Abstract
We investigated metabolizable energy intake (MEI) and milk energy output in European hares throughout gestation and lactation in females raising three young, i.e., close to maximum litter size in this precocial species. We hypothesized that herbivorous hares may face a central limitation of energy turnover during lactation, imposed by maximum capacity of the gastrointestinal tract. Females were provided with low-energy or high-energy diets, either continually, or during lactation only. Unexpectedly, females on either diet reached identical peak MEIs (> 6 times BMR) during late lactation, with females on low-energy diet increasing food intake proportionally. Thus, we reject our hypothesis that in lactating hares, peak MEI is centrally limited. During early lactation, MEI and milk transfer was however significantly impaired in females on the low-energy diet, indicating a temporal central limitation due to a time-lag caused by the readjustment of energy intake capacity. Importantly, irrespective of the diet, females significantly increased peak MEI late in the breeding season. Consequently, earlier in the season, when energy reserves are still high, energy throughput was not limited by physiological constraints at all. We conclude that extreme MEI may have fitness costs, and that females maximize lifetime reproductive success by actively down-regulating MEI whenever possible.
Keywords: sustained metabolic rate, lactation, food quality, Lepus europaeus, milk production, energy turnover
Introduction
In response to high metabolic requirements during lactation, mammals increase rates of food intake and energy assimilation several-fold. However, it has been shown repeatedly that there are limits to energy turnover, as energy intake and milk production are not raised indefinitely, even in presence of unlimited food (Daan and Drent 1980; Weiner 1992; Hammond et al. 1994; Hammond et al. 1996; Hammond and Kristan 2000; Johnson et al. 2001a, b, c; Johnson and Speakman 2001). Thus, lactating mammals may not only be limited by food availability in the environment, but also by so-called maximum sustained metabolic rates (SusMRs, e.g., Hammond and Diamond 1997), or rates of sustained energy intake (SusEI). These findings have raised questions about the underlying causes of metabolic ceilings. One explanation is that SusMR does not actually reflect a physiologically possible upper limit, but rather is an evolved trait that maximizes fitness by limiting energy turnover during each reproductive bout (e.g., Drent and Daan 1980). Alternatively, upper levels of SusMR may indicate intrinsic physiological bottlenecks. One of the oldest ideas on the nature of these bottlenecks, usually summarized under the term “central limitation hypothesis”, suggests that peak energy intake is limited by the capacity of nutrient- processing, visceral organs (intestines, liver, kidneys; see Gross et al. 1985; Hammond and Wunder 1991;Weiner 1992; Koteja 1996; Bacigalupe and Bozinovic 2002). Another explanation is an upper limit to the maximum performance of “peripheral” tissues, such as mammary glands in lactating females (Hammond et al. 1994; McDevitt and Speakman 1994; Hammond et al. 1996; Hammond and Kristan 2000; Speakman et al. 2001). More recently, evidence was accumulating for a third explanation, namely that the maximum rate of heat dissipation sets the limit for lactating females (Speakman and Krol 2005; Krol et al. 2007).
There is, however, no reason to assume that identical physiological constraints, if any, must determine SusMR in different mammalian species. For instance, it has been argued that herbivores, which consume large amounts of low-energy food, may be more prone to central limitation than, for instance, seed-eaters that are specialized on a diet with high energy density (sensu Hackländer et al. 2002a). This question was addressed by a study on herbivorous European hares, a species which has high costs of lactation reaching levels of SusEI that are larger than five times BMR (Hackländer et al. 2002a). In that study, lactating females kept on a diet enriched with fat outperformed those on a low-fat diet in terms of both metabolizable energy intake (MEI) and milk energy output (Hackländer et al. 2002a, b). While these results clearly supported the central limitation hypothesis, they were obtained from females with variable, natural litters sizes (1-3 young) and with very few females actually operating at SusEI. Therefore, the first objective of the present study was to reevaluate whether female hares with close to maximal litter sizes (i.e., three young) are in fact unable to compensate for a low-energy diet by a proportional increase in food intake and MEI during peak lactation. In addition to the assessment of possible effects on MEI, we also intended to determine dietary effects on milk energy output. Fortunately, European brown hares provide an excellent model for measurements of milk transfer. As females nurse their young only once per day (Broekhuizen and Maaskamp 1980), their milk output can be determined easily and accurately by weighing pups before and after suckling.
Typically, any compensatory increase of food intake in response to changes in metabolic demands or food quality is accompanied by adjustments in the size and function of the gastrointestinal tract (e.g. Gross et al. 1985; Hammond and Wunder 1991; Starck 1999; Hammond and Kristan 2000; Piersma and Drent 2003). However, growth of the gastrointestinal tract requires some time (e.g. Piersma and Drent 2003), possibly leading to a temporal central limitation during early lactation. In farm animals, there is indeed evidence for such a limitation during early lactation caused by the time-lag between increasing energy requirements and the adjustment of intake capacity (reviewed in Forbes 2007). These problems will be even amplified if food quality is fluctuating, particularly when a sudden decrease in diet quality occurs during lactation. Therefore, the second objective of the present study was to evaluate the effects of a sudden switch between high and low food quality, at the time of parturition, on MEI and milk energy output.
To address these questions, we fed females which had high energy demands, i.e., with an experimentally manipulated litter size of three young, two diets of different energy content during gestation and lactation. Two control groups were continuously maintained on a high-fat or low-fat diet, respectively. Females in two further experimental groups were either switched from the high-fat to the low-fat diet at parturition, or vice versa. In all groups, MEI was measured continuously throughout gestation and lactation. We predicted that, if a central limitation constrains SusEI in European hares, females kept on the low-fat diet would be unable to fully compensate for its low energy content, and would have significantly reduced rates of MEI and during peak (i.e., late) lactation. We also predicted that a possible temporal central limitation (due to a time lag in the adjustment of intake capacity) should lead to significantly reduced rates of MEI during early lactation, either in all females kept on the low-fat diet, or in the high- to low-fat switch-group only. If, on the other hand, peripheral limitation by mammary gland capacity was limiting for hares, we expected that milk energy output at fixed litter sizes should be constant, irrespective of the diet.
Materials and Methods
Animals and housing
European hares (also called Brown hares) were born and kept in our outbred breeding colony at the Research Institute of Wildlife Ecology, University of Veterinary Medicine Vienna, Austria (48° 14′ N 16° 20′ E). Hares were housed individually in cages as outlined in Hackländer et al. (2002 a, b). All females and their young were provided with water and food ad libitum. Animals were fed either a “low-fat diet”, i.e., standard hare pellets (Raiffeisen, Salzburg, Austria) produced to match the mean chemical composition of stomach contents from free-ranging hares (Brüll 1976; Onderscheka and Tataruch 1982), or a “high-fat diet”, i.e., pellets enriched with sunflower oil (12.5 kg oil per 100 kg pellets). The mean gross energy content of the low-fat diet over the whole study period was 16.7 ± 0.02 kJ g−1 (dry weight) with 16.6 ± 0.05% protein, 70.7 ± 0.09% fibre, and 3.0 ± 0.06% fat. Gross energy content of the high-fat diet was 18.8 ± 0.03 kJ g−1, with 15.4 ± 0.04% protein, 62.8 ± 0.10% fibre and 12.09 ± 0.12% fat. To ensure that the fat and energy content were stable throughout the study, we analysed dietary fat content every time the pellets were mixed with oil. The chemical analyses of the diet were carried out as outlined in Hackländer et al. (2002a). Females were assigned to, and always stayed within, one of four experimental groups. Groups HH and LL were provided high-fat or low-fat diet, respectively, throughout the study. Group LH was maintained at low-fat diet two weeks prior to and during gestation (approximately six weeks), but switched to high-fat diet on the day following parturition. Vice versa, group HL was switched from high-fat to low-fat diet on the day following parturition. To reduce a potential novelty–effect of the newly presented food quality, we added 5 pellets (~2 g) of the familiar food quality to the food rack on the first day of the diet switch.
Data were sampled between February 2004 and October 2007. Data analysis was restricted to a total of 53 mothers and their 91 litters which successfully weaned three young after four weeks (28 days) of lactation. Due to differences in rates of pregnancies, and particularly, rates of success in raising large litters, sample sizes differed between treatments. The number of females (N) for which these data could be obtained was N=16, 17, 13, and 7 for groups HH, HL, LH, and LL, respectively), and the number of lactations (n) in these groups was n= 28, 27, 22, and 14, respectively. Data were obtained during three seasons, see below (n=28, 41, and 22 is spring, summer, and autumn, respectively). Most females contributed either 1 (N=23) or 2 litters (N=23), 6 females had 3 litters each, and 1 female 4 had litters.
All experimental animals were aged between 1 and 5 years and were in good health and condition. Hares were exposed to natural photoperiod and to indoor temperatures in an unheated housing facility, with temperatures ranging from 8 °C to 25° C over the study period. Mean ambient temperature varied by less than 2.5° C among the three seasons studied (see below). During the nine months reproductive period each year, body weight of all animals was determined weekly to the nearest gram. Food intake was determined over bi-weekly feeding trials (three or four day intervals) by weighing offered and uneaten food in all females. Food items spilled from the racks were dried, weighed, and subtracted from food consumption. To minimize effects of changes in humidity on food mass, food pellets were stored next to the cages prior to their usage.
Total faeces produced by the females were collected biweekly over three and four day intervals, dried at 60° C in a drying oven (Heraeus, Germany) for 48 hours and then weighed to the nearest 0.1 g (Ohaus, Germany). Gross energy content of faeces was determined by near infrared spectroscopy (NIRS) as outlined in detail in Valencak et al. (2009). The following parameters were determined: dry matter, protein, fat, ash, acid detergent fiber (ADF) and lignin. For calibration of the NIRS analysis, 80 samples were chemically analyzed using standardized methods for crude protein, crude fat, crude ash and dry matter (Nehring, 1960). ADF and lignin were determined by the Van Soest detergent analyses (Otzelberger, 1983). The NIR calibration results were evaluated by cross validation. Coefficients of determination for fat, protein, ash, lignin and dry matter were 0.93, 0.93, 0.83, 0.87, 0.87, and 0.96, respectively.
Females were paired with males for two days three times per year, i.e., in February-March (spring), May-June (summer) and late July-August (autumn). To allow litter size manipulations, pairings took place synchronously each time. Immediately after birth of the young (40.8 ± 0.13 days after mating), litter sizes were manipulated to achieve a litter size of three for all females investigated. We did not fully cross-foster litters but in most cases, females gave birth to two pups (mean litter sizes: 2.06, 1.85, 2.04, and 2.00 in groups HH, HL, LH, and LL, respectively; P=0.67; repeated measurements ANOVA) and we added one pup from another female which was then left without pups until the next mating. All females readily accepted and nursed additional young. Long-term data from our breeding colony show that only 10.1% of all females have litters larger than three leverets (n=813 litters) and very few females are able to successfully wean more than three young. Thus, by raising three leverets, all females in our experiment had high (see also Hackländer et al. 2002a), and approximately equal energy requirements. During all seasons, small milk samples (< 3 ml, N=53) from a subsample of females of all dietary groups were collected close to peak lactation (at day 10-14 of the lactation period) and each sample was chemically analyzed as outlined in Hackländer et al. (2002a). Mean milk fat, protein and lactose contents were 220.42 ± 20.35 mg g−1, 150.92 ± 12.25 mg g−1, and 13.50 ± 0.98 mg g−1, respectively.
Because young are nursed only once a day in the wild (Broekhuizen and Maaskamp 1980), females were kept separately from their young, except for a short nursing period in the morning (8-9 am). Milk intake of young was measured daily by weighing the leverets before and after the 1 h suckling period to the nearest 0.1 g (and total milk transfer by mothers was computed from the sum of these weight changes). Initial trials showed that weight losses during the nursing period due to faecal and urinary losses in juveniles were negligible (<2 g per juvenile) compared to the milk intake (~60g). Therefore, faecal and urinary losses during these nursing periods were not determined. During suckling, leverets had no access to other food sources. Otherwise, all leverets had ad libitum access to high-fat pellets (irrespective of the mother’s diet), and GEI of each litter was determined at weekly intervals, using the same methods as for adult females.
Computation of energy contents and statistical analyses
Energy content of solid food and faeces was calculated by using energetic values given in Livesey (1984) and Livesey and Marinos (1988). Thus, we used gross energy contents of 23.3 kJ g−1 for protein, 39.6 kJ g−1 for fat, and 17.5 kJ g−1 for fibre/ Nitrogen Free Extracts (NFE). Gross energy intake (GEI) was computed from the amount of food consumed per day multiplied by its energy content. Metabolizable energy intake (MEI) was calculated by (i) correcting GEI for urinary energy losses due to nitrogen excretion by using a metabolizable protein energy content of 19.3 kJ g−1 (Livesey 1984) and (ii) computing the difference between this corrected, utilizable GEI and the energy content of the daily amount of faeces excreted. The estimated average urinary energy loss was 3.3% of GEI. Assimilation efficiency (AE) was computed as MEI / GEI *100. The conversion factors above (using 19.3 kJ g−1 for protein), as well as an energetic value of 16.5 kJ g−1 of lactose (Stubbs et al. 1997), were also used to compute milk energy content. To estimate daily milk energy output per female, individual daily milk mass transfer (see below) was multiplied by mean energy content of the sample from each dietary group (n= 16, 20, 7, 9; for groups HH, HL, LH, and LL, respectively).
All statistical analyses were computed in R (2.8.0.; R Development Core Team 2008). Data on MEI, AE, and milk energy transfer in females, as well as growth of young (weaning, birth weights) and solid food intake of litters were analysed with a repeated measures design, as data within and partly between study years were sampled from the same animals. We fitted linear mixed effect models with separate intercepts for each female included as the random factor, using the R-package nlme (Pinheiro et al. 2008). Fixed effects in full multiple regression models were always diet (either high-fat or low fat-diet during the periods investigated, or dietary group (HH, HL, LH, LL); see Results) time interval (bi-weekly intervals during gestation and lactation), body weight, season (spring, summer, autumn), female age (1-5 years), and mean ambient temperature over the measurement interval. In all analyses concerning gestation, i.e., before diets were switched in two of the subgroups, we differentiate only between high- and low-fat diets. In some cases, (e.g., MEI; Fig. 2) separate linear regression models were computed for the initial (intervals 1-5) and the peak phase of the lactation period (intervals 6-8). Otherwise, time interval was treated as a factor, rather than a continuous variable. Non-significant terms were removed from the final models. In lme models testing for differences in juvenile growth, we used “litter” as the random factor. Solid food intake of juveniles (determined for entire litters only) was compared between dietary groups using simple linear (lm) models, using averages over all four lactation weeks.
Fig. 2.
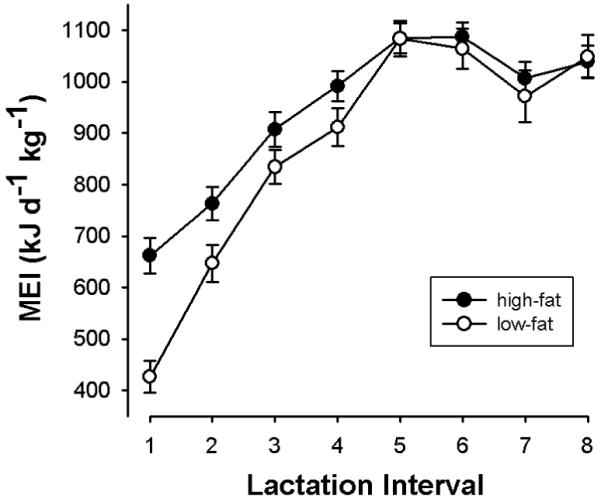
Time course of weight-specific MEI over the lactation period in females fed a high-fat (high; N=28; n=50) or low-fat (low; N=23, N=41) diet. The abscissa shows half-weekly intervals after parturition. MEI in females fed the low-fat diet started at lower values and increased faster (MEI [kJ d−1 kg−1] =312.1 + 156.1 x interval, for intervals 1-5) than in females on the high-fat diet (MEI [kJ d−1 kg−1]=568.4 + 107.3 x interval). Means ± s.e.m. from pooled data over all seasons.
ANOVAs from lme and lm models were computed using marginal (Type III) sums of squares. Residuals from all models were normally distributed and showed no evidence for heterogeneity of variances. Post-hoc comparisons between time intervals or dietary groups were carried out using Tukey contrasts within the R-package multcomp (Hothorn et al. 2008). Data are presented as means ± s.e.m.
In no case had ambient temperature a significant effect on any of the response variables and it is therefore not further mentioned in Results. Effects of body mass differences between females were eliminated by inserting body weight as a fixed covariate in all statistical models for data obtained from females. For graphical representations only, effects of body mass differences were removed by showing weight-specific data.
Results
Gestation
Expectedly, MEI increased with increasing body weight (F1,688=29.37, P<0.0001; N=53, n=91). Mean MEI during gestation, adjusted for differences in body weights, was higher in animals on the low-fat diet than on the high-fat diet (F1,688=38.07, P<0.0001; Fig 1.). As mean body weight in low-fat females (N=23; 3.456 ± .026 kg) was somewhat lower than among high-fat females (N=28; 3.778 ± 0.019 kg; F1,700=0.265, P=0.60) this difference was most pronounced when weight-specific MEIs were compared (Fig. 1; main graph). All females continuously gained weight during gestation, from 3.428 ± 0.082 kg at interval 2 to 3.855 ± 0.050 kg at interval 11. Despite this weight gain, MEI decreased over the last two gestation weeks (interval 8-11; Fig 1; F9,688=18.23, P<0.0001, for overall differences between time intervals). This was reflected by significant decreases in MEI during both intervals 10 and 11 compared to interval 8 (P<0.001 in each case, post hoc comparisons).
Fig. 1.
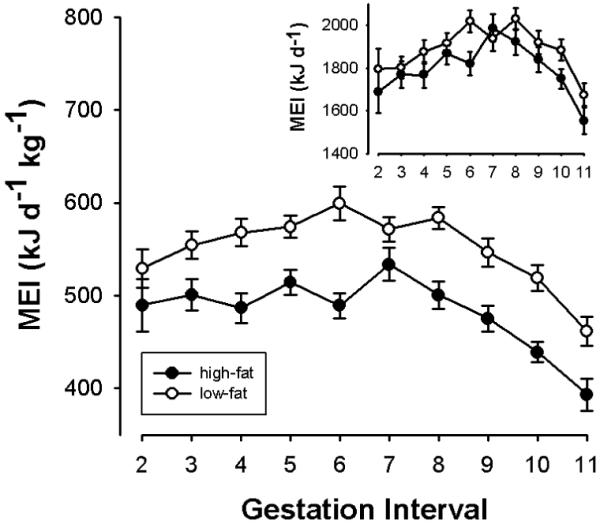
Time course of weight-specific (main graph) and total (inset graph) MEI over the gestation period in females fed a high fat (high; N=28, n= 50) or low fat (low; N=23; n=41) diet. The abscissa shows half-weekly intervals after fertilization. Means ± s.e.m. from pooled data over all seasons from N=53 females and n=91 gestation periods.
MEI during gestation differed between seasons, but this effect was diet-dependent (F[diet:season]2,688 =8.76; P=0.0002): Among females on the high-fat diet, MEI increased from 458.2 ± 8.0 kJ d−1 kg−1 (n=15) to 476.7 ± 9.5 kJ d−1 kg−1 (n=24) and 509.8 ± 9.1 kJ d−1 kg−1 (n=11) in spring, summer, and autumn, respectively. Among females on the low-fat diet, an increase in MEI was observed only in autumn (spring: 549.4 ± 11.0 kJ d−1 kg−1 (n=13); summer: 545.0 ± 6.8 kJ d−1 kg−1 (n=17); autumn 563.9 ± 10.6 kJ d−1 kg−1 (n=11)).
AE during gestation was higher in females on the high-fat diet (67.8 ± 0.21%; n=50) compared to the low-fat diet (63.0 ± 0.20%; n=41; F1,687=38.2, P<0.0001). AE slightly decreased over the gestation period (from 67.7 ± 0.77% to 64.8 ± 0.55%; F=1,687=29.9; P<0.0001). Further, AE was ~3% lower in younger females (age 1-3; n=75; 65.2 ± 0.32%) compared to older females (age 4-5; n=16; 68.3 ± 0.20%; F4, 687=11.59, P<0.0001).
Lactation
MEI and Assimilation Efficiency
The diet provided during gestation had no effect on MEI during the subsequent lactation period, neither on the initial increase in MEI (F1, 369=2.46, P=0.17), nor on peak MEI during lactation (F1, 247= 0.178, P=0.67; n=50 vs. 41). Peak MEI was reached at interval 5, and its overall mean was 1084.8 ± 31.0 kJ d−1 kg−1, which is equivalent to 6.3 times BMR. However, irrespective of the preceding diet, the time course of MEI significantly differed between females fed the low-fat and high-fat diets during lactation (Fig. 2). Among females on the low-fat diet (groups LL and HL), MEI started at lower levels, and increased with significantly larger slopes during lactation intervals 1-5 (F [diet: interval] 1, 370= 20.819, P<.0001) compared to females on the high-fat diet (groups LH and HH). Accordingly, mean MEI was 14 % lower in animals on the low-fat diet during intervals 1-5. MEI of the animals on the two diets converged at interval 5 (Fig. 2), and diet had no further effect on MEI during peak lactation (intervals 5-8; F1, 247= 0.178, P= 0.67).
A potential problem of experiments involving sudden switches of diet are “novelty effects”, i.e., the avoidance or preference of new food items offered, irrespective of their nutrient content. However, comparing the levels of food intake (g d−1) immediately after the diet switch (intervals 1 and 2 of lactation) between groups kept at the same or a new diet, we found no evidence for such a novelty effect (groups LL (n=14) vs. HL (n=27): F1,49=2.236, P=0.14; groups HH (n=28) vs. LH (n=22): F1,57=0.738, P=0.39). Also, there was no significant difference in MEI between groups of hares on a constant diet and diet-switch groups (offered another diet after parturition) during week one of lactation (P>0.12 for both comparisons).
MEI during lactation again increased with body weight (F1, 579=24.084, P<0.0001; n=91). Further, mean MEI was significantly (F2, 579=14.15, P<0.0001) affected by season, with higher mean MEIs in autumn (976.8 ± 21.6 kJ d−1 kg−1; n=22) than in spring (912.8 ± 21.9 kJ d−1 kg−1; n=28) and summer (875.16 ± 15.5 kJ d−1 kg−1; n=41), irrespective of the diet. As illustrated in Fig. 3, this effect of season was also observed during peak lactation (F2, 247=3.87, P=0.022).
Fig. 3.
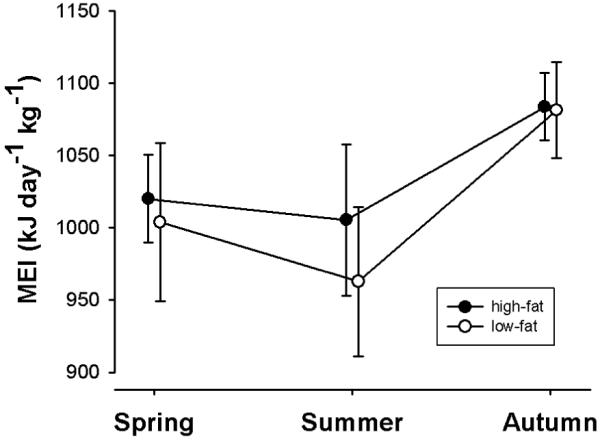
Peak MEI of lactating female hares in spring, summer, and autumn. MEI significantly increased in autumn in both females fed a low-fat and a high-fat diet. Means ± s.e.m from 15, 24, and 11 lactation periods in females on high-fat diet, and 13, 17 and 11 lactation periods in females on the low-fat diet, for spring summer and autumn, respectively.
AE during lactation was significantly higher among females on the high-fat diet (69.8 ± 0.39%; n=50) than on the low-fat diet (62.4 ± 0.59%; n=41; F1,579=127.0, P<0.0001). Also, irrespective of the diet, AE was slightly higher in autumn (68.1 ±0.62%) than in spring (66.5 ± 0.69%) and summer (65.6 ± 0.58%; F2,579=7.81, P<0.001). During lactation, mean AE per interval varied between 62.6% and 71.0%, but did not change systematically.
Milk energy output
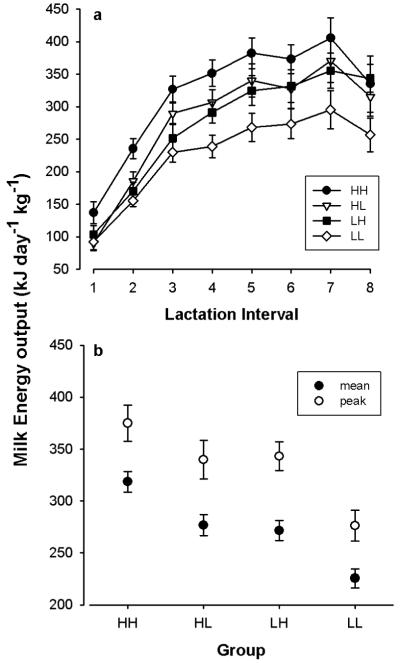
Similar to MEI, milk energy transfer to young was positively associated with body weight (F1, 586=18.27, P<0.0001; n=91). Milk energy output increased significantly during the first half of the lactation period (F7, 586=92.16, P<0.0001) and then leveled off (Fig. 4a). Interestingly, mean milk energy output was affected by both the diet during the preceding gestation and the current diet during lactation, as indicated by significant differences between the experimental groups (F3,586=20.29, P<0.0001; Fig. 4b). Compared to the diet-switch groups LH and HL, which had a similar intermediate milk energy output (P=0.99; post-hoc comparisons), milk energy transfer was significantly decreased in group LL (P<0.001) and increased in group HH (P<0.01). This was due to differences in both milk volume and energy content (Table 1).
Fig. 4.
Milk energy transfer from females to young. a) Time course of milk energy output over the lactation period (half-weekly intervals) in females fed a high-fat diet throughout gestation and lactation (HH), a low-fat diet during both intervals (LL), or females switched from a high- to low-fat diet (HL), or vice versa to a high-fast diet (LH), at parturition. b) Mean milk energy transfer in the dietary groups over the entire lactation period (mean) and during peak lactation (intervals 5-8). Means ± s.e.m from 28, 27, 22, and 14 lactation periods in groups HH, HL, LH, and LL, respectively.
Table 1.
Effects of experimental diets on daily milk mass and milk energy content in lactating females
| HH | HL | LH | LL | |
|---|---|---|---|---|
| Female body mass (kg) | 3.592 | 3.615 | 3.267 | 3.640 |
| Milk mass (g d−1 kg−1) | 24.55 ± 0.72 | 21.67 ± 0.71 | 23.33 ± 0.78 | 21.52 ± 0.82 |
|
| ||||
| Milk energy (kJ g−1) | 12.98 ± 0.12 | 12.85 ± 0.18 | 11.58 ± 0.08 | 10.48 ± 0.12 |
Data are means ± s.e.m from N=16, 17, 13, and 7 females, and n= 28, 27, 22, and 14 lactation periods, in groups
HH, HL, LH, and LL, respectively
HH: High-fat diet throughout study
LL: Low-fat diet throughout study
HL: High-fat diet during gestation, low-fat diet during lactation
LH: Low-fat diet during gestation, high-fat diet during lactation
Milk energy output also differed between seasons (F2,586=43.57, P<0.0001), irrespective of the dietary group. Energy transfer was highest in spring (342.0 ± 11.28 kJ d−1 kg−1; n=28), and lower in both summer (243.4 ± 6.12 kJ d−1 kg−1; n=41) and autumn (265.6 ± 8.32 kJ d−1 kg−1; n=22).
Juveniles
Birth weights and growth
Mean birth weights of young in the four experimental groups were almost identical (125.3 ± 2.1 g (n=28), 125.4 ± 2.3 g (n=27), 123.5 ± 2.4 g (n=22), 122.2 ± 3.5 g (n=14) for groups HH, HL, LH, LL, respectively), and unaffected by diet during gestation (F1,87=0.385, P=0.54). Despite the differences in milk uptake, growth and hence weaning weights (on day 28) were also independent of the mothers diet during lactation (677.7 ± 14.0 g and 690.3 ± 14.1 g, for young of mothers on the high-fat and low-fat diet, respectively; F1,87=0.21, P=0.65).
Energy intake
Notable intake of solid food in juveniles occurred only in weeks three and four of lactation (Fig. 5). Mean GEI from solid food was lowest in offspring of females on the HH diet, intermediate in groups HL and LH, and highest in group LL offspring (Table 2; F3,85 = 5.11, P=0.003). Thus, there was an apparently compensatory increase of GEI from solid food intake (from ~8% to ~20%) as energy intake via milk decreased (Table 2). In contrast to females, GEI in juveniles was unaffected by season (F2,85 = 0.9477, P = 0.39).
Fig. 5.
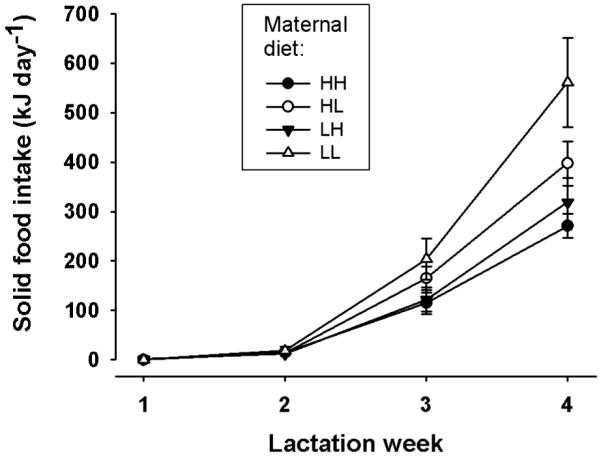
Time course of gross energy intake of juvenile hares from solid food over the 4 week lactation period. All juveniles were fed identical (high-fat) pellets, but their mothers were fed a high fat-diet throughout gestation and lactation (HH), a low-fat diet during both intervals (LL), were switched from a high to low-fat diet (HL), or vice versa to a high-fast diet (LH), at parturition. Means ± s.e.m from 28, 27, 22, and 14 lactation periods in groups HH, HL, LH, and LL, respectively.
Table 2.
Gross energy intake (GEI) from milk and solid food of litters (3 juveniles each) in the four dietary groups
| HH | HL | LH | LL | |
|---|---|---|---|---|
| GEI from milk (kJ day−1) | 1145.9 ± 36.2 | 1003.5 ± 38.1 | 886.5 ± 32.3 | 818.9 ± 32.5 |
| GEI from solid food (kJ day−1) | 97.8 ± 9.8 | 144.1 ± 16.1 | 108.0 ± 16.8 | 195.8 ± 28.6 |
|
| ||||
| % GEI from solid food | 7.8 | 12.5 | 8.9 | 19.3 |
Data are means ± s.e.m for n= 28, 27, 22, and 14 litters from groups HH, HL, LH, and LL, respectively.
HH: High-fat diet throughout study
LL: Low-fat diet throughout study
HL: High-fat diet during gestation, low-fat diet during lactation
LH: Low-fat diet during gestation, high-fat diet during lactation
Discussion
Gestation
Contrary to typical small rodents, which give birth to a large number of altricial young each time they reproduce (e.g., Gilbert 1986), European hares only have few pups, averaging two young in our study (see also Hackländer 2002a, b). Yet, female hares produced much less tissue (mean litter weight at birth was ~7 % of initial maternal weights) than, for instance, laboratory mice (mean litter size 11; litter weight equaling ~65% of maternal weight, e.g., Johnson et al. 2001a) over a gestation period which is twice as long, i.e., 42 versus 21 days. Therefore, one would expect that peak rates of energy intake during gestation are much lower in hares (or other precocial mammals) than in altricial small rodents. This was not the case, however. MEI increased to up to 2.8 times BMR in hares. This is similar to peak rates during gestation in laboratory mice (~2.6 times BMR, Johnson 2001a), and is well within in the range of elevations in other small mammals (reviewed in Thompson 1992), including another precocial species, the guinea pig (2.4 times BMR, see Künkele 2000). We conclude that hares, similar to other mammals, increased MEI during early gestation not just to cover the immediate costs of the production of young, but to accumulate energy reserves to support the subsequent late gestation and the lactation period (see also Künkele 2000; VandeHaar 1999). This conclusion is also supported by the observation that females in our study gained, on average, 82.4g ± 1.1 body weight, from shortly (1-3 days) before gestation until immediately (1 day) after parturition. Increased deposition of energy reserves would also explain why females on the low-fat diet - with presumably lower initial body fat stores – (see also below) showed significantly higher rates of MEI throughout gestation (Fig. 1), although in our sample, this elevated energy intake did not elicit faster body weight gains among low-fat females.
The last (~2 week) period of gestation in hares was characterized by a significant decline of MEI (Fig. 1). This finding seems surprising, since females increased body weight until the end of gestation. However, similar late-gestation declines in food intake have also been observed in small rodents such as mice (e.g., Johnson et al. 2001a; Speakman et al. 2001), guinea pigs (Künkele 2000) or rats (Ingvartsen and Andersen 2000), and are a well-known phenomenon in livestock, e.g., sows, ewes and dairy cattle (Forbes 1970; Ingvartsen and Andersen 2000; Forbes 2007). It has been suggested that this late-gestation recession in food intake is caused by the physical compression of the stomach (or rumen) from the growing uterus, but this hypothesis has been contradicted by experimental evidence showing more pronounced declines in animals on an energy-rich, less voluminous diet (reviews in Ingvartsen and Andersen 2000; Forbes 2007). We suggest that this apparently “programmed” (likely steroid-dependent, c.f. Ingvartsen and Andersen 2000) reduction of appetite may ultimately serve to lower predation risks associated with foraging. In hares, a reduction of food intake during late gestation should be particularly important at a time when high body weight undoubtedly impairs escaping from predators at maximum running speeds which are four times higher than expected from a mammal of this size (Garland 1983).
Lactation
We originally hypothesized that peak MEI during late lactation may depend on food quality, and would be lower in animals on the low-fat diet, as predicted by the central limitation hypothesis (Weiner 1992; Koteja 1996; Hammond and Diamond 1997; reviewed in Speakman and Krol 2005 and Speakman 2008). We found, however, no differences in SusEI between hares from our dietary groups (interval 5-6, Fig. 2). Since the efficiency of assimilation was even higher in animals on the high-fat diet, the peak level of energy intake in females on low-fat diet was entirely achieved by a large (+27.5 %) increase in food intake. Therefore- in contrast to conclusions from earlier studies on females with varying litter size (Hackländer et al. 2002a) our present data (with much larger sample sizes) indicate that European hares flexibly respond to differences in food quality, without facing a central limitation imposed by the capacity of the gastrointestinal tract. This is true, at least, for peak lactation, and for the environmental conditions and differences in food quality tested here. Thus, our study on European hares provides further support for the increasing evidence that gut capacity does not usually set the upper limit in lactating mammals (reviews in Speakman and Krol 2005; Speakman 2008).
During early lactation, on the other hand, MEI was clearly impaired in females kept on the low-fat diet (Fig. 2). This difference was related to the diet provided during lactation only, without detectable carry-over effects of the previous food-type. We interpret this result as an indication for a temporary central limitation of MEI in European hares. As in other mammals (Speakman and McQueenie 1996; Naya et al. 2008), the gastrointestinal tract of lactating hares significantly increases during lactation, compared to non-reproducing controls (TGV, unpublished data). Arguably, it takes time until the size and function of gastrointestinal organs matches elevated energy requirements (Piersma and Drent 2003), and females in our experiments probably had low gut-sizes at the onset of lactation, given the significant reduction of food intake during late gestation. Our measurements of time courses of MEI (Fig. 2) are consistent with the hypothesis that full capacity of the digestive system of females on the low-fat diet was only reached after approximately two weeks. The alternative explanation, that females on the low-fat diet actively down-regulated MEI during this early phase seems unlikely, because these females did eventually increase food intake to match that of mothers on the high-fat diet. Thus, a genuine central limitation during early lactation in females on the low-fat diet appears to be the most parsimonious explanation for this effect.
It has been shown that considerable time is necessary to grow the gastrointestinal tract in response to changing energy demands or diet quality. In birds, for instance, it takes several days to approximately two weeks to fully adjust the mass of their gizzards to changes in food quality (Starck 1999; Dekinga et al. 2001). It seems that in eco-physiological research on mammals, the time necessary to adjust gut function to changing energy demands or fluctuating food composition has received less attention in the past. However, suboptimal food intake during early lactation is another phenomenon that is well known from farm animals, such as ewes, and particularly dairy cows (Weldon et al. 1994; reviews in VandeHaar 1999; Forbes 2007). In cows, endogenous limits to food intake frequently induce a severely negative energy balance and significant losses of body mass in the first lactation phase (e.g., Villa-Godoy et al. 1988; Butler and Smith 1989). For lactating laboratory mice, there is also strong evidence that the adjustment of gut size and function in response to increasing energy demands takes time to develop (Speakman et al. 2001). Both domestic animals and laboratory mice were, however, artificially selected for high reproductive output and, particularly in dairy cows, for maximum milk production. Therefore, one could argue that in these cases the mismatch between energy-intake and –output results from artificial selection for “asymorphosis”, i.e., for high energy output (namely maximum capacity of mammary glands) which is not matched by equal selection for high energy input. This was, however, clearly not the case in our outbred colony of European hares, which has been maintained without selection for any particular trait. Therefore, a temporary central limitation of energy intake may well play an import role in free-living mammals, especially in species exposed to large environmental fluctuations (c.f. Veloso and Bozinovic 2000). In herbivores living in agricultural areas, such as hares, these fluctuations will be further augmented by harvesting of crops, which represents a sudden dramatic decrease in the availability of high-quality food for this species (Marboutin and Aebischer 1996).
Seasonal effects
Recently, we found that MEIs in lactating hares maintained on a high-caloric diet changed over the breeding season with significantly higher rates of energy intake in autumn, compared to spring and summer (Valencak et al. 2009). In this previous study we could directly show, using a marker substance, that females used body fat reserves accumulated in the previous winter (Zörner 1996) to augment milk fat content when breeding early in the year, but not in autumn when fat deposits were already largely depleted (Valencak et al. 2009). This leads to higher total investments into young born early in the year, which have a higher reproductive value (Valencak et al. 2009; see also Speakman and Krol 2005). Our present data extend these observations by showing that this seasonal pattern also occurs during gestation, and is not restricted to females continuously given a high-fat diet, but is also detectable in animals on a low-fat diet (Fig. 3). Higher rates of MEI were independent of ambient temperature, and due to both higher food intake and a slight, but statistically significant increase in AE, indicating that they were accompanied by improved energy assimilation capacity.
The principal inference from this seasonal pattern is that females were able to increase MEI at peak lactation (in autumn), but chose to maintain peak MEI at significantly lower levels during large parts of the breeding season, i.e., spring and summer. These results reinforce our earlier conclusion that peak MEI during lactation in hares is a regulated life-history trait, instead of being imposed by physiological constraints, such as central, peripheral or other limits (Valencak et al. 2009). In other words, energy turnover during late lactation apparently levels off “because exertion beyond this level is detrimental to parent survival” (Drent and Daan 1980). Speakman and Krol 2005 and Speakman 2008 discuss possible mechanisms, such as oxidative stress, involved in this trade-off model, although it seems that we still have to understand how these costs actually translate into fitness trade-offs (Speakman 2008).
Milk energy output
Milk energy output was the only variable investigated that was affected not only by the current diet of lactating mothers but also by the preceding diet (Fig. 4). Milk volume and milk energy content were highest in females on the continuous high-fat diet, lowest in the permanent low-fat group, and intermediate in the diet-switch groups (Table 1). We probably were able to detect these differences in milk energy transfer only because hares, with their brief, once-a-day nursing period, provide an excellent model in which milk output can be quantified precisely. The observed differences between experimental groups indicate that milk energy output of females was affected by both body fat reserves accumulated before parturition and current food quality during lactation. Hares provided with additional fat during gestation were able to transfer more and richer milk to young. Those animals given a low-fat diet during gestation, on the other hand, could not fully compensate for the deficiency in fat reserves by adjusting MEI.
These observations allow several conclusions: (i) Significant differences in milk energy output between dietary groups argue against any limiting role of the capacity of mammary glands (i.e., peripheral constraints) for lactation performance. (ii) Milk transfer is not simply determined by pup demand, as milk energy output differed despite the constant litter size. (iii) Reproductive investment in hares is affected not only by long-term cycles in fat stores (i.e., deposits accumulated in the non-breeding season), but also by short term fat accumulation between reproductive bouts and during gestation. (iv) Short-term changes in food quality immediately affect milk energy output, since females switched to a lower quality diet could not fully compensate by adequately increasing MEI during early lactation. This last conclusion confirms previous results by Hackländer et al. (2002a) who pointed out that the high susceptibility of milk energy output to food quality may be one of the crucial factors for the decline of European hare populations in the past decades (Mitchell-Jones et al. 1999). This decline was paralleled by a strong intensification of agriculture leading to a reduction in the abundance of weeds, which are highly preferred dietary plants of this species (Reichlin et al. 2006).
Juveniles
Juvenile hares were able to fully compensate for impaired milk energy intake, by a proportional increase in solid food intake (Fig. 5). Thus, weaning weights were unaffected by the mother’s diet, as in previous laboratory studies in European hares (Hackländer et al. 2002a; Valencak et al. 2009). However, it remains to be shown whether juvenile milk intake affects their “quality” in terms of, e.g., tissue composition, immunocompetence or other factors which might have long-term effects on fitness. Also, it is clear that this outcome was partly caused by our laboratory setting, in which leverets had ad libitum access to (high-fat) solid food. In free-living juveniles, one would expect both higher energy expenditure and predation risk due to increased foraging in those juveniles receiving less energy from their mothers. Consequently, the lack of differences in growth and weaning weights observed here, does not mean that maternal food quality has no effect on juvenile development, and recruitment in natural populations of European hares.
Conclusions
The results of this study indicate that SusEI in hares during peak lactation was neither limited by gut capacity, nor by maximum capacity of mammary glands, nor determined by pup demand. The fact that females in autumn were able to significantly increase SusEI above previous levels supports our previous view (Valencak et al. 2009) that peak SusEI in European hares should be considered a regulated trait instead of being imposed by any physiological constraint. While there certainly are environmental conditions under which females may reach actual physiologically imposed limits, it appears that typically, maternal investment in this species is actively controlled to maximize lifetime reproductive success. Early during lactation, females on a low-quality diet did face, however, a temporary energetic bottleneck, presumably because time was needed to enlarge the alimentary tract. This kind of temporary central limitation, which represents an example for a ‘lag-time limit’ to phenotypic flexibility (DeWitt et al. 1998), may well also occur in other free-living herbivores which face a low energy content of their diet. We observed that even a short term limitation of MEI affected total milk output, which expectedly has long term consequences on the offspring’s fitness. Thus, for hares, limits to the speed of reactive adjustments of nutrient intake capacity in response to fluctuating food quality have to be considered ecologically relevant.
Acknowledgements
This work was funded by grant P17794-B06 from the Austrian Science Fund, and by the province of Lower Austria. We are grateful to the animal house facility staff (Michaela Salaba, Peter Steiger) and to numerous students who helped with hare welfare and data sampling. We would like to thank Eva Steiger and Le Minh Hien who performed all chemical analyses under direction of Frieda Tataruch. All experiments described here comply with the current laws in Austria, where the experiments were performed.
Glossary
Alphabetical list of abbreviations
- AE
Assimilation Efficiency
- ADF
Acid Detergent Fibre
- BMR
Basal Metabolic Rate
- GEI
Gross Energy Intake
- LM
Linear Models
- LME
Linear Mixed Effects Models
- MEI
Metabolizable Energy Intake
- NFE
Nitrogen Free Extracts
- NIRS
Near Infrared Spectroscopy
- SusEI
Sustained Energy Intake
- SusMR
Sustained Metabolic Rate
References
- Bacigalupe LD, Bozinovic F. Design, limitations and sustained metabolic rate: lessons from small mammals. J Exp Biol. 2002;205:2963–2970. doi: 10.1242/jeb.205.19.2963. [DOI] [PubMed] [Google Scholar]
- Broekhuizen S, Maaskamp F. Behavior of does and leverets of the European hare (Lepus europaeus) whilst nursing. J Zool (Lond) 1980;191:487–501. [Google Scholar]
- Brüll U. Nahrungsbiologische Studien am Feldhasen in Schleswig-Holstein: ein Beitrag zur Äsungsverbesserung. In: Pielowski Z, Pucek Z, editors. Ecology and management of the European hare populations. Polish Hunting Association; Warszawa: 1976. pp. 93–99. [Google Scholar]
- Butler WR, Smith RD. Interrelationships between energy balance and postpartum reproductive function in dairy cattle. J Dairy Sci. 1989;72:767–783. doi: 10.3168/jds.S0022-0302(89)79169-4. [DOI] [PubMed] [Google Scholar]
- Dekinga A, Dietz MW, Koolhaas A, Piersma T. Time course and reversibility of changes in the gizzards of red knots alternately eating hard and soft food. J Exp Biol. 2001;204:2167–2173. doi: 10.1242/jeb.204.12.2167. [DOI] [PubMed] [Google Scholar]
- DeWitt T, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. TREE. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- Drent RH, Daan S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Forbes JM. Voluntary food intake of pregnant ewes. J Anim Sci. 1970;31:1222–1227. [Google Scholar]
- Forbes JM. Reproduction and lactation. In: Forbes JM, editor. Voluntary food intake and diet selection in farm animals. 2nd edition Cab International; London: 2007. pp. 341–364. [Google Scholar]
- Garland T. The relation between maximal running speed and body mass in terrestrial mammals. J Zool (Lond) 1983;199:157–170. [Google Scholar]
- Gilbert AN. Mammary number and litter size in rodentia: The “one-half rule”. Proc Natl Acad Sci USA. 1986;83:4828–4830. doi: 10.1073/pnas.83.13.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JE, Wang Z, Wunder B. Effects of food quality and energy needs: changes in gut morphology and capacity of Microtus ochrogaster. J Mammal. 1985;66:661–667. [Google Scholar]
- Hackländer K, Tataruch F, Ruf T. The effect of dietary fat content on lactation energetics in the European hare (Lepus europaeus) PBZ. 2002a;75:19–28. doi: 10.1086/324770. doi: 10.1086/324770. [DOI] [PubMed] [Google Scholar]
- Hackländer K, Arnold W, Ruf T. Postnatal development and thermoregulation in the precocial european hare (Lepus europaeus) J Comp Biochem Physiol B. 2002b;172:183–190. doi: 10.1007/s00360-001-0243-y. doi: 10.1007/s00360-001-0243-y. [DOI] [PubMed] [Google Scholar]
- Hammond KA, Wunder B. The role of diet quality and energy need in the nutritional ecology of a small herbivore, Microtus ochrogaster. Physiol Zool. 1991;64:541–567. [Google Scholar]
- Hammond KA, Konarzewski M, Torres R, Diamond J. Metabolic ceilings under a combination of peak energy demands. Physiol Zool. 1994;67:1479–1506. [Google Scholar]
- Hammond KA, Lloyd K, Diamond J. Is mammary output capacity limiting to lactational performance in mice? J Exp Biol. 1996;199:337–349. doi: 10.1242/jeb.199.2.337. [DOI] [PubMed] [Google Scholar]
- Hammond KA, Diamond J. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. doi: 10.1038/386457a0. [DOI] [PubMed] [Google Scholar]
- Hammond KA, Kristan D. Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus) PBZ. 2000;73:547–556. doi: 10.1086/317757. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simulataneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Ingvartsen KL, Andersen JB. Integration of metabolism and intake regulation: a review focusing on periparturient animals. J Dairy Sci. 2000;83:1573–1597. doi: 10.3168/jds.S0022-0302(00)75029-6. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Thomson SC, Speakman JR. Limits to sustained energy intake: I. Lactation in the laboratory mouse, Mus musculus. J Exp Biol. 2001a;204:1925–1935. doi: 10.1242/jeb.204.11.1925. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Thomson SC, Speakman JR. Limits to sustained energy intake: II. Inter-relationships between resting metabolic rate, life-history traits and morphology in Mus musculus. J Exp Biol. 2001b;204:1937–1946. doi: 10.1242/jeb.204.11.1937. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Thomson SC, Speakman JR. Limits to sustained energy intake: III. Effects of concurrent pregnancy and lactation in Mus musculus. J Exp Biol. 2001c;204:1947–1956. doi: 10.1242/jeb.204.11.1947. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Speakman JR. Limits to sustained energy intake: V. Effect of cold-exposure during lactation in Mus musculus. J Exp Biol. 2001;204:1967–1977. doi: 10.1242/jeb.204.11.1967. [DOI] [PubMed] [Google Scholar]
- Koteja P. Limits to the energy budget in a rodent, Peromyscus maniculatus: the central limitation hypothesis. Physiol Zool. 1996;69:981–993. [Google Scholar]
- Krol E, Murphy M, Speakman JR. Limits to sustained energy intake X. Effects of fur removal on reproductive performance in laboratory mice. J Exp Biol. 2007;210:4233–4243. doi: 10.1242/jeb.009779. [DOI] [PubMed] [Google Scholar]
- Künkele J. Energetics of gestation relative to lactation in a precocial rodent, the guinea pig (Cavia porcellus) J Zool (Lond) 2000;250:533–539. [Google Scholar]
- Livesey G. The energy equivalents of ATP and the energy values of food proteins and fats. Brit J Nutr. 1984;51:15–28. doi: 10.1079/bjn19840005. [DOI] [PubMed] [Google Scholar]
- Livesey G, Marinos E. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J. Cli Nutr. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- Marboutin E, Aebischer NJ. Does harvesting arable crops influence the behaviour of the European hare, Lepus europaeus ? Wildl Biol. 1996;2:83–91. [Google Scholar]
- Mc Devitt, Speakman JR. Central limits to sustainable metabolic rate have no role in cold acclimation of the short -tailed field vole (Microtus agrestis) Physiol. Zool. 1994;67:1117–1139. [Google Scholar]
- Mitchell-Jones AJ, Amori G, Bogdanowicz W, Krystufek B, Reijnders PJH, Spitzenberger F, Stubbe M, Thissen JBM, Vohralik V, Zima J. Atlas of European mammals. Academic Press; London: 1999. [Google Scholar]
- Naya DE, Ebensperger LA, Sabat P, Bozinovic F. Digestive and metabolic flexibility allows female degus to cope with lactation costs. PBZ. 2008;81:186–194. doi: 10.1086/527453. [DOI] [PubMed] [Google Scholar]
- Nehring K. Agrikulturchemische Untersuchungsmethoden für Dünge- und Futtermittel, Böden und Milch. Parey; Hamburg: 1960. [Google Scholar]
- Onderscheka K, Tataruch F. Ein Versuch zur Erstellung von Normalwerten wildlebender Tiere und die Anwendung dieser Daten in der Wildbiologie. Wien Tierärztl Monschr. 1982;69:274–279. [Google Scholar]
- Otzelberger K. Österreichisches Methodenbuch für die Untersuchung von Futtermitteln, Futterzusatzstoffen und Schadstoffen. Wien; 1983. Arbeitsgemeinschaft der Landwirtschaftlichen Versuchsanstalten in Österreich. [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. TREE. 2003;18:228–233. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3. 2008:1–88. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Reichlin T, Klansek E, Hackländer K. Diet selection by hares (Lepus europaeus) in arable land and its implications for habitat management. Eur J Wildl Res. 2005;51:137–295. [Google Scholar]
- Speakman JR, McQueenie J. Limits to sustained metabolic rate: the link between food intake, basal metabolic rate, and morphology in reproducing mice, Mus musculus. Physiol Zool. 1996;69:746–769. [Google Scholar]
- Speakman JR, Gidney A, Bett J, Mitchell IP, Johnson MS. Limits to sustained energy intake: IV. Effect of variation in food quality on lactating mice, Mus musculus. J Exp Biol. 2001;204:1957–1965. doi: 10.1242/jeb.204.11.1957. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Krol E. Limits to sustained energy intake IX: a review of hypotheses. J Comp Physiol B. 2005;175:375–394. doi: 10.1007/s00360-005-0013-3. doi: 10.1007/s00360-005-0013-3. [DOI] [PubMed] [Google Scholar]
- Speakman JR. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. 2008. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck JM. Phenotypic flexibility of the avian gizzard: rapid, reversible and repeated changes of organ size in response to changes in dietary fibre content. J Exp Biol. 1999;202:3171–3179. doi: 10.1242/jeb.202.22.3171. [DOI] [PubMed] [Google Scholar]
- Stubbs RJ, Prentice AM, James WPT. Carbohydrates and energy balance. Ann. NY Acad. Sci. 1997;819:44–69. doi: 10.1111/j.1749-6632.1997.tb51798.x. [DOI] [PubMed] [Google Scholar]
- Thompson SD. Gestation and lactation in small mammals: Basal metabolic rate and the limits of energy use. In: Tomasi TE, Horton TH, editors. Mammalian energetics. Interdisciplinary views of metabolism and reproduction. Comstock; Ithaca: 1992. pp. 213–259. [Google Scholar]
- Valencak TG, Tataruch F, Ruf T. Peak energy turnover in lactating European hares: the role of fat reserves. J Exp Biol. 2009;212:231–237. doi: 10.1242/jeb.022640. doi: 10.1242/jeb.022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Haar MJ. Nutritional factors and lactation. In: Knobil E, Neill JD, editors. Encyclopedia of reproduction. III. Academic Press; San Diego, USA: 1999. pp. 422–432. [Google Scholar]
- Veloso C, Bozinovic F. Effect of food quality on the energetics of reproduction in a precocial rodent, Octodon degus. J Mammal. 2000;81:971–978. [Google Scholar]
- Weiner J. Physiological limits to sustainable energy budgets in birds and mammals: ecological implications. TREE. 1992;7:384–388. doi: 10.1016/0169-5347(92)90009-Z. doi: 10.1016/0169-5347(92)90009-Z. [DOI] [PubMed] [Google Scholar]
- Villa-Godoy A, Hughes TL, Emery RS, Chapin LT, Fogwell RL. Association between energy balance and luteal function in lactating dairy cows. J Dairy Sci. 1988;71:1963–1072. doi: 10.3168/jds.S0022-0302(88)79653-8. [DOI] [PubMed] [Google Scholar]
- Weldon WC, Lewis AJ, Louis GF, Kovar JL, Giesemann MA, Miller PS. Postpartum hypophagia in primiparous sows: I. Effects of gestation feeding level on feed intake, feeding behavior, and plasma metabolite concentrations during lactation. J Anim Sci. 1994;72:387–394. doi: 10.2527/1994.722387x. [DOI] [PubMed] [Google Scholar]
- Zörner E. Der Feldhase. Spektrum Akademischer Verlag; Heidelberg: 1996. [Google Scholar]