Abstract
Mutations in the adenomatous polyposis coli (APC) tumour suppressor are the key initiating event of colorectal cancer. Although the control of WNT signaling is well established as a central tumour suppressive function, the significance of APC in regulating chromosome instability is less well established. In this study, we test whether APC-deficient cells have a functional spindle assembly checkpoint in vivo by examining the response of these cells to Taxol and Vinorelbine.
Here we show for the first time that APC deficiency compromises the arrest response to Taxol in vivo. This effect is independent of the role APC plays in WNT signaling. At higher levels of Taxol, APC-deficient cells arrest as efficiently as wild-type cells. Importantly, this dose of Taxol strongly suppresses intestinal tumourigenesis in models of benign (APCMin/+ mouse) and invasive (AhCreER+ APCfl/+ PTENfl/fl) cancer.
In contrast to intestinal enterocytes with a general spindle assembly checkpoint defect due to Bub1 deletion, APC-deficient enterocytes arrest equivalently to wild-type when treated with Vinorelbine. This suggests that the failed arrest in response to Taxol is due to a specific defect in microtubule stabilisation following Taxol treatment rather than a general role of the APC protein in the mitotic spindle checkpoint.
In summary, this study clarifies the role of APC as a mitotic spindle checkpoint protein in vivo and shows that APC-deficient cells have a compromised response to Taxol.
Keywords: APC, WNT signaling, Taxol, Mitotic spindle assembly checkpoint
Introduction
Numerous studies have reported that the APC (Adenomatous Polyposis Coli) tumour suppressor plays a critical role in protecting against Chromosome Instability (CIN) (Dikovskaya et al., 2007). This was first established in ES cells lacking APC, which rapidly became aneuploid in vitro (Fodde et al., 2001; Kaplan et al., 2001). Since then recent reports have suggested that APC plays a critical role in the spindle assembly checkpoint (Dikovskaya et al., 2001), kinetochore-microtubule attachment and cytokinesis (Caldwell et al., 2007). However, a number of these phenotypes are relatively subtle (though very reproducible) and others appear to be contentious and possibly cell context specific (Rusan and Peifer, 2008; Tighe et al., 2004). Therefore, assessing whether the role of APC in protecting against chromosomal instability is important for tumour initiation or progression has been difficult. Also it has been difficult to distinguish phenotypes due to specific functions of APC from secondary consequences of WNT signaling deregulation following APC loss.
The main function of APC in chromosomal instability is thought to be due to its ability to bind microtubules (Dikovskaya et al., 2004). APC has been shown to unambiguously localise to microtubules, where it interacts with EB1 (Su et al., 1995), and microtubule kinetochore attachments where it is thought to be important for the spindle assembly checkpoint (possibly thorough an interaction with BubR1 and Bub1) (Dikovskaya et al., 2007). However, there is conflicting evidence about the role of APC in the spindle assembly checkpoint (as measured by the arrest caused by microtubule poisons) with one study showing a reduction (albeit not a block) in arrest after Nocodazole in U2OS cells, whilst another study failed to find an impact of APC depletion (Dikovskaya et al., 2007; Draviam et al., 2006; Green et al., 2005). One of the difficulties in comparing these studies is that many have been performed in cell lines where other genes in the spindle checkpoint could have been mutated and therefore removing APC may be revealing secondary differences. Furthermore, different experimental conditions may have different effects on different cells lines. Also it is clear from studies that cells in culture (even primary cells) have a much greater propensity to acquire chromosome instability than matched cells in vivo (Uetake and Sluder, 2004).
Therefore, we decided to test the response of APC-deficient cells to two mitotic spindle poisons, Taxol and Vinorelbine, (as a measure of the mitotic spindle checkpoint) immediately after APC is lost in vivo. Both Taxol and Vinorelbine bind to the β subunit of tubulin but cause different consequences: Taxol inhibits microtubule depolymerisation hence stabilising microtubules, whilst Vinorelbine depolymerises microtubules therefore destabilising microtubules (Krikorian and Breillout, 1991; Nogales et al., 1999). The phenotype of APC deletion in the intestine is dramatic: co-incident with β-catenin activation, there is a marked increase in enterocyte proliferation (and apoptosis) and decrease in migration and differentiation (Sansom et al., 2004). We define this as a crypt-progenitor cell-like phenotype. In this system, co-deletion of c-Myc rescues all of the phenotypes of APC loss and thus, despite the presence of nuclear β-catenin, the majority of WNT target genes are not activated (Sansom et al., 2007). In conclusion, APC and c-Myc co-deletion allows an investigation of the APC-loss phenotype separate from WNT target gene activation.
In this study we definitively show that, rather than being a general mitotic spindle assembly checkpoint protein like Bub1, APC is only required for the response to Taxol at low doses and is not required for the arrest in response to the microtubule-destabilising agent Vinorelbine. Moreover, this role of APC at low Taxol doses is independent of WNT signaling.
Results and Discussion
APC-deficient cells are resistant to 10mg/kg Taxol
To characterise the spindle assembly checkpoint within intestinal enterocytes in vivo, we injected wild-type mice with 10mg/kg of Taxol and harvested mice at 30 mins, 1, 2, 3, 6, 9, 12 and 24 hours. A significant induction of arrested mitotic figures was seen in wild-type mice from 3 hours (see figure 1A, supplementary figure S1 A). By 24 hours, levels of mitotic figures had returned to normal levels. Furthermore, BrdU labelling at 24 hours shows reduced numbers of BrdU positive cells following Taxol treatment compared to vehicle-treated (supplementary figure S1 C).
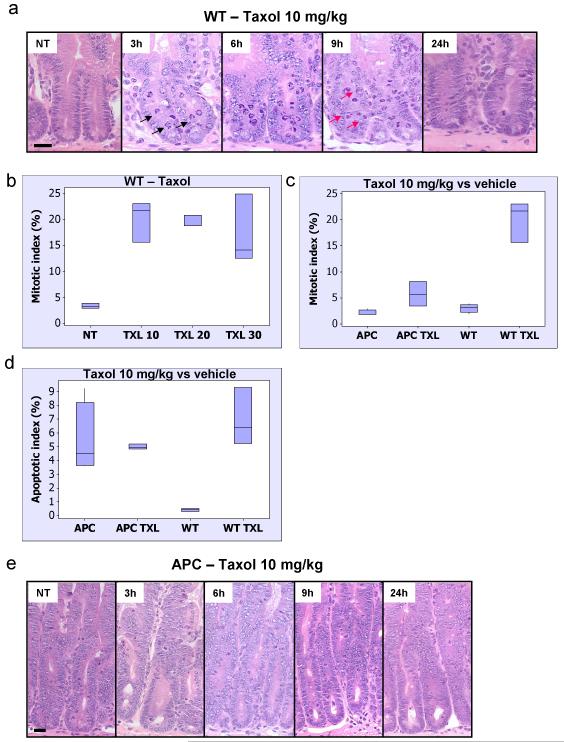
Figure 1. APC-deficient intestinal cells are resistant to low level Taxol treatment.
Mice injected with either vehicle (NT), 10, 20 or 30mg/kg Taxol IP (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored using H+E staining. Mitotic and apoptotic index was expressed as percentage of cells per crypt. As intestinal enterocytes enter G2 phase they move out of the crypt lining and into the lumen where they will undergo mitosis and cytokinesis before retuning back to the single cell layer crypt lining. However enterocytes can undergo apoptosis anywhere in the crypt.
(a) Representative H+E staining of crypts from WT mice treated with Taxol 10mg/kg and sacrificed at 3h, 6h, 9h or 24h post-injection. Black arrows – mitotic figure, red arrow – apoptotic figure. Scale bar 20μm. (b) Mitotic index in WT intestinal crypts from mice treated with vehicle versus increasing concentrations of Taxol, p<0.026. (c) and (d) Mitotic and apoptotic index in intestinal crypts from vehicle or Taxol 10mg/kg treated WT or Cre+ APC fl/fl mice. The induction in mitotic arrest and apoptosis is significant only in WT vehicle versus treated, p<0.026. NT – vehicle-treated. (e) Representative H+E staining of crypts from Cre+ APC fl/fl mice treated with Taxol 10mg/kg and sacrificed at 3h, 6h, 9h or 24h post-injection. Scale bar 20μm.
Given this clear onset of arrest in wild-type mice, we therefore decided to investigate whether the onset and severity of the arrest was affected by APC deficiency. Therefore, control (AhCre+ APC+/+) and experimental (AhCre+ APCfl/fl) mice were given 3 injections of the Cre inducer β–napthoflavone to induce recombination and four days later mice were injected with 10mg/kg Taxol and harvested either 3, 6 or 9 hours later. At all 3 timepoints intestinal enterocytes lacking APC showed significantly fewer mitotic figures than APC proficient tissue treated with Taxol (Figure 1C and E, supplementary figure S2 A).
It should be noted that Taxol treatment also induces a robust apoptotic response in wild-type cells (figure 1D, supplementary figure S1 B). However, APC deletion in itself is a promoter of apoptosis with APC-deficient tissue showing more apoptosis than wild-type tissue (Sansom et al., 2004). Taxol treatment does not promote additional apoptotic figures in APC null tissue suggesting that these cells are truly insensitive to Taxol action, and do not simply have a different response to the drug (death instead of arrest, figure 1D).
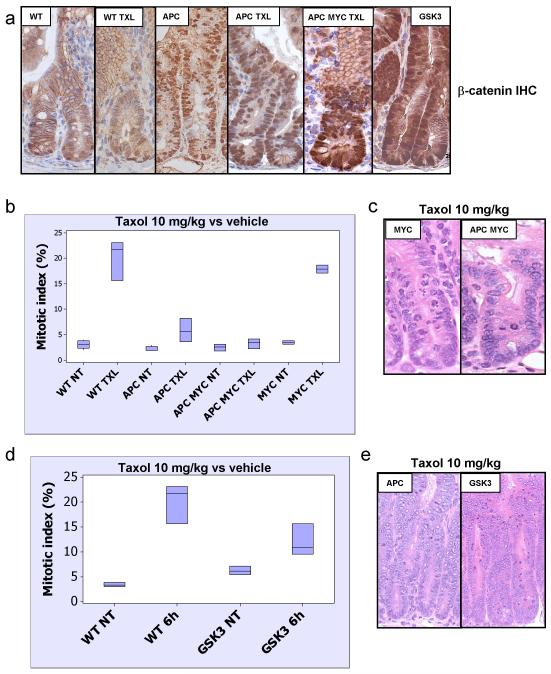
Given the possibility that changes in this mitotic checkpoint may be due to alterations in WNT signaling, we investigated the levels of nuclear β-catenin (the most reliable measure of activated WNT signaling in vivo) in AhCre+ APCfl/fl intestinal enterocytes treated with Taxol and found no difference when compared to vehicle-treated AhCre+ APCfl/fl mice (figure 2A). This suggested that the failure to arrest was not related to changes in WNT signaling following Taxol treatment. A key event downstream of beta-catenin nuclear accumulation is the expression of c-MYC, which drives the hyperproliferation, loss of migration and differentiation and increase in apoptosis seen following APC deletion (Sansom et al., 2007). If APC and c-MYC are co-deleted nuclear beta-catenin is still observed but intestinal crypts look ostensibly normal with wild-type levels of proliferation, apoptosis, migration and differentiation. Hence, to further test our hypothesis we investigated whether doubly mutant AhCre+ APCfl/flMycfl/fl were also resistant to 10mg/kg Taxol treatment. If this were so it would prove that APC-loss has an impact on the cell even in the absence of an active WNT pathway. Figure 2B shows that four days after Cre induction the AhCre+ APCfl/fl Mycfl/fl enterocytes have a comparable defect in arrest to the AhCre+ APCfl/fl Myc+/+ enterocytes. This lack of arrest strongly argues against a role of other WNT target genes in the failed activation of the mitotic checkpoint.
Figure 2. Resistance to Taxol in APC-deficient intestinal crypts is independent of activation of the canonical WNT/beta-catenin pathway.
Mice injected with either vehicle (NT) or 10mg/kg Taxol IP (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored by H+E staining. Mitotic index was expressed as percentage of cells per crypt. (a) IHC detecting beta-catenin showing nuclear localisation in Cre+ APC fl/fl (vehicle or Taxol 10mg/kg treated), Cre+ APC fl/fl MYC fl/fl (10mg/kg Taxol treated) and Cre+ GSK3 alpha fl/fl beta fl/fl mice. Note non-nuclear localisation of beta-catenin in WT vehicle or Taxol 10mg/kg treated intestine. (b) Mitotic index in intestinal crypts from Taxol 10mg/kg or vehicle treated WT, Cre+ MYC fl/fl, Cre+ APC fl/fl, or Cre+ APC fl/fl MYC fl/fl mice. There is no significant difference between the response in Cre+ APC fl/fl and Cre+ APC fl/fl MYC fl/fl mice and between WT and Cre+ MYC fl/fl mice, p>0.05. (c) Representative H+E staining of crypts from Cre+ MYC fl/fl (Taxol 10mg/kg) and Cre+ APC fl/fl MYC fl/fl mice (Taxol 10mg/kg). (d) Mitotic index in intestinal crypts from vehicle or Taxol 10mg/kg treated WT and Cre+ GSK3 alpha fl/fl beta fl/fl mice. (e) Representative H+E staining of crypts from Cre+ APC fl/fl, or Cre+ GSK3 alpha fl/fl beta fl/fl mice treated with Taxol 10mg/kg.
To directly test whether increasing WNT signaling per se leads to an impaired response to Taxol we investigated whether intestinal enterocytes that lacked both GSK3 alpha and GSK3 beta showed a normal response to Taxol. Co-deletion of GSK3 alpha and beta produces an intestinal phenotype comparable to APC deletion (Radulescu et al., in preparation) with enlarged intestinal crypts with nuclear β-catenin. When these mice were treated with 10mg/kg of Taxol they showed a robust arrest to Taxol (figure 2D, 2E), showing unambiguously that deregulated WNT signaling on its own is not sufficient to impair the response to Taxol.
Increased Levels of Taxol overcome the role of APC in cell cycle arrest
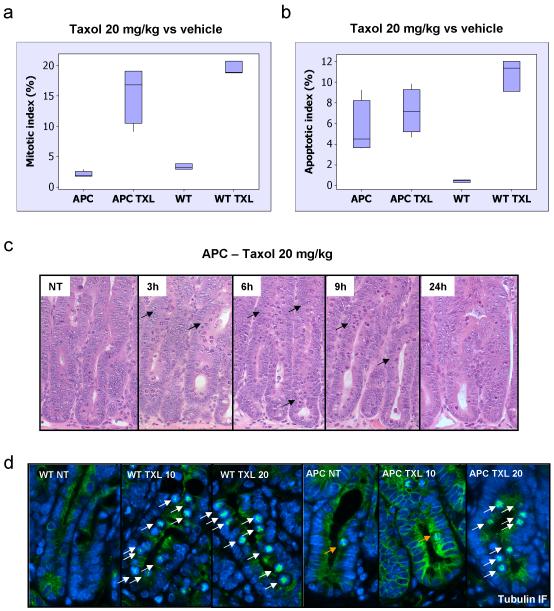
One of the difficulties in delineating the role of APC in the response to MT poisons in the literature relates to the different sensitivities of cell lines to MT poisons. For example, in the study by Draviam et al 2006 (Draviam et al., 2006) which found no impact of APC depletion on arrest by Nocodazole they used a cell line where 100nM Nocodazole induced a robust arrest within 60-80% of cells, whilst in the study which saw an impact of APC loss, much lower levels of arrest were induced and lower doses of drug were used (6-12% cells) (Dikovskaya et al., 2007). Therefore, it is possible that the role of APC may only be revealed in cell lines where the spindle assembly checkpoint has been partially compromised or when working below the threshold where it is engaged. Consequently, to test whether a robust activation of the spindle checkpoint overcomes the requirement of APC we raised the concentration of Taxol to 20mg/kg and investigated the arrest of AhCre+ APCfl/fl at the 6 hours timepoint (figure 3A). In contrast to wild-type cells, which arrest to the same extent following treatment with 10, 20 or 30mg/kg Taxol (figure 1B), APC-deficient cells are only able to arrest at concentrations of 20 or higher (figure 3A and 3C). Moreover, a more extended timecourse showed an efficient arrest with 20mg/kg in the APC-deficient mice across the whole timecourse (supplementary figure S2 B). In terms of apoptosis, Taxol 20mg/kg was still unable to increase the levels of cell death present in APC deleted cells (figure 3B) indicating that perhaps the apoptotic pathway specific for mitosis is already fully activated by the loss of APC.
Figure 3. Resistance to Taxol in APC-deficient intestinal crypts can be reversed by increasing drug concentration.
Mice injected with either vehicle (NT), or 20mg/kg Taxol IP (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored by H+E staining. Mitotic and apoptotic index was expressed as percentage of cells per crypt. (a) and (b) Mitotic and apoptotic index in intestinal crypts from vehicle or Taxol 20mg/kg treated WT or Cre+ APC fl/fl mice. The induction in mitotic arrest is significant in both WT and Cre+ APC fl/fl drug treated compared to vehicle same genotype, p<0.026. The induction in apoptosis is significant only in WT treated versus vehicle with p<0.026. (c) Representative H+E staining of crypts from Cre+ APC fl/fl mice treated with Taxol 20mg/kg and sacrificed at 3h, 6h, 9h or 24h post-injection. NT – vehicle-treated. Arrows indicate mitotic figures as a result of Taxol treatment. (d) Tubulin immunofluorescence (green) was performed on PFA-fixed tissue from WT or Cre+ APC fl/fl mice treated with vehicle (NT), 10 or 20mg/kg Taxol and taken 6h post-injection. Slides were counterstained with DAPI (blue). White arrows indicate bundled microtubules in response to Taxol treatment. Orange arrows indicate normal mitotic figures.
Therefore, these data show that at high levels of Taxol the spindle assembly checkpoint can be established without APC and thus APC is not essential for the mitotic spindle checkpoint.
Given previous data suggesting that microtubules in APC-deficient cells are destabilised (Kroboth et al., 2007) we hypothesised that APC-deficiency might lead to a reduced stabilisation of microtubules in response to Taxol and that this may be one reason for the lack of arrest. Consistent with this theory, mitotic microtubules in wild-type mice were stabilised in response to Taxol at 10 and 20mg/kg (figure 3D, note the bundling of microtubules in the centre of the cell in a star pattern). In contrast, APC-deficient intestine only showed stabilised microtubules in mitosis at 20mg/kg (white arrows), while at 10mg/kg normal mitotic distribution of microtubules was observed (orange arrow). Interestingly, the pattern of the bundles microtubules stabilised by Taxol is very similar to the monopolar spindles observed in vitro in cells treated with a fluorescent Taxol derivative (Abal et al., 2001). It is conceivable that the induction in apoptosis by Taxol in the intestine is mediated by the formation of a monopolar spindle instead of bipolar one. In cell culture this resulted in apoptosis from M-phase, as opposed to death in the subsequent G1 phase.
APC-deficient intestinal cells arrest robustly in response to Vinorelbine and do not phenocopy deletion of BUB1
If indeed the failed response to low levels of Taxol is due to destabilised microtubules in APC-deficient mice, this leads to two predictions: first, APC-deficient cells should arrest robustly with microtubule-destabilising agent Vinorelbine. Secondly, inactivation of a key component of the mitotic spindle assembly checkpoint should show a different response to mitotic poisons than APC deletion, namely failed arrest in response to both agents independent of drug concentration.
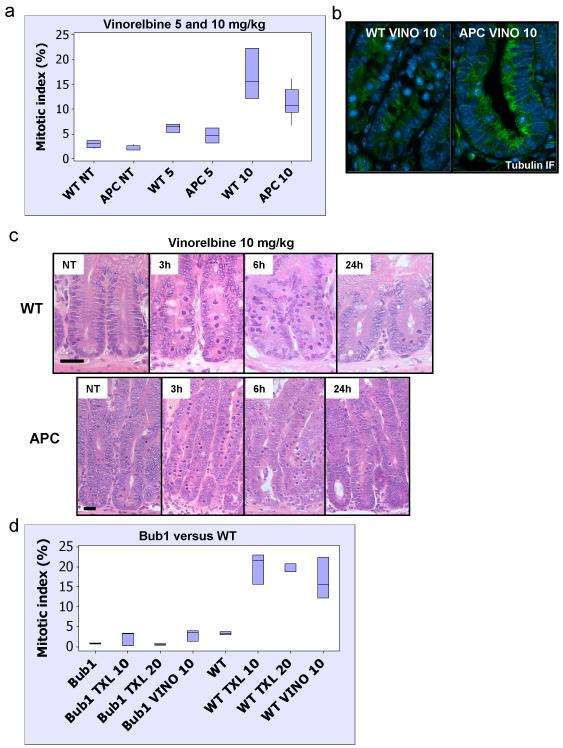
To test the first possibility we investigated the arrest in wild-type and APC-deficient intestines following two doses of Vinorelbine: 5 and 10mg/kg. 5mg/kg Vinorelbine induces a weak arrest whilst 10mg/kg induces a robust arrest at 6 hours following iv injection (figure 4A, 4C and supplementary figure S3). As predicted, even at low doses, the level of arrest was equivalent between wild-type and APC-deficient intestines. Moreover, staining for microtubules showed destabilised microtubules in mitotic figures of both genotypes after treatment (figure 4B). This data clearly shows that APC is not required as a general mitotic spindle checkpoint protein (as determined by spindle poisons).
Figure 4. APC is not required as a general mitotic spindle checkpoint protein.
Mice injected with either vehicle (NT), 5 or 10mg/kg Vinorelbine IV (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored by H+E staining. Mitotic and apoptotic index was expressed as percentage of cells per crypt. (a) Mitotic index in intestinal crypts from WT or Cre+ APC fl/fl mice treated with vehicle, 5 or 10mg/kg Vinorelbine. The induction in mitosis is significant for both 5 and 10mg/kg Vinorelbine treated versus untreated p<0.026. (b) Tubulin immunofluorescence (green) was performed on PFA-fixed tissue from WT or Cre+ APC fl/fl mice treated with 10mg/kg Vinorelbine and taken 6h post-injection. Slides were counterstained with DAPI (blue). (c) Representative H+E staining of crypts from WT or Cre+ APC fl/fl mice treated with Vinorelbine 10mg/kg and sacrificed at 3h, 6h or 24h post-injection. NT – vehicle-treated. Scale bar 20μm. (d) Deletion of the bona fide mitotic spindle checkpoint protein Bub1 (Cre+ Bub1 fl/fl mice) leads to complete abrogation of mitotic arrest in intestinal crypts following treatment with either 10, 20mg/kg Taxol or 10mg/kg Vinorelbine.
Next to ensure that the mitotic spindle assembly checkpoint is required for arrest in vivo in response to both Taxol and Vinorelbine, we investigated whether intestines deficient for Bub1 can arrest in response to these drugs. Bub1 deficiency has a profound effect on intestinal homeostasis at later timepoints (Radulescu et al in preparation); however, 2 days following Bub1 deletion no marked phenotypes are observed. Figure 4D and supplementary figure S4 show that intestinal enterocytes lacking Bub1 are unable to undergo mitotic arrest following either low or high dose Taxol or Vinorelbine. Therefore, this shows that while inactivating a bona fide SAC protein abrogates mitotic arrest in response to either mitotic poison, inactivating APC does not. In turn, this implies that APC is not a bona fide SAC protein.
High doses of Taxol suppress tumourigenesis in the APCMin/+ mouse
Finally, we wished to address if the resistance to Taxol of the APC-deficient intestine has functional consequences for tumourigenesis. To this end we investigated whether Taxol affects tumourigenesis in the APCMin/+ mouse (Moser et al., 1990). On a purebred C57BL6J background these mice develop over 100 adenomas by 100 days of age, with tumour initiation occurring by loss of the remaining APC wild-type allele. Therefore, we aged 3 cohorts of 20 APCMin/+ mice: vehicle-treated, 10mg/kg (every two weeks) Taxol and 20mg/kg (every two weeks) Taxol. These mice were harvested at 100 days of age and tumour number and size was scored. As shown in figure 5A, 10mg/kg did not cause a significant reduction in adenoma multiplicity at 100 days. Figure 5B shows in turn that 20mg/kg caused a significant reduction in tumour multiplicity. Tumour size was also significantly reduced in mice treated with 20mg/kg Taxol (Figure 5D). Therefore in this study we show that the ability to arrest in response to Taxol directly predicts whether Taxol will suppress adenoma formation. This result is in agreement with in vitro data showing that activation of the mitotic checkpoint is required following Taxol treatment in order to kill cancer cells (Gascoigne and Taylor, 2008; Huang et al., 2009). Given that APCMin/+ mice were only injected with Taxol every 2 weeks we also investigated whether a more frequent Taxol treatment regime had a more dramatic impact of tumourigenesis. Figure 5C shows a strong reduction in tumour number when mice were treated 3 times every 2 weeks, which was significantly lower than mice that had been treated only once every 2 weeks.
Figure 5. High Taxol concentrations dramatically reduce tumour number and size in benign and invasive mouse models of intestinal cancer.
Cohorts of APCMIN/+ mice (45 days of age, n≥16) were treated every 2 weeks with either 10, 20mg/kg Taxol or vehicle until they reached 100 days of age. The most efficient treatment, 20mg/kg, was then increased to 3 injections a week every other week (Txl 20 x3). The same regime was used in an invasive model of tumourigenesis using Cre+ APCfl/+ PTEN fl/fl mice. Mice started treatment 2 weeks after induction and were sacrificed at 60 days post-induction. (a) Tumour number is not decreased in APCMIN/+ mice treated with Taxol 10mg/kg once every 2 weeks (p=0.48). (b) Taxol 20mg/kg treatment (once every 2 weeks) reduces significantly the number of tumours in APCMIN/+ (p=0.0007). (c) Taxol 20mg/kg administered 3 times a week every 2 weeks further reduces the number of tumours in APCMIN/+ (p=0.0001). (d) All Taxol regimes reduce the average tumour size in APCMIN/+ mice. Vehicle versus 10/mg/kg p=0.015, vehicle versus 20mg/kg p≤0.006. (e) Taxol reduces tumour number in Cre+ APCfl/+ PTEN fl/fl mice, p=0.001.
Finally, we investigated whether this regime of Taxol could suppress more aggressive intestinal tumours induced by combined APC and PTEN deficiency (Marsh et al., 2008). Cohorts of AhCreER+ APCfl/+ PTEN fl/fl were treated with 20mg/kg Taxol or vehicle 3 times every 2 weeks and harvested 60 days following Cre induction. In this model, both β-napthoflavone and Tamoxifen are required to activate Cre recombinase and tumours are formed after loss of the APC allele. In these mice too, intestinal tumourigenesis was strongly suppressed by treatment with Taxol (figure 5E). These data raise the possibility that doses of taxanes that induce arrest in colorectal cancer in vivo may be a useful treatment option. However, within colorectal cancer the multiple drug resistance (MDR) pump is often deregulated, which may prevent Taxol concentration from reaching sufficiently high levels in tumours to cause arrest (Linn and Giaccone, 1995). In the future, this may not present a problem with new microtubule-stabilising agents (now in clinical trials) coming on line that are not recognised by MDR pumps e.g. Patupilone (Novartis). Thus, it will be of great interest to determine if this agent has chemopreventative effects in mouse models of intestinal tumourigenesis like those used in this study.
In summary, using an in vivo model where we synchronously deleted APC, we defined a role for APC in the response to MT poisons. Given this response is a good indicator of the spindle assembly checkpoint in vivo, we have shown that, unlike Bub1, APC is not a general mediator of the spindle assembly checkpoint. Instead it is required for the efficient arrest at lower doses of Taxol suggesting that APC is involved in the sensitivity of the spindle assembly checkpoint activation. This reconciles published data because it confirms that the amount of drug used to treat cells and their inherent threshold for activation of this checkpoint is crucial when making comparisons between different experiments. Importantly, our data with GSK3 loss suggest that this function of APC is independent of its role in Wnt signaling, though we cannot absolutely rule out a contribution from activation of Tcf-regulated transcription. Instead may be due to the destabilisation of microtubules in APC-deficient cells. Clinically important is our finding that the onset of arrest in APC-deficient cells directly predicts responsiveness of the APCMin/+ mice to Taxol.
Methods
Ethics Statement
All mouse experiments were performed under the UK Home Office guidelines.
Mouse colonies
Outbred male mice from 6-12 weeks of age were used, which were segregating for the C57BL6J and S129 genomes (5 generations C57Bl6J). The alleles used were as follows: APC580S (Shibata et al., 1997), Mycfl/fl (Baena et al., 2005), AhCre (Ireland et al., 2004), AhCreER (Kemp et al., 2004), PTENfl/fl (Suzuki et al., 1998; Suzuki et al., 2003), Bub1 fl/fl (Perera et al., 2007), GSK3 alphafl/fl and GSK3 betafl/fl (Patel et al., 2008). All APCMin/+ experiments were performed on mice that were 100% C57BL6J (Moser et al., 1990).
Tissue isolation
To induce recombination, mice were given 3 injections (i.p.) spaced four hours apart of β-napthoflavone (Sigma) at 80mg/kg concentration (Sansom et al., 2004). At the appropriate time-point, mice were killed and the small intestine removed and flushed with water (at least 3 mice were used for each experiment). A number of studies have suggested that Cre Recombinase expression may induce DNA damage. In this study as the Cre is only induced on the first day of the experiment (within the intestine crypt and stem cells), and by the time the mice are treated with MT poisons (4 days later), Cre is no longer expressed (Ireland et al., 2004).
Microtubule poison experiments
Taxol (Paclitaxel, Mayne Pharma), a microtubule stabiliser, and Vinorelbine (Navelbine, Pierre Fabre), a microtubule destabiliser, were used as microtubule poisons. All experiments were performed on group sizes of at least 3 mice each. For short term experiments, APC was deleted from the intestinal epithelium by Cre mediated recombination and mice were treated with Taxol 10, 20 or 30mg/kg (i.p.) and harvested 30min, 1, 2, 3, 6, 9, 12, 15 or 24h later. Vinorelbine was used at 5 or 10mg/kg concentration and delivered i.v. For long-term experiments cohorts APCMin/+ mice (n=20) were given one Taxol injection (i.p.) of 10 or 20mg/kg every 2 weeks from 45 days of age. Mice were harvested at 100 days of age and the number of tumours was scored in both the small and large intestine. For the dose which showed the highest impact on tumour number the treatment was increased to 3 Taxol injections a week (every other day) every 2 weeks. For the invasive intestinal tumour model AhCreER+ APCfl/+ PTENfl/fl mice were given 4 daily injections of β-napthoflavone and Tamoxifen (Sigma) at 80mg/kg concentration each (Kemp et al., 2004). Taxol 20mg/kg treatment (3 i.p. injections a week on alternative days) started 2 weeks after the last injection and mice were sacrificed 60 days after induction.
Assaying crypt size, apoptosis and mitosis
Apoptosis, crypt size and mitotic index were scored from H+E stained sections as previously described (Sansom et al., 2004). For each analysis, 25 full crypts were scored from at least 3 mice of each genotype.
Immunohistochemistry (IHC) and immunofluorescence (IF)
IHC was performed as described previously (Gregorieff et al., 2005; Sansom et al., 2004). Primary antibodies used for IHC were: anti-β-catenin (mouse monoclonal, 1:50; Transduction Labs) and anti-BrdU (mouse monoclonal, 1:500, BD Biosciences). IHC imaging was performed using a Zeiss Axio Imager A1 microscope (EC PLAN-NEOFLUAR 40x/0,75 ∞/0.17), AxioCam MRC camera (Zeiss) and Axiovision Rel 4.7 (Zeiss) acquisition software.
For IF PFA-fixed gut tissue was stained with anti-α tubulin (YL 1/2 rat monoclonal, 1:50, kind gift of Dr. Paul Appleton), Alexa488 goat anti-rat antibody (Invitrogen) was used as secondary antibody and slides were mounted with Prolong Gold (Invitrogen). For imaging a Leica DMIRB microscope was used (Leica HCX PL APO 40X NA 0.85 with 1.5X auxiliary magnification). Pictures were taken at room temperature using a Hamamatsu C4742-95 monochrome camera and Openlab 5.5 (Improvision) acquisition software.
Supplementary Material
Acknowledgements
This work was funded by CR-UK project and program grants to Inke Näthke and programme grant to Owen Sansom. Sorina Radulescu is funded by an AICR grant. Help from Colin Nixon with histology, Derek Miller and Tom Hamilton with transgenic work was much appreciated.
Footnotes
Conflict of interest.
The authors declare no conflict of interest.
REFERENCES
- Abal M, Souto AA, Amat-Guerri F, Acuna AU, Andreu JM, Barasoain I. Centrosome and spindle pole microtubules are main targets of a fluorescent taxoid inducing cell death. Cell Motil Cytoskeleton. 2001;49:1–15. doi: 10.1002/cm.1016. [DOI] [PubMed] [Google Scholar]
- Baena E, Gandarillas A, Vallespinos M, Zanet J, Bachs O, Redondo C, et al. c-Myc regulates cell size and ploidy but is not essential for postnatal proliferation in liver. Proc Natl Acad Sci U S A. 2005;102:7286–91. doi: 10.1073/pnas.0409260102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell CM, Green RA, Kaplan KB. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J Cell Biol. 2007;178:1109–20. doi: 10.1083/jcb.200703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovskaya D, Newton IP, Nathke IS. The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts. Mol Biol Cell. 2004;15:2978–91. doi: 10.1091/mbc.E03-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovskaya D, Schiffmann D, Newton IP, Oakley A, Kroboth K, Sansom O, et al. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176:183–95. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovskaya D, Zumbrunn J, Penman GA, Nathke IS. The adenomatous polyposis coli protein: in the limelight out at the edge. Trends Cell Biol. 2001;11:378–84. doi: 10.1016/s0962-8924(01)02069-4. [DOI] [PubMed] [Google Scholar]
- Draviam VM, Shapiro I, Aldridge B, Sorger PK. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 2006;25:2814–27. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–8. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–22. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol Biol Cell. 2005;16:4609–22. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–38. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16:347–58. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–46. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–32. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Kemp R, Ireland H, Clayton E, Houghton C, Howard L, Winton DJ. Elimination of background recombination: somatic induction of Cre by combined transcriptional regulation and hormone binding affinity. Nucleic Acids Res. 2004;32:e92. doi: 10.1093/nar/gnh090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian A, Breillout F. Vinorelbine (Navelbine). A new semisynthetic vinca alkaloid. Onkologie. 1991;14:7–12. doi: 10.1159/000216938. [DOI] [PubMed] [Google Scholar]
- Kroboth K, Newton IP, Kita K, Dikovskaya D, Zumbrunn J, Waterman-Storer CM, et al. Lack of adenomatous polyposis coli protein correlates with a decrease in cell migration and overall changes in microtubule stability. Mol Biol Cell. 2007;18:910–8. doi: 10.1091/mbc.E06-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn SC, Giaccone G. MDR1/P-glycoprotein expression in colorectal cancer. Eur J Cancer. 1995;31A:1291–4. doi: 10.1016/0959-8049(95)00278-q. [DOI] [PubMed] [Google Scholar]
- Marsh V, Winton DJ, Williams GT, Dubois N, Trumpp A, Sansom OJ, et al. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet. 2008;40:1436–44. doi: 10.1038/ng.256. [DOI] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–28. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera D, Tilston V, Hopwood JA, Barchi M, Boot-Handford RP, Taylor SS. Bub1 maintains centromeric cohesion by activation of the spindle checkpoint. Dev Cell. 2007;13:566–79. doi: 10.1016/j.devcel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Rusan NM, Peifer M. Original CIN: reviewing roles for APC in chromosome instability. J Cell Biol. 2008;181:719–26. doi: 10.1083/jcb.200802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–9. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–90. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–3. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, et al. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–7. [PubMed] [Google Scholar]
- Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–78. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Itami S, Ohishi M, Hamada K, Inoue T, Komazawa N, et al. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer Res. 2003;63:674–81. [PubMed] [Google Scholar]
- Tighe A, Johnson VL, Taylor SS. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J Cell Sci. 2004;117:6339–53. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- Uetake Y, Sluder G. Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a “tetraploidy checkpoint”. J Cell Biol. 2004;165:609–15. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.