Abstract
A number of synaptogenic factors induce presynaptic or postsynaptic differentiation when presented to axons or dendrites. Many such factors participate in bidirectional trans-synaptic adhesion complexes. Axonal neurexins interacting in an isoform-specific code with multiple dendritic partners (neuroligins, LRRTMs, or Cbln-GluRδ), and axonal protein tyrosine phosphatase receptors interacting with dendritic NGL-3, nucleate local networks of high affinity protein-protein interactions leading to aligned presynaptic and postsynaptic differentiation. Additional secreted target-derived factors such as fibroblast growth factors and glial-derived factors such as thrombospondin bind specific axonal or dendritic receptors stimulating signal transduction mechanisms to promote selective aspects of synapse development. Together with classical adhesion molecules and controlled by transcriptional cascades, these synaptogenic adhesion complexes and secreted factors organize the molecular composition and thus functional properties of central synapses.
Introduction
Synapses are the basic units of communication in the brain. Synaptic transmission relies on the coordinated development of highly specialized structures spanning both participating cell surface membranes and cytoplasms. Synaptic specializations on both sides of the cleft involve membranous organelles, cytoskeleton, and vast protein networks. Minimally, synaptic function requires that postsynaptic neurotransmitter receptors with associated scaffolding and signaling molecules be precisely aligned on the dendrite opposite chemically matched presynaptic vesicles with regulated release and recycling machinery in the axon.
We discuss in this review 'synaptogenic' proteins for vertebrate central neuron synapses, defined here as proteins that induce presynaptic or postsynaptic differentiation when presented to axons or dendrites, respectively. Clearly there are also other molecules that contribute in essential ways to synaptogenesis. For example, cadherin and immunoglobulin superfamily proteins are key mediators of synaptic adhesion [1], and transcription factors such as MEF2 and Npas4 control synaptogenesis by regulating expression of many genes including some discussed here [2]. We focus here on recent advances related to synaptogenic cell surface and cleft proteins that induce synaptic differentiation, also commonly known as synaptic organizing proteins.
Classes of synaptic organizing proteins
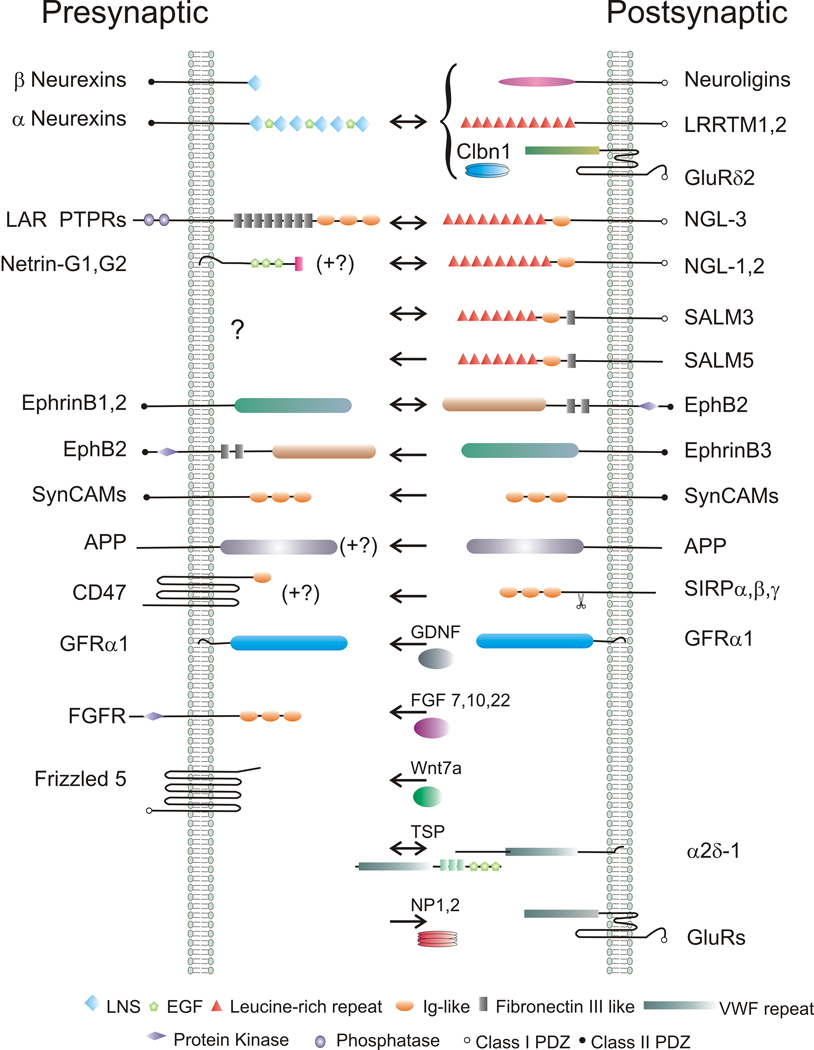
Synaptic organizing proteins exist in two main classes: (i) synaptic adhesion complexes, and (ii) secreted factors. An inventory of synaptogenic proteins is presented in Figure 1.
Figure 1.
An inventory of synaptogenic molecules, defined here as proteins that induce presynaptic (←) or postsynaptic (→) differentiation when presented to axons or dendrites, respectively. Many of the adhesion complexes have bidirectional synaptogenic activity (↔). The main receptors are also shown for the secreted synaptogenic factors. PDZ domain binding sites and common protein domains are indicated.
The synaptogenic adhesion complexes are composed of transmembrane presynaptic and postsynaptic partners that bind in trans across the cleft, a classic example being presynaptic neurexin and postsynaptic neuroligin [3–5]. Such cleft-spanning synaptic organizing complexes often have bidirectional activity, inducing presynaptic and postsynaptic differentiation, and by their nature mediate cell adhesion and alignment of the pre- and post-synaptic specializations. At least initially, synaptogenic activity mediated by synaptic adhesion complexes does not involve enzymatic activity but rather recruitment via high affinity protein-protein interactions (Figure 2A). Three particularly interesting findings and principles have emerged recently.
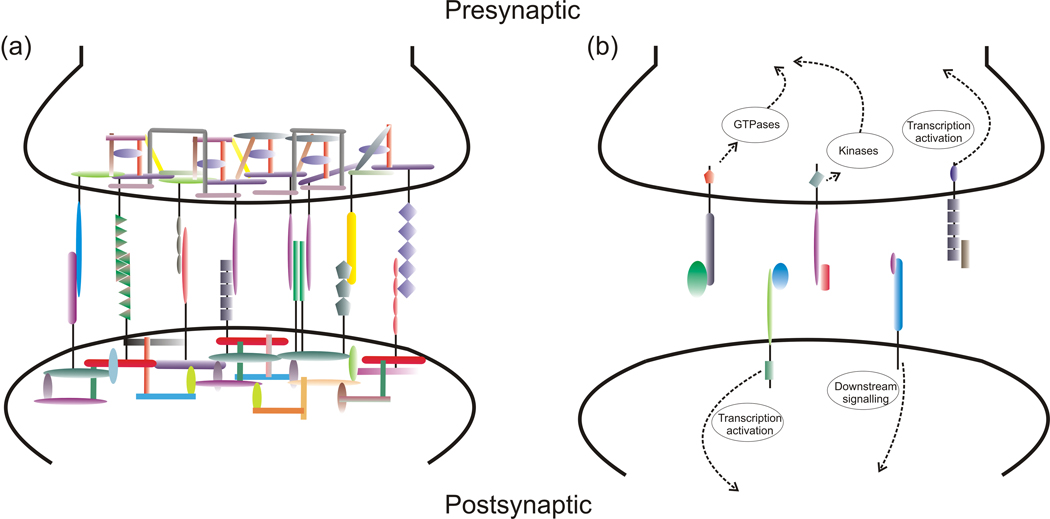
Figure 2.
Different initial mechanisms by which synaptogenic molecules promote synaptic differentiation. (a) Many synaptogenic adhesion complexes function primarily by nucleating a dynamic network of local high affinity protein-protein interactions in which each component interacts with multiple other components. (b) Many of the secreted factors directly activate signal transduction cascades involving kinases and GTPases, and perhaps regulate transcription. However, there are no strict boundaries, synaptogenic adhesion proteins can be kinases (e.g. Ephs) and secreted factors can act by local aggregation (e.g. NP1/2). These initial mechanisms are likely to converge on common downstream pathways mediating aspects of synaptic differentiation.
First, neurexins bind multiple, structurally diverse partners across the cleft (Table 1). The 4–5 mammalian neuroligins were the first characterized neurexin binding partners [3–5]. Neuroligin-1 with an insert at its B splice site is the major glutamatergic neuroligin and binds only β-neurexins. Neuroligin-2 functions specifically at GABAergic synapses and appears to bind all neurexins. Leucine-rich repeat transmembrane neuronal proteins LRRTMs, initially discovered to be synaptogenic molecules in an unbiased expression screen [6••], were recently identified in three independent studies to be trans-synaptic partners for neurexins [7••, 8••,–9••]. LRRTM1 and LRRTM2 are glutamatergic postsynaptic proteins and bind α and β neurexins specifically lacking an insert at splice site 4 (−S4) [6••,9••]. LRRTM2 and neuroligin-1 compete with similar affinity for an overlapping face of β-neurexin(−S4) [9••]. Given their broadly overlapping expression patterns [10,11], neuroligin-1, LRRTM1, and LRRTM2 are likely to coexist at many glutamatergic postsynaptic sites..
Table 1.
Isoform-specific interactions between neurexins and postsynaptic partners
| Neurexin isoform: | β (−S4) | β (+S4) | α (−S4) | α (+S4) |
|---|---|---|---|---|
| LRRTM 1,2 | Yes | No | Yes | No |
| Neuroligin 1(+B) | Yes | Yes | No | No |
| Neuroligin 1(−B),2,3,4 | Yes | Yes | Yes | Yes |
| Cbln1-GluRδ2 | No | Yes | ? | ? |
In a recent study, neurexins were identified as the trans-synaptic binding partners for another structurally distinct ligand pair: the complex of Cbln1-GluRδ2 [12••]. Cbln1 was shown to bind β-neurexins containing but not lacking the insert at splice site 4 (+S4). The neurexin(+S4)-Cbln1-GluRδ2 trans-synaptic triad, found to be essential for normal bidirectional parallel fiber-Purkinje cell synaptogenesis, illustrates the second principle: use of a secreted bridging protein (Cbln) to link transmembrane presynaptic and postsynaptic components [12••,13••]. Cbln1, a member of the C1q tumor necrosis factor superfamily secreted by granule cells, and GluRδ2, a member of the ionotropic glutamate receptor family, were suspected to function in the same pathway since Cbln1−/− and GluRδ2−/− mice had remarkably similar phenotypes 14,15]. Common defects include ataxia, deficient long-term depression, reduced numbers of parallel fiber-Purkinje synapses, increased numbers of free spines, and mismatches in the length of postsynaptic density relative to active zone at many remaining synapses. While Cbln1 and GluRδ2 function selectively in parallel fiber synaptogenesis between cerebellar granule cells and Purkinje cells [12••,13••], related Cbln and GluRδ proteins are more widely expressed potentially resulting in considerable overlap with neuroligins and LRRTMs.
A third key finding was the discovery of an independent bidirectional synaptic organizing complex with high potency like neurexins and its partners: postsynaptic netrin G ligand NGL-3 and presynaptic LAR family protein tyrosine phosphatase receptors PTPRs [16••,17]. NGLs were studied in the context of synaptogenesis based on their isolation as PSD-95 interacting proteins [18]. Like LRRTMs [6], the leucine-rich repeats of NGL-3 mediate presynaptic induction [16••]. However, a different family of presynaptic players are involved; NGL-3 binds the first two fibronectin domains of axonal LAR, PTPσ and PTPδ [16••,17]. NGL-3 and the PTPR partners are broadly expressed in brain [17], suggesting substantial co-existence at synapses with neurexins and partners.
An unbiased cell-based screen to isolate the set of proteins able to trigger presynaptic differentiation identified LRRTMs, re-isolated neuroligins and NGLs and revealed the existence of cDNA pools containing novel potent synaptogenic factors [6••]. Thus, we have yet to identify the full complement of synapse organizing proteins. While this cell-based expression screen appears biased towards identifying transmembrane synaptic organizers, biochemical screens fractionating brain extracts [19] or glial conditioned media [20] have yielded several secreted synaptic organizing proteins.
Secreted synaptogenic factors can be derived from the target dendrite, the presynaptic cell, neighboring neurons, or neighboring astrocytes. They can selectively promote aspects of presynaptic differentiation or of postsynaptic differentiation, in part depending on the location of their receptor. Mechanisms of synaptic differentiation promoted by secreted factors can require activation of kinases, signaling pathways [21,22] and perhaps transcription (Figure 2B). It is not yet clear whether secreted synaptogenic factors are locally instructive, like the synaptic adhesion complexes, or perhaps more permissive in promoting synapses to form but not specifying their exact sites. Recent findings in this area include the differential roles of FGF22 and FGF7 in glutamatergic and GABAergic presynaptic differentiation [23•], and identification of dendritic surface receptors for the glial-derived synaptogenic factor thrombospondin [24•,25].
Presynaptic differentiation
Local instruction by synaptic adhesion complexes
The simplest and most likely mechanism whereby neuroligins, LRRTMs, and Cbln-GluRδ2 induce presynaptic differentiation is by locally aggregating neurexins on the axon surface. Indeed, competition with neurexin1β ectodomain reduced synaptogenic activity for each of these partners [7••,12••,26], mutation of LRRTM2 abolishing neurexin interaction abolished its synaptogenic activity [9], and RNAi-mediated knock-down of all neurexins reduced synaptogenic activity of GluRδ2-Cbln1 [12••]. Moreover, artificially aggregating recombinant neurexin1β on the axon surface is sufficient to cluster synaptic vesicles by a mechanism requiring the neurexin cytoplasmic domain [27]. A recent study suggested that binding to β-neurexins is not sufficient for neuroligin-1 to induce presynaptic differentiation and that binding to a-neurexins is necessary [28]. However, such a result contrasts with the findings from multiple groups that showed neuroligin-1(+B) binds only to β, not α, neurexins and robustly induces presynaptic differentiation [9••,26,29]. Most data suggest that aggregation of either α or β neurexins on the axon can mediate presynaptic differentiation. Considering that α-neurexins are required for presynaptic calcium channel function [30], induction of functional presynaptic differentiation by aggregation of β-neurexins may involve recruitment of α-neurexins.
Neurexins participate in both glutamatergic and GABAergic synaptogenesis. It will be important to determine exactly which neurexin isoforms, including splice variants, are expressed by specific GABAergic and glutamatergic neurons. We can make some educated guesses about the roles of neurexin variants in GABAergic versus glutamatergic synaptogenesis based on the synaptogenic activities and binding specificities of neurexin partners. Neuroligin-1(+B), LRRTM1, and LRRTM2 selectively induce glutamatergic presynaptic differentiation and collectively bind all neurexins except the α-neurexin(+S4) variants [6••,9••,29,31]. Neuroligin-2 selectively induces GABAergic presynaptic differentiation [31] and also binds α-neurexin(+S4) variants [29] thus suggesting that α-neurexin(+S4) variants may be selectively expressed by GABAergic neurons. A specific role of α-neurexins(+S4) in GABAergic synaptogenesis is also supported by coculture studies [32] and by the finding that in vivo knockout of all α-neurexins, leaving β-neurexin expression intact, did not alter the number of morphologically defined glutamatergic synapses in cortex but reduced GABA synapse numbers by nearly half [30]. Interaction of α but not β neurexins with dystroglycan [33], which is present along with neuroligin-2 at a subset of mature GABAergic postsynaptic sites [34,35], may also contribute to long term stabilization of GABAergic synapses. Different glutamatergic synapses also express selective neurexin variants. Cerebellar neurons express mainly neurexin(+S4) variants, matching the cerebellar-specific expression of the (+S4)-selective Cbln1-GluRδ2 partners, whereas (+S4) and (−S4) neurexins are highly expressed in hippocampus and cortex [12••]. The neurexin isoform partner code (Table 1), (+S4) with Cbln-GluRδ, (−S4) with LRRTMs, β with neuroligin-1(+B), and all neurexins with other neuroligins is intriguing, although the full physiological significance is not yet understood.
Aggregation of axonal neurexins likely induces presynaptic differentiation by nucleating a network of high affinity protein-protein interactions to recruit synaptic vesicles and active zone machinery. It is controversial whether neuroligin-1 dimerization is required for presynaptic inducing activity [27,28], but one can imagine how dimerization would facilitate nucleation by aggregated neurexins. The neurexin class II PDZ domain interaction site is presumed to be essential for mediating synaptogenic activity via binding to scaffolding proteins such as CASK [36], Mints [37], and/or syntenins [38]. Other PDZ-independent interactions of the neurexin intracellular domain may be important, including binding to protein 4.1N to promote local assembly of actin filaments [39] and binding to synaptotagmin to promote vesicle recruitment [40]. It is remarkable that aggregating neurexins by presenting neuroligins or LRRTMs appears sufficient to induce extensive differentiation of presynaptic sites capable of spontaneous and evoked transmitter release [6••,41,42]. Although the initial trigger is purely protein-protein interactions, it seems likely that catalytic processes must be invoked at some point to generate such complete presynaptic specializations. Whether phosphorylation of neurexins by CASK [43] or other kinases contributes to synaptic differentiation has not yet been explored. Artificial presynaptic differentiation induced by binding of poly-D-lysine coated beads to isolated axons, which presmably hijacks some of the normal cellular machinery, occurred as rapidly as one hour and required F-actin reorganization [44].
The most potent known synaptogenic complex independent of neurexins, postsynaptic NGL-3 acting on presynaptic LAR family PTPRs [16••], induces presynaptic specializations by a slightly different mechanism. These PTPRs do not terminate in PDZ domain binding sites. Intracellularly, they are composed of an active phosphatase domain and a phosphatase-like domain that binds α-liprins [45]. Given the role of liprins in presynaptic differentiation in Drosophila and C. elegans, and their ability to directly bind CASK, RIMs, and ERC/ELKS/CAST [46,47], recruitment of liprins is likely essential for presynaptic differentiation mediated by LAR family PTPRs. Whether the phosphatase activity plays a role has not yet been determined. NGL-1 and NGL-2 do not bind LAR. Their less potent presynaptic inducing activities may be mediated in part by binding to the selective ligands netrin-G1 and netrin-G2, but must involve additional factors since direct aggregation of the GPI-anchored netrin-Gs on the axon surface did not induce presynaptic differentiation [18].
Also less potent than NGL-3 or the neurexin partners, SynCAMs/Necls [48], EphB2 [49], and ephrinB3 [50,51] are reported to induce presynaptic differentiation in co-culture. Like neurexin, the corresponding presynaptic partners, SynCAMs, ephrinB1/2, and EphB2, respectively, all terminate in class II PDZ domain binding sites and bind syntenins and/or CASK. However, initial analyses of knockout mice suggest that SynCAMs may be as or more important for axon guidance and myelination [52,53]. A surprising new player reported to induce presynaptic differentiation is amyloid precursor protein (APP), by a mechanism requiring APP or APLP2 in the contacting axons [54•]. Although APP does not terminate in a PDZ domain binding site, its direct interaction with Mint1 was suggested to mediate presynaptic differentiation. SALM3/Lrfn4 and SALM5/Lrfn5 also induce presynaptic differentiation by mechanisms not yet identified [55], perhaps involving transcellular association of SALM5 homophilically or with SALM4/Lrfn3 [56]. The immunoglobulin domain protein SIRPα induces presynaptic differentiation by a mechanism at least partially involving axonal CD47 [22]. However, SIRPα may be more like the secreted factors in a couple of ways, in that it is active even in cleaved ectodomain form, and its mode of action involves G protein signaling [22].
In the co-culture or bead-induced hemi-synapse formation assay, where a single protein such as a neuroligin or LRRTM or NGL-3, or just the corresponding ectodomain, induces presynaptic differentiation in contacting axons, the triggering protein is presented at high local concentration. How are such high local concentrations achieved at bona fide synapses, or are they? Two additional mechanisms are likely important. First, other proteins such as cadherin and immunoglobulin superfamily proteins contribute to adhesion of the axon-dendrite contact site. Such adhesion mechanisms may be necessary to allow the axonal and dendritic synaptogenic partners to come in proximity. In support of such cooperative function, the ability of neuroligin-1 overexpression to increase mEPSC frequency in neurons differentiated from ES cells was lost by targeted deletion of N-cadherin [57]. Second, evidence is accumulating that none of these synaptogenic proteins acts alone at synapses. At a single glutamatergic synapse, we envision dendritic neuroligins, LRRTMs, and perhaps GluRδ-Cbln binding to overlapping sets of neurexin variants, dendritic NGL-3 binding to axonal LAR family PTPRs, and additional cell adhesion and soluble synaptogenic factors acting in concert to achieve presynaptic differentiation (Figure 2). The recently discovered overlapping interactions and the multiple sets of synaptogenic factors may be necessary to drive synaptogenesis by a dynamic network of local high affinity protein-protein interactions. For example, the interactions of neurexins with CASK, LAR with liprin, and CASK with liprin may reinforce each other. Multiple individual synaptogenic adhesion proteins may serve as nucleating factors, and thus loss of any single synaptogenic protein may result in only partial defects in synaptogenesis. Indeed, relatively subtle defects have been found in individual neuroligin-1, neuroligin-2 and LRRTM1 knockout mice [6••,10,58, 59••,60,61].
Promotion by secreted factors
Growth factors and neurotrophic factors comprise one class of secreted synaptogenic proteins. BDNF and NT-3, not discussed in detail here, have long been appreciated to promote aspects of synaptogenesis through activating their tyrosine kinase receptors TrkB and TrkC, respectively [62]. GDNF in a complex with GFRα1 was reported to locally induce presynaptic differentiation by a mechanism mediated in part by GFRα1 in the axons [63]. Since GFRα1 is GPI-anchored, presumably a coreceptor must be involved to achieve presynaptic differentiation. GDNF could also mediate adhesion between cells expressing GFRα and was proposed to be a bridging molecule [63], like Cbln1. FGFs have been a focus of recent research. FGF22 was isolated from an unbiased biochemical screen for proteins that induce vesicle clustering in cultured motoneurons, and multiple FGFs were found to be active [19]. FGF2 was also previously found to increase clustering of presynaptic and postsynaptic markers and their apposition [21]. Analyses of knockout mice revealed selective roles for target-derived FGF22 in glutamatergic synaptogenesis and FGF7 in GABAergic synaptogenesis onto hippocampal CA3 neurons [23•].
The research on FGFs illustrates apparent differences in the mode of action of secreted synaptogenic proteins compared with synaptogenic adhesion complexes. Whereas the adhesion complexes trigger relatively complete synaptic differentiation, FGF22 and FGF7 selectively mediated synaptic vesicle clustering and not active zone formation or postsynaptic differentiation [23•]. Thus, secreted factors may be utilized to promote selective aspects of synaptogenesis. FGFRs are tyrosine kinases, and the effects of FGF2 and FGF22 on synaptic vesicle accumulation were blocked by kinase inhibitors [21,22]. Thus, whereas the synaptogenic adhesion complexes act primarily through nucleating networks of high affinity protein-protein interactions, secreted synaptogenic factors may act primarily by stimulating catalytic signaling pathways.
An unresolved issue is how these secreted factors act in soluble form to promote development of highly localized structures. Several mechanisms can be envisioned. One, the secreted factors may bridge presynaptic and postsynaptic components, like Cbln1. Two, they may bind to presynaptically localized receptors, implying that other mechanisms have already generated a partial presynaptic site with the localized receptor. Three, they may be secreted in a highly localized manner, with the secretion site controlling the zone of action. Alternatively, secreted factors may promote synaptogenesis in a more permissive way, for example by increasing the aggregation of synaptic vesicles along the axon but not instructing the precise site of synapse formation. FGF22 and FGF7 increase axon branching as well as vesicle clustering in cultured neurons [19], suggesting non-localized actions, and yet they can localize specifically to excitatory or inhibitory synapses [23•], whether by localized secretion or binding to specific receptors is not yet known.
Wnts are another class of secreted factors that promote synaptogenesis. Wnt7a is involved transiently in cerebellar glomerular development in vivo [64] by a mechanism involving Dishevelled [65]. In hippocampal cultures, Wnt7a increases synaptic vesicle clustering and mEPSC frequency without altering postsynaptic properties [66] by a mechanism involving Frizzled5 [67]. Additional Wnt family members contribute specific synaptogenic activities through multiple signaling cascades [68,69]. Importantly, as shown in C. elegans, Wnts can also act as anti-synaptogenic factors, creating regions of a neuron refractory for synaptogenesis [70]. Such anti-synaptogenic factors are likely to play important roles in directing synaptogenesis but have not yet been well studied in mammals.
Postsynaptic differentiation
Local instruction by synaptic adhesion complexes
Just as aggregation of neurexins on the axon surface mediates presynaptic differentiation, aggregation of the neurexin postsynaptic partners, or NGL-3, on the dendrite surface mediates postsynaptic differentiation. Direct aggregation of recombinant neuroligins [71], LRRTM2 [6••], GluR52 [13••], or NGL-3 [16••] with antibody-coated beads was sufficient to recruit multiple postsynaptic proteins including PSD-95/93, Shank, GKAP, Homer, SynGAP, and NMDA receptor NR1. As for the presynaptic side, the mechanism of postsynaptic differentiation induced by these synaptogenic adhesion complexes occurs initially, and perhaps completely, by nucleating a dynamic network of high affinity protein-protein interactions. Neuroligins, LRRTMs, GluRδ, and NGLs all terminate in PDZ domain binding sites which can bind PSD-95, PSD-93, SAP102, and/or SAP97 and other glutamatergic scaffolding proteins [6••,18,72–74]. All bear consensus class I PDZ domain binding sites except for LRRTMs for which the atypical - ECEV mediates the interaction. It seems likely that non-PDZ domain interaction sites in the intracellular domains of these postsynaptic adhesion proteins are also important in postsynaptic differentiation; for example, GluRδ2 directly binds and recruits Shank2 [75], and neuroligin-1 and LRRTM2 can each target to glutamatergic postsynaptic sites via intracellular sequences distinct from the PDZ domain binding sites [6••,76].
Whether AMPA receptors are recruited by these synaptogenic adhesion complexes is not entirely clear, and may vary among complexes. AMPA receptor recruitment has not been demonstrated by simple coculture with LAR family PTPRs or neurexins, but was reported for neurexin coculture with neurons overexpressing PSD-95 upon treatment with glutamate [77]. Direct aggregation of NGL-3 but not NGL-2 on dendrites recruited surface GluR2 [16••,78]. Whether such direct aggregation of neuroligin-1 or LRRTM2 recruits AMPA receptors has not been tested, although recombinant LRRTM2 was reported to co-immunoprecipitate individually with GluR1, GluR2, and NR1 [8••]. SALM 1–3 also terminate in class I PDZ domain binding sites, SALM 2 and 3 but not 5 can recruit postsynaptic proteins [55,78], and SALM 1 coimmunoprecipitates with NR1 [79]. EphB2 interacts with AMPA receptors indirectly through a class II PDZ domain intermediate such as GRIP or PICK1 [49], and the extracellular domain of EphB2 directly binds the extracellular domain of NR1 [80].
Postsynaptic differentiation that is artificially induced by any of the above synaptogenic factors is incomplete in another major way. These synaptogenic adhesion complexes are not sufficient to induce dendritic spine morphogenesis. Yet many of these proteins contribute to spine morphogenesis, since overexpression, knock-down, or knockout alters spine numbers or spine morphology [7••,18,81]. Signaling through EphB-ephrinB complexes in particular may be more important for spine morphogenesis [82•,83] than for the other aspects of synaptic differentiation that are the focus of this review. Perhaps the coculture or bead induction assays preclude spine morphogenesis, or perhaps no individual molecule presented to dendrites will be sufficient to trigger the full signaling cascade necessary for spine morphogenesis; it may prove fruitful to test co-presentation of multiple factors.
Another incompletely resolved issue is the role of neuroligins in selective GABAergic versus glutamatergic postsynaptic differentiation. In fact, the roles of neuroligins may be even broader, given the evidence for a role of neuroligin-1 in ciliary ganglion cholinergic synaptogenesis [84]. In the central nervous system, neuroligin-2 localizes specifically to GABAergic synapses [71,85], neuroligin-1 localizes selectively to glutamatergic synapses [86], and individual knockout mice exhibit selective deficits in GABAergic/glycinergic or glutamatergic transmission, respectively [58,59••]. Yet the phenotype of neuroligin-1,2,3 triple knockout mice is more severe than any of the single knockouts [10], indicating some redundancy among neuroligins. All neuroligins terminate in class I PDZ domain binding sites and can bind PSD-95 [72], and a recent study showed that all neuroligins can bind the GABAergic scaffolding protein gephyrin via a conserved cytoplasmic motif [59••]. Importantly, only neuroligin-2 also bound collybistin/ArhGEF9 and could recruit gephyrin bound to collybistin [59••]. The importance of this interaction is supported by defects in GABAergic synaptogenesis in collybistin knockout mice [87]. All neurexins were recently reported to interact directly with GABAA receptors, although neurexin knock-down did not alter GABAergic transmission [88]; further studies will be required to determine whether this low affinity interaction has physiological significance. The potential interaction of all neuroligins with common glutamatergic and GABAergic scaffolding proteins may be an important design feature. Overexpressing PSD-95 not only enhances excitatory transmission but also reduces inhibitory transmission via translocating neuroligin-2 from GABAergic to glutamatergic synapses; such mechanisms may be utilized for controlling the balance of excitatory and inhibitory input onto neurons [89]. It is becoming clear that chemical matching of presynaptic and postsynaptic components cannot be achieved by neurexins and known partners; there must be additional GABA-specific and glutamate-specific adhesion complexes.
Promotion by secreted factors
One of the earliest identified synaptogenic factors for glutamatergic synapses was the secreted neuronal pentraxin NP2/NARP [90]. NP2 and the related NP1 interact via their pentraxin domain with the extracellular domain of all AMPA receptor subunits, and NP1/2 expressing cells recruit AMPA receptors to contact sites [90–92]. This mechanism of direct aggregation is rather like that of the synaptogenic adhesion complexes. Recent analyses of NP1/2 knockout mice have revealed a developmentally restricted role for NP1/2 in AMPA receptor clustering in vivo. During early postnatal stages, NP1/2 knockouts exhibited reduced clustering of GluR4 in hippocampus [92] and reduced AMPA-mediated but normal NMDA-mediated retinogeniculate transmission [93]. AMPA-mediated transmission in the knockouts subsequently surpassed that in wild type, possibly due to later-developing compensatory mechanisms. Perhaps in part due to the altered transmission, deficits were also found in segregation of eye-specific retinogeniculate projections [93,94].
Thrombospondins (TSPs), glial derived synaptogenic factors, act in a complementary manner to NP1/2, promoting formation of ultrastructurally normal synapses that are functional presynaptically but lack AMPA receptors [20]. We consider TSPs in the postsynaptic section because they act via binding receptors on dendrites. It was recently found that the 3 EGF repeats of TSPs mediate the synaptogenic activity by binding the α2δ-1 auxiliary calcium channel subunit, a target of GABApentin [24•]. The mechanism of TSP action appears to be independent of calcium channel function, but the events downstream of binding to the GPI-anchored α2δ-1 and potential coreceptors for intracellular signal transduction have not yet been delineated. Interestingly, α2δ-3 was found to be necessary presynaptically in Drosophila for bouton morphogenesis, also by a mechanism independent of calcium channel activity [95]. Another report suggested that TSPs accelerate synaptogenesis by binding to and aggregating neuroligin-1 on dendrites [25], although whether neuroligin-1 with bound TSP could simultaneously bind neurexins to induce apposing presynaptic differentiation is not clear. Based on the several day period required to see the effects of TSPs, along with the broad distribution and developmental window of action, it was suggested that TSPs may induce neurons to alter the expression of genes involved in synaptogenesis, such as the locally acting factors discussed here, rather than necessarily acting locally themselves [20].
Beyond basic synaptic differentiation
Synaptic development is a protracted process involving axon and dendrite contact, presynaptic and postsynaptic differentiation, morphogenesis, maturation, maintenance, and plasticity. In addition to their roles in differentiation, the synaptogenic proteins discussed here may function in other aspects of synaptogenesis, including synapse specificity. Thorough analyses have not been performed for most of the synaptogenic adhesion proteins, except the cerebellar-specific Cbln1-GluRδ2 as discussed above, but the initial analyses suggest that there may be considerable synaptic selectivity. A role of neurexins in synaptic partner choice was first suggested upon the discovery of the possibility of thousands of neurexin splice variants. Although neuroligin-2 is localized to nearly all GABAergic synapses [85], its function appears to be more selective. Neuroligin-2 knockout mice show selective deficits at perisomatic synapses in hippocampus [59••] and at pyramidal cell synapses made by fast-spiking interneurons but not by somatostatin-positive interneurons in cortex [60]. LRRTMs, NGLs, and LAR family PTPRs show interesting laminar-specific distributions [6••,11,17]. NGL-1 and NGL-2 concentration in specific dendritic layers was dependent on their axonal netrin-G partners [96]. Conditional knockout of GluRδ2 in adult cerebellum revealed an essential function in synapse maintenance [97], but the role of most other proteins in synapse maintenance is not known. The contribution of each of the individual synaptogenic adhesion complex proteins to cellular partner choice and synapse maintenance are major questions that will require careful in vivo analyses of localization and effects of targeted deletion to resolve.
Synaptic organizing proteins are well-suited to mediate plasticity because they integrate presynaptic and postsynaptic domains and influence the size, stability and morphology of synapses. A role of ephrins and EphRs in synaptic plasticity has been well documented [83], TSPs were implicated in experience dependent plasticity in mouse barrel cortex [24•], and Wnts in experience dependent plasticity at hippocampal mossy fiber synapses [98]. Clear evidence for neurexins and partners as mediators of synaptic plasticity has not yet emerged, but activity regulation of neurexin splicing [32] hints at such roles, and altering postsynaptic levels of neuroligin-1 can retrogradely modulate release probability [99]. Clever strategies may be required to test roles in plasticity separately from roles in synaptic differentiation for these potent synaptogenic adhesion complexes. Cerebellar long-term depression, which is abolished by loss of either Cbln1 or GluRδ2, surprisingly was rescued by viral expression of GluRδ2 lacking the N-terminal Cbln1-binding domain, even without rescue of the structural synaptic defects associated with loss of GluRδ2 [100]. Perhaps high level expression in the absence of Cbln1 binding allowed sufficient postsynaptic accumulation of the GluRδ2 C-terminal domain to rescue plasticity.
Since the initial linkage of mutations in neuroligins 3 and 4 to autism in 2003 [101], evidence has rapidly accumulated for contribution of neuroligin and neurexin variants to the neurodevelopmental disorders autism, schizophrenia, and mental retardation [5,102]. Although occurring in only a small fraction of patients, alterations include copy number variants, protein-truncating frameshifts, and function-altering missense variants, some de novo, thus constituting strong evidence of contribution to the disease. Mimicking these variants in mice can phenocopy some aspects of the disease; for example, neuroligin-4 knockout mice exhibit selective deficits in social behavior and ultrasonic communication [103]. Most of the other synaptogenic proteins have not been systematically studied in this context, although associations were also found for LRRTM1 and GluRδ1 with schizophrenia [104,105] and SynCAM1 with autism [106]. Based on their function to recruit components to control synaptic properties in circuits important for cognitive processing, we suspect that many of these synaptogenic genes are at high risk for predisposing to neurodevelopmental disorders. Further understanding of the molecular pathways and circuit events down-stream of these synaptogenic molecules, and assessment of the validity of genetically-based mouse models of these disorders, may eventually contribute to effective targeted therapies.
Conclusions
An often-asked question is, "why are there so many synaptogenic proteins"? A recent variation of this question might be, "why are there multiple neurexin postsynaptic partners"? One can imagine several reasons. (1) Redundancy is one obvious reason, so that synaptogenesis will not be so susceptible to single-gene deleterious events. (2) The nature of the initial events in synaptic differentiation, nucleation by high affinity protein-protein interactions rather than catalytic events, may indicate another reason, a sort of mechanical stability. A network of such high affinity interactions where each protein connects with multiple other proteins may be necessary to drive local assembly. Such a network of multiply interacting proteins is thought to maintain both the presynaptic density and the glutamatergic postsynaptic density. Cross-interactions of neurexins with multiple postsynaptic partners as well as parallel systems may help stabilize interactions across the cleft by tying into the presynaptic and postsynaptic networks at multiple points. (3) Convergence onto signaling pathways and signal amplification may be another reason. Multiple signaling pathways and cytoskeletal networks must be invoked to achieve complete presynaptic and postsynaptic differentiation including morphogenesis in a physiologically relevant time-frame. (4) These synaptogenic proteins may contribute to synaptic specificity, pairing specific presynaptic and postsynaptic cells, controlling exactly where and when synapses form. Specificity may apply not only to partner choice but may also direct synapses to subcellular domains. (5) Different synaptogenic proteins may confer differences in molecular composition, structure, and functional properties. Although the cell culture experiments with synaptogenic adhesion proteins revealed surprisingly little variability in such properties, loss of function in vivo may reveal more variability in the synaptic properties conferred by each molecule. (6) A primary function of some of these proteins may be in synaptic plasticity. The need for differential regulation by activity and signaling pathways would create a need for diversity in secreted and adhesion complex synaptic organizing proteins.
Acknowledgements
We thank members of the Craig lab for helpful discussions, and Frederick Dobie for comments on the manuscript. T.J.S. is supported by a Michael Smith Foundation for Health Research Postdoctoral Fellowship, A.M.C. is supported by a Canada Research Chair, and related research in our laboratory is supported by Canadian Institutes of Health Research MOP-84241, National Institutes of Health R01-MH070860, and the Mind Foundation of British Columbia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 2.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 4.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017.. The authors performed an unbiased expression screen for molecules that induce presynaptic differentiation, re-isolating neuroligin-2 and NGL-3 and identifying and characterizing a new synaptogenic family for glutamatergic synapses, LRRTMs. The screen revealed additional synaptogenic pools, indicating that our current inventory is far from complete.
- 7. Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64:791–798. doi: 10.1016/j.neuron.2009.12.012.. Along with [8••, 9••], these authors found a direct interaction between LRRTM2 and neurexins, here using affinity chromatography. LRRTM2 bound neurexins competitively with neuroligin-1, and selectively bound neurexins 1 α and β (−S4) and not (+S4). The authors also showed that this interaction mediates cell adhesion.
- 8. de Wit J, Sylwestrak E, O'Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019.. Along with [7••, 9••], these authors found a direct interaction between LRRTM2 and neurexins, here using affinity chromatography. Interaction of LRRTM2 was reported specifically with neurexin 1. shRNA against LRRTM2 reduced excitatory synapse density in culture and reduced AMPA and NMDA evoked transmission in slices following viral introduction of shRNA in vivo.
- 9. Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30:7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010.. Along with [7••, 8••], these authors found a direct interaction between LRRTM2 and neurexins, here using a candidate binding screen. LRRTM2 and LRRTM1 bound and were recruited by neurexins-1,2,3 α and β (−S4) and not (+S4) isoforms. LRRTM2 and neuroligin-1(+B) bound competitively to neurexin1β(−S4) at a highly overlapping surface, and when co-expressed in neurons cooperated to promote presynaptic differentiation.
- 10.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Lauren J, Airaksinen MS, Saarma M, Timmusk T. A novel gene family encoding leucine- rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics. 2003;81:411–421. doi: 10.1016/s0888-7543(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 12. Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–1079. doi: 10.1016/j.cell.2010.04.035.. Along with [13••], this study showed that a direct interaction between the presynaptically secreted Cbln1 and postsynaptic GluRδ2 mediates parallel fiber synapse formation between cerebellar granule cells and Purkinje cells. These authors further identified neurexins (+S4) as the presynaptic transmembrane protein that participates in a synaptic triad neurexin-Cbln1-GluRδ2. This was an elegant study with many supporting experiments in culture and in vivo.
- 13. Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, et al. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 2010;328:363–368. doi: 10.1126/science.1185152.. Along with [12••], this study showed that a direct interaction between the presynaptically secreted Cbln1 and postsynaptic GluRδ2 mediates parallel fiber synapse formation between cerebellar granule cells and Purkinje cells. This was an elegant study with many supporting experiments in culture and in vivo.
- 14.Hirai H, Pang Z, Bao D, Miyazaki T, Li L, Miura E, Parris J, Rong Y, Watanabe M, Yuzaki M, et al. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci. 2005;8:1534–1541. doi: 10.1038/nn1576. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 16. Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279.. This study identified a potent bidirectional synaptogenic adhesion complex independent from neurexin and partners: postsynaptic NGL-3 and presynaptic protein tyrosine phosphatase receptor LAR. Supporting data included co-culture induction of presynaptic and postsynatpic differentiation, and shRNA knock-down of NGL-3 resulting in reduced excitatory synapse density and mEPSC frequency in hippocampal culture.
- 17.Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J Biol Chem. 2010;285:13966–13978. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, Kim K, Kim H, Weinberg RJ, Kim E. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- 19.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 22.Umemori H, Sanes JR. Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J Biol Chem. 2008;283:34053–34061. doi: 10.1074/jbc.M805729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–787. doi: 10.1038/nature09041.. These authors studied the roles of distinct FGFs as target-derived factors in synaptogenesis onto hippocampal CA3 neurons. FGF22 −/− mice showed normal synapse numbers but deficits in glutamatergic presynaptic differentiation: reductions in VGLUT1 clustering, docked vesicles, mEPSC frequency, and susceptibility to seizure. FGF7 −/− mice showed similarly selective defects in GABAergic presynaptic differentiation.
- 24. Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025.. This study identified a receptor on dendrites that mediated the synaptogenic activity of astrocyte-derived thrombospondin: the a2δ1 auxiliary calcium channel subunit. siRNA knock-down of a2δ1 reduced synaptogenic activity of TSP or astrocytes, and overexpression in vivo increased excitatory synapse number in postnatal cortex. a2δ1 ligand GABApentin regulated TSP-mediated synaptogenesis and barrel cortex plasticity.
- 25.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- 26.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 27.Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko J, Zhang C, Arac D, Boucard AA, Brunger AT, Sudhof TC. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. Embo J. 2009;28:3244–3255. doi: 10.1038/emboj.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;424:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 31.Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Kang Y, Zhang X, Dobie F, Wu H, Craig AM. Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J Biol Chem. 2008;283:2323–2334. doi: 10.1074/jbc.M703957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- 35.Levi S, Grady RM, Henry MD, Campbell KP, Sanes JR, Craig AM. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J Neurosci. 2002;22:4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J Biol Chem. 2000;275:39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- 38.Grootjans JJ, Reekmans G, Ceulemans H, David G. Syntenin-syndecan binding requires syndecan-synteny and the co-operation of both PDZ domains of syntenin. J Biol Chem. 2000;275:19933–19941. doi: 10.1074/jbc.M002459200. [DOI] [PubMed] [Google Scholar]
- 39.Biederer T, Sudhof TC. CASK and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- 40.Hata Y, Davletov B, Petrenko AG, Jahn R, Sudhof TC. Interaction of synaptotagmin with the cytoplasmic domains of neurexins. Neuron. 1993;10:307–315. doi: 10.1016/0896-6273(93)90320-q. [DOI] [PubMed] [Google Scholar]
- 41.Fu Z, Washbourne P, Ortinski P, Vicini S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol. 2003;90:3950–3957. doi: 10.1152/jn.00647.2003. [DOI] [PubMed] [Google Scholar]
- 42.Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Sudhof TC, Kavalali ET. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Sudhof TC, Wahl MC. CASK Functions as a Mg2+-independent neurexin kinase. Cell. 2008;133:328–339. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucido AL, Suarez Sanchez F, Thostrup P, Kwiatkowski AV, Leal-Ortiz S, Gopalakrishnan G, Liazoghli D, Belkaid W, Lennox RB, Grutter P, et al. Rapid assembly of functional presynaptic boutons triggered by adhesive contacts. J Neurosci. 2009;29:12449–12466. doi: 10.1523/JNEUROSCI.1381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pulido R, Serra-Pages C, Tang M, Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc Natl Acad Sci U S A. 1995;92:11686–11690. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stryker E, Johnson KG. LAR, liprin alpha and the regulation of active zone morphogenesis. J Cell Sci. 2007;120:3723–3728. doi: 10.1242/jcs.03491. [DOI] [PubMed] [Google Scholar]
- 47.Sigrist SJ, Schmitz D. Structural and functional plasticity of the cytoplasmic active zone. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2010.08.012. In press. [DOI] [PubMed] [Google Scholar]
- 48.Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClelland AC, Hruska M, Coenen AJ, Henkemeyer M, Dalva MB. Trans-synaptic EphB2-ephrin-B3 interaction regulates excitatory synapse density by inhibition of postsynaptic MAPK signaling. Proc Natl Acad Sci U S A. 2010;107:8830–8835. doi: 10.1073/pnas.0910644107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, Chen L. Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation. J Neurosci. 2007;27:7508–7519. doi: 10.1523/JNEUROSCI.0705-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park J, Liu B, Chen T, Li H, Hu X, Gao J, Zhu Y, Zhu Q, Qiang B, Yuan J, et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci. 2008;28:12815–12819. doi: 10.1523/JNEUROSCI.2665-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niederkofler V, Baeriswyl T, Ott R, Stoeckli ET. Nectin-like molecules/SynCAMs are required for post-crossing commissural axon guidance. Development. 2010;137:427–435. doi: 10.1242/dev.042515. [DOI] [PubMed] [Google Scholar]
- 54. Wang Z, Wang B, Yang L, Guo Q, Aithmitti N, Songyang Z, Zheng H. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J Neurosci. 2009;29:10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009.. This study explored the role of amyloid precursor protein (APP) in synaptogenesis using conditional knockout mice. APP induced presynaptic differentiation in coculture by a mechanism requiring APP/APLP2 in the hippocampal neurons. APP/APLP2 were also needed both in muscle and in motoneurons for normal neuromuscular synapse development.
- 55.Mah W, Ko J, Nam J, Han K, Chung WS, Kim E. Selected SALM (synaptic adhesion-like molecule) family proteins regulate synapse formation. J Neurosci. 2010;30:5559–5568. doi: 10.1523/JNEUROSCI.4839-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seabold GK, Wang PY, Chang K, Wang CY, Wang YX, Petralia RS, Wenthold RJ. The SALM family of adhesion-like molecules forms heteromeric and homomeric complexes. J Biol Chem. 2008;283:8395–8405. doi: 10.1074/jbc.M709456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, Lessmann V, Dresbach T, Gottmann K. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc Natl Acad Sci U S A. 2010;107:11116–11121. doi: 10.1073/pnas.0914233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023.. In this study exploring the role of neuroligin-2 in GABAergic synaptogenesis, all neuroligins were found to bind gephyrin but only neuroligin-2 also bound collybistin and recruited collybistin and gephyrin. Furthermore, neuroligin-2 −/− mice exhibited deficits in GABAergic and glycinergic but not glutamatergic transmission, and exhibited selective deficits in perisomatic GABAergic synapses in hippocampus, revealing subcellular specificity.
- 60.Gibson JR, Huber KM, Sudhof TC. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J Neurosci. 2009;29:13883–13897. doi: 10.1523/JNEUROSCI.2457-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 63.Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- 64.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 65.Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- 67.Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137:2215–2225. doi: 10.1242/dev.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- 69.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 70.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 71.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 73.Roche KW, Ly CD, Petralia RS, Wang YX, McGee AW, Bredt DS, Wenthold RJ. Postsynaptic density-93 interacts with the delta2 glutamate receptor subunit at parallel fiber synapses. J Neurosci. 1999;19:3926–3934. doi: 10.1523/JNEUROSCI.19-10-03926.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyer G, Varoqueaux F, Neeb A, Oschlies M, Brose N. The complexity of PDZ domainmediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology. 2004;47:724–733. doi: 10.1016/j.neuropharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 75.Uemura T, Mori H, Mishina M. Direct interaction of GluRdelta2 with Shank scaffold proteins in cerebellar Purkinje cells. Mol Cell Neurosci. 2004;26:330–341. doi: 10.1016/j.mcn.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Dresbach T, Neeb A, Meyer G, Gundelfinger ED, Brose N. Synaptic targeting of neuroligin is independent of neurexin and SAP90/PSD95 binding. Mol Cell Neurosci. 2004;27:227–235. doi: 10.1016/j.mcn.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ko J, Kim S, Chung HS, Kim K, Han K, Kim H, Jun H, Kaang BK, Kim E. SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron. 2006;50:233–245. doi: 10.1016/j.neuron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Wang CY, Chang K, Petralia RS, Wang YX, Seabold GK, Wenthold RJ. A novel family of adhesion-like molecules that interacts with the NMDA receptor. J Neurosci. 2006;26:2174–2183. doi: 10.1523/JNEUROSCI.3799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 81.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 82. Kayser MS, Nolt MJ, Dalva MB. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59:56–69. doi: 10.1016/j.neuron.2008.05.007.. Using cultures from knockout mice and shRNA knock-down and rescue, the authors showed that EphBs contribute to dendritic filopodia motility and synaptogenesis in a particular time window. Constitutively active PAK to increase filopodia motility and kinase-deficient EphB2 to mediate adhesion with presynaptic ephrins rescued synaptogenesis only when co-expressed.
- 83.Lai KO, Ip NY. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol. 2009;19:275–283. doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Conroy WG, Nai Q, Ross B, Naughton G, Berg DK. Postsynaptic neuroligin enhances presynaptic inputs at neuronal nicotinic synapses. Dev Biol. 2007;307:79–91. doi: 10.1016/j.ydbio.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 85.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 86.Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey RJ, Harvey K, O'Sullivan GA, Laube B, Hulsmann S, et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. Embo J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang C, Atasoy D, Arac D, Yang X, Fucillo MV, Robison AJ, Ko J, Brunger AT, Sudhof TC. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010;66:403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levinson JN, El-Husseini A. Building Excitatory and Inhibitory Synapses: Balancing Neuroligin Partnerships. Neuron. 2005;48:171–174. doi: 10.1016/j.neuron.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 90.O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate- early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 91.Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–528. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- 92.Sia GM, Beique JC, Rumbaugh G, Cho R, Worley PF, Huganir RL. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron. 2007;55:87–102. doi: 10.1016/j.neuron.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 93.Koch SM, Ullian EM. Neuronal pentraxins mediate silent synapse conversion in the developing visual system. J Neurosci. 2010;30:5404–5414. doi: 10.1523/JNEUROSCI.4893-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishimura-Akiyoshi S, Niimi K, Nakashiba T, Itohara S. Axonal netrin-Gs transneuronally determine lamina-specific subdendritic segments. Proc Natl Acad Sci U S A. 2007;104:14801–14806. doi: 10.1073/pnas.0706919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takeuchi T, Miyazaki T, Watanabe M, Mori H, Sakimura K, Mishina M. Control of synaptic connection by glutamate receptor delta2 in the adult cerebellum. J Neurosci. 2005;25:2146–2156. doi: 10.1523/JNEUROSCI.4740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 99.Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kakegawa W, Miyazaki T, Kohda K, Matsuda K, Emi K, Motohashi J, Watanabe M, Yuzaki M. The N-terminal domain of GluD2 (GluRdelta2) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivo. J Neurosci. 2009;29:5738–5748. doi: 10.1523/JNEUROSCI.6013-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Francks C, Maegawa S, Lauren J, Abrahams BS, Velayos-Baeza A, Medland SE, Colella S, Groszer M, McAuley EZ, Caffrey TM, et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol Psychiatry. 2007;12:1129–1139. 1057. doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhiling Y, Fujita E, Tanabe Y, Yamagata T, Momoi T, Momoi MY. Mutations in the gene encoding CADM1 are associated with autism spectrum disorder. Biochem Biophys Res Commun. 2008;377:926–929. doi: 10.1016/j.bbrc.2008.10.107. [DOI] [PubMed] [Google Scholar]