Abstract
Chlamydia trachomatis and smoking are major risk factors for tubal ectopic pregnancy (EP), but the underlying mechanisms of these associations are not completely understood. Fallopian tube (FT) from women with EP exhibit altered expression of prokineticin receptors 1 and 2 (PROKR1 and PROKR2); smoking increases FT PROKR1, resulting in a microenvironment predisposed to EP. We hypothesize that C. trachomatis also predisposes to EP by altering FT PROKR expression and have investigated this by examining NFκB activation via ligation of the Toll-like receptor (TLR) family of cell-surface pattern recognition receptors. PROKR2 mRNA was higher in FT from women with evidence of past C. trachomatis infection than in those without (P < 0.05), and was also increased in FT explants and in oviductal epithelial cell line OE-E6/E7 infected with C. trachomatis (P < 0.01) or exposed to UV-killed organisms (P < 0.05). The ability of both live and dead organisms to induce this effect suggests ligation of a cell-surface-expressed receptor. FT epithelium and OE-E6/E7 were both found to express TLR2 and TLR4 by immunohistochemistry. Transfection of OE-E6/E7 cells with dominant-negative TLR2 or IκBα abrogated the C. trachomatis–induced PROKR2 expression. We propose that ligation of tubal TLR2 and activation of NFκB by C. trachomatis leads to increased tubal PROKR2, thereby predisposing the tubal microenvironment to ectopic implantation.
Tubal ectopic pregnancy occurs in 1%–2% of all pregnancies in Europe and the United States.1 In the Western world, it remains the most common cause of maternal mortality in the first trimester of pregnancy.1, 2
Epidemiological studies indicate that Chlamydia trachomatis infection is a risk factor for tubal ectopic pregnancy, although there is a paucity of solid evidence for the long-term reproductive sequelae of C. trachomatis infection.3, 4 The extent to which C. trachomatis infection accounts for the adhesions, tubal alteration, damage, or occlusion that predispose to ectopic pregnancy or infertility in humans remains largely unknown. However, the mechanisms by which these occur are believed to be primarily immunologically mediated and not a direct consequence of tissue destruction by the organism,5, 6 although more recent evidence does support a cytotoxic effect of C. trachomatis on ciliated epithelium.7 C. trachomatis has been shown to initiate innate immune responses by ligating members of the Toll-like receptor (TLR) family of cell-surface pattern recognition receptors, in particular TLR2 and TLR4.8, 9 TLR ligation initiates downstream signaling, leading to the activation of transcription factors, such as NFκB (nuclear factor kappa-light-chain enhancer of activated B cells), resulting in transcription of immune and inflammatory mediators.10
Experimental animal models (mainly in rodent species) of genital C. trachomatis infection provide clues to disease pathogenesis; however, these experimental infections are usually conducted using defined infectious doses under highly controlled conditions for relatively short periods of time, and in animals that have limited genetic variability and with different C. trachomatis immune evasion strategies compared with humans.11, 12, 13, 14
Using human ex vivo and in vitro models, we have recently investigated how another risk factor for ectopic pregnancy, cigarette smoking, leads to tubal implantation. Tubal ectopic pregnancy is thought to be a consequence of embryo retention within the fallopian tube due to impaired smooth muscle contractility and alterations in the tubal microenvironment.15 Our studies demonstrate that cigarette smoking increases transcription of prokineticin receptor 1 (PROKR1), a G-protein coupled receptor.16 The PROKRs are receptors for PROK1, a molecule known for its angiogenic properties, control of smooth muscle contractility, and regulation of genes important for intrauterine implantation.17, 18, 19 Both PROKR1 and PROKR2 expression are altered in fallopian tube from women with ectopic pregnancy, where implantation has already occurred.20
In this study, we hypothesized that, like cigarette smoking, C. trachomatis infection alters fallopian tube PROKR expression and thereby predisposes the tubal microenvironment to ectopic pregnancy.
Materials and Methods
Tissue Collection
Human fallopian tube biopsies and sera were collected from women during hysterectomy for benign gynecological conditions (n = 38). The median age of the women in this study was 41 years (range, 27–49 years). Approval for this study was obtained from the Lothian research ethics committee (LREC 04/S1103/20), and informed, written consent was obtained from each patient. Tissues were divided into three portions and were (1) stored in Ambion RNAlater stabilization solution (Applied Biosystems, Austin, TX) overnight, after which they were frozen at −80°C for RNA extraction, or (2) fixed in neutral-buffered formalin for 24 hours and mounted in paraffin for immunohistochemical analysis, or (3) stored fresh in PBS for explant culture. Sera were stored frozen at −20°C until use.
Enzyme-Linked Immunosorbent Assay for Evidence of Past C. trachomatis Infection
Past C. trachomatis infection was determined by an enzyme-linked immunosorbent assay that detects serum antibodies to PgP3 protein, as described previously.21 A cutoff value for absorbance at 450 nm of ≥0.473 gave a specificity of ≥96% for identifying positive sera.21
C. trachomatis Detection in Fallopian Tube Biopsies by PCR
All fallopian tube biopsies included in this study were screened for current chlamydial infection by PCR. DNA was extracted from whole fallopian tube biopsies from the ampullary region of the tube, as detailed in the manufacturers' protocol (Qiagen, West Sussex, UK). The PCR protocol used a well-validated, in-house, plasmid-based methodology (kindly developed and designed by the West of Scotland Specialist Virology Centre, Glasgow).
RNA Extraction, cDNA Preparation, and Quantitative Real-Time PCR
RNA was extracted from fallopian tube tissue using TRIzol reagent (Invitrogen, Paisley, UK) according to the manufacturer's recommended method. After extraction, RNA was treated with DNase and was purified using RNeasy (Qiagen, West Sussex, UK). RNA was extracted from cells using the Qiagen RNeasy kit, which included a DNase treatment step. RNA concentrations were quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). Two hundred nanograms of RNA was reverse-transcribed into cDNA using random hexamers, according to the recommended method (Applied Biosystems [ABI], Foster City, CA). TaqMan real-time PCR was used to quantify PROKR mRNA transcript levels. Reactions were performed using an ABI Prism 7900 system, using standard conditions. Previously validated primer and FAM (6-carboxyfluorescein) labeled probe sequences are listed in Table 1. All reactions were performed in triplicate. Gene expression was normalized to RNA loading using primers and VIC labeled probe (Applied Biosystems) for ribosomal 18S as an internal standard and expressed as relative to a positive RNA standard (cDNA from a single mid-secretory endometrial tissue) that was included in all reactions.
Table 1.
qRT-PCR Primer and Probe Sequences
| Primer/probe (label) | Sequence |
|---|---|
| PROKR1 forward | 5′-TCTTACAATGGCGGTAAGTCCA-′3 |
| PROKR1 reverse | 5′-CTCTTCGGTGGCAGGCAT-′3 |
| PROKR1 probe (FAM) | 5′-TGCAGACCTGGACCTCAAGACAATTGG-′3 |
| PROKR2 forward | 5′-GCTCTGTGCCTCCGTCAACT-′3 |
| PROKR2 reverse | 5′-CCAGCAAGGCATTGGTGG-′3 |
| PROKR2 probe (FAM) | 5′-CCTGCGCACCGTCTCCCTCTACG-′3 |
| 18S forward | 5′-CGGCTACCACATCCAAGGAA-′3 |
| 18S reverse | 5′-GCTGGAATTACCGCGGCT-′3 |
| 18S probe (VIC) | 5′-TGCTGGCACCAGACTTGCCCTC-′3 |
Immunohistochemistry for TLR2 and TLR4
Standard immunohistochemistry procedures were used to localize TLR2 and TLR4 in fallopian tube tissue, as described previously.20 A TLR2-specific goat polyclonal antibody (Abcam, Cambridge, UK) was diluted 1:250 in normal horse serum and a TLR4-specific rabbit polyclonal antibody (Abcam) was diluted 1:100 in normal goat serum. Normal goat IgG and rabbit IgG diluted in the same way as the primary antibodies were used as negative controls for TLR2 and TLR4 staining, respectively.
For immunocytochemistry, cells of oviductal epithelial cell line OE-E6/E7 were seeded at 500,000 cells/chamber on BD Falcon chamber slides (BD Biosciences, Oxford, UK) in Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12) (Invitrogen) and incubated overnight at 37°C, 5% CO2. Medium was then removed and adherent cells fixed in 90% acetone, 10% methanol. Fixed cells were blocked in 3% hydrogen peroxide in methanol for 30 minutes, then washed twice in phosphate-buffered saline (PBS). Avidin and biotin blocks were then performed (Vector Laboratories, Peterborough, UK) according to the manufacturer's recommended method. Cells were then washed and blocked in antibody diluent (normal horse serum for TLR2 and normal goat serum for TLR4) for 30 minutes. TLR2 goat polyclonal antibody (Abcam) was diluted 100-fold in antibody diluent for immunocytochemistry; TLR4 rabbit polyclonal antibody (Abcam) was diluted 100-fold. Immunocytochemistry was completed as described above for immunohistochemistry.
Oviductal Epithelial OE-E6/E7 Cell Culture
Oviductal epithelial OE-E6/E7 cells22 were maintained in DMEM/F12 containing 10% fetal bovine serum at 37°C, 5% CO2. For experiments, cells were seeded at 500,000 cells per well of a 12-well dish (BD Biosciences) and cultured for 24 hours, after which the medium was then removed, the cells were washed once with PBS and serum-free DMEM/F12 was added, and the cells were incubated overnight. Cells were exposed either to C. trachomatis (serovar E) as previously described23 or to synthetic TLR ligands, as described below. C. trachomatis exposures included UV-killed C. trachomatis (multiplicity of infection [MOI] 1.0) or live C. trachomatis at MOI values of 0.1 and 1.0 (all diluted in serum-free DMEM/F12). Synthetic ligands were triacetylated lipoprotein (Pam3 CSK4; 100 ng/ml) for TLR2 or monophosphoryl lipid A (MPLA; 100 ng/ml) for TLR4 (InvivoGen, San Diego, CA). Control cells were cultured in medium alone. Cells were treated for 8, 12, 24, or 48 hours, after which the medium was removed and the cells were treated with Qiagen RLT buffer, then frozen at −80°C before RNA extraction.
Fallopian Tube Explant Culture
Fallopian tube biopsies were cultured as described previously.20 Tissues were exposed to C. trachomatis, as described above. After treatment, tissues were treated with TRIzol reagent (Invitrogen) and frozen at −80°C before RNA extraction.
Transient Transfection of OE-E6/E7 Cells with Dominant Negative TLR2
Cells were transiently transfected by electroporation with empty p-zero plasmid (control) or p-zero plasmid containing a dominant negative form of TLR2 in which the conserved Toll-like TIR domain has been removed, rendering TLR2 unable to signal downstream (InvivoGen, Toulouse, France). For electroporation, 400 μL of cells at a concentration of 2.2 × 106 cells/ml in DMEM/F12 + 10% fetal bovine serum were added to each 0.4-cm cuvette (Bio-Rad, Hemel Hempstead, UK) on ice, and 20 μg of plasmid in a volume of 40 μL was added to the cells in each cuvette. Cells were electroporated using a Bio-Rad Gene Pulser system at a voltage of 0.26 kV with infinite internal resistance and maximum capacitance. After electroporation, 200 μL of cells were added to 1 ml of DMEM/F12 + 10% fetal bovine serum in a 12-well dish. Cells were incubated for 48 hours at 37°C, 5% CO2. The medium was then replaced with serum-free DMEM/F12 overnight before cells were treated with C. trachomatis or TLR2 ligand, as described above. After treatments, cells were treated with Qiagen RLT buffer and frozen at −80°C before RNA extraction.
Transformation of OE-E6/E7 Cells with Dominant Negative IκBα
Cells were seeded at 2.5 × 105 cells/ml in a 12-well dish and were cultured for 24 hours at 37°C, 5% CO2. Medium was then removed and replaced with serum-free DMEM/F12, and the cells were cultured overnight. Cells were then treated with either control adenovirus (Ad-d1703) or the same virus but containing a dominant negative IκBα mutant, as described previously.24, 25 Adenovirus was used at an MOI of 100 and the cells were cultured for 24 hours, after which they were treated with either C. trachomatis or with synthetic TLR2 ligand, as described above. After these treatments, cells were treated with Qiagen RLT buffer and were frozen at −80°C before RNA extraction.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software (version 4.02; La Jolla, CA). Differences between groups were analyzed using two-tailed, unpaired t-tests and differences were considered significant when P < 0.05.
Results
PROKR2 mRNA Levels Are Increased in Fallopian Tube from Women with Evidence of Past C. trachomatis Infection
Study participants were divided into two groups on the basis of the ≥0.473 cutoff for absorbance at 450 nm. Fourteen of the women in this study were identified as seropositive for past C. trachomatis infection (mean absorbance, 1.133). The remaining 24 women were identified as seronegative for past infection (mean absorbance, 0.084). The levels of mRNA encoding PROK1, PROK2, PROKR1, and PROKR2 were measured in fallopian tube from patients using quantitative real-time PCR. There were no differences in PROK1 or PROK2 levels in fallopian tube from women with evidence of past C. trachomatis infection, compared with women with no evidence of past infection (data not shown). In one patient sample, PROKR1 levels were undetermined; in the remaining samples, PROKR1 levels were no different between women with and without evidence of past C. trachomatis infection (Figure 1A). PROKR2 levels, however, were significantly higher in fallopian tube from women with evidence of past C. trachomatis infection, compared with women with no evidence of past infection (Figure 1B, P < 0.05). All fallopian tube biopsies were screened for current C. trachomatis infection by PCR and were shown to be negative (data not shown). In addition, all fallopian tube samples were routinely examined under hematoxylin and eosin staining by a gynecological histopathologist. None of the samples showed microscopic evidence of chronic inflammation.
Figure 1.
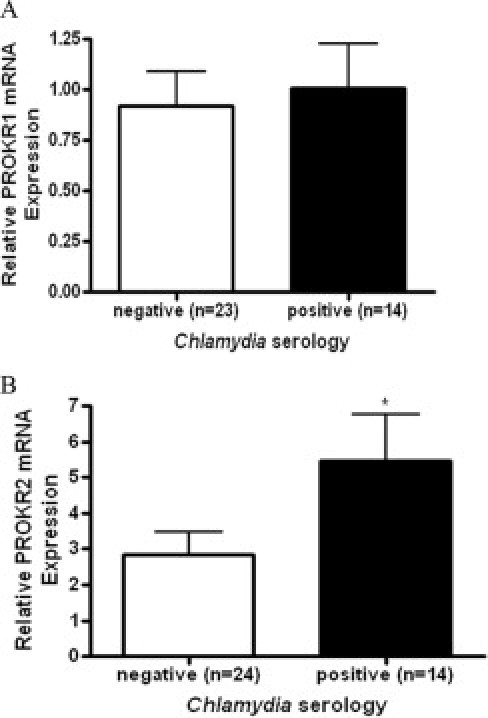
PROKR1 and PROKR2 mRNA levels in women with and without serological evidence of past C. trachomatis infection. Anti-PgP antibodies in patient sera were measured by enzyme-linked immunosorbent assay as evidence of past C. trachomatis infection. Patients were divided into two groups, negative (n = 24) and positive (n = 14), based on a predetermined cutoff value. Quantitative real-time PCR (qRT-PCR) was used to measure PROKR1 (A) and PROKR2 (B) transcript levels in fallopian tube from patients in these two groups, as described under Materials and Methods. *P < 0.05. Error bars indicate the SEM.
Oviductal Epithelial OE-E6/E7 Cell and Fallopian Tube Expression of PROKR2 mRNA Is Increased with Exposure to C. trachomatis
There were no changes in PROKR1 mRNA expression in OE-E6/E7 cells or fallopian tube explants treated with C. trachomatis (data not shown). However, PROKR2 mRNA expression levels were significantly increased after 8 hours in OE-E6/E7 cells (Figure 2) (n = 5). To confirm that the increases seen with the UV-treated C. trachomatis were not due to surviving organisms infecting the cells, we tested the infectious ability of the live and UV-inactivated C. trachomatis using the OE-E6/E7 cell line. We infected the cells with various levels of C. trachomatis for varying lengths of time, from 8 hours to a maximum of 48 hours. By 48 hours, we demonstrated infection of OE-E6/E7 cells by live C. trachomatis, evidenced by the formation of inclusion bodies when cells were fixed and stained with a C. trachomatis--specific lipopolysaccharide antibody (infectivity of approximately 24%; see Supplemental Figure S1A at, http://ajp.amjpathol.org). No infection of cells was demonstrated by the same concentration of UV-killed C. trachomatis even after 48 hours (infectivity of <1%; see Supplemental Figure S1B at, http://ajp.amjpathol.org).
Figure 2.

PROKR2 mRNA levels in OE-E6/E7 cells and fallopian tube explants treated with C. trachomatis in vitro.A: OE-E6/E7 cells (n = 5) were infected with C. trachomatis (MOI 1.0) or exposed to UV-killed organisms (MOI 1.0 equivalent), for 8 hours. qRT-PCR was used to measure PROKR2 mRNA levels in the treated cells. B: Fallopian tube explants (n = 4) were infected with C. trachomatis (MOI 0.1 and 1.0) or exposed to UV-killed organisms (MOI 1.0 equivalent), and PROKR2 mRNA levels were measured using qRT-PCR. Error bars indicate the SEM. *P < 0.05; **P < 0.01.
TLR2 and TLR4 Localize to the Epithelium in Human Fallopian Tube and Are Expressed in Oviductal Epithelial OE-E6/E7 Cells
Both TLR2 and TLR4 were found to be expressed in the fallopian tube epithelium by immunohistochemistry (Figure 3, A and B). In OE-E6/E7 cells, TLR2 was shown to be expressed by immunocytochemistry (Figure 3C), but we could not identify any TLR4 staining (Figure 3D). We have no explanation for the discrepancy between the tissue and OE-E6/7 cell-line expression of TLR4, other than to say that alterations in gene expression can occur in vitro during the immortalization of cells. In addition, changes in the expression of certain proteins may also be the result of long-term culture conditions associated with the use of immortalized cell lines.
Figure 3.
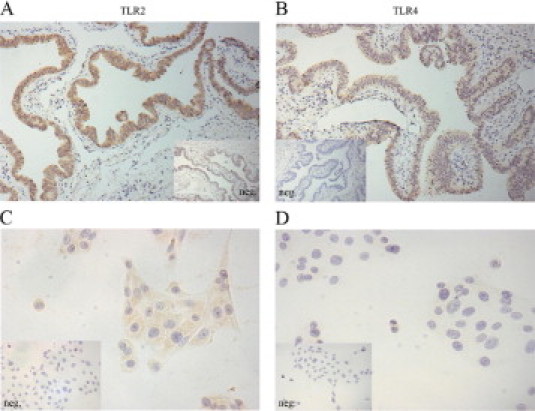
Immunohistochemical localization of TLR2 and TLR4 in fallopian tube tissue and OE-E6/E7 cells. Immunohistochemical staining for TLR2 and TLR4 in human fallopian tube explants and in OE-E6/E7 cells, as described under Materials and Methods. TLR2 (A, C) and TLR4 (B, D). Inset: Negative control, for each primary stain. Magnification, X40.
PROKR2 mRNA Expression in OE-E6/E7 Cells Can Be Induced by TLR2 Activation
Promoter analysis of prokineticin genes has highlighted that they can be activated by TLR signaling.26 Hence, we investigated the effect of treatment with TLR ligands on PROKR2 expression. PROKR2 levels were significantly increased in OE-E6/E7 cells treated with TLR2 ligand (Figure 4A; P < 0.01). In contrast, PROKR2 levels were not altered in cells treated with a synthetic ligand for TLR4 (Figure 4B). Given this finding, the absence of accessory molecules necessary for TLR4 activation in the OE-E6/E7 cells, such as MD2 and CD14, cannot be ruled out. The lack of response to TLR4 ligand may also be due to the lack of TLR4 protein expression identified in OE-E6/E7 cells by immunocytochemistry (Figure 3D).
Figure 4.
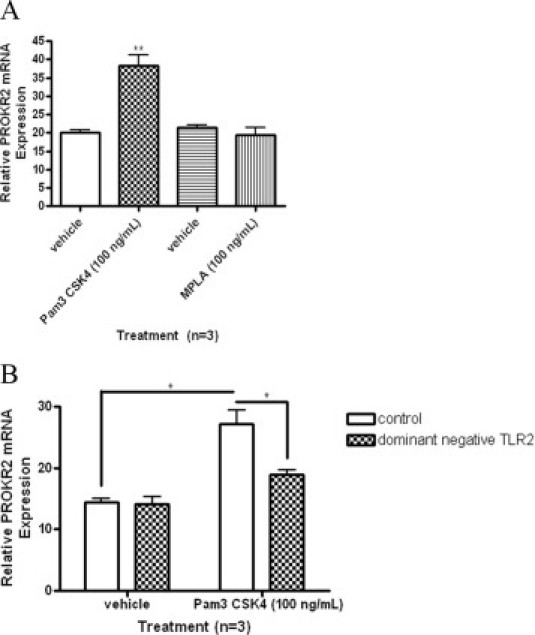
PROKR2 mRNA levels in OE-E6/E7 cells treated with synthetic TLR2 and TLR4 ligands and in cells transfected with dominant negative TLR2. A: OE-E6/E7 cells (n = 3) were treated with a TLR2 ligand (Pam3 CSK4, 100 ng/ml) and a TLR4 ligand (MPLA, 100 ng/ml) for 8 hours. PROKR2 mRNA levels were measured using qRT-PCR, as described under Materials and Methods. B: OE-E6/E7 cells were transiently transfected with a dominant-negative TLR2 construct using electroporation. Transfected cells were treated with synthetic TLR2 ligand for 8 hours and PROKR2 mRNA levels were measured by qRT-PCR. Error bars indicate the SEM. *P < 0.05; **P < 0.01.
To confirm that the increase in PROKR2 expression observed in OE-E6/E7 cells in response to treatment with a TLR2 ligand was mediated through TLR2, cells were transiently transfected with either a control plasmid or a plasmid containing a dominant negative form of TLR2. Transfection of OE-E6/E7 cells with dominant negative TLR2 prevented the increase in PROKR2 expression after treatment with the ligand. No effect was observed in cells transfected with the empty plasmid (Figure 4B). These results confirm that PROKR2 expression can be increased in response to TLR2 ligation in OE-E6/E7 cells. As for the treatment with synthetic TLR ligand, transient expression of the dominant negative TLR2 negated the increase in PROKR2 mRNA expression induced by C. trachomatis infection or by exposure to UV-killed organisms, confirming that C. trachomatis induces PROKR2 expression through activation of TLR2 (Figure 5).
Figure 5.

PROKR2 mRNA levels in OE-E6/E7 cells transiently expressing dominant-negative TLR2 and treated with C. trachomatis. OE-E6/E7 cells (n = 4) were transiently transfected with a dominant-negative form of TLR2 using electroporation. Cells were infected with C. trachomatis (MOI 1.0) or exposed to UV-killed organisms for 8 hours and PROKR2 levels were measured by qRT-PCR, as described under Materials and Methods. Error bars indicate the SEM. *P < 0.05; **P < 0.01.
C. trachomatis-Induced PROKR2 mRNA Expression in OE-E6/E7 Cells Is Partially Mediated by NFκB Activation
OE-E6/E7 cells were transformed with adenovirus containing an empty vector or containing a dominant negative form of IκBα with point mutations at two serine residues required for its phosphorylation. After transformation with adenovirus for 24 hours, cells were infected with C. trachomatis or exposed to UV-killed organisms for 8 hours (n = 3). The increase in PROKR2 mRNA expression induced by either live or UV-killed C. trachomatis was reversed in cells transformed with dominant negative IκBα (Figure 6), indicating that NFκB activates transcription of PROKR2 in response to C. trachomatis infection of OE-E6/E7 cells (although levels of PROKR2 were lower than those reported in Figure 2).
Figure 6.
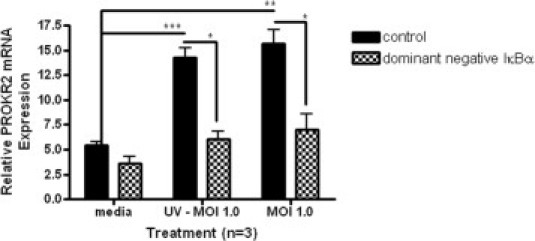
PROKR2 mRNA levels in OE-E6/E7 cells transformed with Adenovirus expressing dominant negative IκBα and exposed to C. trachomatis. OE-E6/E7 cells (n = 3) were transformed with a control adenovirus or adenovirus expressing a dominant-negative form of the NFκB inhibitor, IκBα. Cells were infected with C. trachomatis (MOI 1.0) or exposed to UV-killed organisms (MOI 1.0 equivalent) and PROKR2 mRNA levels were measured by qRT-PCR, as described under Materials and Methods. Error bars indicate the SEM. ***P < 0.001; **P < 0.01; *P < 0.05.
Discussion
Here we present a mechanism that may explain the epidemiological link between C. trachomatis infection and tubal ectopic pregnancy. Alterations in PROK signaling have been previously shown to promote a microenvironment conducive to embryo implantation.20, 27, 28 We propose that C. trachomatis infection increases tubal expression of PROKR2 mRNA, thus predisposing to ectopic implantation in the fallopian tube, and that this effect is mediated by TLR2 and NFκB.
To support this hypothesis, we report that expression levels of PROKR2 mRNA are higher in fallopian tube from women with serological evidence of past C. trachomatis infection, compared with women with no serological evidence of past infection. In addition, we demonstrate that exposure of fallopian tube explants and an oviductal epithelial cell line (OE-E6/E7) to C. trachomatis in vitro causes an upregulation of PROKR2 mRNA expression. We show that this effect is rapid (within 8 hours) and is seen also with UV-killed C. trachomatis, suggesting involvement of a pattern recognition receptor. We demonstrate that the pattern recognition receptor TLR2 is expressed in the fallopian tube epithelium and that PROKR2 mRNA levels are increased in OE-E6/E7 cells treated with a synthetic TLR2 ligand. We also show that transient expression of a dominant negative TLR2 by OE-E6/E7 cells reduces the induction of PROKR2 expression caused by TLR2 ligand or C. trachomatis treatment. Finally, we demonstrate that transformation of OE-E6/E7 cells with an adenovirus expressing the inhibitor protein IκBα (a dominant negative inhibitor of NFκB) partially negates the induction of PROKR2 expression resulting from TLR2 ligand or C. trachomatis treatment. This suggests that activation of TLR2 signaling by C. trachomatis results in the activation of NFκB, which is partially responsible for induction of PROKR2 transcription in the fallopian tube.
Our data suggest that TLR2 ligation by C. trachomatis in the human fallopian tube is an important feature of the early host immune response to infection, comparable to results from studies in mice.29, 30 Macrophages and fibroblasts from homozygous TLR2 knockout mice have been found to produce less inflammatory cytokines than heterozygous TLR2 knockouts in response to C. trachomatis infection.31 Similar results were also reported in in vitro studies where mouse dendritic cells and macrophages were infected with C. pneumoniae.29, 30 Furthermore, oviducts of homozygous TLR2 knockout mice showed no evidence of chronic inflammatory responses after C. trachomatis infection was resolved, compared with mice expressing wild-type TLR2.31 These results, in addition to our findings in human fallopian tube, suggest that TLR2 may be responsible for initiating the long-term immunological responses associated with C. trachomatis infection.
TLR2 ligation by C. trachomatis can occur on the cell surface as well as intracellularly, where TLR2 recruitment to C. trachomatis inclusion bodies has been reported.9 Our data demonstrating activation of TLR2 signaling by both live and UV-killed C. trachomatis suggests cell surface ligation and does not rule out intracellular ligation in oviductal epithelial cells. The precise components of C. trachomatis that ligate TLR2 in OE-E6/E7 cells are unknown. Previous studies have demonstrated activation of TLR2 by both endogenous heat-shock protein 60 (HSP60)32 and HSP60 produced by C. pneumoniae.33 Results from the current study allow us to speculate that HSP60 from C. trachomatis may also activate TLR2 signaling.
We also demonstrate novel regulation of PROKR2 mRNA expression either directly or indirectly by NFκB. Regulation of PROK and PROKR expression has not been studied extensively. However, these genes have been shown to be hormonally regulated in the endometrium (PROK1) and fallopian tube (PROK2, PROKR1)34, 20; regulated by hypoxia in human trophoblast (PROK1 and PROKR1)35; and regulated by cytokines, such as granulocyte colony-stimulatory factor and granulocyte-macrophage colony-stimulatory factor, in human immune cells (PROK2).36 Regulation by NFκB is relevant in ectopic pregnancy, because NFκB signaling plays a central role in controlling expression of proinflammatory genes and genes important for activating immune responses, such as cytokines, chemokines, and adhesion molecules; in the endometrium, expression of active NFκB components, such as p65 and p50, has been shown to be increased during the window of implantation.37, 38, 39
We believe that our findings not only are important in the context of ectopic pregnancy but may have wider implications in helping to explain the association of C. trachomatis with other pathologies. C. trachomatis infection is also associated with pelvic inflammatory disease40 and ocular trachoma.41 Pelvic inflammatory disease is thought to be caused by C. trachomatis in 20% to 40% of cases and accounts for 18% of cases of infertility42 and 5% of cases of chronic pelvic pain.43 These statistics have led to the introduction of a national C. trachomatis screening program in England (http://www.chlamydiascreening.nhs.uk). Ocular trachoma, caused by C. trachomatis infection of the conjunctival epithelium, has blinded >1 million people in the developing world.44 The most recent estimate from the World Health Organization placed the burden of disease at approximately 1.3 million disability-adjusted life-years.41 Thus, further understanding of the effects of C. trachomatis on epithelial cell function, inflammation, and the microenvironment should lead to improvements in disease control.
Footnotes
This work was supported by a Wellbeing of Women Project Grant (RG993) (A.W.H., H.N.J., G.E., H.O.D.C.). H.N.J. was supported by Medical Research Council (MRC) core funding (U.1276.00.004.00002.02). G.E. was funded by the Scottish Government Rural and Environmental Research and Analysis Directorate (RERAD). A.W.H. is supported by an MRC Clinician Scientist Fellowship.
Supplemental material for this article can be found on http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2010.11.019.
Supplementary data
Supplemental Figure 1.
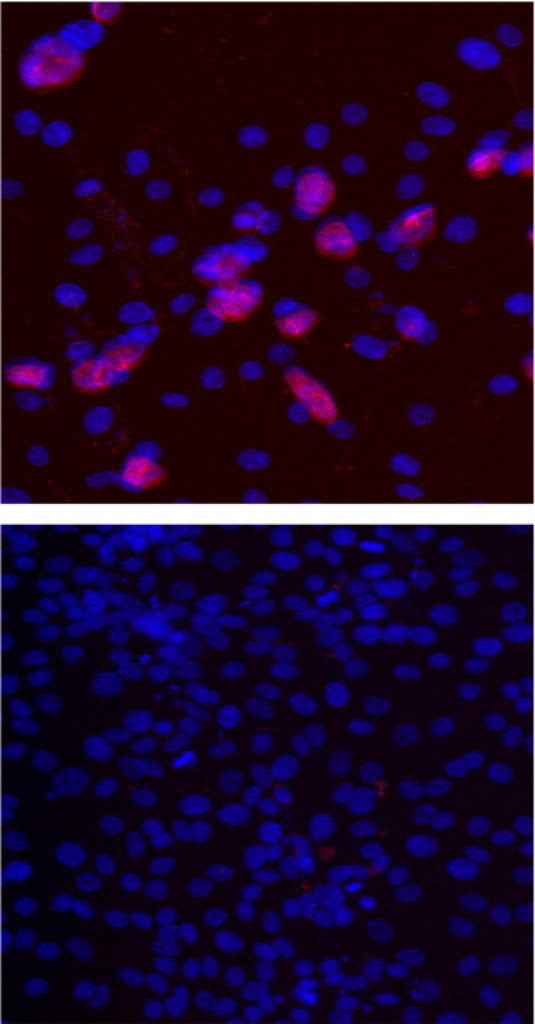
Chlamydia trachomatis inclusions in OE-E6/E7 cells treated with live and UV-killed C. trachomatis. Oviductal epithelial cell line OE-E6/E7 cells were infected with live and UV-killed C. trachomatis (MOI 1.0) for 48 hours. Inclusion bodies formed in fixed cells were stained with C. trachomatis-specific lipopolysaccharide antibody (indicated by red fluorescence). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue staining).
References
- 1.Farquhar C.M. Ectopic pregnancy. Lancet. 2005;366:583–591. doi: 10.1016/S0140-6736(05)67103-6. [DOI] [PubMed] [Google Scholar]
- 2.Walker J.J. Ectopic pregnancy. Clin Obstet Gynecol. 2007;50:89–99. doi: 10.1097/GRF.0b013e31802f4f79. [DOI] [PubMed] [Google Scholar]
- 3.Ankum W.M., Mol B.W., Van der Veen F., Bossuyt P.M. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril. 1996;65:1093–1099. [PubMed] [Google Scholar]
- 4.Bakken I.J., Skjeldestad F.E., Nordbø S.A. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case-control study. Sex Transm Dis. 2007;34:166–169. doi: 10.1097/01.olq.0000230428.06837.f7. [DOI] [PubMed] [Google Scholar]
- 5.Rice P.A., Schachter J. Pathogenesis of pelvic inflammatory disease: What are the questions? JAMA. 1991;266:2587–2593. [PubMed] [Google Scholar]
- 6.Beatty W.L., Morrison R.P., Byrne G.I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hvid M., Baczynska A., Deleuran B., Fedder J., Knudsen H.J., Christiansen G., Birkelund S. Interleukin-1 is the initiator of fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol. 2007;9:2795–2803. doi: 10.1111/j.1462-5822.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 8.Joyee A.G., Yang X. Role of Toll-like receptors in immune responses to chlamydial infections. Curr Pharm Des. 2008;14:593–600. doi: 10.2174/138161208783885344. [DOI] [PubMed] [Google Scholar]
- 9.O'Connell C.M., Ionova I.A., Quayle A.J., Visintin A., Ingalls R.R. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions: Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem. 2006;281:1652–1659. doi: 10.1074/jbc.M510182200. [DOI] [PubMed] [Google Scholar]
- 10.Fukata M., Vamadevan A.S., Abreu M.T. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Rey-Ladino J., Koochesfahani K.M., Zaharik M.L., Shen C., Brunham R.C. A live and inactivated Chlamydia trachomatis mouse pneumonitis strain induces the maturation of dendritic cells that are phenotypically and immunologically distinct. Infect Immun. 2005;73:1568–1577. doi: 10.1128/IAI.73.3.1568-1577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajonuma L.C., Chan P.K., Ng E.H., Fok K.L., Wong C.H., Tsang L.L., Tang X.X., Ho L.S., Lau M.C., Chung C.M., He Q., Huang H.Y., Yang D.Z., Rowlands D.K., Chung Y.W., Chan H.C. Involvement of cystic fibrosis transmembrane conductance regulator (CFTR) in the pathogenesis of hydrosalpinx induced by Chlamydia trachomatis infection. J Obstet Gynaecol Res. 2008;34:923–930. doi: 10.1111/j.1447-0756.2008.00826.x. [Erratum appeared in J Obstet Gynaecol Res 2009, 35:833, and in J Obstet Gynaecol Res 2009, 35:1004] [DOI] [PubMed] [Google Scholar]
- 13.Meoni E., Faenzi E., Frigimelica E., Zedda L., Skibinski D., Giovinazzi S., Bonci A., Petracca R., Bartolini E., Galli G., Agnusdei M., Nardelli F., Buricchi F., Norais N., Ferlenghi I., Donati M., Cevenini R., Finco O., Grandi G., Grifantini R. CT043, a protective antigen that induces a CD4+ Th1 response during Chlamydia trachomatis infection in mice and humans. Infect Immun. 2009;77:4168–4176. doi: 10.1128/IAI.00344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey A.J., Cunningham K.A., Hafner L.M., Timms P., Beagley K.W. Effects of inoculating dose on the kinetics of C. muridarum genital infection in female mice. Immunol Cell Biol. 2009;87:337–343. doi: 10.1038/icb.2009.3. [DOI] [PubMed] [Google Scholar]
- 15.Shaw J.L., Dey S.K., Critchley H.O., Horne A.W. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update. 2010;16:432–444. doi: 10.1093/humupd/dmp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw J.L., Oliver E., Lee K.F., Entrican G., Jabbour H.N., Critchley H.O., Horne A.W. Cotinine exposure increases fallopian tube PROKR1 expression via nicotinic AChRα-7: a potential mechanism explaining the link between smoking and tubal ectopic pregnancy. Am J Pathol. 2010;177:2509–2515. doi: 10.2353/ajpath.2010.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado-Pérez D., Evans J., Denison F., Millar R.P., Jabbour H.N. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab. 2007;18:66–72. doi: 10.1016/j.tem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans J., Catalano R.D., Morgan K., Critchley H.O., Millar R.P., Jabbour H.N. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877–2887. doi: 10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Bullock C.M., Knauer D.J., Ehlert F.J., Zhou Q.Y. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 20.Shaw J.L., Denison F.C., Evans J., Durno K., Williams A.R., Entrican G., Critchley H.O., Jabbour H.N., Horne A.W. Evidence of prokineticin dysregulation in fallopian tube from women with ectopic pregnancy. Fertil Steril. 2010;94:1608–1608.e1. doi: 10.1016/j.fertnstert.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills G.S., Horner P.J., Reynolds R., Johnson A.M., Muir D.A., Brown D.W., Winston A., Broadbent A.J., Parker D., McClure M.O. Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin Vaccine Immunol. 2009;16:835–843. doi: 10.1128/CVI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y.L., Lee K.F., Xu J.S., Wang Y.L., Tsao S.W., Yeung W.S. Establishment and characterization of an immortalized human oviductal cell line. Mol Reprod Dev. 2001;59:400–409. doi: 10.1002/mrd.1046. [DOI] [PubMed] [Google Scholar]
- 23.King A.E., Wheelhouse N., Cameron S., McDonald S.E., Lee K.F., Entrican G., Critchley H.O., Horne A.W. Expression of secretory leukocyte protease inhibitor and elafin in human fallopian tube and in an in-vitro model of Chlamydia trachomatis infection. Hum Reprod. 2009;24:679–686. doi: 10.1093/humrep/den452. [DOI] [PubMed] [Google Scholar]
- 24.Jobin C., Haskill S., Mayer L., Panja A., Sartor R.B. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol. 1997;158:226–234. [PubMed] [Google Scholar]
- 25.Henriksen P.A., Hitt M., Xing Z., Wang J., Haslett C., Riemersma R.A., Webb D.J., Kotelevtsev Y.V., Sallenave J.M. Adenoviral gene delivery of elafin and secretory leukocyte protease inhibitor attenuates NF-kappa B-dependent inflammatory responses of human endothelial cells and macrophages to atherogenic stimuli. J Immunol. 2004;172:4535–4544. doi: 10.4049/jimmunol.172.7.4535. [DOI] [PubMed] [Google Scholar]
- 26.Catalano R.D., Lannagan T.R., Gorowiec M., Denison F.C., Norman J.E., Jabbour H.N. Prokineticins: novel mediators of inflammatory and contractile pathways at parturition? Mol Hum Reprod. 2010;16:311–319. doi: 10.1093/molehr/gaq014. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado-Pérez D., Brown P., Morgan K., Millar R.P., Thompson E.A., Jabbour H.N. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochim Biophys Acta. 2009;1793:1315–1324. doi: 10.1016/j.bbamcr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans J., Catalano R.D., Brown P., Sherwin R., Critchley H.O., Fazleabas A.T., Jabbour H.N. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Netea M.G., Kullberg B.J., Galama J.M., Stalenhoef A.F., Dinarello C.A., Van der Meer J.W. Non-LPS components of C. pneumoniae stimulate cytokine production through Toll-like receptor 2-dependent pathways. Eur J Immunol. 2002;32:1188–1195. doi: 10.1002/1521-4141(200204)32:4<1188::AID-IMMU1188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Prebeck S., Kirschning C., Dürr S., da Costa C., Donath B., Brand K., Redecke V., Wagner H., Miethke T. Predominant role of Toll-like receptor 2 versus 4 in C. pneumoniae-induced activation of dendritic cells. J Immunol. 2001;167:3316–3323. doi: 10.4049/jimmunol.167.6.3316. [DOI] [PubMed] [Google Scholar]
- 31.Darville T., O'Neill J.M., Andrews C.W., Jr, Nagarajan U.M., Stahl L., Ojcius D.M. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 32.Tsan M.F., Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 33.Costa C.P., Kirschning C.J., Busch D., Dürr S., Jennen L., Heinzmann U., Prebeck S., Wagner H., Miethke T. Role of chlamydial heat shock protein 60 in the stimulation of innate immune cells by C. pneumoniae. Eur J Immunol. 2002;32:2460–2470. doi: 10.1002/1521-4141(200209)32:9<2460::AID-IMMU2460>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Battersby S., Critchley H.O., Morgan K., Millar R.P., Jabbour H.N. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann P., Feige J.J., Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- 36.Zhong C., Qu X., Tan M., Meng Y.G., Ferrara N. Characterization and regulation of bv8 in human blood cells. Clin Cancer Res. 2009;15:2675–2684. doi: 10.1158/1078-0432.CCR-08-1954. [DOI] [PubMed] [Google Scholar]
- 37.Page M., Tuckerman E.M., Li T.C., Laird S.M. Expression of nuclear factor kappa B components in human endometrium. J Reprod Immunol. 2002;54:1–13. doi: 10.1016/s0165-0378(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 38.King A.E., Collins F., Klonisch T., Sallenave J.M., Critchley H.O., Saunders P.T. An additive interaction between the NFkappaB and estrogen receptor signalling pathways in human endometrial epithelial cells. Hum Reprod. 2010;25:510–518. doi: 10.1093/humrep/dep421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 40.Manavi K. A review on infection with Chlamydia trachomatis. Best Pract Res Clin Obstet Gynaecol. 2006;20:941–951. doi: 10.1016/j.bpobgyn.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Hu V.H., Harding-Esch E.M., Burton M.J., Bailey R.L., Kadimpeul J., Mabey D.C. Epidemiology and control of trachoma: systematic review. Trop Med Int Health. 2010;15:673–691. doi: 10.1111/j.1365-3156.2010.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haggerty C.L., Gottlieb S.L., Taylor B.D., Low N., Xu F., Ness R.B. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(Suppl 2):S134–S155. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 43.Paavonen J., Eggert-Kruse W. Chlamydia trachomatis: impact on human reproduction. Hum Reprod Update. 1999;5:433–447. doi: 10.1093/humupd/5.5.433. [DOI] [PubMed] [Google Scholar]
- 44.Burton M.J., Mabey D.C. The global burden of trachoma: a review. PLoS Negl Trop Dis. 2009;3:e460. doi: 10.1371/journal.pntd.0000460. [DOI] [PMC free article] [PubMed] [Google Scholar]


