition. This is true in normal subjects, but not in patients with focal hand dystonia (Tinazzi et al., 2000). The pathophysiology of the temporal discrimination abnormality has been explored using SEP recovery curves (Tamura et al., 2008). If two SEPs are produced at short interval, components of the second SEP are inhibited by the first one. One study showed loss of inhibition in several SEP components at several intervals (Frasson et al., 2001). A highly specific abnormality was seen in patients with focal hand dystonia in another study. Only for the P27 component and only for the 5 ms interval was there a loss of inhibition (Figure 2) (Tamura et al., 2008). Moreover, both in normal subjects and in patients, the behavioral capability of temporal discrimination correlated with the amount of inhibition of the P27 at the 5 ms interval. Thus, it seems that the abnormality of short latency inhibition in sensory function might be similar to the abnormality in motor function.
Figure 2.
Study of sensory inhibition in the temporal domain in normal subjects (healthy volunteers, HV) and patients with focal hand dystonia (FHD). Recovery curve of the P27 component of the median nerve somatosensory evoked potential (SEP) from both right (Rt) and left (Lt) hands showing the amplitude of the second SEP compared with the first one. Patients showed less inhibition at the 5 ms interval. *p<0.05. Modified from (Tamura et al., 2008) with permission.
A direct look at inhibitory processes in sensory function is possible with a measure of the high frequency oscillations (HFO) at about the time of the N20 component of the SEP. This is because HFOs might reflect a series of inhibitory postsynaptic potentials (IPSPs) (Ozaki and Hashimoto, 2005). The possibility of decreased HFOs in focal dystonia was first demonstrated in cervical dystonia (Inoue et al., 2004), but this has also been shown in (female) patients with writer's cramp (Cimatti et al., 2007).
Sensorimotor integration
In addition to abnormalities that might be described as pure motor or pure sensory, there are also abnormalities that are best described as those of sensorimotor integration. The clinical phenomenon of the sensory trick is certainly an indication of a sensory influence on the motor system, but its physiology is not understood.
In some circumstances vibration of the affected arm will induce dystonia in patients with focal hand dystonia (Kaji et al., 1995b). This phenomenon can be blocked with dilute lidocaine indicating that it is mediated by spindle afferents. As described above, vibration leads to abnormal perception in patients as well. Moreover, in normal subjects, vibration of a muscle leads to an augmentation of its MEP and a reduction of its SICI; these effects are lacking in patients with writer's cramp (Rosenkranz et al., 2005).
A possible measure of sensorimotor integration is the contingent negative variation (CNV), which is the EEG activity between two sensory stimuli (first, warning, and, second, commanding) that trigger a movement. This potential can be compared to the Bereitschaftspotential (BP) which is the EEG activity prior to a self-paced voluntary movement. There may be a minor abnormality of the BP (Deuschl et al., 1992; Zeuner et al., 2009), but there is a clear reduction in amplitude of the CNV. Specifically, the CNV is abnormal prior to hand movements in patients with focal hand dystonia, but not prior to neck movements (Hamano et al., 1999; Ikeda et al., 1996). On the other hand, the CNV is abnormal prior to neck movements in patients with cervical dystonia, but not prior to hand movements (Kaji et al., 1995a).
There is also a specific abnormality of the SEP during the reaction time of a sensory triggered movement (Murase et al., 2000). While the N30 component was gated (reduced in amplitude) for normal subjects, it was not for patients with writer's cramp. On the other hand, the P22 was gated in the patients, but not for normal subjects. (An increased N30 amplitude has been described in the literature (Reilly et al., 1992), but this has not been a regular abnormality in other studies (Tamura et al., 2008).)
The studies reviewed above about SAI and LAI relate to this topic, as they reveal the influence of peripheral sensory inputs onto motor circuits. Another effect of peripheral stimuli is to decrease SICI. This influence is diminished in focal hand dystonia (McDonnell et al., 2007b; Simonetta-Moreau et al., 2006).
Plasticity
Another abnormality in focal dystonia is that of plasticity. This issue will be described at length in another article in this series. It is relevant here, however, to note several aspects of the abnormal plasticity. First, since plasticity depends on the amount of inhibition (Di Lazzaro et al., 2006; McDonnell et al., 2007a), so an abnormality of plasticity could be secondary to the abnormality of inhibition. Second, not only is plasticity increased in magnitude in several paradigms, but there is also abnormal spread of the plasticity. That is, if the plasticity is focused on the APB by stimulating the median nerve, there are abnormal increases in ulnar nerve muscles, FDI (Quartarone et al., 2003) and ADM (Weise et al., 2006). This is another abnormality of loss of lateral or surround inhibition.
Approaches to therapy by augmenting inhibition
If dystonia is characterized by a loss of inhibition, then augmenting inhibition might be helpful. In this regard, it is reassuring that several drugs that can be useful have GABAergic actions. These include the benzodiazepines which promote GABA-A receptor mediated transmission, and baclofen, an agonist at GABA-B receptors. Physiological techniques have also been employed. Deep brain stimulation can be effective, and it is interesting in this regard that there are improvements in physiological inhibition that correlate with clinical improvement (Tisch et al., 2006a; Tisch et al., 2006b).
Since repetitive TMS (rTMS) delivered over M1 at slow rates (between 0.2 and 1 Hz) can induce an increase in inhibition (as assessed, for example, by a reduction in MEP amplitude), this too might be helpful. A study at 1 Hz showed normalization of SICI and some modest improvement in performance (Siebner et al., 1999). This approach might be restricted, however, since there is evidence that 1 Hz rTMS over M1 has only limited physiological effects (Stinear and Byblow, 2004c). Another target could be the premotor cortex since rTMS at 1 Hz can ameliorate the deficit in reciprocal inhibition in dystonia (Huang et al., 2004). Nine patients with writer's cramp and seven age-matched control subjects were studied using subthreshold 0.2 Hz rTMS applied to M1, supplementary motor area (SMA) or premotor cortex (Murase et al., 2005). Stimulation of the premotor cortex but not M1 significantly improved the rating of handwriting in the patient group. rTMS over the other sites or using a sham coil in the patient group or trials in the control group revealed no clinical changes. 1 Hz rTMS was applied at 90% of resting motor threshold for 20 minutes over the left premotor cortex in an open study of three patients with severe, generalized, secondary dystonia (Lefaucheur et al., 2004). After treatments for 5 consecutive days, painful spasms were reduced for 3-8 days.
Although the detailed influence of 1 Hz stimulation of the primary sensory cortex (S1) is not known, such stimulation for 30 minutes daily for 5 days produced some improvement in writing in patients with writer's cramp (Havrankova et al., 2010).
It might be possible to employ training procedures to normalize the motor system by employing strategies to augment surround inhibition or somatosensory discrimination. Attempts have been made in this regard, but they have not generally produced substantial results. Various types of motor training have been utilized that try to individuate finger movements (Candia et al., 2002; Zeuner et al., 2008; Zeuner et al., 2005). One difficulty with this is that normal subjects show more of an increase of SICI with selective practice than do patients with focal hand dystonia (Butefisch et al., 2005). Sensory training has included teaching patients to read Braille (Zeuner et al., 2002; Zeuner and Hallett, 2003). A study with a single session of asynchronous afferent stimulation produced some physiological evidence both for improved sensorimotor cortical representation and improved writing (Schabrun et al., 2009). Improvements so far have not been sustained, and this might be due to the underlying abnormal physiology. It is likely, however, that efforts over many years will be necessary to reverse a process that took many years to create, and this has not yet been undertaken.
Therapies remain suboptimal, and botulinum toxin injection is currently the most effective approach for the focal dystonias (Hallett et al., 2009).
Role of the basal ganglia
This review has been written largely describing abnormalities at the cortical level, but dystonia is generally thought be derived from dysfunction of the basal ganglia. There is evidence for basal ganglia abnormalities from imaging and clinicopathological correlation of secondary dystonia. However, it is not unreasonable to ask how the abnormalities described here can be related to the basal ganglia. The basal ganglia do have an important influence on the motor cortex, but how a basal ganglia abnormality could produce dystonia is not completely clear. In the classic view, there are two anatomical pathways through the basal ganglia. The direct pathway (striatum-internal division of the globus pallidus-thalamus) is facilitatory, and the indirect pathway (striatum-external division of the globus pallidus-subthalamic nucleus-internal division of the globus pallidus-thalamus) is inhibitory. One notion is that the direct pathway helps command the desired movement, while the indirect pathway inhibits unwanted movements (Mink, 1996). A number of investigators have felt that there is an imbalance in the direct and indirect pathways so that the direct pathway is relatively overactive (or that the indirect pathway is relatively underactive) (Hallett, 2004; Hallett, 2006). The postulated imbalance should lead to excessive movement and, in particular, a loss of surround inhibition.
A hypothesis
A possible mechanism that could explain most of the abnormalities is a deficiency in numbers or a specific class of inhibitory interneurons, although there is no evidence for this as yet. As noted above, there is some evidence for decreased GABA in the brain in patients and that increasing GABA can be therapeutic. A loss of striatal parvalbumin-reactive GABAergic interneurons was found in a hamster model of paroxysmal dystonia (dt(sz)) (Gernert et al., 2000). The loss of inhibition would be a primary consequence, and altered sensory and motor representations and function would be secondary results. Also as noted earlier, altered plasticity can also result from loss of inhibition. The loss of inhibition might be widespread, might involve the sensory and motor cortex directly, or just involve the basal ganglia directly and the sensory and motor cortex indirectly. Such a loss of inhibition could be a consequence of the abnormal genetic background in patients with dystonia and would predispose them to develop dystonia if there are other factors, such as repetitive activity in focal hand dystonia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research Highlights:
Dystonia is characterized by loss of inhibition throughout the neuraxis.
Loss of surround inhibition leads to overflow and muscle spasms.
Mild sensory abnormalities in dystonia may also be due to loss of inhibition.
References
- Abbruzzese G, et al. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–45. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Baker RS, et al. Maladaptive neural compensatory mechanisms in Bell's palsy-induced blepharospasm. Neurology. 1997;49:223–9. doi: 10.1212/wnl.49.1.223. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, et al. Abnormal somatosensory homunculus in dystonia of the hand. Annals of Neurology. 1998;44:828–831. doi: 10.1002/ana.410440520. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, et al. Spatial discrimination is abnormal in focal hand dystonia. Neurology. 2000a;55:1869–73. doi: 10.1212/wnl.55.12.1869. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, et al. Sensory discrimination capabilities in patients with focal hand dystonia. Ann Neurol. 2000b;47:377–80. [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clin Neurophysiol. 2010;121:98–103. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, et al. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci. 2008;28:10363–9. doi: 10.1523/JNEUROSCI.3564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, et al. Surround inhibition depends on the force exerted and is abnormal in focal hand dystonia. J Appl Physiol. 2009a;107:1513–8. doi: 10.1152/japplphysiol.91580.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, et al. Inter-hemispheric inhibition is impaired in mirror dystonia. Eur J Neurosci. 2009b;29:1634–40. doi: 10.1111/j.1460-9568.2009.06710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, et al. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985;108:593–608. doi: 10.1093/brain/108.3.593. [DOI] [PubMed] [Google Scholar]
- Bertolasi L, et al. Impaired heteronymous somatosensory motor cortical inhibition in dystonia. Mov Disord. 2003;18:1367–73. doi: 10.1002/mds.10514. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, et al. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Brighina F, et al. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: a preliminary report. Exp Brain Res. 2009;192:651–6. doi: 10.1007/s00221-008-1572-9. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, et al. Task-dependent intracortical inhibition is impaired in focal hand dystonia. Mov Disord. 2005;20:545–51. doi: 10.1002/mds.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth S, et al. Abnormal cortical sensory activation in dystonia: an fMRI study. Mov Disord. 2003;18:673–82. doi: 10.1002/mds.10416. [DOI] [PubMed] [Google Scholar]
- Byl N, et al. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology. 1996;47:508–520. doi: 10.1212/wnl.47.2.508. [DOI] [PubMed] [Google Scholar]
- Candia V, et al. Sensory motor retuning: a behavioral treatment for focal hand dystonia of pianists and guitarists. Arch Phys Med Rehabil. 2002;83:1342–8. doi: 10.1053/apmr.2002.35094. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, et al. Bell's palsy-induced blepharospasm relieved by passive eyelid closure and responsive to apomorphine. Clin Neurophysiol. 2005;116:2348–53. doi: 10.1016/j.clinph.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Chen R, et al. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Chuke JC, et al. Bell's Palsy-associated blepharospasm relieved by aiding eyelid closure. Annals of Neurology. 1996;39:263–268. doi: 10.1002/ana.410390217. [DOI] [PubMed] [Google Scholar]
- Cimatti Z, et al. Time-frequency analysis reveals decreased high-frequency oscillations in writer's cramp. Brain. 2007;130:198–205. doi: 10.1093/brain/awl259. [DOI] [PubMed] [Google Scholar]
- Classen J, et al. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Defazio G, et al. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–93. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- Deuschl G, et al. The movement related cortical potential is abnormal is patients with writer's cramp. Movement Disorders. 1992;7(Suppl 1):127. [Google Scholar]
- Di Lazzaro V, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000a;111:794–9. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. Reduced cerebral cortex inhibition in dystonia: direct evidence in humans. Clin Neurophysiol. 2009;120:834–9. doi: 10.1016/j.clinph.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000b;135:455–61. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268–73. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118:2207–14. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575:721–6. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, et al. Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol. 2006;59:825–34. doi: 10.1002/ana.20837. [DOI] [PubMed] [Google Scholar]
- Fiorio M, et al. Temporal processing of visuotactile and tactile stimuli in writer's cramp. Ann Neurol. 2003;53:630–5. doi: 10.1002/ana.10525. [DOI] [PubMed] [Google Scholar]
- Frasson E, et al. Somatosensory disinhibition in dystonia. Mov Disord. 2001;16:674–82. doi: 10.1002/mds.1142. [DOI] [PubMed] [Google Scholar]
- Frima N, et al. Abnormal vibration-induced illusion of movement in idiopathic focal dystonia: an endophenotypic marker? Mov Disord. 2008;23:373–7. doi: 10.1002/mds.21838. [DOI] [PubMed] [Google Scholar]
- Fuhr P, et al. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalography and Clinical Neurophysiology. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gernert M, et al. Deficit of striatal parvalbumin-reactive GABAergic interneurons and decreased basal ganglia output in a genetic rodent model of idiopathic paroxysmal dystonia. J Neurosci. 2000;20:7052–8. doi: 10.1523/JNEUROSCI.20-18-07052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald RA, et al. Idiopathic focal dystonia: a disorder of muscle spindle afferent processing? Brain. 1997;120:2179–85. doi: 10.1093/brain/120.12.2179. [DOI] [PubMed] [Google Scholar]
- Hallett M. Dystonia: abnormal movements result from loss of inhibition. Adv Neurol. 2004;94:1–9. [PubMed] [Google Scholar]
- Hallett M. Pathophysiology of writer's cramp. Hum Mov Sci. 2006;25:454–63. doi: 10.1016/j.humov.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hallett M, et al. Treatment of focal dystonias with botulinum neurotoxin. Toxicon. 2009;54:628–33. doi: 10.1016/j.toxicon.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano T, et al. Abnormal contingent negative variation in writer's cramp. Clin Neurophysiol. 1999;110:508–15. doi: 10.1016/s1388-2457(98)00045-5. [DOI] [PubMed] [Google Scholar]
- Hanajima R, et al. Difference in intracortical inhibition of the motor cortex between cortical myoclonus and focal hand dystonia. Clin Neurophysiol. 2008;119:1400–7. doi: 10.1016/j.clinph.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Hanajima R, et al. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509(Pt 2):607–18. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrankova P, et al. Repetitive TMS of the somatosensory cortex improves writer's cramp and enhances cortical activity. Neuro Endocrinol Lett. 2010;31:73–86. [PubMed] [Google Scholar]
- Herath P, et al. In Vivo Neurochemistry of Primary Focal Hand Dystonia: An Magnetic Resonance Spectroscopic Neurometabolite Profiling Study at 3 T. Movement Disorders. 2010 doi: 10.1002/mds.23306. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, et al. One-Hz repetitive transcranial magnetic stimulation of the premotor cortex alters reciprocal inhibition in DYT1 dystonia. Mov Disord. 2004;19:54–9. doi: 10.1002/mds.10627. [DOI] [PubMed] [Google Scholar]
- Huang YZ, et al. Restoration of motor inhibition through an abnormal premotor-motor connection in dystonia. Mov Disord. 2010;25:689–96. doi: 10.1002/mds.22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, et al. Abnormal sensorimotor integration in writer's cramp: study of contingent negative variation. Movement Disorders. 1996;11:638–690. doi: 10.1002/mds.870110614. [DOI] [PubMed] [Google Scholar]
- Inoue K, et al. Disinhibition of the somatosensory cortex in cervical dystonia-decreased amplitudes of high-frequency oscillations. Clin Neurophysiol. 2004;115:1624–30. doi: 10.1016/j.clinph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kaji R, et al. Physiological study of cervical dystonia. Task-specific abnormality in contingent negative variation. Brain. 1995a;118:511–522. doi: 10.1093/brain/118.2.511. [DOI] [PubMed] [Google Scholar]
- Kaji R, et al. Tonic vibration reflex and muscle afferent block in writer's cramp: Implications for a new therapeutic approach. Annals of Neurology. 1995b;38:155–162. doi: 10.1002/ana.410380206. [DOI] [PubMed] [Google Scholar]
- Kessler KR, et al. Short latency afferent inhibition and facilitation in patients with writer's cramp. Mov Disord. 2005;20:238–42. doi: 10.1002/mds.20295. [DOI] [PubMed] [Google Scholar]
- Kimberley TJ, et al. Establishing the definition and inter-rater reliability of cortical silent period calculation in subjects with focal hand dystonia and healthy controls. Neurosci Lett. 2009;464:84–7. doi: 10.1016/j.neulet.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, et al. Altered dorsal premotor-motor interhemispheric pathway activity in focal arm dystonia. Mov Disord. 2008;23:660–8. doi: 10.1002/mds.21881. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, et al. Low-frequency repetitive TMS of premotor cortex can reduce painful axial spasms in generalized secondary dystonia: a pilot study of three patients. Neurophysiol Clin. 2004;34:141–5. doi: 10.1016/j.neucli.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Levy LM, Hallett M. Impaired brain GABA in focal dystonia. Ann Neurol. 2002;51:93–101. [PubMed] [Google Scholar]
- Lourenco G, et al. Impaired modulation of motor cortex excitability by homonymous and heteronymous muscle afferents in focal hand dystonia. Mov Disord. 2007;22:523–7. doi: 10.1002/mds.21312. [DOI] [PubMed] [Google Scholar]
- Matsumura M, et al. GABAergic inhibition of neuronal activity in the primate motor and premotor cortex during voluntary movement. Journal of Neurophysiology. 1992;68:692–702. doi: 10.1152/jn.1992.68.3.692. [DOI] [PubMed] [Google Scholar]
- Matsumura M, et al. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. Journal of Neurophysiology. 1991;65:1542–1553. doi: 10.1152/jn.1991.65.6.1542. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, et al. Suppression of LTP-like plasticity in human motor cortex by the GABA(B) receptor agonist baclofen. Exp Brain Res. 2007a doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, et al. The effect of cutaneous input on intracortical inhibition in focal task-specific dystonia. Mov Disord. 2007b;22:1286–92. doi: 10.1002/mds.21508. [DOI] [PubMed] [Google Scholar]
- Meunier S, et al. Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann Neurol. 2001;50:521–7. doi: 10.1002/ana.1234. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Molloy FM, et al. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126:2175–82. doi: 10.1093/brain/awg219. [DOI] [PubMed] [Google Scholar]
- Murase N, et al. Abnormal premovement gating of somatosensory input in writer's cramp. Brain. 2000;123(Pt 9):1813–29. doi: 10.1093/brain/123.9.1813. [DOI] [PubMed] [Google Scholar]
- Murase N, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain. 2005;128:104–15. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- Nakashima K, et al. Reciprocal inhibition in writer's and other occupational cramps and hemiparesis due to stroke. Brain. 1989;112:681–697. doi: 10.1093/brain/112.3.681. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, et al. Digit-specific aberrations in the primary somatosensory cortex in Writer's Cramp. Ann Neurol. 2009;66:146–154. doi: 10.1002/ana.21626. [DOI] [PubMed] [Google Scholar]
- O'Dwyer JP, et al. Sensory abnormalities in unaffected relatives in familial adult-onset dystonia. Neurology. 2005;65:938–40. doi: 10.1212/01.wnl.0000176068.23983.a8. [DOI] [PubMed] [Google Scholar]
- Ozaki I, Hashimoto I. Neural mechanisms of the ultrafast activities. Clin EEG Neurosci. 2005;36:271–7. doi: 10.1177/155005940503600406. [DOI] [PubMed] [Google Scholar]
- Panizza M, et al. H-reflex recovery curve and reciprocal inhibition of H-reflex in different kinds of dystonia. Neurology. 1990;40:824–828. doi: 10.1212/wnl.40.5.824. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, et al. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. Journal of Neuroscience. 1997;17:843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirio Richardson S, et al. Long-latency afferent inhibition during phasic finger movement in focal hand dystonia. Exp Brain Res. 2009;193:173–9. doi: 10.1007/s00221-008-1605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzki N, et al. Kinesthesia is impaired in focal dystonia. Mov Disord. 2006;21:754–60. doi: 10.1002/mds.20799. [DOI] [PubMed] [Google Scholar]
- Quartarone A, et al. Corticospinal excitability during motor imagery of a simple tonic finger movement in patients with writer's cramp. Mov Disord. 2005;20:1488–95. doi: 10.1002/mds.20626. [DOI] [PubMed] [Google Scholar]
- Quartarone A, et al. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–96. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Reilly JA, et al. The N30 component of somatosensory evoked potentials in patients with dystonia. Electroencephalography and Clinical Neurophysiology. 1992;84:243–247. doi: 10.1016/0168-5597(92)90005-v. [DOI] [PubMed] [Google Scholar]
- Richardson SP, et al. Changes in short afferent inhibition during phasic movement in focal dystonia. Muscle Nerve. 2007 doi: 10.1002/mus.20943. [DOI] [PubMed] [Google Scholar]
- Ridding MC, et al. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. Journal of Neurology, Neurosurgery and Psychiatry. 1995;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, et al. Pathophysiological differences between musician's dystonia and writer's cramp. Brain. 2005;128:918–31. doi: 10.1093/brain/awh402. [DOI] [PubMed] [Google Scholar]
- Sanger TD, et al. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–17. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabrun SM, et al. Normalizing motor cortex representations in focal hand dystonia. Cereb Cortex. 2009;19:1968–77. doi: 10.1093/cercor/bhn224. [DOI] [PubMed] [Google Scholar]
- Schicatano EJ, et al. Animal model explains the origins of the cranial dystonia benign essential blepharospasm. J Neurophysiol. 1997;77:2842–6. doi: 10.1152/jn.1997.77.5.2842. [DOI] [PubMed] [Google Scholar]
- Scontrini A, et al. Somatosensory temporal discrimination in patients with primary focal dystonia. J Neurol Neurosurg Psychiatry. 2009;80:1315–9. doi: 10.1136/jnnp.2009.178236. [DOI] [PubMed] [Google Scholar]
- Shin HW, et al. Hemispheric asymmetry of surround inhibition in the human motor system. Clin Neurophysiol. 2009;120:816–9. doi: 10.1016/j.clinph.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Siebner HR, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology. 1999;52:529–37. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Simonetta-Moreau M, et al. Lack of inhibitory interaction between somatosensory afferent inputs and intracortical inhibitory interneurons in focal hand dystonia. Mov Disord. 2006;21:824–34. doi: 10.1002/mds.20821. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004a;56:595–9. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004b;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- Sohn YH, et al. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res. 2003;148:176–85. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Elevated threshold for intracortical inhibition in focal hand dystonia. Mov Disord. 2004a;19:1312–7. doi: 10.1002/mds.20160. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired inhibition of a pre-planned response in focal hand dystonia. Exp Brain Res. 2004b;158:207–12. doi: 10.1007/s00221-004-1891-4. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired modulation of corticospinal excitability following subthreshold rTMS in focal hand dystonia. Hum Mov Sci. 2004c;23:527–38. doi: 10.1016/j.humov.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired modulation of intracortical inhibition in focal hand dystonia. Cereb Cortex. 2004d;14:555–61. doi: 10.1093/cercor/bhh017. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Task-dependent modulation of silent period duration in focal hand dystonia. Mov Disord. 2005;20:1143–51. doi: 10.1002/mds.20514. [DOI] [PubMed] [Google Scholar]
- Syed NA, et al. Blink reflex recovery in facial weakness: an electrophysiologic study of adaptive changes. Neurology. 1999;52:834–8. doi: 10.1212/wnl.52.4.834. [DOI] [PubMed] [Google Scholar]
- Tamura Y, et al. Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov Disord. 2008;23:558–65. doi: 10.1002/mds.21870. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, et al. Task-specific impairment of motor cortical excitation and inhibition in patients with writer's cramp. Neurosci Lett. 2005;378:55–8. doi: 10.1016/j.neulet.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, et al. Deficits of temporal discrimination in dystonia are independent from the spatial distance between the loci of tactile stimulation. Mov Disord. 2002;17:333–8. doi: 10.1002/mds.10019. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, et al. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain. 2000;123:42–50. doi: 10.1093/brain/123.1.42. [DOI] [PubMed] [Google Scholar]
- Tisch S, et al. Changes in blink reflex excitability after globus pallidus internus stimulation for dystonia. Mov Disord. 2006a;21:1650–5. doi: 10.1002/mds.20899. [DOI] [PubMed] [Google Scholar]
- Tisch S, et al. Changes in forearm reciprocal inhibition following pallidal stimulation for dystonia. Neurology. 2006b;66:1091–3. doi: 10.1212/01.wnl.0000204649.36458.8f. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, et al. Magnetic stimulation over the cerebellum in humans. Annals of Neurology. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, et al. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalography and Clinical Neurophysiology. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Weise D, et al. The two sides of associative plasticity in writer's cramp. Brain. 2006;129:2709–21. doi: 10.1093/brain/awl221. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, et al. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol (Lond) 1999;517:591–7. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y, et al. Abnormal perception of the tonic vibration reflex in idiopathic focal dystonia. Eur J Neurol. 2000;7:529–33. doi: 10.1046/j.1468-1331.2000.t01-1-00102.x. [DOI] [PubMed] [Google Scholar]
- Zeuner KE, et al. Sensory training for patients with focal hand dystonia. Ann Neurol. 2002;51:593–8. doi: 10.1002/ana.10174. [DOI] [PubMed] [Google Scholar]
- Zeuner KE, Hallett M. Sensory training as treatment for focal hand dystonia: a 1-year follow-up. Mov Disord. 2003;18:1044–7. doi: 10.1002/mds.10490. [DOI] [PubMed] [Google Scholar]
- Zeuner KE, et al. Slow pre-movement cortical potentials do not reflect individual response to therapy in writer's cramp. Clin Neurophysiol. 2009;120:1213–9. doi: 10.1016/j.clinph.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Zeuner KE, et al. Motor re-training does not need to be task specific to improve writer's cramp. Mov Disord. 2008;23:2319–27. doi: 10.1002/mds.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner KE, et al. Motor training as treatment in focal hand dystonia. Mov Disord. 2005;20:335–41. doi: 10.1002/mds.20314. [DOI] [PubMed] [Google Scholar]
- Ziemann U, et al. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol (Lond) 1996;496:873–81. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]