Abstract
By using antibodies against mouse F3/contactin, we found immunologically related glycoproteins expressed in the nervous tissue of the snail Helix pomatia. Helix contactin-related proteins (HCRPs) include different molecules ranging in size from 90 to 240 kD. Clones isolated from a cDNA expression library allowed us to demonstrate that these proteins are translated from a unique 6.3-kb mRNA, suggesting that their heterogeneity depends on posttranslational processing. This is supported by the results of endoglycosidase F treatment, which indicate that the high-molecular-weight components are glycosylation variants of the 90-kD chain. In vivo and in cultures, HCRPs antibodies label neuronal soma and neurite extensions, giving the appearance of both cytoplasmic and cell surface immunostaining. On the other hand, no expression is found on nonneural tissues. Functionally, HCRPs are involved in neurite growth control and appear to modulate neurotransmitter release, as indicated by the inhibiting effects of specific antibodies on both functions. These data allow the definition of HCRPs glycoproteins as growth-promoting molecules, suggesting that they play a role in neurite development and presynaptic terminal maturation in the invertebrate nervous system.
Keywords: axonal glycoproteins, neurite growth, neurotransmitter release, F3/contactin
In vertebrates, axonal growth and pathfinding control depend on mechanisms involving a large set of neuronal surface glycoproteins, which belong to distinct families (Dickson, 2002; De Castro, 2003; Wen and Zheng 2006; Maness and Schachner, 2007). In particular, molecules built by the association of immunoglobulin type C2 and fibronectin type III (FNIII) domains play a relevant role in basic events of neuronal differentiation as axonal growth, fasciculation, and pathfinding (Brummendorf and Lemmon, 2001), a role also played by several extracellular matrix components (Galtrey and Fawcett, 2007). Depending on the mechanism of membrane association, axonal surface glycoproteins may be classified into two groups, i.e., transmembrane, e.g., L1/NgCAM and NrCAM (Walsh and Doherty, 1997), and glycosylphosphatidylinositol (GPI)-anchored, e.g., F3/contactin (Gennarini et al., 1989, 1991) and TAG-1 (Furley et al., 1990).
Most of these molecules display a wide species distribution, and some of them have been detected in invertebrates such as Aplysia and leech, where NCAM and L1 homologues, called ApCAM and tractin, have been described (Thompson et al., 1996; Xu et al., 2003). The functional role of these molecules is also likely conserved, as shown for the ApCAM ability to mediate the formation of synaptic contacts (Mayford et al., 1992) and to modulate synaptic function (Zhu et al., 1995; Schacher et al., 2000).
Based on the stereotyped organization of its neuronal connections, the nervous tissue of the land snail Helix pomatia represents a helpful model for elucidating the mechanisms underlying neuronal connectivity and synaptic function and for addressing in a comprehensive way the significance of adhesive glycoprotein expression in neuronal function. In this context, we have previously shown that cultured neurons from the cerebral ganglion are able to regenerate neurites and to modulate neurotransmitter release in a target-dependent manner (Ghirardi et al., 2000, 2001) and that target-bound adhesion molecules play a fundamental role in these processes (Ghirardi et al., 2001). Only few reports are available in this field (Keller and Schacher, 1990; Alenius and Bohm, 1997; Ghirardi et al., 2001), so the present study is aimed at expanding the repertoire of neuronal glycoproteins potentially involved in mediating such functions in invertebrate nervous tissues.
In particular, we focus on F3/contactin as a potentially useful molecular candidate. Originally identified in the chick (Ranscht, 1988) and mouse (Gennarini et al., 1989), F3/contactin was subsequently detected also in humans (Berglund and Ranscht, 1994), ox (Watanabe et al., 1995), rat (Shimazaki et al., 1998), fish (Haenisch et al., 2005), and Drosophila (Faivre-Sarrailh et al., 2004). The molecule is known to modulate several developmental events, including cell adhesion, axonal growth, pathfinding, and myelination, and is involved in synaptic function (Gennarini et al., 1991; Pesheva et al., 1993; Berglund et al., 1999; Murai et al., 2002; Bizzoca et al., 2003; Hu et al., 2003). Deletion studies indicated that such functions map to different domains within the Ig-like and FNIII-like regions (Durbec et al., 1994). Here, we use mouse F3/contactin antibodies, previously demonstrated to affect basic neuronal functions in rodents (Durbec et al., 1994; Buttiglione et al., 1996), to identify and partially to characterize related molecules expressed in Helix nervous tissue. The rationale for this study is to gain a broad view of the potential functional significance of this glycoprotein class through the use of a simple neuronal system.
MATERIALS AND METHODS
Cell Culture
The technique for culturing neurons from Helix pomatia has been described previously (Ghirardi et al., 1996). Briefly, C1 and C3 neurons from the cerebral ganglion and B2 from the buccal ganglion were dissociated by a thin glass micropipette, plated on poly-L-lysine (0.5 mg/ml in 0.1 M sodium tetraborate, pH 8.2; Sigma; St. Louis, MO), preincubated in Aplysia hemolymph for 24 hr, and cultured in L15 Leibovitz medium (Sigma) at 18°C for 3–5 days.
Antisera
Two rabbit antisera were used: 1) the 24III serum, raised against a mouse F3/contactin-β-galactosidase fusion protein encompassing the whole immunoglobulin region and the first FNIII repeat (Gennarini et al., 1991) and 2) the Lim antiserum, raised against a synthetic peptide spanning the 20 N-terminal amino acids of mouse F3/contactin, coupled to keyhole limpet hemocyanin (KLH; Roche, Mannheim, Germany).
Immunostaining Procedures
Living cells
Three to five days after plating, the cells were rinsed with Helix saline and incubated for 1 hr at room temperature (RT) with the 24III or Lim antisera [1:125 or 1:1,000, respectively, diluted in 5% bovine serum albumin (BSA) in 0.1 M phosphate-buffered saline (PBS)], using the preimmune sera at the same dilution as controls. After rinsing, TRITC-labelled goat anti-rabbit secondary antibodies were applied for 1 hr at RT. The stained cells were observed with an inverted microscope equipped with fluorescent optics, and images were acquired with an intensified CCD camera (Hamamatsu, Iwata-City, Japan), processed in Image Pro Plus (Media Cybernetics, Gleichen, Germany) and stored in a Pentium IV PC.
Fixed cells
Alternatively, the cells were fixed with 4% paraformaldehyde in 0.1 M PBS for 40 min at RT and, after bleaching with 0.6% H2O2 in ethanol, incubated in saturation buffer for 45 min at RT, followed by the above primary antisera in the presence or absence of 0.25% saponin (1 hr at RT or ON at 4°C). The 24III or Lim antisera and the corresponding preimmune sera were used at 1:125 or 1:1,000 dilutions, respectively, in saturation buffer. After a 1-hr incubation with TRITC-conjugated secondary antibodies (1:100 dilution; Jackson Laboratories; Bar Harbor, ME), labelled cells were washed and observed with fluorescence optics. Images were analyzed digitally.
Immunohistochemistry
Immediately after dissection, cerebral ganglia were fixed by immersion in 75% picric acid containing 6.25% glutaraldeyde and 1% acetic acid, then embedded in paraffin, and 5-μm-thick microtome sections were generated. For immunohistochemical staining, sections were first incubated with 0.3% H2O2 in PBS to counteract endogenous peroxidases and then with blocking buffer [3% BSA + 5% fetal calf serum (FCS) in PBS] for 30 min. Primary antibodies (1:1,000 dilution in blocking buffer) were then applied ON at 4°C, and, after rinsing with PBS, the sections were incubated for 1 hr at RT with biotinylated goat anti-rabbit IgG (2 μg/ml in blocking buffer; Vector, Burlingame, CA), followed by streptavidin-peroxidase (Vector; 5 μg/ml in blocking buffer). Finally, the sections were treated with 3-amino-9-etylcarbazole (ABC kit; Vector) for 10 min at RT. Pictures were taken with a Spot RTKE camera (Diagnostic Instruments; Sterling Heights, MI).
Western Blot
Extracts from either mouse brain or whole Helix ganglia were obtained by direct suspension of the ganglia in 5 volumes of Laemmli electrophoresis sample buffer, boiled for 2 min, and then centrifuged at the highest speed in a microcentrifuge to remove genomic DNA and insoluble debris. Alternatively, tissues were homogenized in 5 volumes of RIPA buffer [50 mM Tris/HCl, pH 7.4, containing 150 mM NaCl, 0.1% NP40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and 5 mM iodoacetamide, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 10 U/ml aprotinin, 5 μM pepstatin, 40 μM leupeptin, 5 μg/ml α2-macroglobulin (Roche, Mannheim, Germany) as protease inhibitors]. After 10 min of incubation on ice, the homogenate was centrifuged (highest speed in a microcentrifuge at 4°C) to remove debris and nuclei, and the supernatant was boiled for 2 min in an equal volume of Laemmli electrophoresis sample buffer. Samples containing 100 μg protein were separated on a 7% polyacrylamide gel transferred to nylon membranes and probed with the primary antisera (24III and Lim 1:1,000 dilution) or a rabbit preimmune serum (same dilution), followed by horseradish peroxidase (HRP)-labelled goat anti-rabbit IgG and detected by the chemiluminescent ECL system (GE Healthcare, Uppsala, Sweden).
N-Glycosidase F Digestion
Tissues homogenized in RIPA buffer as above were subjected to N-deglycosylation with N-glycosidase F (Roche) as described by Gennarini et al. (1989). For controls, duplicate aliquots of the extracts were incubated in the absence of the enzyme. Equivalent protein amounts from digested and undigested samples were then analyzed by Western blotting by using the above antisera.
Immunoaffinity Purification of Antibodies
Whole extracts from Helix ganglia (about 5 mg protein) were separated on 7% polyacrylamide gels and blotted onto nitrocellullose. After location of F3/contactin antibody-reactive bands, the corresponding nitrocellulose region was dissected out, saturated for 30 min at 37°C and incubated with F3/contactin antisera (1:500 dilution in saturation buffer) ON at 4°C. After several washes, bound antibodies were eluted in a 0.2 M glycine buffer, pH 2.8, and immediately neutralized with unbuffered 1 M Tris. Finally, BSA was added to the eluate to a 10% final concentration.
Measurements of Neurite Length
To measure neurite length, phase-contrast images of the cultures were acquired in Image Pro Plus with a Sony videocamera. Measurement of neurite length was made on extensions originating from the axonal stumps. For this, a circumferential line centered at the tip of the stump and interconnecting the tips of the three longest neurites was drawn in a semiautomatic way on the computer screen in order to demarcate the area covered by the growing neurites. The radius of such a circumferential line was then taken as a measure of the extent of neurite growth. Measures were made in the presence of the 24III anti-mouse F3/contactin or the corresponding preimmune (taken as control) serum. Statistical analysis was performed using the two-way repeated-measures ANOVA, followed by multiple-comparisons Newman Keuls test.
Detection of Neurotransmitter Release
The secretory properties of the C1 terminals were analyzed by adding a freshly dissociated assay neuron B2 to 3-5-day-old C1–C3 cocultures. The assay neuron was impaled with a glass micropipette filled with 2.5 M KCl (10–15 MΩ) connected to an Axoclamp 2A preamplifier (Axon Instruments, Union City, CA) in bridge mode. This neuron was micromanipulated in close contact with C1 neurites, and left in situ throughout the experiment. Neurotransmitter release was evoked by electrical stimulation of C1 (10 Hz for 10 sec) every 5 min and measured as the amount of depolarization recorded in the B2 assay neuron, previously hyperpolarized 30 mV below its resting potential. The results were expressed as means ± SEM, and statistical significance was determined by a two-way ANOVA followed by a post hoc test of Newman Keuls for multiple comparisons.
Isolation of HCRP cDNAs From a Lambda Expression Library
A custom cDNA λTriplEx2 expression library from Helix ganglia mRNA by oligo(dT) priming (Clontech, Mountain View, CA) was screened by using the 24III serum previously adsorbed on Escherichia coli lysates to counteract the background. After isolation, positive phages were converted in pTriplEx2 plasmids by cre-LoxP recombination mediated at LoxP sites of λTriplEx2 and sequenced by using both 5′-λTriplEx2 and T7 sequencing primers at the MWG Biotech (Ebersberg, Germany) and then analyzed by using the BLAST program on the NCBI web pages (http://www.ncbi.nlm.nih. gov/BLAST).
RNA Preparation and Analysis
After dissection, mouse brain and Helix ganglia were either immediately used for RNA preparation or frozen in liquid nitrogen and stored at −80°C until use. Total RNA was prepared by tissue homogenization in Trizol reagent (Invitrogen, Milan, Italy) according to the manufacturer’s instructions. For poly(A)+ preparation, the Message Maker mRNA isolation system (Invitrogen) was used. For Northern blotting, 16 μg of total or 2 μg of poly(A)+ RNA was separated on formaldehyde-containing 0.8% agarose gels, transferred to nylon membranes (Hybond C; GE Healthcare), and hybridized to the 960-bp insert from the isolated 9.1 clone using standard protocols (Gennarini et al., 1989).
RESULTS
Mouse F3/Contactin-Related Immunoreactivity Is Observed on Sections From Helix Ganglia
To look for mouse F3/contactin antibodies-reacting molecules expressed in Helix nervous tissue, sections from paraffin-embedded ganglia were processed with the 24III antiserum (Gennarini et al., 1991). In cerebral ganglia, this serum decorated the perikarya of neuronal cells and their axonal extensions (arrows in Fig. 1A,B), mostly giving the appearance of a cytoplasmic immunostaining (Fig. 1A–C). However, labelling of cell membranes, including the axolemma, was also observed (arrow in Fig. 1B). On the other hand, no labelling was observed within the nuclei nor when the preimmune serum was used (not shown), both findings supporting the specificity of the immunostaining profile. A variable labelling intensity was observed within different cells belonging to the same ganglion, strongly expressing cells being flanked by cells bearing lower amounts of the antigen (Fig. 1A). In some instances, on the perikarya, the immunostaining was concentrated on adjacent cells membranes (black arrows in Fig. 1D), and heavily stained granules were detected in the cytoplasm (Fig. 1D, white arrowheads), indicating that the underlying antigen was associated with intracellular organelles, besides being expressed at the cell membrane and within the neuronal cytoplasm.
Fig. 1.
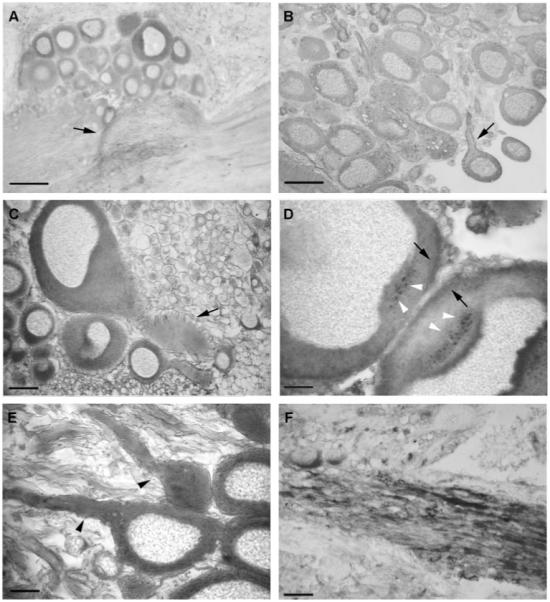
A–F: F3/contactin-related immunoreactivity on sections from Helix pomatia cerebral ganglia. Low-power pictures (A,B) demonstrated variable but relevant expression on both cell bodies and fiber tracts. Expression was observed on neurites (arrows in A,B) with both axoplasmatic and membrane (arrow in B) staining. High-power pictures (C–E) confirmed strong expression within the cytoplasm, in particular on intracellular organelles (D, white arrowheads), whereas low or absent antigen location was found within the nuclei (C–E). Antigen expression was also associated with fibers, on which a nonhomogeneous distribution was observed (A,E,F). In E, arrowheads point to low- and high-antigen-expressing axons. No expression was observed within the trophospongium (arrow in C). Scale bars = 100 μm in A,B; 20 μm in C,F; 10 μm in D,E.
As for the perikarya, also in the neuropil the distribution of the immunostaining was not homogeneous (Fig. 1A), strongly expressing fibers being flanked by fibers expressing lower amounts of the antigens (Fig. 1E,F). Lower expression in the trophospongium, the domain of nerve cells where glial cells contact axons (arrow in Fig. 1C), supported the neuronal derivation of the immunoreactive molecules.
Different F3/Contactin-Related Proteins Might Be Identified by Western Blots on Helix Ganglia Extracts
To identify the molecular components underlying F3/contactin immunoreactivity, extracts from Helix ganglia were probed by Western blot with the 24III antiserum, using a mouse brain extract as the positive control (Fig. 2). Although the typical 135-kD band was detected in mouse brain, two main proteins of 240- and 200-kD were identified in Helix ganglia, together with a lower Mr component around 90 kD, which was inconsistently detected in Helix ganglia sample buffer extracts (Fig. 2). Comparable results were obtained irrespective of whether the whole F3/contactin antiserum or antibodies immunopurified from the higher MW bands were used. No protein band was detected when the 24III preimmune serum was used (not shown). Although the 90-, 200-, and 240-kD chains were identified by antibodies against mouse F3/contactin, none of them corresponded in size to it. Therefore, we called them Helix contactin-related proteins (HCRPs).
Fig. 2.
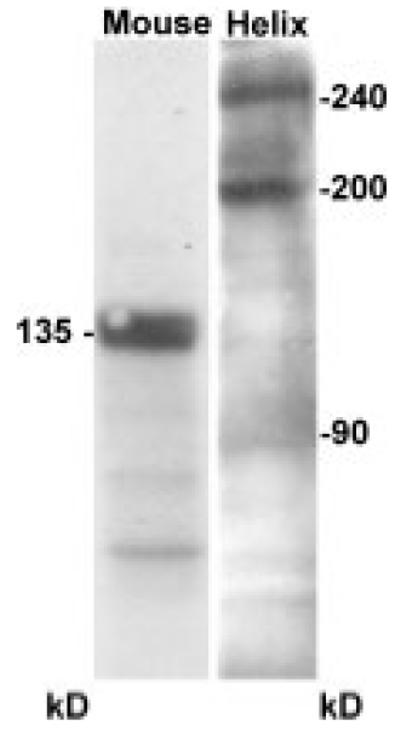
Detection of F3/contactin-related molecules on Helix pomatia cerebral ganglia extracts. Western blots with the 24III rabbit anti-mouse F3/contactin antiserum revealed two main bands at 240 and 200 kD, together with a minor Mr component around 90 kD. The canonical 135-kD F3/contactin band was found on mouse brain extracts.
Cloning of HCRP cDNA and Identification of Its mRNA
The identification of three different HCRP protein bands raised the question of their derivation. They could arise from transcription of different genes or, alternatively, by the posttranscriptional processing of a pre-mRNA arising from a single gene. In both cases, the different isoforms should represent the translation products of different mRNAs. A further possibility, however, was that the observed protein heterogeneity arose from posttranslational processing, which would predict a single mRNA chain. To address these possibilities, we isolated HCRP-cDNA clones to identify the corresponding mRNA by Northern hybridization. For this, mouse F3/contactin antibodies were used for isolating HCRP-related cDNAs from a Helix nervous tissue expression library (see Materials and Methods). Of three independent clones, we focused on 9.1 cDNA, containing a 960-bp insert whose sequence is shown in Figure 3A. This cDNA was used in turn to probe Northern blots of total or poly(A)+ RNA prepared from the whole ganglia. As shown in Figure 3B, a 6.3-kb band was identified by this means in both cases, indicating that the different HCRP isoforms most likely arise from posttranslational processing of a single protein backbone.
Fig. 3.

Molecular heterogeneity of HCRPs. A: Isolation of a HCRP cDNA. The nucleotide sequence of clone 9.1, isolated from a Helix ganglia expression library with the mouse F3/contactin antiserum, is shown. B: Identification of the HCRP mRNA. Total and poly(A)+ RNAs from Helix ganglia were probed by Northern blot with a 32P-labelled 9.1 cDNA probe. A main 6.3-kb chain was detected in both cases. C: N-deglycosylation of HCRPs. Detergent extracts from Helix pomatia ganglia in RIPA buffer were treated with N-glycosidase F and probed with the 24III antiserum. In digested samples (+), the 240-kD band disappeared, and the 200-kD chain become thinner. Minor differences were found for the 90-kD isoform, which appeared as a doublet of closely spaced bands.
To verify whether differences in glycosylation could account for HCRP heterogeneity, extracts from Helix ganglia in RIPA buffer were treated with N-glycosidase F, which cleaves N-linked sugars, and probed with the 24III serum by Western blotting. As shown in Figure 3C, under these conditions, the 240-kD band could not be detected, and the 200-kD one was much fainter than in controls. On the other hand, the 90-kD isoform appeared to be predominant and composed of a doublet of closely spaced bands. These data suggested that the higher Mr HCRP isoforms arose by differential N-glycosylation of the 90-kD chain, which therefore was likely to represent the main HCRP protein backbone.
Mouse F3/Contactin Antibodies Label the Surface of Helix Neurons in Culture
These data indicated that HCRPs were glycoprotein components associated with cytoplasmic compartments and intracellular organelles of Helix neurons and also suggested expression at the membrane level. To verify whether HCRPs were exported at the cell surface, living cultures of Helix neurons were immunostained with the 24III and the Lim antisera. At the same time, both sera were used on fixed cells. A couple of days after plating, Helix neurons developed an extensive neurite network from the initial axonal stump, and incubation with F3/contactin antibodies labelled the main axons and the branching neurites (Fig. 4A–C). A strong signal was observed on the growth cones and on the varicosity swellings (Fig. 4D–F, arrowheads). Patchy distribution of the immunostaining along the neurites indicated clustering of immunoreactive molecules. However, some distinct regions appeared immunonegative along the labelled axons (arrows in Fig. 4D–F). On living cells, similar results were obtained (Fig. 4G–I).
Fig. 4.
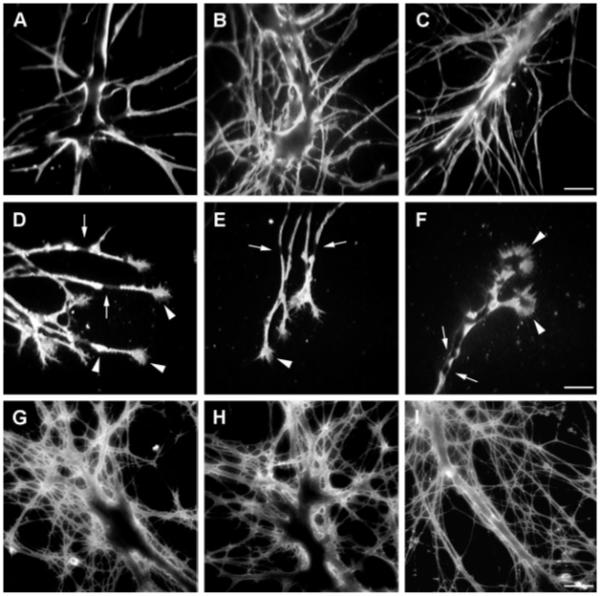
HCRP-related immunoreactivity on cultured neurons from the cerebral ganglia. A–F: On fixed cells, F3/contactin antibodies stained both the main axonal stump (A–C) and the arising branching neurites. A membrane-like profile and a patchy distribution of the immunostaining were observed, indicating clustering of the corresponding surface molecules; however, some distinct regions appeared immunonegative along the labelled axons (arrows). Strong expression was also found on the growth cones and varicosities along the neurites (white arrowheads in D–F). G–I: HCRPs-related immunoreactivity on living cultures from the cerebral ganglia. Scale bars = 50 μm in C (applies to A–C); 50 μm in F (applies to D–F); 50 μm in I (applies to G–I).
Together, these data indicated HCRPs expression both within the neuronal cytoplasm and at the cell surface of Helix neurons. The demonstration that antibodies against mouse F3/contactin recognized HCRPs in living cells allowed us to use them for functional studies.
Functional Characterization of the HCRP Glycoproteins
We next wanted to address the potential significance of HCRPs expression in neurite growth control and in neurotransmitter release modulation in identified neurons from Helix pomatia ganglia (Ghirardi et al., 2001).
Role of HCRPs in neurite growth
To check the involvement of HCRPs in neurite growth control, cultures of Helix neurons plated with their initial axonal segment were used. Neurons were left to adhere to the substrate for 6 hr, when a large growth cone formed from the tip of the axonal stump. Then, the 24III (Fig. 5A) or preimmune (Fig. 5B) sera were added to the medium (time 0), and axonal growth was measured over 48 hr. As shown in Figure 5C, measurements of neurite elongation at 24 hr indicated that, in the presence of the F3/contactin antibodies, neurite growth was only 41.285% ± 5.3% compared with control preparations treated with preimmune serum (P < 0.001, n = 6; Newman Keuls multiple-comparisons test). In the antibody-treated preparations, the neurite elongation stopped, and retraction with formation of thick neurites occurred at the 48-hr time point (Fig. 5A). In fact, quantitative analysis indicated that neurite elongation was reduced to 17.63% ± 5.69% compared with the value measured in control cultures at 24 hr, whereas the value of 149.94% ± 8.416% was measured in control neurons (P < 0.001; Fig. 5B,C). These data showed that neurite elongation of Helix neurons was affected by treatment with F3/contactin antibodies.
Fig. 5.
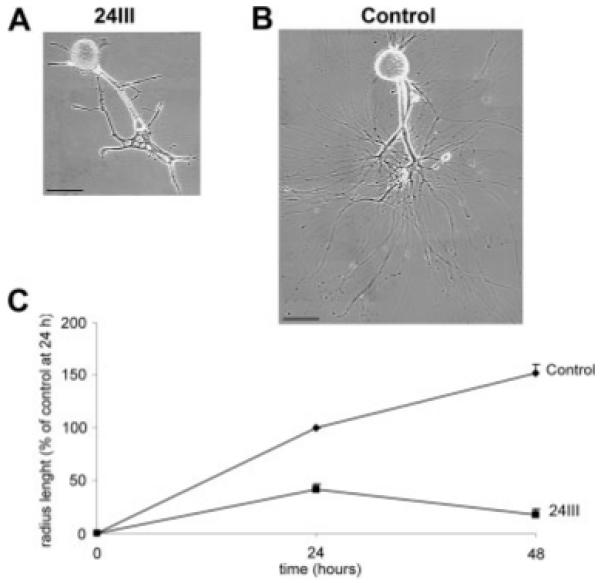
Effects of HCRPs on neurite growth. A,B: Neurite growth profile of cultured Helix neurons in the presence of the mouse F3/contactin antiserum (24III) or in the presence of the corresponding preimmune serum (control) at 48 hr. C: Graphic representation of neurite growth of Helix neurons in the presence of mouse F3/contactin antiserum (24III) or in the presence of the corresponding preimmune serum (control). Neurite elongation was followed over 48 hr and is shown at 24 and 48 hr. Each point represents the percentage mean value (±SEM) of radius length of a circumferential line interconnecting the tips of the three longest neurites, normalized to the control mean value measured at 24 hr (567.2 μm). Two-way analysis of variance (repeated measures) indicates a significant effect of treatments (F1,9 = 276.93, P < 0.001) and time (F1,9 = 6.205, P < 0.05) and a significant interaction between time and treatments (F1,9 = 43.69, P < 0.001). Scale bars = 100 μm.
Involvement of HCRPs in modulation of neurotransmitter release
We previously found that the secretory capabilities of the presynaptic C1 neuron were markedly reduced when it was cocultured with an inappropriate target such as the C3 neuron (Ghirardi et al., 2000, 2001). On the other hand, when the appropriate target B2 was inserted by micromanipulation within the network made by intermingled C3 and C1 neurites, the transmitter release from C1 increased within 30 min to the level observed in these neurons cultured in the presence of the appropriate target. By using such a model, we wanted to verify whether the positive effect of the appropriate B2 target on neurotransmitter release could be, at least in part, mediated by HCRPs expressed by these neurons. To this aim, the B2 neuron was incubated with either the 24III (1:125 dilution) or the Lim (1:1,000 dilution) antisera for 2 hr before being added to C1-C3 cocultures. Then, the amplitude of the B2 depolarization evoked by C1 electrical stimulation was measured. Immediately after the addition of either the 24III or the Lim sera-treated B2 neurons, the mean evoked depolarization was of 6.33 mV (±0.33 mV, n = 6) and 7.5 mV (±1.8 mV, n = 6), respectively. These values did not differ from those obtained with untreated (4.9 ± 2.2 mV, n = 5) or preimmune-serum-treated (5.1 ± 1.1 mV, n = 5) B2 neurons (Fig. 6). However, at the next time points, although a progressive increase in the evoked depolarisation was observed in untreated (14 ± 3.03 mV at 20 min and 13.6 ± 2.04 mV after 30 min) and in preimmune-serum-treated (17.1 ± 8.2 mV at 20 min and 21.4 ± 7.2 mV after 30 min) B2 cells, a significant decrease was found in 24III antibody treated B2 cells at 20 min (4.7 ± 0.55 mV) and at 30 min (5.08 ± 0.35 mV; P < 0.01 and P < 0.001) compared with the preimmune-serum-treated cells, respectively (Newman Keuls multiple-comparisons test). Similar results were obtained at the same time points in Lim antibody-treated cells (3.05 ± 1.0mV at 20 min, and 2.88 ± 1.29 mV after 30 min of contact, P < 0.01 compared with the control conditions). The above effects were unlikely to arise from a reduced responsivity of B2 to 5-HT, insofar as neither 24III nor Lim antibodies interfered with the response of B2 to bath application of exogenous serotonin (10−5 M; data not shown). Together, the functional studies indicated the HCRPs involvement in both axonal growth control and neurotransmitter release.
Fig. 6.
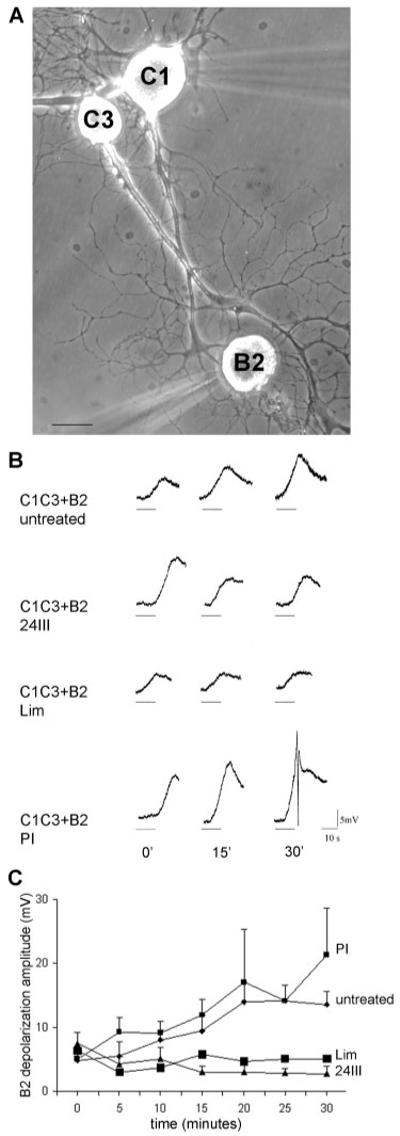
Effects of F3/contactin antibodies on neurotransmitter release from Helix neurons in culture. Preincubation of the appropriate target B2 neuron with either 24III or Lim antisera inhibits its inductive effect on neurotransmitter release evoked by C1 stimulation in the presence of the wrong target. A: Coculture of the presynaptic neuron C1 with the wrong target C3 and the assay neuron B2 (appropriate target). The freshly dissociated neuron B2 was added to the 3-day-old C1–C3 coculture at the time of recording. The C1 and B2 neurons were then impaled with microelectrodes (out of focus) for intracellular stimulation and recording. B: Examples of depolarization evoked in the assay neuron B2 by a 10-Hz 10-sec train of spikes elicited in C1 (horizontal bars) immediately (0), 15 min, and 30 min after B2 addition to C1–C3 coculture in different experimental conditions (untreated, in the presence of 24III or Lim antibodies, in the presence of the preimmune serum PI). C: Mean amplitude (±SEM) of the depolarization recorded in the assay neuron B2 following C1 stimulation at the indicated time points. Diamonds and small squares represent the build up of the evoked depolarization of B2 preincubated in culture medium or preimmune serum, respectively, from 0 to 30 min after establishment of contact with neurites of C1–C3 coculture. This progressive increase of the evoked depolarization does not occur when the B2 neuron has been preincubated with 24III (triangles) or Lim (large squares) antibodies for 2 hr. Analysis of variance indicates a significant effect of treatments (F3,126 = 19.78, P < 0.001) and time (F6,126 = 2.69, P < 0.02) and a significant interaction between time and treatments (F18,126 = 1.92, P < 0.01). Scale bar = 100 μm.
DISCUSSION
Here, we report on the distribution and function of F3/contactin immunologically related glycoproteins (HCRPs) expressed in the nervous tissue of the snail Helix pomatia, showing that they are involved in neurite growth and modulation of neurotransmitter release.
HCRP Structural Properties
HCRPs include three main 240-, 200-, and 90-kD glycoproteins, which differ in their sugar content. Indeed, after enzymatic N-deglycosylation, the 240-kD component disappears, whereas the 200-kD chain becomes much thinner, suggesting that these chains differ by their content in N-linked sugars. On the other hand, the 90-kD band becomes predominant in the deglycosylated sample, which suggests that it could represent the main HCRPs deglycosylated form from which the 200-kD and 240-kD chains arise by differential addition of N-linked sugars.
Further posttranslational processing may be responsible for this protein appearing as a doublet both in native and in N-deglycosylated extracts. The existence of a unique HCRP protein backbone is consistent with the demonstration of a unique HCRP mRNA.
Based on the large carbohydrate content, HCRPs could represent nervous tissue proteoglycans. This is supported by their similarity in cellular distribution to known nervous tissue proteoglycans such as receptor protein tyrosine phosphatase (RPTP) beta and its phosphacan derivative (Garwood et al., 2003; Hayashi et al., 2005). Indeed, HCRP expression recalls most the observations for the above-mentioned vertebrate RPTP family components, being found within the cytoplasm and in particular within the Golgi complex, which suggests association with organelles (Hayashi et al., 2005). As for their cellular distribution, although RPTP beta and phosphocan were originally identified on glia (Engel et al., 1996); they are also expressed on neurons (Snyder et al., 1996; Hayashi et al., 2005). Similarly to HCRPs, the RPTP family includes isoforms arising from addition of complex carbohydrate chains to the protein backbone (Barnea et al., 1994; Maeda et al., 1994; Maurel et al., 1994). In vertebrates, such proteoglycans interact with known adhesion receptors such as NCAM, NgCAM/L1, NrCAM, and TAG-1 (Milev et al., 1996); with contactin itself (Peles et al., 1995); and with some of its ECM receptors, such as tenascin C (Grumet et al., 1994) and tenascin R (Milev et al., 1998). These molecular complexes are thought to be of key relevance for early neural developmental events, including neuronal migration (Ohyama et al., 2004) as well as axonal growth (Friedlander et al., 1994; Milev et al., 1994; Maeda and Noda, 1996).
HCRP Distribution in Helix Nervous Tissue
Immunohistochemical staining revealed high HCRP expression within the neuronal soma and in neurite extensions. Expression in the former may correspond to forms transiting through the trans-Golgi secretory pathways, which may be relevant both for protein glycosylation and for export toward the growing axon, where HCRPs were found to be expressed. This suggests that the molecules could undergo axonal transport and, therefore, that there is a large HCRP intracellular pool, which feeds the axonally expressed components.
Within the same ganglion, HCRP expression varies from cell to cell, indicating differential regulation within different neurons. However, a heterogeneity in the staining pattern is also observed within the same cell along the neurites, resulting in discrete segments lacking these molecules. Such expression profile could depend on either regulated changes in the rate of axonal transport or on selective interactions of the molecules with components expressed along specific axonal segments as shown for interactions of the Sema 3A semaphorin component with proteoglycan receptors (De Wit et al., 2005). Finally, the demonstrated HCRP expression at the level of the axonal branching points could support a potential role for HCRPs in directional axonal growth, as demonstrated also for other vertebrates glycoprotein components such as Nogo (Richard et al., 2005).
HCRP Functional Role
To address the functional role of HCRPs, we first explored the possibility that HCRPs mediate the control of neurite growth, in which both F3/contactin and brain proteoglycans are involved in vertebrates (Gennarini et al., 1991; Margolis et al., 1996; Margolis and Margolis, 1997; Bizzoca et al., 2003; Schwartz and Domowicz, 2004). Indeed, neurite growth from Helix neurons was strongly inhibited by mouse F3/contactin antibodies, as shown in the case of several vertebrate neural adhesive molecules (see, e.g., Jones et al., 2003). Our data also provide indications that HCRPs could be involved in modulation of neurotransmitter release, which suggests complex interactions with the nerve cell signalling machinery responsible for modulating exocytosis (Rosenmund et al., 2003). In this respect, a role has been also proposed for extracellular matrix components such as the astrocyte-derived thrombospondins, involved in maintaining adhesion of pre- and postsynaptic specializations (Ullian et al., 2001; Washbourne et al., 2004; Ziv and Garner, 2004).
In conclusion, by using antibodies against mouse F3/contactin, we have found a set of glycoproteins (HCRPs) that share its role in neurite growth control in Helix pomatia nervous tissue. In addition, the use of a simple neuronal system has allowed us to propose a role for these glycoproteins in the regulation of exocytotic events that preside over neurotransmitter release.
ACKNOWLEDGMENTS
The technical assistance of Domenico Buonsanti, Dr. Claudio Franchino, and Petra Kieslinger is acknowledged. Dr. L. De Benedictis is thanked for help with cDNA cloning techniques.
Contract grant sponsor: Italian Ministry of University FIRB; Contract grant number: NE01WY7P; Contract grant sponsor: COFIN; Contract grant number: 2005055095; Contract grant sponsor: Fondazione Pierfranco e Luisa Mariani; Contract grant sponsor: Telethon Italy; Contract grant number: 1101; Contract grant sponsor: Austrian Science Foundation; Contract grant number: P17874B05.
REFERENCES
- Alenius M, Bohm S. Identification of a novel neural cell adhesion molecule-related gene with a potential role in selective axonal projection. J Biol Chem. 1997;272:26083–26086. doi: 10.1074/jbc.272.42.26083. [DOI] [PubMed] [Google Scholar]
- Barnea G, Grumet M, Milev P, Silvennoinen O, Levy JB, Sap J, Schlessinger J. Receptor tyrosine phosphatase beta is expressed in the form of proteoglycan and binds to the extracellular matrix protein tenascin. J Biol Chem. 1994;269:14349–14352. [PubMed] [Google Scholar]
- Berglund EO, Ranscht B. Molecular cloning and in situ localization of the human contactin gene (CNTN1) on chromosome 12q11-q12. Genomics. 1994;21:571–582. doi: 10.1006/geno.1994.1316. [DOI] [PubMed] [Google Scholar]
- Berglund EO, Murai KK, Fredette B, Sekerkova G, Marturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. doi: 10.1016/s0896-6273(00)81126-5. [DOI] [PubMed] [Google Scholar]
- Bizzoca A, Virgintino D, Lorusso L, Buttiglione M, Yoshida L, Polizzi A, Tattoli M, Cagiano R, Rossi F, Kozlov S, Furley A, Gennarini G. Transgenic mice expressing F3/contactin from the TAG1 promoter exhibit developmentally regulated changes in the differentiation of cerebellar neurons. Development. 2003;130:29–43. doi: 10.1242/dev.00183. [DOI] [PubMed] [Google Scholar]
- Brummendorf T, Lemmon V. Immunoglobulin superfamily receptors: cis-interactions, intracellular adapters and alternative splicing regulate adhesion. Curr Opin Cell Biol. 2001;13:611–618. doi: 10.1016/s0955-0674(00)00259-3. [DOI] [PubMed] [Google Scholar]
- Buttiglione M, Revest JM, Rougon G, Faivre-Sarrailh C. F3 neuronal adhesion molecule controls outgrowth and fasciculation of cerebellar granule cell neurites: a cell-type-specific effect mediated by the Ig-like domains. Mol Cell Neurosci. 1996;8:53–69. doi: 10.1006/mcne.1996.0043. [DOI] [PubMed] [Google Scholar]
- De Castro F. Chemotropic molecules: guides for axonal pathfinding and cell migration during CNS development. News Physiol Sci. 2003;18:130–136. doi: 10.1152/nips.01414.2002. [DOI] [PubMed] [Google Scholar]
- De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Durbec P, Gennarini G, Buttiglione M, Gomez S, Rougon G. Different domains of the F3 neuronal adhesion molecule are involved in adhesion and neurite outgrowth promotion. Eur J Neurosci. 1994;6:461–472. doi: 10.1111/j.1460-9568.1994.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Engel M, Maurel P, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. I. Cellular sites of synthesis of neurocan and phosphacan. J Comp Neurol. 1996;366:34–43. doi: 10.1002/(SICI)1096-9861(19960226)366:1<34::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Garwood J, Heck N, Reichardt F, Faissner A. Phosphacan short isoform, a novel non-proteoglycan variant of phosphacan/receptor protein tyrosine phosphatase-beta, interacts with neuronal receptors and promotes neurite outgrowth. J Biol Chem. 2003;278:24164–24173. doi: 10.1074/jbc.M211721200. [DOI] [PubMed] [Google Scholar]
- Gennarini G, Cibelli G, Rougon G, Mattei MG, Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989;109:775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G, Durbec P, Boned A, Rougon G, Goridis C. Transfected F3/F11 neuronal cell surface protein mediates intercellular adhesion and promotes neurite outgrowth. Neuron. 1991;6:595–606. doi: 10.1016/0896-6273(91)90062-5. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Casadio A, Santarelli L, Montarolo PG. Aplysia hemolymph promotes neurite outgrowth and synaptogenesis of identified Helix neurons in cell culture. Invert Neurosci. 1996;2:41–49. doi: 10.1007/BF02336659. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Casadio A, Naretto G, Levi R, Montarolo PG. Influence of the target on distribution and functioning of the varicosities of Helix pomatia metacerebral cell C1 in dissociated cell culture. Neuroscience. 2000;96:843–853. doi: 10.1016/s0306-4522(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Naretto G, Fiumara F, Vitiello F, Montarolo PG. Target-dependent modulation of neurotransmitter release in cultured Helix neurons involves adhesion molecules. J Neurosci Res. 2001;65:111–120. doi: 10.1002/jnr.1134. [DOI] [PubMed] [Google Scholar]
- Grumet M, Milev P, Sakurai T, Karthikeyan L, Bourdon M, Margolis RK, Margolis RU. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J Biol Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- Haenisch C, Diekmann H, Klinger M, Gennarini G, Kuwada JY, Stuermer CA. The neuronal growth and regeneration associated Cntn1 (F3/F11/contactin) gene is duplicated in fish: expression during development and retinal axon regeneration. Mol Cel. Neurosci. 2005;28:361–374. doi: 10.1016/j.mcn.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Miyata S, Yamada M, Kamei K, Oohira A. Neuronal expression of the chondroitin sulfate proteoglycans receptor-type protein-tyrosine phosphatase beta and phosphacan. Neuroscience. 2005;131:331–348. doi: 10.1016/j.neuroscience.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, Takeda Y, Chia W, Sankar N, Ng YK, Ling EA, Maciag T, Small D, Trifonova R, Kopan R, Okano H, Nakafuku M, Chiba S, Hirai H, Aster JC, Schachner M, Pallen CJ, Watanabe K, Xiao ZC. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F, Schacher S. Neuron-specific membrane glycoproteins promoting neurite fasciculation in Aplysia californica. J Cell Biol. 1990;111:2637–2650. doi: 10.1083/jcb.111.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Noda M. 6B4 proteoglycan/phosphacan is a repulsive substratum but promotes morphological differentiation of cortical neurons. Development. 1996;122:647–658. doi: 10.1242/dev.122.2.647. [DOI] [PubMed] [Google Scholar]
- Maeda N, Hamanaka H, Shintani T, Nishiwaki T, Noda M. Multiple receptor-like protein tyrosine phosphatases in the form of chondroitin sulfate proteoglycan. FEBS Lett. 1994;354:67–70. doi: 10.1016/0014-5793(94)01093-5. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans as mediators of axon growth and pathfinding. Cell Tissue Res. 1997;290:343–348. doi: 10.1007/s004410050939. [DOI] [PubMed] [Google Scholar]
- Margolis RK, Rauch U, Maurel P, Margolis RU. Neurocan and phosphacan: two major nervous tissue-specific chondroitin sulfate proteoglycans. Perspect Dev Neurobiol. 1996;3:273–290. [PubMed] [Google Scholar]
- Maurel P, Rauch U, Flad M, Margolis RK, Margolis RU. Phosphacan, a chondroitin sulfate proteoglycan of brain that interacts with neurons and neural cell-adhesion molecules, is an extracellular variant of a receptor-type protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1994;91:2512–2516. doi: 10.1073/pnas.91.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Barzilai A, Keller F, Schacher S, Kandel ER. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256:638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Maurel P, Haring M, Margolis RK, Margolis RU. TAG1/axonin1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-zeta/beta, and NCAM. J Biol Chem. 1996;271:15716–15723. doi: 10.1074/jbc.271.26.15716. [DOI] [PubMed] [Google Scholar]
- Milev P, Chiba A, Haring M, Rauvala H, Schachner M, Ranscht B, Margolis RK, Margolis RU. High affinity binding and overlap-ping localization of neurocan and phosphacan/protein-tyrosine phosphatase-zeta/beta with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J Biol Chem. 1998;273:6998–7005. doi: 10.1074/jbc.273.12.6998. [DOI] [PubMed] [Google Scholar]
- Murai KK, Misner D, Ranscht B. Contactin supports synaptic plasticity associated with hippocampal long-term depression but not potentiation. Curr Biol. 2002;12:181–190. doi: 10.1016/s0960-9822(02)00680-2. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Ikeda E, Kawamura K, Maeda N, Noda M. Receptor-like protein tyrosine phosphatase zeta/RPTP beta is expressed on tangentially aligned neurons in early mouse neocortex. Brain Res Dev Brain Res. 2004;148:121–127. doi: 10.1016/j.devbrainres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, Grumet M, Schlessinger J. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron. 1993;10:69–82. doi: 10.1016/0896-6273(93)90243-k. [DOI] [PubMed] [Google Scholar]
- Ranscht B. Sequence of contactin, a 130 kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988;107:1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Giannetti N, Saucier D, Sacquet J, Jourdan F, Pellier-Monnin V. Neuronal expression of Nogo-A mRNA and protein during neurite outgrowth in the developing rat olfactory system. Eur J Neurosci. 2005;22:2145–2158. doi: 10.1111/j.1460-9568.2005.04418.x. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Rettig J, Brose N. Molecular mechanisms of active zone function. Curr Opin Neurobiol. 2003;13:509–519. doi: 10.1016/j.conb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Schacher S, Wu F, Sun ZY, Wang D. Cell-specific changes in expression of mRNAs encoding splice variants of Aplysia cell adhesion molecule accompany long-term synaptic plasticity. J Neurobiol. 2000;45:152–161. doi: 10.1002/1097-4695(20001115)45:3<152::aid-neu3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Schwartz NB, Domowicz M. Proteoglycans in brain development. Glycoconj J. 2004;21:329–341. doi: 10.1023/B:GLYC.0000046278.34016.36. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Hosoya H, Takeda Y, Kobayashi S, Watanabe K. Age-related decline of F3/contactin in rat hippocampus. Neurosci Lett. 1998;245:117–120. doi: 10.1016/s0304-3940(98)00179-7. [DOI] [PubMed] [Google Scholar]
- Snyder SE, Li J, Schauwecker PE, McNeil TH, Salton SR. Comparison of RPTP zeta/beta, phosphacan, and trkB mRNA expression in the developing and adult rat nervous system and induction of RPTP zeta/beta and phosphacan mRNA following brain injury. Brain Res Mol Brain Res. 1996;40:79–96. doi: 10.1016/0169-328x(96)00039-3. [DOI] [PubMed] [Google Scholar]
- Thompson C, Lin CH, Forscher P. An Aplysia cell adhesion molecule associated with site-directed actin filament assembly in neuronal growth cones. J Cell Sci. 1996;109:2843–2854. doi: 10.1242/jcs.109.12.2843. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Ambrosini M, D’Errico P, Bertossi M, Papadaki C, Karagogeos D, Gennarini G. Regional distribution and cell type-specific expression of the mouse F3 axonal glycoprotein: a developmental study. J Comp Neurol. 1999;413:357–372. doi: 10.1002/(sici)1096-9861(19991025)413:3<357::aid-cne1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, El-Husseini A. Cell adhesion molecules in synapse formation. J Neurosci. 2004;24:9244–9249. doi: 10.1523/JNEUROSCI.3339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Shimazaki K, Hosoya H, Fukamauchi F, Takenawa T. Cloning of the cDNA encoding neural adhesion molecule F3 from bovine brain. Gene. 1995;160:245–248. doi: 10.1016/0378-1119(95)00062-b. [DOI] [PubMed] [Google Scholar]
- Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16:52–58. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Xu YZ, Ji Y, Zipser B, Jellies J, Johansen KM, Johansen J. Proteolytic cleavage of the ectodomain of the L1 CAM family member tractin. J Biol Chem. 2003;278:4322–4330. doi: 10.1074/jbc.M210775200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wu F, Schacher S. Changes in expression and distribution of Aplysia cell adhesion molecules can influence synapse formation and elimination in vitro. J Neurosci. 1995;15:4173–4183. doi: 10.1523/JNEUROSCI.15-06-04173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]


