Abstract
Premarin™ is the most commonly prescribed estrogenic component of hormone therapy, given since 1942. The current study is the first examining cognitive effects of tonic Premarin treatment in an animal model. Middle-aged ovariectomized (Ovx) rats received vehicle or one of three doses of Premarin (12, 24 or 36 μg daily). Rats were tested on a spatial working and reference memory maze battery. Both Medium- and High- dose Premarin enhanced memory retention, while Low-dose Premarin impaired learning and memory retention. Correlations with serum hormone levels showed that as the ratio of estrone:17β-estradiol increased, animals tended to show better working memory performance. Taken together with the dissociation of dose-specific estrogenic profiles, results suggest that higher levels of estrone, in the presence of 17β-estradiol concentrations higher than that of Ovx levels, may be beneficial for memory. Moreover, Premarin exerted dose and brain-region specific effects on BDNF and NGF protein levels, with most marked changes in cingulate and perirhinal cortices. Hippocampal gene expression profiling demonstrated significant Premarin-induced transcriptional changes in genes linked to plasticity and cognition. These findings indicate that Premarin can impact memory and the brain, and that dosing should be recognized as a clinically relevant factor possibly affecting the direction and efficacy of cognitive outcome.
Keywords: Premarin, estrogen, hormone replacement, working memory, spatial memory, neurotrophins, gene expression
1. Introduction
Conjugated equine estrogen, trade name Premarin (Wyeth Pharmaceuticals, Philadelphia, PA), has been administered since 1942 and is the most widely used estrogenic component of hormone therapy in North America (Segal, 1997; Sitruk-Ware, 2002). Premarin is given unopposed to women who have undergone surgical menopause including uterus removal (Farquhar et al., 2009; The North American Menopause Society, 2003). As well, Premarin is the estrogenic component of Prempro, the most prescribed combination hormone therapy for women with a uterus (Segal, 1997; Sitruk-Ware, 2002). Clinical findings assessing cognitive effects of Premarin-containing therapies have been inconclusive. Premarin-containing therapy has been reported to improve memory in case studies (Ohkura et al., 1995), non-randomized small quasi-experimental designs (Carlson and Sherwin, 1998) and small double-blind placebo-controlled studies (Campbell and Whitehead, 1977; Kantor et al., 1973). Also, a randomized, double-blind placebo controlled crossover trial showed that Premarin treatment altered brain activation patterns in women during memory task performance (Shaywitz et al., 1999). Yet, findings from the large placebo-controlled WHI Memory Study (WHIMS), conducted by the National Institutes of Health, showed that Premarin treatment yielded a non-significant increased incidence of probable dementia and mild cognitive impairment in women 65 and over (Espeland et al., 2004; Shumaker et al., 2004). Further, there was an elevated probable dementia risk, and no effect on mild cognitive impairment, in women taking Premarin+medroxyprogesterone (Shumaker et al., 2003). This combination therapy also had a negative effect on verbal memory, but a trend for positive effects on figural memory, in women 65 and over that were free of probable dementia (WHI Study of Cognitive Aging, WHISCA, Resnick et al., 2006). Together, the clinical studies indicate that Premarin-containing therapy can result in both beneficial and detrimental actions on cognition in women.
Cognitive effects of estrogen replacement have been evaluated in animal models. In young and middle-aged ovariectomized (Ovx) rodents, 17β-estradiol enhances spatial working memory (Bimonte and Denenberg, 1999; Daniel et al., 1997; Daniel et al., 2005; Fader et al., 1999; Gibbs, 1999; Hruska and Dohanich, 2007; Luine and Rodriguez, 1994) and spatial reference memory (Bimonte-Nelson et al., 2006; El-Bakri et al., 2004; Feng et al., 2004; Frick et al., 2002; Markham et al., 2002). Like the clinical findings testing Premarin, not all animal studies testing 17β-estradiol have shown positive effects (Chesler and Juraska, 2000; Fernandez and Frick, 2004; Galea et al., 2001; Galea et al., 2002; Holmes et al., 2002; Singh et al., 1994). To date, 17β-estradiol has been the primary type of estrogen used to test cognitive effects of hormone therapy in the animal model. 17β-estradiol is the most potent naturally-circulating estrogen, followed by estrone and estriol, in order of receptor affinity (Kuhl, 2005; Sitruk-Ware, 2002). Premarin is derived from the urine of pregnant mares, and is comprised of a complex mixture of estrogen sulfates that have been conjugated by the horse’s liver before excretion in urine; many of the estrogens present in Premarin are unique to horses (Bhavnani, 1998). Premarin contains the sulfates of at least ten estrogens, is over 50% estrone sulfate, 20-25% equilin sulfate, and has only trace amounts of 17β-estradiol; after metabolism, the resulting biologically active circulating hormones are primarily estrone and, after estrone’s conversion, 17β-estradiol, as well as equilin (Bhavnani, 2003; Sitruk-Ware, 2002). It is hypothesized that these three metabolites are primarily responsible for the estrogenic effects of Premarin (Sitruk-Ware, 2002). It is noted that there are other estrogens and related metabolites present in Premarin that could alter efficacy of 17β-estradiol effects, and may initiate effects on their own; these hormones include, but are not limited to, delta 8,9 dehydroestrone, dihydroequilin-17β and equilenin (Kuhl, 2005). Therefore, the animal studies done thus far testing the cognitive effects of 17β-estradiol cannot be directly compared to potential effects of Premarin.
Like the cognitive enhancements seen after 17β-estradiol treatment given via subcutaneous injection (Bimonte-Nelson et al., 2006; Chesler and Juraska, 2000; Daniel and Dohanich, 2001; Luine et al., 2003; Sandstrom and Williams, 2001), we recently showed cognitive enhancements after Premarin treatment given via cyclic, intermittent subcutaneous injections in middle-aged Ovx rats (Acosta et al., 2009). Specifically, with this regimen Premarin improved spatial working memory delayed-match-to-sample (DMS) plus-maze performance and attenuated overnight forgetting on the spatial reference memory Morris water maze (MWM). However, cyclic intermittent versus tonic estrogen administration may influence realization of memory benefits. With tonic estrogen treatment, estrogen receptors become downregulated, while with cyclic intermittent estrogen treatment, estrogen receptor recycling and other physiological changes occur that may enhance ultimate responsiveness for many parameters, including learning and memory (Blaustein, 1993; Brown et al., 1996; Kassis and Gorski, 1981; Rosser et al., 1993). Women, including those enrolled in the WHI study, typically take hormone therapy as a daily oral tonic regimen, not intermittent in nature. The cognitive effects of tonic Premarin treatment have not been evaluated in an animal model.
In vitro studies provide evidence that Premarin, or components thereof, has positive effects on the brain. Premarin enhances neuronal growth and increases neuronal survival after experimentally-induced insult in in vitro preparations, including in cognitive brain regions (Brinton et al., 2000; Diaz Brinton et al., 2000; Zhao and Brinton, 2006). While these in vitro experiments provide compelling evidence that Premarin could result in brain changes ultimately leading to enhancement in brain functions such as learning and memory, direct evaluations testing tonic Premarin’s effects on cognition have not been done in an animal model.
Discerning the mechanism of the potentially cognitive enhancing effects of Premarin could have wide implications for future research and treatments for optimizing hormone therapy. Neurotrophins may be one mechanism of estrogen-induced neuroprotection or mnemonic changes. Survival and maintenance of cholinergic neurons are dependent upon neurotrophins, including nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF); age-related neurotrophin changes have been reported in animal models, and NGF and BDNF have been associated with cognitive function (Bimonte et al., 2003, Bimonte-Nelson et al., 2008; Granholm, 2000; Hall et al., 2000; Kesslak et al., 1998; Ma et al., 1998; Siegal and Chauhan, 2000). 17β-estradiol treatment significantly impacts neurotrophin systems in young and aged Ovx rats, increasing neurotrophin and its receptor mRNA levels in basal forebrain, frontal cortex and hippocampus (McMillan et al., 1996; Pan et al., 1999) and elevating NGF and BDNF protein levels in cognitive brain regions (Bimonte-Nelson et al., 2004). Whether Premarin induces cognitive change, and whether such changes are related to neurotrophin alterations, has not yet been evaluated.
From a broader perspective, gene expression experiments can yield insight into mechanistic and molecular processes involved in nootropic hormone therapies. Evaluations have been performed to determine whether 17β-estradiol influences hippocampal gene expression. While these constitute a small handful of studies, and work has only been done in young mice, this nonetheless provides evidence that estrogens can impact gene expression (Malyala et al., 2004), with effects most notably recognized within domains of synaptic plasticity in the hippocampus (Aenlle et al., 2007; Pechenino and Frick, 2009). The present experiment capitalized on similar gene expression array procedures in the hippocampus to yield insight into potential mechanisms of action of Premarin in cognitively-characterized middle-aged Ovx rats.
The present study examined three Premarin doses in middle-aged rats after surgical menopause. The doses were based on the 0.625 mg/day dose commonly prescribed to women, and used in the WHI studies, altered only for body weight of the rat. We tested whether Premarin altered learning and memory using a battery of tasks designed to tap several memory domains, followed by BDNF, NGF and gene expression assays. Since this is the first cognitive study to tonically administer Premarin to the rodent, we also obtained vaginal smears, uterine weights and hypothalamic weights to confirm endocrine responsiveness. Further, since 17β-estradiol and estrone levels are increased in menopausal women after Premarin treatment (Sitruk-Ware, 2002), we measured 17β-estradiol and estrone blood levels in our surgically menopausal rats. This allowed for treatment verification and determination of circulating levels that could be correlated with maze scores to aid in interpretation of potentially effective treatment regimens. Indeed, while higher circulating 17β-estradiol replacement levels have been correlated with better spatial reference memory MWM performance (Talboom et al., 2008), correlations have not been evaluated for working memory. Furthermore, relationships between maze performance and circulating estrone, or the ratio of estrone:17β-estradiol, have not been assessed in the rodent, but have correlated with neuropsychological test scores in menopausal women (Lebrun et al., 2005; Phillips and Sherwin, 1992; Sherwin, 1988; Wolf and Kirschbaum, 2002).
2. Materials and methods
2.1 Subjects
At the start of the experiment there were 37 thirteen-month old Fischer-344 female rats born and raised at the aging colony of the National Institute on Aging at Harlan Laboratories (Indianapolis, IN). Prior to surgery, rats were acclimated for several days, and were pair housed with an identical treatment assigned cage-mate. Animals had access to food and water ad lib, and were maintained on a 12-h light/dark cycle. All procedures were approved by the local IACUC committee and adhered to NIH standards.
2.2 Ovariectomy and pump insertion surgeries
Fourteen days before behavioral testing, all rats were anesthetized with acute isoflourene inhalation and received Ovx. Bilateral dorsolateral incisions were made in the skin and peritoneum, and the ovaries and tips of uterine horns were ligatured and removed. The muscle was then sutured and the skin stapled. At the time of Ovx, Alzet osmotic pumps (model 2ML4, DURECT Corporation, Cupertino, CA) containing either proplyene glycol (Ovx-Vehicle), or proplyene glycol plus one of three doses of Premarin (Ovx-Premarin-Low, Ovx-Premarin-Medium, Ovx-Premarin-High), were implanted dorsally into the scruff of the neck. Premarin was manufactured by Wyeth Pharmaceuticals (Philadelphia, PA) but obtained from a commercial pharmacy via veterinary prescription. The doses used in the current study were based on the daily 0.625 mg CEE dose commonly taken by women, and used in the WHIMS. Using the average female weight of 70 kg (www.halls.md) for calculations, resulting in 0.00893 mg drug/kg body weight woman, we determined the Premarin-Medium dose (24 μg Premarin powder, which is 10.53% Premarin) to be the rat body weight equivalent of what is clinically prescribed (Espeland et al., 2004; Shumaker et al., 2003; Shumaker et al., 2004). To approximate the injected doses we previously found to influence memory (Acosta et al., 2009), we included 12 μg (Ovx-Premarin-Low) and 36 μg (Ovx-Premarin-High) doses as well. Upon completion of both surgical procedures, rats were given rimadyl for pain and 2 ml of saline. Two weeks following initial pump insertion and two days before behavioral testing began, the first pump was removed and a second pump, filled with the identical substrate to that given previously, was inserted in each rat in the same manner as the first pump insertion. Behavioral testing began 16 days after hormone administration was first initiated via the first pump insertion.
2.3 Vaginal smears and uterine weights
To confirm Ovx and Premarin treatment, vaginal smears were performed at various time intervals throughout the study. Smears were classified as either proestrus, estrus, metestrus or diestrus (Goldman et al., 2007). To examine the effects of Premarin on uterine and pituitary tissues, at sacrifice the uteri of subjects were removed, trimmed of visible fat and immediately weighed (wet weight), and pituitaries were collected and weighed (wet weight).
2.4 Water radial arm maze: testing spatial working and reference memory
Subjects were tested on the water radial arm maze (WRAM). This is a complex task which requires spatial working and reference memory. This win-shift task utilizes water-escape onto hidden platforms as the reinforcer (Bimonte and Denenberg, 1999, 2000; Bimonte et al., 2000, Bimonte et al., 2002; Hyde et al., 1998, Hyde et al., 2000). The 8-arm maze was filled with room temperature water tinted black with nontoxic paint. Four arms had hidden platforms at their ends. Each subject had different platform locations that remained fixed throughout the experiment. A subject was released from the start arm and had 3 min to locate a platform. Once a platform was found, the animal remained on it for 15 sec, and was returned to its heated cage for 30 sec until its next trial. During the inter-trial-interval (ITI), the just-chosen platform was removed from the maze. The animal was placed again into the start alley and allowed to locate another platform. A daily session consisted of these events repeated until all four platforms were located. Consequently, for each animal a daily session consisted of four trials per session, with the number of platformed arms reduced by one on each subsequent trial, and one more item of information to be remembered after every trial. Hence, the working memory system was increasingly taxed as trials progressed.
Error quantification and blocking procedures are based upon previous studies using the WRAM (Bimonte and Denenberg, 1999, 2000; Bimonte et al., 2000, Bimonte et al., 2002; Bimonte et al., 2003; Hyde et al., 1998, Hyde et al., 2000). Each subject was given one session a day for 11 consecutive days. Day 1 was considered training because the animal had no previous experience in the maze. Days 2-11 were testing sessions, blocked into two phases: the initial phase (days 2-6), and the latter phase (days 7-11). Behavioral testing took place between 0800 and 1400 h. An arm entry was counted when the tip of a rat’s snout reached a mark delineated on the outside of the arm (11 cm into the arm). Errors were quantified for each daily session using the orthogonal measures of working and reference memory errors (Jarrard et al., 1984), as done previously in WRAM studies (Bimonte et al., 2000; Bimonte et al., 2002; Hyde et al., 2000). Working Memory Correct (WMC) errors were first and repeat entries into any arm from which a platform had been removed during that session. Reference Memory (RM) errors were first entries into any arm that never contained a platform. Working Memory Incorrect (WMI) errors were repeat entries into reference memory arms. During initial and latter phases, performance was measured for each type of error separately, as well as the 3 error subtypes combined (total errors). On day 12, a 4-hour delay was imposed between trials 2 and 3 to assess retention of multiple items of spatial information (Luine and Rodriguez, 1994). The dependent measure for performance on the delay day was total errors on trials 3 and 4, the trials after the 4-hour delay.
2.5 Morris water maze: testing spatial reference memory
The MWM evaluates spatial reference memory. This win-stay task consisted of a round tub (188 cm in diameter) filled with room temperature water made opaque with black non-toxic paint, with a hidden platform (10 cm wide) (Bimonte-Nelson et al., 2006; Morris et al., 1982). A video camera above the maze tracked the rat’s path during each trial and a tracking system (Ethovision, Noldus Instruments, Leesburg, VA) analyzed each path. The rat was placed in the maze from any of four locations (North, South, East, or West) and had 60 sec to locate the platform, which remained in a fixed location (Northeast quadrant). Once the rat found the platform, the trial was terminated. After 15 sec on the platform, the rat was removed from the maze and placed into its heated cage until the next trial. The rats were given 5 trials a day for 3 days. The approximate ITI was 5-8 min. Swim distance (cm) was the dependent variable for the testing, non-probe trial portion of this task. To evaluate whether rats localized the platform to the spatial location, after all test trials were completed on day 3, a sixth trial was given. This sixth trial was a 60 sec probe trial whereby the platform was removed. Since rats that learned the platform location were expected to spend the greatest percent distance in the target quadrant (Bimonte-Nelson et al., 2006), we analyzed probe trial data by assessing group differences in percent distance (cm) in the target (where the platform was previously located) and opposite (quadrant diagonally opposite to where the platform was previously located) quadrants. To further evaluate search strategy, the probe trial was divided into two 30 sec epochs. The platform crossings, e.g. the number of times an animal swam over the previously platformed location, was quantified for each epoch. This analysis strategy was chosen to yield insight into whether animals knew the general vicinity of the platform location (via targeting the platform quadrant) and/or the exact platform location (via crossing over the platform location, quantified as platform crossings). Additionally, we assessed swim speed (distance swum/trial time) during both 30 sec epochs during the probe trial, as it could be a measure of motor ability and partially account for group differences in performance.
2.6 Delayed-match-to-sample plus maze: testing spatial working and recent memory
After MWM testing, rats were trained in a water-escape DMS plus maze task, a task that assesses spatial working and recent memory. The apparatus had four arms (each 38.1 cm long and 12.7 cm wide) and was filled with room temperature water made opaque with black non-toxic paint. The maze had a hidden escape platform at the end of one of the four arms. The platform location changed every day, but was fixed within a day. Rats received six consecutive trials within a daily session. The first trial was the information trial where the rat was exposed to that day’s platform location, the second trial was the working memory test trial, and trials three through six were recent memory test trials (Frick et al., 1995). Rats were dropped off in a semi-randomly chosen start arm location, and were given a maximum of 90 sec to swim to the platform. Once on the platform, the rat remained on it for 15 sec, followed by placement into a heated cage for a 30 sec ITI. An arm entry was counted when the tip of a rat’s snout reached a mark delineated on the outside of the arm (11 cm into the arm). Entry into an arm with no platform counted as an error, the dependent variable. After four days of testing with a 30 sec ITI between all trials, rats were tested with a 6-hour delay (day 5) between the information trial (trial 1) and the working memory test trial (trial 2) to assess delayed memory retention. There were no additional trials after the post-delay trial (e.g. trial 2). Errors were defined as entry into an arm with no platform, and were quantified for trial 2. In addition, to determine whether each group was affected by this delay test, performance of each group on trial 2 was compared to trial 2 at baseline (day 4 was used as baseline) via a repeated measures ANOVA with Day (test trial for delay day vs. test trial for baseline day) as the repeated measure.
2.7 Brain tissue collection
All rats were sacrificed the day after testing ended. Animals were anesthetized with isoflurane (Vetone, Meridian, Indiana) and decapitated according to NIH euthanasia guidelines. Brains were rapidly dissected by the same experimenter who was blind to treatment group status. Referring to Paxinos and Watson (1998), from the left hemisphere, frontal cortex, cingulate cortex, entorhinal cortex, dorsal and ventral hippocampus (CA1/2), perirhinal cortex, and temporal cortex were dissected for neurotrophin quantification; from the right hemisphere the dorsal hippocampus (CA1/2) was dissected for gene expression profiling. ELISA procedures were carried out on the left hemisphere, and gene expression profiling was carried out on the right hemisphere, to reduce potential inter-hemispheric variability, a procedure used by our laboratory and others (Bimonte-Nelson et al., 2008; French, et al., 2006; Gobbo and O’Mara, 2004). Tissues were placed in pre-weighed microcentrifuge tubes, quickly weighed, frozen on dry ice and stored in at −70 °C until analysis.
2.8 Hormone assays
After decapitation, serum was collected and stored at −4 °C until analysis. Estrone and 17β-estradiol levels were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) according to previously published methods (Nelson et al., 2004). After dansyl chloride derivatization, samples were separated by fast gradient chromatography and then were injected in a tandem mass spectrometer after formation of positive ions with atmospheric pressure chemical ionization. Limits of quantification for estrone and 17β-estradiol were 0.2 and 0.5 pg/ml, respectively, with interassay CV’s of 15% or less at the concentrations obtained for these steroids.
2.9 Neurotrophin level quantification
BDNF and NGF levels were assessed using commercially available assay kits from Promega (Madison, WI). Neurotrophin assay procedures were done as previously described (Bimonte et al., 2003; Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004; Bimonte-Nelson et al., 2008; French, et al., 2006). In brief, flat-bottom plates were coated with the corresponding capture antibody, which binds the neurotrophin of interest. The captured neurotrophin was bound by a second specific antibody, which was detected using a species-specific antibody conjugated to horseradish peroxidase as a tertiary reactant. All unbound conjugates were removed by subsequent wash steps according to the Promega protocol. After incubation with chromagenic substrate, color change was measured in an ELISA plate reader at 450 nm. Using these kits, BDNF and NGF can be quantified in the range of 4.7-300 pg/ml and 7.8-500 pg/ml, respectively. For each assay kit, cross-reactivity with other trophic proteins is < 2-3%.
2.10 Gene expression
These gene expression procedures have been used in previous studies (Basu, et al., 2006; Long et al., 2009). For gene expression, dorsal hippocampal tissues from the Ovx-Vehicle, Ovx-Premarin-Low and Ovx-Premarin-High rats that were used for maze testing were taken. However, for the final data analysis, three subjects from the Ovx-Vehicle group, three from the Ovx-Premarin-Low group and seven from the Ovx-Premarin-High group were used. This was due to a number of samples not progressing forward to data analysis because of stringent quality control measures. These samples did not meet the minimum cRNA amplification requirements for expression profiling, or they were dropped after evaluating quality control reports following array scanning. These samples did not pass at least one of the following criteria: a maximum 3′/5′ GAPDH ratio of 3.0, at least 30% present calls, and/or a maximum scaling factor of 5.0. Values falling outside of these thresholds are indicative of sample degradation and thus a lower level of sample quality. Thus some groups suffered more attrition than others and individuals represented in the groups compared varied. Total RNA was isolated from dorsal hippocampal samples using the Qiagen (Germantown, MD) RNeasy Mini Kit. Total RNA was eluted with 20 μL of RNase-free water. RNA was precipitated by the addition of 1/10 volume 3M NaOAc (pH 5.2) and 2.5 volumes absolute ethanol. After mixing and incubation at −20 °C for 1 hour, the samples were spun at ≥12,000 g for 20 m at 4 °C. The resulting pellet was washed twice with 80% ethanol and air dried. Pellets were resuspended in 9.1 μL of DEPC-treated water. Isolated total RNA from each sample was amplified, cleaned, and biotin-labeled using Affymetrix’s (Santa Clara, CA) GeneChip Once-Cycle Target Labeling kit with a T7 promoter as per manufacturer’s protocol. Amplified and labeled cRNA was quantitated on a spectrophotometer and run on a 1% TAE gel to check for an evenly distributed range of transcript sizes. 20 μg of cRNA was fragmented to approximately 35 to 200 bp by alkaline treatment (200mM Tris-acetate, pH 8.2, 500 mM KOAc, 150 mM MgOAc) and run on a 1% TAE gel to verify fragmentation. Separate hybridization cocktails are made using 15 μg of fragmented cRNA from each sample as per Affymetrix’s protocol. Two hundred μL of each cocktail was separately hybridized to an Affymetrix Rat Genome 230 2.0 Array for 16 hours at 45 °C in the Hybridization Oven 640. Arrays were washed on Affymetrix’s upgraded GeneChip Fluidics Station 450 using a primary streptavidin phycoerythrin (SAPE) stain, subsequent biotinylated antibody stain, and secondary SAPE stain. Arrays were scanned on Affymetrix’s GeneChip Scanner 3000 7G with AutoLoader. Scanned images obtained by the Affymetrix GeneChip Operating Software (GCOS) v1.2 were used to extract raw signal intensity values per probe set on the array and calculate detection calls as absent, marginal, or present. Assignment of detection calls was based on probe-pair intensities for which one probe was a perfect match of the reference sequence and the other was a mismatch probe for which the thirteenth base (of the 25 oligonucleotide reference sequence) was changed. Signals are calculated using the One-Step Tukey’s Biweight Estimate and all raw chip data was scaled in GCOS to 150 using MAS5.0 to normalize signal intensities for inter-array comparisons.
2.11 Statistical analyses
Since our interest was to determine whether each dose enhanced performance relative to the vehicle group, all of our two-group comparisons were planned. Uterine, serum, and brain analyses were run via t-tests set a priori. Because the group comparisons represented a priori planned contrasts, each comparison was evaluated using an alpha level of 0.05 except when noted otherwise (Keppel and Wickens, 2004, p. 115). For behavior assessments, for each dependent variable described above, data were analyzed separately for each maze with an omnibus repeated measures ANOVA with Treatment as the between variable, and Days and/or Trials as the within variable, as appropriate for the specific maze test.
Using Pearson r correlations, we correlated serum estrone and 17β-estradiol levels (pg/ml), and the ratio of estrone:17β-estradiol (estrone divided by 17β-estradiol), with the following measures: WRAM total errors for initial and latter phases, WRAM total errors on post-delay trials, MWM swim distance (cm), target quadrant percent distance (cm) on the MWM probe trial, DMS WM trial total errors on day 1-4, and DMS errors after the delay. We also correlated these serum hormone levels to each other to aid interpretation of hormone profiles.
Gene expression analysis consisted of two comparisons: (1) Ovx-Vehicle versus Ovx-Premarin-Low animals, and (2) Ovx-Vehicle versus Ovx-Premarin-High animals. Within each comparison, all samples in the comparison were first evaluated based on Affymetrix detection calls. For comparison 1, those genes demonstrating at least one present call out of a total of six (three Ovx-Vehicle animals and three Ovx-Premarin-Low animals) calls were extracted in order to remove genes that did not show measurable levels of expression across both sample groups. Similarly, for comparison 2, those genes demonstrating at least one present call out of a total of ten calls (three Ovx-Vehicle animals and seven Ovx-Premarin-High) were identified. Following this detection call filter, the average expression signals were calculated for each group in both comparisons; those genes that have both average expression signals less than 100 in a comparison are removed (e.g. if the Ovx-Vehicle average signal and the Ovx-Premarin-Low average signal for a gene are both less than 100 in comparison 1, the gene is removed) because changes at consistently low levels overlap with background. The ratio of average expression signals for each probe represents the fold change. The Student’s t-test (heteroscedastic, two-tailed) was used to calculate p-values for transcriptomic changes for each gene. MetaCore GeneGo software was used for pathway analysis of significant genes. This software takes an input list of genes and evaluates the genes against an annotated database of genes/proteins and maps, which represent known relationships between genes/proteins. The software database also incorporates Gene Ontology (GO) processes under which the input list of genes can also be organized. This tool supports the identification of processes and systems that are likely to be affected by changes in expression of specific genes. In this study, genes that demonstrate the most significant changes (p < 0.01) are input into the pathway analysis software to determine what mechanisms are likely affected by respective transcriptomic changes. For comparison 1, 962 genes were input into GeneGo, whereas for comparison 2, 120 genes were evaluated. The top ten processes for each comparison are listed in Table 2 and represent the processes most likely affected by changes in gene expression. The numerator of the ratio represents the number of genes from the input list that are contained in the process, while the denominator represents the total number of genes in the process.
Table II. Significant transcriptomic changes in the dorsal hippocampi (right hemisphere) of low and high Premarin dosed treatment groups.
Gene expression profiling was performed on dorsal hippocampi collected from the Ovx-Vehicle group, Ovx-Premarin-Low group, and Ovx-Premarin-High group. Comparative analyses led to the generation of multiple gene lists: (1) Ovx-Vehicle group versus Ovx-Premarin-Low group and (2) Ovx-Vehicle group versus Ovx-Premarin-High group. The statistically significant genes (P<0.01) demonstrating the greatest fold changes are shown.
| List 1: Ovx-Vehicle group versus Ovx-Premarin-Low group | ||||
|---|---|---|---|---|
| Probe Set ID | Gene Symbol | Gene Title | P-value | Fold |
| 1370751_at | LOC257642 | rRNA promoter binding protein | 4.61E-03 | 19.21 |
| 1375422_at | --- | ---- | 5.48E-03 | 14.07 |
| 1398594_at | --- | Transcribed locus | 9.88E-03 | 12.10 |
| 1375212_at | Ankrd52_pred | ankyrin repeat domain 52 (predicted) | 8.72E-03 | 8.75 |
| 1379101_at | Dhx36_pred | DEAH box polypeptide 36 (predicted) | 2.87E-03 | 6.32 |
| 1376707_at | C1qtnf4_pred | C1q and tumor necrosis factor related protein 4 (predicted) |
7.66E-05 | −2.53 |
| 1368465_at | Accn1 | amiloride-sensitive cation channel, neuronal (degenerin) |
8.38E-03 | −2.59 |
| 1368701_at | Atp1a3 | ATPase, Na+/K+ transporting, alpha 3 polypeptide |
4.71E-03 | −3.22 |
| 1371376_at | RGD1565596 | similar to Gene model 461 (predicted) | 6.50E-03 | −3.57 |
| 1385825_at | RGD1559930 | similar to mKIAA0256 protein (predicted) |
5.25E-03 | −3.69 |
| List 2: Ovx-Vehicle group versus Ovx-Premarin-High group | ||||
|---|---|---|---|---|
| Probe Set ID | Gene Symbol | Gene Title | P-value | Fold |
| 1377532_at | RGD1305020 | similar to Hepatocellularcarcinoma- associated antigen 58 homolog |
7.08E-05 | 1.83 |
| 1370454_at | Homer1 | homer homolog 1 (Drosophila) | 3.95E-03 | 1.73 |
| 1392319_at | RGD1564983 | similar to leucine rich repeat containing 10 (predicted) |
5.36E-03 | 1.57 |
| 1386975_at | Pdk2 | pyruvate dehydrogenase kinase, isoenzyme 2 |
8.82E-04 | 1.46 |
| 1390647_at | Phtf2_pred | putative homeodomain transcription factor 2 (predicted) |
2.90E-03 | 1.36 |
| 1392613_at | --- | Transcribed locus | 5.98E-03 | −1.29 |
| 1373092_at | --- | Transcribed locus | 5.84E-04 | −1.31 |
| 1391605_at | --- | Transcribed locus | 5.06E-03 | −1.34 |
| 1372702_at | PRP-2 | proline-rich protein | 9.12E-04 | −1.36 |
| 1375122_at | LOC690262 | similar to YY1-associated factor 2 | 4.52E-04 | −1.40 |
3. Results
3.1 Vaginal smears, uterine weights, pituitary weights and blood hormone levels
With the exception of one Ovx-Premarin treated rat, all Ovx-Premarin treated rats exhibited estrous vaginal smears that had many cornified cells. The animal with the non-estrous smear was excluded from the study. All Ovx-Vehicle rats showed continuous diestrus smears. Ovx-Premarin-Low, Ovx-Premarin-Medium and Ovx-Premarin-High treatments increased uterine weights relative to Ovx-Vehicle animals [Ovx-Vehicle vs Ovx-Premarin-Low t(16)=8.52; p < 0.0001; Ovx-Vehicle vs. Ovx-Premarin-Medium t(15)=7.29; p < 0.0001; Ovx-Vehicle vs Ovx-Premarin-High t(14)=8.77; p < 0.0001] (Figure 1a). Premarin also dose dependently influenced pituitary weights (Figure 1b). Pituitary weights in the Ovx-Premarin-High group were significantly elevated compared to the Ovx-Vehicle treated group [t(13)=3.05; p < 0.01].
Figure 1.
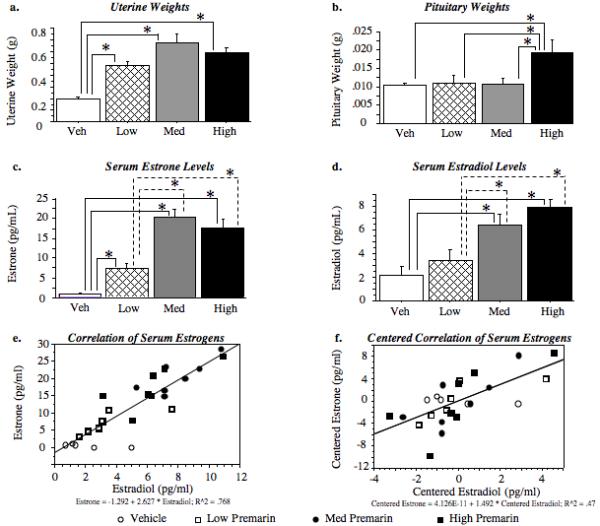
Physiologial measures of Premarin stimulation. a) Mean (±SEM) uterine weights (g). All doses of Premarin increased uterine weights relative to untreated controls (p < 0.0001). b) Mean (±SEM) pituitary weights (g). The highest dose of Premarin increased pituitary weights as compared to all other groups (p < 0.05). c) Mean (±SEM) serum estrone (pg/ml). All doses of Premarin increased serum estrone levels relative to controls (p < 0.05). Medium (dashed lines: p < 0.0005) and high (dashed lines: p < 0.01) Premarin doses produced significantly higher estrone levels than the low dose. d) Mean (±SEM) serum 17β-estradiol (pg/ml). Medium and high, but not low, dose Premarin increased serum 17β-estradiol as compared to vehicle controls (p < 0.05). Medium (dashed lines: p < 0.01) and high (dashed lines: p < 0 .05) Premarin doses produced significantly higher 17β-estradiol levels than the low dose. e) Scattergram of serum estrogens. 17β-estradiol and estrone were significantly correlated (r = 0.885, p < 0.0001). f) Centered scattergram of serum estrogens. The correlation remained significant after mean group differences were removed, suggesting that the significant correlation was not due to group membership (r = 0.710, p < 0.0001).
Premarin treatment dose-dependently increased circulating hormone levels. All doses of Premarin increased estrone levels as compared to vehicle controls [Ovx-Vehicle vs Ovx-Premarin-Low t(7)=3.23; p < 0.05; Ovx-Vehicle vs. Ovx-Premarin-Medium t(8)=6.92; p < 0.0001; Ovx-Vehicle vs. Ovx-Premarin-High t(8)=4.62; p < 0.005] (Figure 1c). Since there appeared to be differences between the Premarin-dosed groups, and knowing whether these groups differed would aid interpretation of the behavior findings, we performed post-hoc comparisons comparing the three Premarin doses to each other for estrone and 17β-estradiol. Both medium [Ovx-Premarin-Low vs Ovx-Premarin-Medium t(11)=5.66; p < 0.0001] and high [Ovx-Premarin-Low vs Ovx-Premarin-High t(11)=3.69; p < 0.0036] Premarin doses had higher estrone levels than low dose Premarin. Only the two higher doses significantly increased circulating 17β-estradiol as compared to Ovx animals [Ovx-Vehicle vs. Ovx-Premarin-Medium t(10)=5.54; p < 0.0005; Ovx-Vehicle vs. Ovx-Premarin-High t(10)=3.41; p < 0.0001] (Figure 1d). For estrone, both medium (Ovx-Premarin-Low vs Ovx-Premarin-Medium t(11)=4.11; p < 0.0017) and high (Ovx-Premarin-Low vs Ovx-Premarin-High t(11)=2.37; p < 0.037) Premarin doses elevated 17β-estradiol levels significantly more than low dose Premarin. Post-hoc comparisons indicated that Ovx-Premarin-Medium and Ovx-Premarin-High groups did not differ from each other for estrone or 17β-estradiol levels.
Estrone and 17β-estradiol levels were positively correlated when all animals were included [r (24) =.885, p < 0.0001] (Figure 1e), as well as when the correlation was run with only the three Premarin treated groups [r (19) =.894, p < 0.0001]. To ensure that this significant correlation was not attributable to group differences in estrone and 17β-estradiol levels due to the experimental manipulations, we centered the data by subtracting each animal’s score from the mean of the treatment group to which they belonged (Enders and Tofighi, 2007; Hallahan and Rosenthal, 2000). We then replaced the original serum hormone measures with the centered values in the correlation analyses. The correlation remained significant [r (24) =.710, p < 0.0001], suggesting that the relationship between estrone and 17β-estradiol was not being carried by group membership (Figure 1f).
3.2 Water radial arm maze
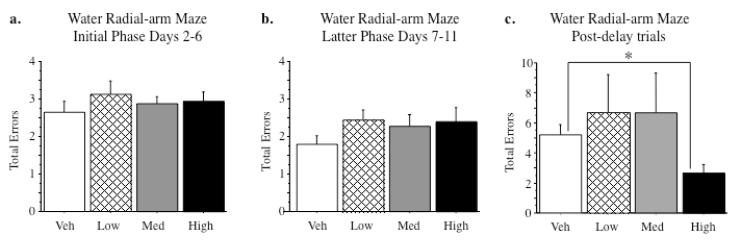
For the Initial (Figure 2a) and Latter (Figure 2b) phases, there were no Treatment main effects, nor were there interactions with Treatment for any variable (WMC, RM, WMI, Total Errors). On day 12, after all animals had been trained on the task, a four hour delay was instilled between trials 2 and 3, placing a higher memory demand for trials 3 and 4. The Ovx-Premarin-High group exhibited better performance than the Ovx-Vehicle group for total errors committed across the post-delay trials [t(16)=2.86; p < 0.05] (Figure 2c). No other Premarin treated group differed in post-delay performance compared to animals receiving vehicle.
Figure 2.
a) Mean (±SEM) number of errors for total errors (WMC, WMI, and RM combined) on the initial phase (D2-6) of the WRAM. No dose of Premarin affected performance. b) Mean (±SEM) number of errors for total errors on the latter phase (D7-11) of the WRAM. No dose of Premarin affected performance. c) Mean (±SEM) number of total errors on WRAM post delay trials, after a four hour delay was imposed between trials two and three. High-dose Premarin animals made fewer errors than Vehicle-treated animals on the post-delay trials (p < 0.05).
3.3 Morris water maze
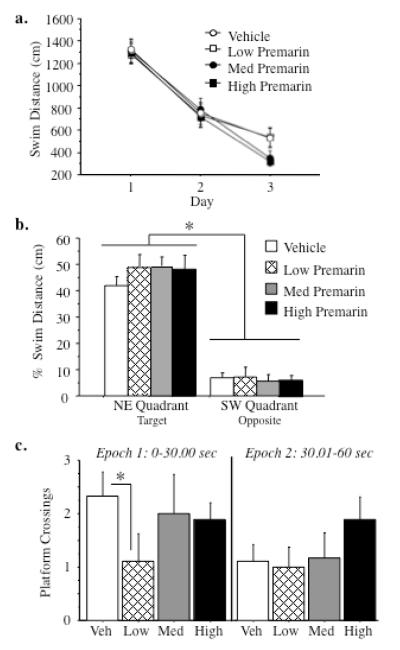
Figure 3a shows the mean distance scores±SE for each treatment group across the three days of MWM testing. Premarin treatment did not alter overall spatial reference memory performance; there was no main effect of Treatment, nor did Treatment interact with Days or Trials for any comparison. For the probe trial, there was a Quadrant main effect for each Ovx-Premarin-treated vs. Ovx-Vehicle comparison, with a higher percent distance spent in the target quadrant versus the opposite quadrant [Ovx-Vehicle vs Ovx-Premarin-Low: F(1,1)=81.79; p < 0.0001; Ovx-Vehicle vs. Ovx-Premarin-Medium: F(1,1)=180.42; p < 0.0001; Ovx-Vehicle vs Ovx-Premarin-High: F(1,1)=109.87; p < 0.0001] (Figure 3b), thereby indicating that all groups, regardless of hormone status, localized the platform location by the end of testing.
Figure 3.
a) Mean (±SEM) swim distance (cm) during Morris maze testing. There were no differences between Premarin- and vehicle- treated groups on learning across days. b) Mean (±SEM) probe trial percent distance in the target and opposite quadrants. All animals, regardless of treatment, swam a higher percent distance in the quadrant where the platform had been previously located, indicating that all animals localized the platform location (p < 0.0001). c) Mean (±SEM) platform crossings on the probe trial. During the first 30 seconds of the probe trial, animals given low dose Premarin made fewer platform crossings than those given vehicle (p<0.01). During the second 30 seconds, there were no group differences in number of platform crossings.
The frequency of platform crossings was dose dependently influenced by Premarin treatment. Ovx-Premarin-Low treated rats made fewer platform crossings than the Ovx-Vehicle controls [Treatment main effect: (15)=2.36; p < 0.05], suggesting that Ovx-Premarin-Low animals were less able to localize the platform location on the probe trial. However, neither the Ovx-Premarin-Medium nor High groups differed from Ovx-Vehicle controls in number of platform crossings. To assess potential changes in platform search strategy across the 60 sec probe trial, we divided the probe trial into two 30 sec epochs and assessed the frequency of platform crossings across these epochs (Figure 3c). A significant Treatment × Epoch interaction was found between the Ovx-Vehicle and Ovx-Premarin-Low groups [F(1,15)=6.54; p <0 .05]. For the first 30 sec epoch, Ovx-Premarin-Low rats made fewer platform crossings than Ovx-Vehicle rats [t(15)=3.42; p < 0.01]; there were no group differences on the second 30 sec epoch. Neither the Ovx-Premarin-Medium nor the Ovx-Premarin-High animals differed from the Ovx-Vehicle animals during the first or second 30 sec epochs. There were no significant comparisons between any Premarin-treated group and vehicle group for swim speed during the total 60 second probe trial, nor for the first or second 30 sec epoch analyzed separately.
3.4 Delayed match-to-sample plus maze
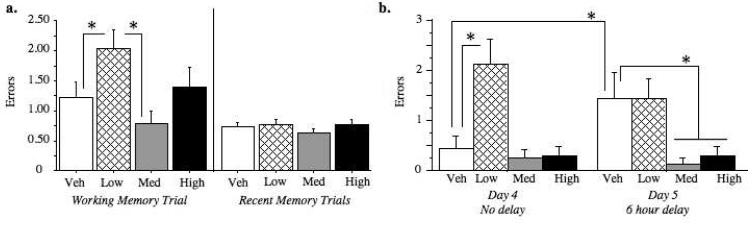
For the working memory test trial (trial 2) collapsed across acquisition days (days 1-4), the Ovx-Premarin-Low group made more errors than the Ovx-Vehicle group [Treatment main effect: F(1,16)=6.24; p < 0.05] (Figure 4a). There were no group differences between the Ovx-Vehicle group and either of the two higher Premarin dose groups for the working memory trial. For recent memory trials (trials 3-6), there was a Treatment × Day interaction for the Ovx-Vehicle and Ovx-Premarin-Low comparison [F(1,3)=4.67, p < 0.01] (Figure 4a), an effect due to Ovx-Premarin-Low animals committing more errors than Ovx-Vehicle animals on day 3 [Treatment effect for Day 3: F(1,16)= 5.23; p < 0.05], but not on days 1, 2, or 4.
Figure 4.
a) Mean (±SEM) total errors during DMS testing. Animals given low dose Premarin made more working memory errors than those given vehicle or medium dose Premarin (ps < 0.05). There were no group differences for recent memory. b) Mean (±SEM) errors on trial two of the baseline day (day 4) and on trial two after the six hour delay (day 5). Animals given low dose Premarin were impaired on day 4 compared to those given vehicle (p < 0.01), and thus were not further impaired by the added challenge of a delay (day 4 compared to day 5, p > 0.36). While all Premarin groups were unchanged from day 4 to day 5 suggested they were not affected by the delay, the Ovx-Vehicle group was significantly impaired after the delay (p < 0.05). On the post-delay trials, the combined Medium and High group made significantly fewer errors compared to the Vehicle group (p<0.01).
For the 6 hour delay, Ovx-Premarin-Medium rats committed fewer errors after the delay than Ovx-Vehicle rats [t(15)=2.41; p < 0.05], and Ovx-Premarin-High rats made somewhat fewer errors than Ovx-Vehicle rats, with a marginal effect [t(14)=1.94; p = 0.07; Figure 4b]. When animals receiving the two highest Premarin doses were combined, this Ovx-Premarin-Medium+High group committed significantly fewer errors on the test trial after the 6-hour delay compared to the Ovx-Vehicle group [t(22)=3.05; p < 0.01] (Figure 4b). When we evaluated baseline performance (trial 2 of day 4, the day before the delay) relative to post-delay performance (trial 2 of day 5) to determine which groups were affected by the delay, it became clear that low-dose Premarin treatment impaired performance independent of the delay. Indeed, on this baseline day Ovx-Premarin-Low rats made more Total errors than Ovx-Vehicle rats [t(16)=2.94; p < 0.01] and performance of the Ovx-Premarin-Low group did not change with the delay (pre-delay Total errors on trial 2 vs. post-delay Total errors on trial 2: p > .36). In contrast, Ovx-Vehicle rats were impaired by the delay [pre-delay Total errors on trial 2 vs. post-delay Total errors on trial 2: F(1,8)=6.00; p < 0.05], while the delay did not impair performance in Ovx-Premarin-Medium and -High rats (ps > .59).
3.5 Circulating estrogens correlate with maze performance
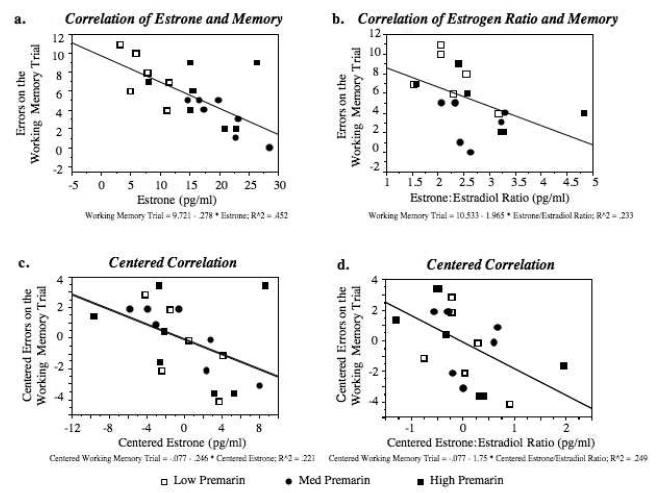
Premarin-treated animals that had higher levels of estrone [r = −0.672, p = .0008](Figure 5a), or a higher estrone:17β-estradiol ratio [r = −0.483, p = .03] (Figure 5b), tended to make fewer errors on the working memory trial of the initial block of DMS testing. These significant correlations were not due to mean differences between treatment groups, as correlations each held up after data were centered to obviate effects of group membership [r = −0.470, p = .04; r = −0.499, p = .023, respectively](Figure 5c and 5d). There were no other significant relationships between estrone, 17β-estradiol and maze performance.
Figure 5.
a) Estrone levels were negatively correlated with DMS errors on the working memory trial (r = −0.672, p < 0.001), indicating that animals with higher estrone levels tended to exhibit better working memory scores. b) The ratio of estrone:17β-estradiol was also negatively correlated with DMS performance on the working memory trial. c) Centered scattergram of estrone and memory. The correlation of estrone and total DMS errors committed on the working memory trial remained significant after mean group differences were removed, indicating that the significant correlation was not due to group differences in hormone levels or maze scores. (r = −0.470, p < 0.05). d) Centered scattergram of the estrogen ratio and memory. The correlation of the estrone:17β-estradiol ratio and total DMS errors committed on the working memory trial remained significant after mean group differences were removed, suggesting that the significant correlation was not due to group membership (r = −0.499, p < 0.05)
3.6 Neurotrophin levels
The effect of Premarin treatment on neurotrophins was assessed in cingulate cortex, frontal cortex, entorhinal cortex, dorsal and ventral hippocampus, perirhinal cortex and temporal cortex (Table I). All doses of Premarin significantly increased BDNF in the cingulate cortex compared to vehicle [Ovx-Vehicle vs. Ovx-Premarin-Low t(16)=2.32; p < 0.05; Ovx-Vehicle vs. Ovx-Premarin-Medium t(15)=2.39; p < 0.05; Ovx-Vehicle vs. Ovx-Premarin-High t(14)=2.36; p < 0.05]. Similarly, the two highest Premarin doses increased NGF in the cingulate cortex; Ovx-Premarin-Medium and Ovx-Premarin-High groups had higher NGF protein levels in cingulate cortex as compared to the Ovx-Vehicle group [Ovx-Vehicle vs. Ovx-Premarin-Medium t(15)=3.80; p < 0.01; Ovx-Vehicle vs Ovx-Premarin-High t(14)=4.00; p < 0.01]. Premarin-High treatment, but no other dose, significantly decreased BDNF levels in the perirhinal cortex compared to vehicle controls [t(14)= 2.20; p< 0.05]. No dose of Premarin altered perirhinal NGF levels, or BDNF or NGF levels in frontal cortex, temporal cortex, entorhinal cortex, dorsal hippocampus or ventral hippocampus.
Table I.
NGF and BDNF protein levels for each treatment group, in each brain region analyzed.
| BDNF (pg/ml; mean±standard error) |
NGF (pg/ml; mean±standard error) |
|
|---|---|---|
|
Cingulate
Gyrus |

|

|
|
Frontal
Cortex |
Ovx-Vehicle = 0.97±0.10 Ovx-Prem-Low = 1.05±0.09 Ovx-Prem-Med= 1.01±0.09 Ovx-Prem-High= 0.98±0.11 |
Ovx-Vehicle = 0.72±0.06 Ovx-Prem-Low = 0.78±0.05 Ovx-Prem-Med= 0.74±0.04 Ovx-Prem-High= 0.69±0.05 |
|
Entorhinal
Cortex |
Ovx-Vehicle = 1.88±0.23 Ovx-Prem-Low = 1.67±0.17 Ovx-Prem-Med= 2.17±0.39 Ovx-Prem-High= 1.80±0.23 |
Ovx-Vehicle = 0.89±0.15 Ovx-Prem-Low = 0.71±0.06 Ovx-Prem-Med= 0.88±0.08 Ovx-Prem-High=0.73±0.08 |
|
Dorsal
Hippo |
Ovx-Vehicle = 4.93±0.85 Ovx-Prem-Low = 5.44±0.80 Ovx-Prem-Med= 5.26±0.56 Ovx-Prem-High= 5.26±0.83 |
Ovx-Vehicle = 2.50±0.27 Ovx-Prem-Low = 2.98±0.34 Ovx-Prem-Med= 3.11±0.32 Ovx-Prem-High= 2.71±0.31 |
|
Ventral
Hippo |
Ovx-Vehicle = 1.64±0.19 Ovx-Prem-Low = 1.94±0.12 Ovx-Prem-Med= 1.93±0.22 Ovx-Prem-High= 1.66±0.12 |
Ovx-Vehicle = 1.12±0.13 Ovx-Prem-Low = 1.44±0.14 Ovx-Prem-Med= 1.44±0.13 Ovx-Prem-High= 1.27±0.10 |
|
Perirhinal
Cortex |

|
Ovx-Vehicle = 1.15±0.14 Ovx-Prem-Low = 1.13±0.22 Ovx-Prem-Med= 0.88±0.12 Ovx-Prem-High= 0.92±0.12 |
|
Temporal
Cortex |
Ovx-Vehicle = 0.34±0.11 Ovx-Prem-Low = 0.25±0.04 Ovx-Prem-Med= 0.26±0.02 Ovx-Prem-High= 0.25±0.04 |
Ovx-Vehicle = 0.58±0.06 Ovx-Prem-Low = 0.56±0.08 Ovx-Prem-Med= 0.54±0.05 Ovx-Prem-High= 0.57±0.05 |
P < 0.05, significantly different from Ovx-Premarin-Vehicle
P < 0.01, significantly different from Ovx-Premarin-Vehicle
3.7 Gene expression
Expression profiling analysis of dorsal hippocampi led to identification of genes that were differentially expressed across treatment groups. In a comparison of the Ovx-Vehicle and the Ovx-Premarin-Low groups, 962 genes demonstrated statistically significant (p < 0.01) changes, whereas in a comparison of the Ovx-Vehicle and the Ovx-Premarin-High groups, only 120 genes showed significant (p < 0.01) changes. Top genes are shown in Table II. For the Ovx-Premarin-Low group, primarily uncharacterized or predicted genes demonstrated the greatest changes (Table II: List 1). For the Ovx-Premarin-High group, top genes (p < 0.01, greatest folds) include Homer1 (homer homolog 1), Pdk2 (pyruvate dehydrogenase kinase, isoenzyme 2), and Prkd2 (protein kinase D2) (Table II: List 2). MetaCore GeneGo pathway analysis of significant genes also pinpointed cellular processes that may be affected by transcriptomic changes. The top 10 processes for each comparative analysis are listed in Table III.
Table III. Genego pathway analysis of significant genes.
Metacore Genego pathway analysis was performed on significant (P<0.01) genes from the following comparisons: Ovx-Vehicle group versus Ovx-Premarin-Low group & Ovx-Vehicle group versus Ovx-Premarin-High group. The top ten processes identified for each comparison are listed. ‘Ratio’ represents the number of genes from the significant list compared to the total number of genes in the process database.
| Ovx-Vehicle group versus Ovx-Premarin-Low group | ||
|---|---|---|
| Process | Ratio | P-value |
| Cellular component organization & biogenesis | 255/3593 | 5.50E-13 |
| Protein metabolic process | 271/3921 | 1.48E-12 |
| Transport | 220/3124 | 1.28E-10 |
| Synaptic transmission | 62/557 | 4.12E-10 |
| Translation | 64/595 | 8.69E-10 |
| Establishment of localization | 224/3262 | 8.82E-10 |
| Cellular protein metabolic process | 242/3602 | 1.04E-09 |
| Macromolecule localization | 97/1092 | 1.46E-09 |
| Protein localization | 94/1046 | 1.50E-09 |
| Cellular macromolecule metabolic process | 243/3660 | 3.01E-09 |
| Ovx-Vehicle group versus Ovx-Premarin-Highgroup | ||
|---|---|---|
| Process | Ratio | P-value |
| Behavioral fear response | 4/25 | 1.05E-05 |
| Behavioral defense response | 4/25 | 1.05E-05 |
| Detection of abiotic stimulus | 6/93 | 1.31E-05 |
| Fear response | 4/31 | 2.54E-05 |
| Negative regulation of glucose import | 3/11 | 2.68E-05 |
| Locomotory behavior | 9/317 | 6.70E-05 |
| Regulation of synaptic transmission | 5/81 | 8.83E-05 |
| Regulation of transmission of nerve impulse | 5/86 | 1.17E-04 |
| Intracellular signaling cascade | 24/1966 | 1.28E-04 |
| Negative regulation of protein catabolic process | 3/20 | 1.79E-04 |
4. Discussion
The current study is the first to evaluate tonic Premarin treatment for memory and potentially associated brain variables using the rodent model. Here, we demonstrate that tonic Premarin treatment affects memory, neurotrophin protein and gene expression in the middle-aged Ovx rat. Confirming peripheral endocrine responsiveness, all three Premarin doses resulted in positive estrous vaginal smears and increased uterine weights, and the highest Premarin dose increased pituitary weights. There were also dose-related increases in serum estrone and 17β-estradiol levels. These levels were within low physiological range for ovary-intact young and middle-aged rodents (Lerner et al., 1990; Page and Butcher, 1982). Accordingly, estrone and 17β-estradiol levels increased to the low physiological range in women after 0.625 and 1.25 mg/tablet daily oral Premarin treatment (O’Connell, 1995). Premarin is largely estrone sulfate, which gets converted to estrone, and then to 17β-estradiol. Therefore, the Premarin-induced elevations in 17β-estradiol correspond with the expected sequence of steroid conversion, even though Premarin itself contains only trace amounts of 17β-estradiol (Kronenberg et al., 2008). In the current study, circulating estrone levels significantly increased with low-, medium- or high- dose Premarin treatment, while 17β-estradiol levels significantly increased only after the medium- and high- dose treatments. Further, the medium- and high- dose regimens resulted in estrone and 17β-estradiol levels that were significantly higher than the low-dose regimen. This suggests that the ratio of these two estrogens varies with Premarin dose, and provides a dissociation of hormone profiles of the subjects in this study. This affords us the opportunity to evaluate whether the ratio of estrone:17β-estradiol correlates with the assessed cognitive and brain variables.
The dose-dependent cognitive effects of Premarin in this study are likely related to the resulting circulating hormone levels. Relative to vehicle, the lowest dose tested, 12 μg daily, impaired spatial learning on two maze tasks, even though the tasks evaluated different types of spatial memory. Specifically, low-dose Premarin treatment impaired learning the MWM (spatial reference memory) and the DMS plus maze (spatial working memory), but had no effect on the WRAM (spatial working and reference memory). These effects are especially noteworthy given that the low-dose Premarin regimen was the only treatment that did not elevate circulating 17β-estradiol levels relative to Ovx-Vehicle animals, while it did increase estrone (Figure 1). These estrone levels were very low physiological (Lerner et al., 1990; Page and Butcher, 1982); significantly lower than those resulting from the medium- or high- doses of Premarin given in the current study. It is therefore plausible that these low circulating estrone levels, in the presence of very low 17β-estradiol levels (comparable to that of Ovx animals), impairs performance on tasks that assess only reference memory place learning, or only working memory place learning, but not a task that is more complex such as the WRAM. This more difficult WRAM task likely challenged the Ovx group more so than the tasks solely testing reference or working memory, resulting in less discrimination of low-dose Premarin effects. The positive correlation we found between performance on the DMS working memory trial and estrone, and with the estrone:17β-estradiol ratio, further supports this tenet. Indeed, as levels of estrone increased, and the ratio of estrone:17β-estradiol increased, animals tended to show better working memory performance on this measure. Taken together with the dissociation of dose-specific estrogenic profiles, results suggest that higher levels of estrone, in the presence of 17β-estradiol concentrations higher than that of Ovx levels, are beneficial for memory. To our knowledge there is no animal study evaluating the cognitive effects of circulating estrone levels, neither endogenous nor exogenous after hormone treatment, although some work has been done in humans in this regard. Higher circulating estrone and 17β-estradiol levels both correlated with better verbal recall scores in oopherectomized women given estrogen-containing hormone therapy (Phillips and Sherwin, 1992), and several cognitive measures improved after estrogen or estrogen-androgen therapy in oopherectomized women, concordant with increases in circulating estrone and 17β-estradiol levels (Sherwin, 1988). Other studies have correlated estrone and 17β-estradiol with cognitive measures in menopausal women that have not been given estrogen therapy. Findings range from higher estrone or 17β-estradiol levels corresponding to better cognitive scores or a lower frequency of mild cognitive impairment (Lebrun et al., 2005; Wolf and Kirschbaum, 2002) to no correlation (Almeida et al., 2005), to higher 17β-estradiol levels corresponding to worse cognitive scores (Barrett-Connor and Goodman-Gruen, 1999). Interestingly, in the latter study, higher endogenous estrone levels were marginally related to better performance on a verbal memory test in menopausal women not on hormone therapy (p=.07, Barrett-Connor and Goodman-Gruen, 1999, p. 1291). While these studies provide support that circulating estrone and 17β-estradiol levels relate to cognition, there has been no methodical assessment of whether the balance between estrone and 17β-estradiol impacts the direction or efficacy of estrone’s cognitive effects. 17β-estradiol’s presence should be presumed when referring to estrone, and vice versa, due to interconversion of these two estrogens by oxidoreductase 17β-hydroxysteroid dehydrogenase (Khan et al., 2004). Thus, it could be argued one can truly not dissociate them. However, this does not preclude estrogenic effects on brain functions due to: (1) total steroid level and/or (2) the balance of estrone and 17β-estradiol.
In vitro, Premarin induced neuroprotection against β-amyloid, hydrogen peroxide and glutamate-induced toxicity in neurons derived from cognitive brain regions including the hippocampus, basal forebrain and cortex; in several cases Premarin was effective at multiple doses (Brinton et al., 2000). Other in vitro work showed that high nanomolar to micromolar estrone concentrations exerted dose-dependent neuroprotective effects on cultured neurons (Bae et al., 2000; Green et al., 1997; Regan and Guo, 1997; Zhao and Brinton, 2006; Brinton et al., 1997), although other components of Premarin were more effective than estrone (Brinton et al., 1997). We do not know the physiological concentrations of estrone or the estrone:17β-estradiol ratio in the brains of our animals, or how the effects may be distributed across various brain networks mediating our effects. However, collectively, the findings suggest that estrone can exert neurotrophic properties and neuroprotection (Prokai and Simpkins, 2007), which could translate to enhanced brain function, at least in the presence of 17β-estradiol concentrations that are higher than Ovx levels.
Using 17β-estradiol treatment, dose dependent mnemonic effects are shown in rodent studies. High physiological 17β-estradiol levels enhanced learning a place strategy on a plus maze in young rats (Korol and Kolo, 2002) and on the MWM in young and middle-aged rats (Talboom et al., 2008). The WRAM, MWM, and DMS tests used in the current study were spatial tasks. While no Premarin dose used in this study enhanced learning of these tasks, it is possible that a higher Premarin dose would have resulted in higher 17β-estradiol levels that could have improved spatial task acquisition. Indeed, the 17β-estradiol levels resulting from even our highest Premarin dose treatment were low physiological. The spatial learning enhancements noted previously were seen with 17β-estradiol levels in the higher physiological range (Korol and Kolo, 2002; Talboom et al., 2008). In the current study, the two highest Premarin doses tested, 24 and 36 μg daily, enhanced memory retention when subjected to an extended temporal challenge. Specifically, high-dose Premarin treatment improved retention of numerous items of information across a 4-hour delay on the WRAM, and the two highest Premarin doses enhanced 6-hour retention of one item of information on the DMS task. Both of these tasks require working- or short- term memory. There was no effect of an overnight delay on the spatial reference memory MWM, suggesting that tonic Premarin does not influence overnight forgetting. These findings suggest that the memory enhancing effects of Premarin are task specific and moreover, require a mnemonic challenge across hours to be manifested. Our findings that the two highest doses of Premarin improved memory retention correspond with other studies showing that a higher 17β-estradiol dose may be necessary to enhance memory, especially in rats approaching old age. Specifically, we have shown that higher circulating 17β-estradiol replacement levels correlate with better spatial reference memory in young and middle-aged Ovx rodents (Talboom et al., 2008). The necessity of a higher dose during aging may be especially poignant for memory retention (Foster et al., 2003). While findings are not yet reconciled, studies report that higher levels of 17β-estradiol given via daily injection (Holmes et al., 2002), or an intermediate, but not high, 17β-estradiol dose given via drinking water (Fernandez and Frick, 2004), impairs spatial maze performance. Also of note, while it is hypothesized that 17β-estradiol and estrone are two Premarin components largely responsible for the estrogenic effects of Premarin (Sitruk-Ware, 2002), there are other estrogens and metabolites present in Premarin that could alter efficacy of estrone effects and/or initiate effects on their own. Thus, although we found correlations between memory performance and estrone, and the estrone:17β-estradiol ratio, cognitive effects due to Premarin treatment could also be related to metabolites of Premarin such as delta 8,9 dehydroestrone, dihydroequilin-17β or equilin (Kuhl, 2005).
Many factors other than dose likely also play a role in estrogenic effectiveness on memory and the brain. The amount of time between hormone loss and subsequent treatment likely impacts efficacy of estrogenic therapy. Women who participated in the WHIMS were between 65-79 years old, and many had experienced ovarian hormone deprivation for a substantial amount of time before receiving Premarin-containing treatment (Shumaker et al., 1998). In the rodent, 17β-estradiol replacement initiated immediately after Ovx enhanced spatial memory performance in middle-aged rats, but imparted no benefit when given 5 months after Ovx (Daniel et al., 2006). Age-related changes in responsiveness may also influence the effectiveness of estrogen treatment. Aged Ovx rats were not responsive to the 17β-estradiol replacement regimen that was effective in young and middle-aged Ovx rats (Talboom et al., 2008), concurring with Age × 17β-estradiol replacement interactions for spatial memory shown by others (Foster et al., 2003). The current study controlled for these factors since time after Ovx and age were constant for all groups. However, whether Premarin-induced memory enhancements would have completely reversed any observed age-related memory retention decrements cannot be determined from the current experiment, as young animals were not assessed for comparison. Future studies incorporating this comparison group would be helpful in determining extent of Premarin-induced improvements during aging.
Interestingly, we have previously shown in middle-aged Ovx rats that 10 μg of Premarin, given via two injections 24 hours apart, followed by 48 hours without injection, enhanced learning of the DMS task used in the current study (Acosta et al., 2009). In contrast, the 12 μg tonic Premarin dose used herein impaired performance on this same measure. Intermittent cyclic vs. tonic regimens are a plausible explanation for the difference in findings. Differences in estrogen receptor expression, with cyclic estrogen treatment facilitating estrogen receptor recycling, and tonic estrogen treatment down-regulating estrogen receptors, indicate divergent neural mechanisms of action for cyclic and tonic administration that likely impact learning and memory changes (Blaustein, 1993; Brown et al., 1996; Kassis and Gorski, 1981; Rosser et al., 1993). There are age-related alterations in the number and activity of estrogen receptors, which could influence responsivity as aging ensues (Chakraborty and Gore, 2004). The animals in our current and prior (Acosta et al., in 2009) Premarin studies were middle-aged. Data suggest an estrogen-receptor dependent mechanism of 17β-estradiol-induced benefits on spatial memory (Zurkovsky et al., 2006). Thus, changes in estrogen receptors with age and with type of estrogen regimen could influence responsiveness to estrogen for spatial memory. Further, for 17β-estradiol, tonic treatment only enhanced memory when cyclic treatment was initiated first in older Ovx rats (Markowska and Savonenko, 2002).
In addition to Premarin-induced dose-dependent effects on cognition, we found dose-dependent effects on neurotrophin protein levels in the cingulate and perirhinal cortices. In the cingulate cortex, all Premarin doses increased BDNF, while only the two highest doses increased NGF. In the perirhinal cortex, only the highest Premarin dose affected neurotrophin levels, decreasing BDNF. BDNF and NGF proteins are implicated in learning and memory (Backman et al., 1996; Fischer et al., 1987; Frick et al., 1997; Mizuno et al., 2000. Scali et al., 1994). Neurotrophins may play a role in estrogenic-induced memory changes, as indicated by the current study using Premarin and prior studies using 17β-estradiol. 17β-estradiol replacement increased BDNF and NGF proteins in the entorhinal cortex in aged Ovx rats (Bimonte-Nelson et al., 2004) and increased levels of TrkA, the high-affinity neurotrophin receptor, in the basal forebrain (McMillan et al., 1996; Singer et al., 1998). The neurotrophin findings in the current study also implicate the cingulate gyrus and perirhinal cortex as potential sites of action for Premarin treatment. These brain regions play critical roles for cognition in rodents, including for spatial and object memory (Bachevalier and Nemanic, 2008; Cain et al., 2006; Ennacuer et al., 1997; Lee et al., 2006; Lukoyanov et al., 2005; Ramos, 2008), and in humans as shown for spatial tasks (Kindermann et al., 2004; Moffat et al., 2006) and for degenerative changes with Alzheimer’s disease (Hirono et al., 1998; Liang et al., 2008; Reiman et al., 2004). It is currently unknown how or whether Premarin-induced growth factor changes are related to the altered memory functions seen after treatment. Hypotheses set forth include compensatory relational changes in the hippocampal/basal forebrain retrograde transport system, which could account for upregulation in some brain regions, but downregulation in others (Granholm, 2000).
In the current report, gene expression profiling of dorsal hippocampus identified mechanisms possibly involved with Premarin-induced memory changes. The dorsal hippocampus was chosen as the region of analysis since it has well-known links with learning and memory, especially regarding spatial navigation (Morris et al., 1982; Jarrard, 1993). While implications of gene expression changes identified in the current study have yet to be determined, the genes listed in Table I represent molecular clues about the processes relating to Premarin-effects on the rat hippocampus. Of these, it is noted that high-dose Premarin treatment increased Homer1 expression. Homer1, which binds metabotropic glutamate receptors (Brakeman et al., 1997), is particularly interesting due to its previously implicated role in memory functions (Jaubert et al., 2007; Lominac et al., 2005; Szumlinski et al., 2005). Since this study is the first to assess Premarin effects on gene expression in the brain after cognitive testing, it is recognized that further studies are necessary to distinguish those transcripts that may be altered by Premarin treatment alone, versus those transcripts that are regulated by a physiological cascade of the improved memory due to Premarin treatment.
In conclusion, this is the first study testing tonic Premarin, the estrogen component of the most commonly utilized hormone therapy given to women since 1942, on a cognitive battery in an animal model. We found that Premarin can affect cognition, with divergent effects depending on dose. In middle-aged Ovx rats, Premarin enhanced memory retention on two tasks at higher doses. Low-dose Premarin impaired some aspects of performance, specific to spatial platform localization and learning a working memory task, but had no effect on memory retention. Premarin-induced cognitive changes may relate to the ratio of estrone to 17β-estradiol, with higher levels associated with better performance, although it is recognized that other components of Premarin could account for Premarin-induced memory changes. Gene expression profiling identified Premarin-associated transcriptomic changes, which likely includes Homer1, and provides a foundation for delineating the molecular processes affected by Premarin. These findings suggest that Premarin can impact memory and the brain, and that dosing should be recognized as a clinically relevant factor possibly affecting the direction and efficacy of cognitive outcome.
Acknowledgements
This research was funded by grants awarded to HAB-N from the National Institute on Aging (MGS 0126), Evelyn F. McKnight Brain Research Foundation, state of Arizona, ADHS, Alzheimer’s Disease Core Center Pilot grant program and the Institute for Mental Health Research. We wish to express our sincerest appreciation to Cynthia Zay, Ian Crain, Blair Braden, and Lisa Castillo for excellent technical assistance. We are also grateful to Dr. Roberta Diaz-Brinton and Dr. Jon Valla for valuable discussion regarding the in vitro literature. The authors have no commercial, or other, relationship with Wyeth Pharmaceuticals.
Footnotes
Disclosure Statement for Authors: There are no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta J, Mayer L, Talboom J,S, Zay C, Scheldrup M, Castillo J, Demers L,M, Enders C,K, Bimonte-Nelson H,A. Premarin enhances memory and prevents scopolamine induced amnesia in middle aged surgically menopausal rats. Hormones and Behavior. 2009;55(3):454–464. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida O,P, Lautenschlager N, Vasikaram S, Leedman P, Flicker L. Association between physiological serum concentration of estrogen and the mental health of community-dwelling postmenopausal women age 70 years and over. American Journal of Geriatric Psychiatry. 2005;13:142–149. doi: 10.1176/appi.ajgp.13.2.142. [DOI] [PubMed] [Google Scholar]
- Aenlle K,K, Kumar A, Cui L, Jackson T,C, Foster T,C. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.09.004. doi:1016/j.neurobioaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differentially processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Backman C, Rose G,M, Hoffer B,J, Henry M,A, Bartus R,T, Friden P, Granholm A. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. Journal of Neuroscience. 1996;16(17):5437–5442. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. Journal of the American Geriatrics Society. 1999;47(11):1289–1293. doi: 10.1111/j.1532-5415.1999.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Basu G,D, Liang W,S, Stephan D,A, Wegner L,T, Conley C,R, Mukherjee P. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Research. 2006;8(6):R69. doi: 10.1186/bcr1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y, Hwang J, Kim Y, Koh J. Anti-oxidative neuroprotection by estrogens in mouse cortical cultures. Journal of Korean Medical Science. 2000;15:327–336. doi: 10.3346/jkms.2000.15.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavnani BR. Pharmacokinetics and pharmacodynamics of conjugated equine estrogens: chemistry and metabolism. Proceedings of the Society of Experimental Biology and Medicine. 1998;217(1):6–16. doi: 10.3181/00379727-217-44199. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer’s. Journal of Steroid Biochemistry and Molecular Biology. 2003;85:473–482. doi: 10.1016/s0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiology and Behavior. 2000;68(4):495–499. doi: 10.1016/s0031-9384(99)00201-2. [DOI] [PubMed] [Google Scholar]
- Bimonte H,A, Granholm A,C, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neuroscience Letters. 2002;328(1):50–54. doi: 10.1016/s0304-3940(02)00442-1. [DOI] [PubMed] [Google Scholar]
- Bimonte H,A, Hyde L,A, Hoplight B,J, Denenberg V,H. In two species, females exhibit superior working memory and inferior reference memory on the water radial arm maze. Physiology and Behavior. 2000;70(3-4):311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bimonte H,A, Nelson M,E, Granholm A,C. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiology of Aging. 2003;24(1):37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson H,A, Hunter C,L, Nelson. M,E, Granholm A,E. Frontal cortex BDNF levels correlate with working memory in an animal model of Down sydrome. Behavioral Brain Research. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson H,A, Nelson M,E, Granholm A,E. Progesterone counteracts estrogen-induced increased in neurotrophins in the aged female rat brain. Neurorepor. 2003;15(17):2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson H,A, Singleton R,S, Nelson M,E, Eckman C,B, Barber. J, Scott., Granholm A,E. Testosterone, but not nonaromatizable dihydrotestosterone improves working memory and alters nerve growth factor levels in aged male rats. Experimental Neurology. 2003;181:301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. European Journal of Neuroscience. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson H,A, Granholm A,C, Nelson M,E, Moore A,B. Neurotrophin proteins levels in male and female Fischer 344 rats from adulthood to senescence: how young is young and how old is old? Experimental Aging Research. 2008;34(1):13–26. doi: 10.1080/03610730701761908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson H,A, Nelson M,E, Granholm A,E. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15(17):2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Blaustein J,D. Estrogen receptor immunoreactivity in rat brain: rapid effects of estradiol injection. Endocrinology. 1993;132(3):1218–1224. doi: 10.1210/endo.132.3.7679973. [DOI] [PubMed] [Google Scholar]
- Brakeman P,R, Lanahan A,A, O’Brien R, Roche K, Barnes C,A, Hunganir R,L, Worley P,F. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386(6622):284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J. The estrogen replacement therapy of the Women’s Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer’s disease. Maturitas. 2000;34:S35–52. doi: 10.1016/s0378-5122(00)00107-9. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu H. The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiology of Aging. 2000;21:475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Proffitt P, Tran J, Luu R. Equilin, a principal component of the estrogen replacement therapy premarin, increases the growth of cortical neurons via an NMDA receptor-dependent mechanism. Experimental Neurology. 1997;147:211–220. doi: 10.1006/exnr.1997.6619. [DOI] [PubMed] [Google Scholar]
- Brown T,J, Yu J, Gagnon M, Sharma M, MacLusky N,J. Sex differences i estrogen receptor and progestin receptor induction in the guinea pig hypothalamus and preoptic area. Brain Research. 1996;725(1):37–48. doi: 10.1016/0006-8993(96)00241-7. [DOI] [PubMed] [Google Scholar]
- Cain D,P, Humpartzoomian R, Boon F. Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat. Behavioural Brain Research. 2006;170:316–325. doi: 10.1016/j.bbr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Campbell S, Whitehead M. Oestrogens for menopausal flushing. British Medical Journal. 1977;1:104–105. doi: 10.1136/bmj.1.6053.104-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson L,E, Sherwin B,B. Steroid hormones, memory and mood in a healthy elderly population. Psychoneuroendocrinology. 1998;23(6):683–603. doi: 10.1016/s0306-4530(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Chakraborty T,R, Gore A,C. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Experimental Biology and Medicine. 2004;229(10):977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Hormones and Behavior. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Daniel J,M, Dohanich G,P. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. Journal of Neuroscience. 2001;21(17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu HP. The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiology of Aging. 2000;21:475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. Journal of Cellular and Molecular Medicine. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C,K, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychological Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton J,P. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacology, Biochemistry, and Behavior. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Farquhar C, Marjoribanks J, Lethaby A, Suckling J,A, Lamberts Quirine. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews. 2009;2:1–281. doi: 10.1002/14651858.CD004143.pub3. [DOI] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang JT. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. Journal of Pineal Research. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behavioral Neuroscience. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Wictorin K, Bjorklund A, Williams L,R, Varon S, Gage F,H. Amelioration of cholinergic neuron atrophy and spatial mempory impairment in aged rats by nerve growth factor. Nature. 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiology of Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- French K,L, Granholm A,E, Moore A,B, Nelson M,E, Bimonte-Nelson H,A. Chronic nicotine improves working and reference memory performance and reduces hippocampal NGF in aged female rats. Behavioural Brain Research. 2006;169:256–262. doi: 10.1016/j.bbr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiology of Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frick K,M, Price D,L, Koliatsos V,E, Markowska A,L. The effects of nerve growth factor on spatial recent memory in aged rats persist after discontinuation of treatment. Journal of Neuroscience. 1997;17(7):2543–2550. doi: 10.1523/JNEUROSCI.17-07-02543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Lee TT, Kostaras X, Sidhu JA, Barr AM. High levels of estradiol impair spatial performance in the Morris water maze and increase ‘depressive-like’ behaviors in the female meadow vole. Physiology of Behavior. 2002;77:217–225. doi: 10.1016/s0031-9384(02)00849-1. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behavioral Brain Research. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs R,B. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones and Behavior. 1999;36(3):222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gobbo O,L, O’Mara S,M. Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischmia. Behavioural Brain Research. 2004;152:231–241. doi: 10.1016/j.bbr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Goldman J,M, Murr A,S, Cooper R,L. The rodent estrous cycle:characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Granholm A. Oestrogen and nerve growth factor-neuroprotection and repair in Alzheimer’s disease. Expert Opinion on Investigational Drugs. 2000;9(4):685–694. doi: 10.1517/13543784.9.4.685. [DOI] [PubMed] [Google Scholar]
- Green P,S, Bishop J, Simpkins J,W. 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells. Journal of Neuroscience. 1997;17(2):511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas K,L, Everitt B. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nature Neuroscience. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hallahan M, Rosenthal R. Interpreting and reporting results. In: Tinsley HEA, Brown SD, editors. Handbook of multivariate statistics and mathematical modeling. Academic Press; San Diego, CA: 2000. pp. 183–208. [Google Scholar]
- Hirono N, Mori E, Ishii K, Ikejiri Y, Imamura T, Shimomura T, Hashimoto M, Yamashita H, Sasaki M. Hyposunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 1998;64:552–554. doi: 10.1136/jnnp.64.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behavioral Neuroscience. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Hruska Z, Dohanich GP. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1-42) and ibotenic acid. Hormones and Behavior. 2007;52:297–306. doi: 10.1016/j.yhbeh.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Hyde L,A, Hoplight B,J, Deneberg V,H. Water version of the radial - arm maze: learning in three inbred strains of mice. Brain Research. 1998;785(2):236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Hyde L,A, Sherman G,F, Deneberg V,H. Non-spatial water radial-arm maze learning in mice. Brain Research. 2000;863(1-2):151–159. doi: 10.1016/s0006-8993(00)02113-2. [DOI] [PubMed] [Google Scholar]
- Jarrad LE. On the role of the hippocapus in learning and memory in the rat. Behavioral and Neural Biology. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Jarrad L,E, Okaichi H, Steward O, Goldschmidt R,B. On the role of hippocampal connections in the performance of place and cue tasks: comparison with damage to hippocampus. Behavioral Neuroscience. 1984;98(6):946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Jaubert P,J, Golub M,S, Lo Y,Y, Germann S,L, Dehoff M,H, Worley P,F, Kang S,H, Schwarz M,K, Seeburg P,H, Berman R,F. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes, Brain, and Behavior. 2007;6:141–154. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Kantor HI, Michael CM, Shore H. Estrogen for older women. American Journal of Obstetrics and Gynecology. 1973;116:115–118. doi: 10.1016/0002-9378(73)90894-6. [DOI] [PubMed] [Google Scholar]
- Kassis J,A, Gorski J. Estrogen receptor replenishment: evidence for receptor recycling. Journal of Biological Chemistry. 1981;256(14):7378–7382. [PubMed] [Google Scholar]
- Keppel G, Wickens TD, editors. Design and analysis: a researcher’s handbook. Pearson; Upper Saddle River: 2004. [Google Scholar]
- Kesslak J,P, So V, Choi J, Cotman C,W, Gomex-Pinilla F. Learning upregrulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behavioral Neuroscience. 1998;112(4):1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Khan N, Sharma KK, Andersson S, Auchus RJ. Human 17 beta-hydroxysteroid dehydrogenases types 1, 2, and 3 catalyze bi-directional equilibrium reactions, rather than unidirectional metabolism, in HEK-293 cells. Archives of Biochemistry and Biophysics. 2004;429:50–59. doi: 10.1016/j.abb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Kinderman S,S, Brown G,G, Zorrilla L,E, Olsen R,K, Jeste D,V. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophrenia Research. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Korol D,L, Kolo L,L. Estrogen-induced changes in place and response learning in young adult female rats. Behavioral Neuroscience. 2002;116(3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM, Melmed S, Polonsky K,S, Larsen P,R. Williams textbook of endocrinology. Saunders Elsevier; Philadelphia: 2008. [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;(8 Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Lebrun C,E, van der Schouw Y,T, de Jong F,H, Pols H,A, Grobbee D,E, Lamberts S,W. Endogneous oestrogens are related to cognition in healthy elderly women. Clinical Endocrinology. 2005;63(1):50–55. doi: 10.1111/j.1365-2265.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- Lee A,C,H, Bandelow S, Schwarzbauer C, Henson R,N,A, Graham K,S. Perirhinal cortex activity during visual object discrimination: an event-related fMRI study. Neuroimage. 2006;33:362–373. doi: 10.1016/j.neuroimage.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Lerner SP, Meredith S, Thayne W,V, Butcher RL. Age-related alterations in follicular development and hormonal profiles in rats with 4-day estrous cycles. Biology of Reproduction. 1990;42(4):633–638. doi: 10.1095/biolreprod42.4.633. [DOI] [PubMed] [Google Scholar]
- Liang W,S, Reiman E,M, Valla J, Dunckley T, Beach T,G, Grover A, Niedzielko T,L, Schneider L,E, Mastroneni D, Caselli R, Kukull. W, Morris J,C, Hulette C,M, Schmechel. D, Rogers J, Stephan D,A. Alzheirmer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. PNAS. 2008;105(11):4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J,E, Swan C, Liang W,S, Cobos I, Potter G,B, Rubenstein J,L. D1x1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. Journal of Comparative Neurology. 2009;512(4):556–572. doi: 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Jacome L,F, Maclusky N,J. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144(7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Lukoyanov N,V, Lukoyanova E,A, Andrade J,P, Paula-Barbosa M,M. Impaired water maze navigation of Wistar rats with retrosplenial cortex lesions: effect of nonspatial pretraining. Behavioural Brain Research. 2005;158:175–182. doi: 10.1016/j.bbr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- MacLusky N,J, Naftolin F, Krey L,C, Franks S. The catechol estrogens. Journal of Steroid Biochemistry. 1981;15:111–124. doi: 10.1016/0022-4731(81)90265-x. [DOI] [PubMed] [Google Scholar]
- Malyala A, Pattee P, Nagalla S,R, Kelly M,J, Ronnekleiv O,K. Suppression subtractive hybridization and microarray identification of estrogen-regulated hypothalamic genes. Neurochemical Research. 2004;29(6):1189–1200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- Markowska A,L, Savonenko A,V. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawl in aging rats. Journal of Neuroscience. 2002;22(24):10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Hormones and Behavior. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- McMillan P,J, Singer C,A, Dorsa D,M. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA in the basal forebrain of the adult female sprague-dawley rat. Journal of Neuroscience. 1996;16(5):1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Menopause Society Role of progestogen in hormone therapy for postmenopausal women: position statement of The North American Menopause Society. Menopause. 2003;10(2):113–132. doi: 10.1097/00042192-200310020-00003. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. Journal of Neuroscience. 2000:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat S,D, Elkins W, Resnick S,M. Age differences in neural systems supporting human allocentric spatial navigation. Neurobiology of Aging. 2006;27:965–972. doi: 10.1016/j.neurobiolaging.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Grebe SK, O’Kane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clinical Chemistry. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- O’Connell M,B. Pharmacokinetic and pharmacologic variation between different estrogen products. Journal of Clinical Pharmacology. 1995;35(9):185–245. doi: 10.1002/j.1552-4604.1995.tb04143.x. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia. 1995;6:99–107. doi: 10.1159/000106929. [DOI] [PubMed] [Google Scholar]
- Page RD, Butcher RL. Follicular and plasma patterns of steroids in young and old rats during normal and prolonged estrous cycles. Biology of Reproduction. 1982;27:383–392. doi: 10.1095/biolreprod27.2.383. [DOI] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Clarkson T. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Society for Experimental Biology and Medicine. 1999;221:118–125. doi: 10.1046/j.1525-1373.1999.d01-64.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Pechenino A,S, Frick K,M. The effects of acute 17β-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiology of Learning and Memory. 2009;91(3):315–322. doi: 10.1016/j.nlm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Prokai L, Simpkins J,W. Structure-nongenomic neuroprotection relationship of estrogen and estrogen-derived compounds. Pharmacology and Therapeutics. 2007;114(1):1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J,M. Perirhinal cortex lesions produce retrograde amnesia for spatial information in rats: consolidation or retrieval? Learning and Memory. 2008;15(8):587–596. doi: 10.1101/lm.1036308. [DOI] [PubMed] [Google Scholar]
- Regan R,F, Guo Y. Estrogens attenuate neuronal injury due to hemoglobin, chemical hypokia, and excitatory amino acids in murine cortical cultures. Brain Research. 1997;764:133–140. doi: 10.1016/s0006-8993(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Reiman E,M, Chen K, Alexander G,E, Caselli R,J, Bandy D, Osborne D, Saunders A,M, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. PNAS. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. Journal of Clinical Endocrinology and Metabolism. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Rosser M, Chorich L, Howard E, Zamorano P, Mahesh V,B. Changes in rat uterine estrogen receptor messenger ribonucleic acid levels during estrogen- and progesterone- induced estrogen receptor depletion and replenishment. Biology of Reproduction. 1993;48(1):89–98. doi: 10.1095/biolreprod48.1.89. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behavioral Neuroscience. 2001;115:384–393. [PubMed] [Google Scholar]
- Scali C, Casamenti F, Pazzagli M, Bartolini L, Pepeu G. Nerve growth factor increases extracellular acetylcholine levels in the parietal cortex and hippocampus of aged rats and resotres object recognition. Neuroscience Letters. 1994;170:117–120. doi: 10.1016/0304-3940(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Segal M. FDA statement on generic premarin. U.S. Food and Drug Administration; [Retrieved November 26, 2007]. 1997. from http://www.fda.gov/CDER/news/cepressrelease.htm. [Google Scholar]
- Shaywitz S,E, Shaywitz B,A, Pugh K,R, Fulbright R,K, Skudlarski P, Mencl W,E, Constable R,T, Naftolin F, Palter S,F, Marchione K,E, Katz L, Shankweiler D,P, Fletcher J,M, Lacadie C, Keltz N, Gore J,C. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, Bowen D, Terrell T, Jones BN. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Seigal G,J, Chauhan H,B. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Research. Brain Research Reviews. 2000;33(2-3):199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Singer C,A, McMillan P,J, Bobie D,J, Dorsa D,M. Effects of estrogen replacement on choline acetyltransferase and trkA mRNA expression in the basal forebrain of aged rats. Brain Research. 1998;789:343–346. doi: 10.1016/s0006-8993(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Research. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. Hormonal replacement therapy. Reviews in Endocrine and Metabolic Disorders. 2002;3:243–256. doi: 10.1023/a:1020028510797. [DOI] [PubMed] [Google Scholar]
- Szumlinski K,K, Lominac K,D, Kleschen M,J, Oleson E,B, Dehoff M,H, Schwartz M,K, Seeberg P,H, Worely P,F, Kalivas P,W. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes, Brain, and Behavior. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Talboom J, Williams BJ, Baxley E,R, West S,G, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiology of Learning and Memory. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf O,T, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Hormones and Behavior. 2002;41(3):259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neuroscience. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown S,L, Korol D,L. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiology of Learning and Memory. 2006;86(3):336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]