Abstract
Immunohistological and elution studies of the human placenta revealed the presence of IgG on the trophoblastic basement membrane (TBM) which demonstrated specificity for placental but not lung, thyroid, or kidney basement membranes, suggesting the presence of a placenta-specific antigen in TBM. IgG comprised the bulk of immunoglobulin in eluates, and small amounts of IgA, trace amounts of IgM, but no IgE or IgD were identified in eluates. The distribution of IgG subclasses in eluate was not unusual as compared to maternal and neonatal sera, and Gm and Inv typing of eluates indicated that it was of maternal origin. Small amounts of eluate-IgG effectively inhibited the blastogenic response of unrelated lymphocytes to old tuberculin, phytohemagglutinin, and in one- or two-way mixed lymphocyte culture reactions. The inhibition was distinct from nonspecific inhibitors, and dose-response analysis indicated that eluate was very much more potent as an inhibitor than were the nonspecific inhibitors. Inhibition was shown to not be due to anti-HL-A activity, and was probably not due to aggregated IgG or immune complexes. Binding of eluate to lymphocytes was very loose as shown by washing experiments, and no binding could be shown by immunofluorescence. The capacity of eluate IgG to inhibit MLC was retained after pepsin digestion to F(ab′)2, suggesting that the inhibition reactions were immunological. It is suggested that eluate-IgG is maternal blocking antibody to a hitherto uncharacterized trophoblast antigen, and it is speculated that either abnormal antigen or aberrant responses to antigen could result in fetal wastage.
Full text
PDF



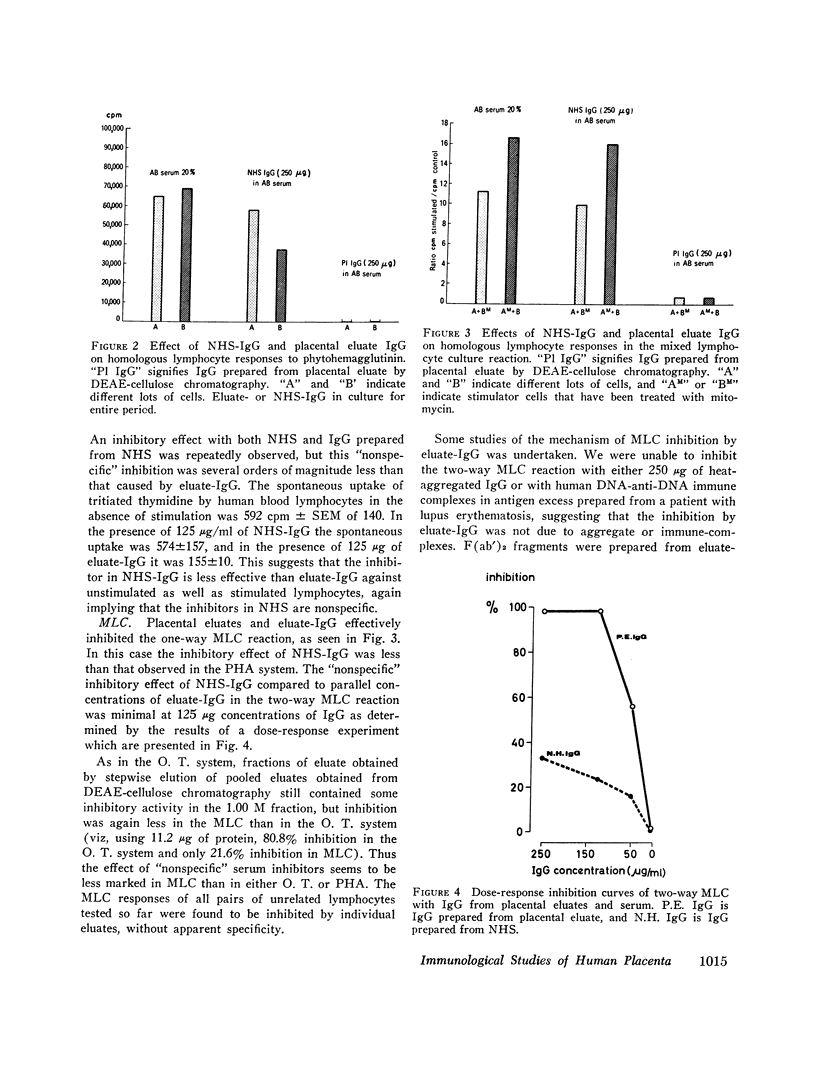
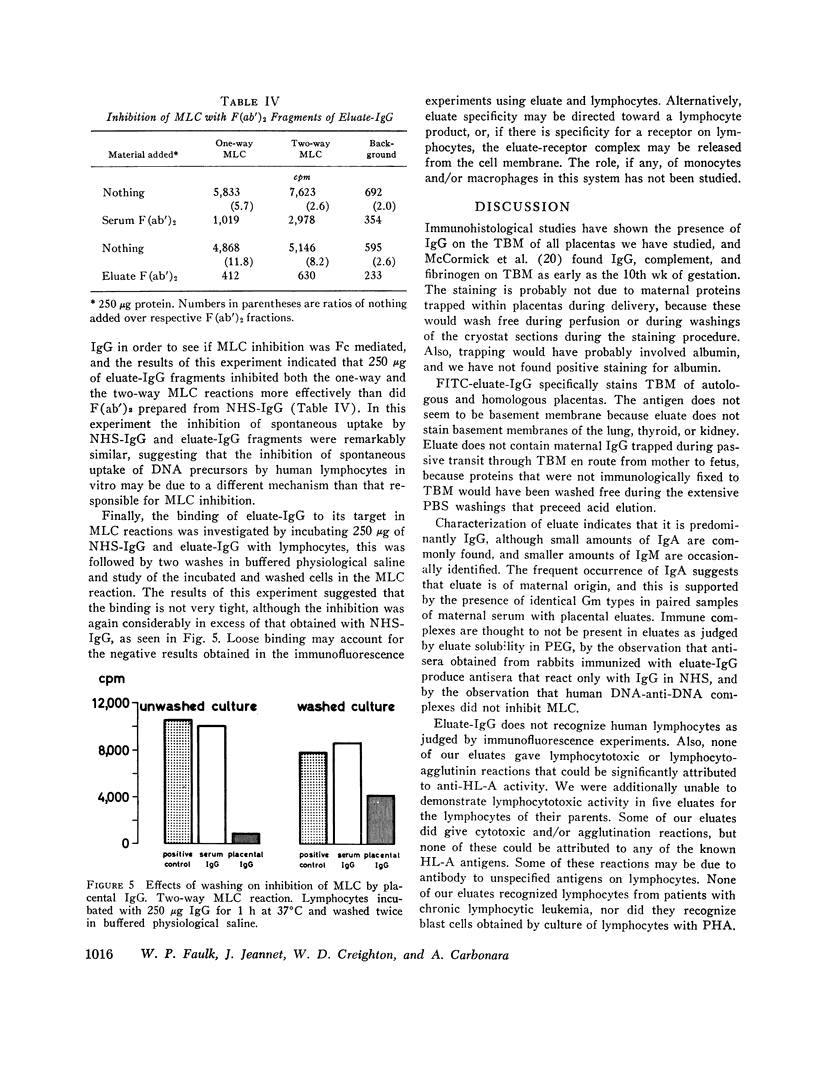



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub J., Kasakura S. In vitro response of foetal lymphocytes to PHA, and a factor plasma which suppresses the PHA response of adult lymphocytes. Clin Exp Immunol. 1971 Mar;8(3):427–434. [PMC free article] [PubMed] [Google Scholar]
- BILLINGHAM R. E. TRANSPLANTATION IMMUNITY AND THE MATERNAL-FETAL RELATION. N Engl J Med. 1964 Mar 26;270:667–CONTD. doi: 10.1056/NEJM196403262701306. [DOI] [PubMed] [Google Scholar]
- Beer A. E., Billingham R. E. Immunobiology of mammalian reproduction. Adv Immunol. 1971;14:1–84. doi: 10.1016/s0065-2776(08)60283-7. [DOI] [PubMed] [Google Scholar]
- Beer A. E., Billingham R. E., Yang S. L. Further evidence concerning the autoantigenic status of the trophoblast. J Exp Med. 1972 May 1;135(5):1177–1184. doi: 10.1084/jem.135.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau M., Latour M., Revillard J. P., Robert M., Traeger J. Blocking antibodies eluted from human placenta. Transplant Proc. 1973 Mar;5(1):589–592. [PubMed] [Google Scholar]
- Bradbury S., Billington W. D., Kirby D. R., Williams E. A. Surface mucin of human trophoblast. Am J Obstet Gynecol. 1969 Jun 1;104(3):416–418. doi: 10.1016/s0002-9378(16)34195-3. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Schiff R. I., Amos D. B. Blocking of autologous and homologous leukocyte responses by human alloimmune plasmas: a possible in vitro correlate of enhancement. J Immunol. 1972 Jan;108(1):34–44. [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Campion P. D., Currey H. L. Cell-mediated immunity in pregnancy. Lancet. 1972 Oct 14;2(7781):830–830. doi: 10.1016/s0140-6736(72)92201-5. [DOI] [PubMed] [Google Scholar]
- Ceppellini R., Bonnard G. D., Coppo F., Miggiano V. C., Pospisil M., Curtoni E. S., Pellegrino M. Transplantation antigens: introductory symposium. Mixed leukocyte cultures and HL-A antigens. I. Reactivity of young fetuses, newborns and mothers at delivery. Transplant Proc. 1971 Mar;3(1):58–63. [PubMed] [Google Scholar]
- Creighton W. D., Lambert P. H., Miescher P. A. Detection of antibodies and soluble antigen-antibody complexes by precipitation with polyethylene glycol. J Immunol. 1973 Oct;111(4):1219–1227. [PubMed] [Google Scholar]
- Eijsvoogel V. P., van Rood J. J., Du Toit E. D., Schellekens P. T. [Position of a locus determining mixed lymphocyte reaction distinct from the known HL-A loci]. Eur J Immunol. 1972 Oct;2(5):413–418. doi: 10.1002/eji.1830020506. [DOI] [PubMed] [Google Scholar]
- FUDENBERG H. H., FUDENBERG B. R. ANTIBODY TO HEREDITARY HUMAN GAMMA-GLOBULIN (GM) FACTOR RESULTING FROM MATERNAL-FETAL INCOMPATIBILITY. Science. 1964 Jul 10;145(3628):170–171. doi: 10.1126/science.145.3628.170. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Hijmans W. Recent developments in immunofluorescence. Prog Allergy. 1972;16:9–39. doi: 10.1159/000393067. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., van Loghem E., Stickler G. B. Maternal antibody to fetal light chain (Inv) antigens. Am J Med. 1974 Mar;56(3):393–397. doi: 10.1016/0002-9343(74)90621-4. [DOI] [PubMed] [Google Scholar]
- Harrington J. C., Fenton J. W., 2nd, Pert J. H. Polymer-induced precipitation of antigen-antibody complexes: "precipiplex" reactions. Immunochemistry. 1971 May;8(5):413–421. doi: 10.1016/0019-2791(71)90504-0. [DOI] [PubMed] [Google Scholar]
- Hay F. C., Torrigiani G., Roitt I. M. The binding of human IgG subclasses to human monocytes. Eur J Immunol. 1972 Jun;2(3):257–261. doi: 10.1002/eji.1830020312. [DOI] [PubMed] [Google Scholar]
- Hulka J. F., Mohr K. Trophoblast antigenicity demonstrated by altered challenge graft survival. Science. 1968 Aug 16;161(3842):696–698. doi: 10.1126/science.161.3842.696. [DOI] [PubMed] [Google Scholar]
- Kasakura S. A factor in maternal plasma during pregnancy that suppresses the reactivity of mixed leukocyte cultures. J Immunol. 1971 Nov;107(5):1296–1301. [PubMed] [Google Scholar]
- Kirby D. R. The immunological consequences of extrauterine development of allogeneic mouse blastocysts. Transplantation. 1968 Dec;6(9):1005–1009. doi: 10.1097/00007890-196812000-00007. [DOI] [PubMed] [Google Scholar]
- Levine B. B., Stember R. H., Fotino M. Ragweed hay fever: genetic control and linkage to HL-A haplotypes. Science. 1972 Dec 15;178(4066):1201–1203. doi: 10.1126/science.178.4066.1201. [DOI] [PubMed] [Google Scholar]
- Linscott W. D. Effect of cell surface antigen density on immunological enhancement. Nature. 1970 Nov 28;228(5274):824–827. doi: 10.1038/228824a0. [DOI] [PubMed] [Google Scholar]
- Loke Y. W., Joysey V. C., Borland R. HL-A antigens on human trophoblast cells. Nature. 1971 Aug 6;232(5310):403–405. doi: 10.1038/232403a0. [DOI] [PubMed] [Google Scholar]
- MCDEVITT H. O., PETERS J. H., POLLARD L. W., HARTER J. G., COONS A. H. PURIFICATION AND ANALYSIS OF FLUORESCEIN-LABELED ANTISERA BY COLUMN CHROMATOGRAPHY. J Immunol. 1963 Apr;90:634–642. [PubMed] [Google Scholar]
- McCormick J. N., Faulk W. P., Fox H., Fudenberg H. H. Immunohistological and elution studies of the human placenta. J Exp Med. 1971 Jan 1;133(1):1–18. doi: 10.1084/jem.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtilo D. T., Hallgren H. M., Yunis E. J. Depressed maternal lymphocyte response to phytohaemagglutinin in human pregnancy. Lancet. 1972 Apr 8;1(7754):769–771. doi: 10.1016/s0140-6736(72)90522-3. [DOI] [PubMed] [Google Scholar]
- Ramseier H., Lindenmann J. Similarity of cellular recognition structures for histocompatibility antigens and of combining sites of corresponding alloantibodies. Eur J Immunol. 1972 Apr;2(2):109–114. doi: 10.1002/eji.1830020203. [DOI] [PubMed] [Google Scholar]
- Riggio R. R., Parrillo J. e., Jr, Bull F. G., Schwartz G. H., Stenzel K. H., Rubin A. L. Inhibition of lymphocyte transformation by a placental glycoprotein. Transplantation. 1971 Nov;12(5):400–401. doi: 10.1097/00007890-197111000-00011. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Zuckerman J. E., Alpert E., David J. R. Effect of multiparity on human maternal hypersensitivity to foetal antigen. Nature. 1973 Jan 12;241(5385):130–131. doi: 10.1038/241130a0. [DOI] [PubMed] [Google Scholar]
- Tongio M. M., Berrebi A., Pfeiffer B., Mayer S. Serological studies on lymphocytotoxic antibodies in primiparous women. Tissue Antigens. 1971;1(6):243–257. doi: 10.1111/j.1399-0039.1971.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Youtananukorn V., Mantangkasombut P. Specific plasma factors blocking human maternal cell-mediated immune reaction to placental antigens. Nat New Biol. 1973 Mar 28;242(117):110–111. doi: 10.1038/newbio242110a0. [DOI] [PubMed] [Google Scholar]
- Youtananukorn V., Matangkasombut P. Human maternal cell mediated immune reaction to placental antigens. Clin Exp Immunol. 1972 Aug;11(4):549–556. [PMC free article] [PubMed] [Google Scholar]
- Yunis E. J., Amos D. B. Three closely linked genetic systems relevant to transplantation. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3031–3035. doi: 10.1073/pnas.68.12.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loghem E., Mårtensson L. Genetic (Gm) determinants of the gamma-2c (Vi) subclass of human IgG immunoglobulins. A study with special reference to Gm(c3) and Gm(c5), and their relationship with the Gm(b) determinants. Vox Sang. 1967 Nov;13(5):369–392. doi: 10.1111/j.1423-0410.1967.tb03782.x. [DOI] [PubMed] [Google Scholar]




