SUMMARY
It has been suggested that maximum sustained metabolic rates (SusMR) in mammals as reached, for instance, during lactation, are due to limited capacity for heat dissipation. Here, we experimentally test whether heat dissipation limitation (HDL) also constrains energy turnover in lactating European hares. Experimentally, we made use of the fact that hares nurse their young only once per day, which allowed us to keep females and young either at the same or at different ambient temperatures. During the last lactation week (week 4) females kept at thermoneutrality (22° C), irrespective of the cold load of their young, had significantly lower rates of metabolisable energy intake (MEI) than cold exposed mothers (5° C), as predicted by the HDL hypothesis. However, in week 2 of lactation females at thermoneutrality rearing cold-exposed young were able to increase MEI to levels indistinguishable from cold-exposed females. Thus, even at thermoneutral temperature females reached maximum rates of energy turnover, which was inconsistent with the HDL hypothesis. We conclude that SusMR in lactating European hares typically does not result not from physiological constraints but from an active restriction of their energy turnover in order to maximise lifetime reproductive success.
Keywords: Sustained metabolic rate, maternal investment, reproduction, lactation, heat dissipation limit, Lepus europaeus, metabolisable energy intake, milk production
INTRODUCTION
Mammals frequently encounter upper limits to sustained energy turnover (SusMR) which are reached during prolonged periods of cold exposure, high long-term physical activity, or lactation. There are two major types of explanations for these limits. The first concept suggests that life-history explains the upper levels of energy turnover selected (Williams, 1966; Drent and Daan, 1980; Valencak et al., 2009; Valencak und Ruf, 2009). According to this idea, sustained metabolic rate is determined by variable, reproductive life-history tactics, and mammals might actively cap the upper level of energy turnover (possibly well below physiologically feasible levels) to maximise lifetime reproductive success (Drent and Daan, 1982; see also Speakman and Krol, 2005). The other framework available to explain SusMR suggests anatomically or physiologically imposed constraints. Such boundaries could be the extent of the gastrointestinal tract, the capacity of energy expending organs such as skeletal muscles or mammary glands when operating at peak metabolic rates (recently reviewed in Speakman, 2008; Speakman and Krol, 2005).
With regard to this physiological constraint concept, many studies over the last two decades have attempted to identify the proximate factor(s) which determine the upper limits of energy budgets in lactating laboratory mice (e.g., Hammond and Diamond, 1992; Koteja, 1996; Johnson et al., 2001 a, b, c; Speakman et al., 2001; Krol and Speakman, 2003 a, b; Krol et al., 2007). Originally, the debate has focused on whether SusMR is limited ”centrally“, by the capacity of nutrient-processing, visceral organs like intestines, liver, kidneys (Weiner, 1992; Koteja, 1996), or ”peripherally“, by the capacity of energy expending organs such as skeletal muscles or mammary glands (Hammond and Diamond, 1994; Hammond et al., 1996; Hammond and Kristan, 2000; Johnson et al., 2001 a, b, c; Speakman et al., 2001). More recently, Krol and Speakman (2003a, b; see also Krol et al., 2003; Krol et al., 2007) found convincing evidence for the so called “heat dissipation limit hypothesis”, as they observed that mice raising offspring at thermoneutrality were apparently limited by the capacity to dissipate heat under peak metabolic demands. There are two possible mechanisms by which heat dissipation could be limiting for lactating females (Speakman, 2008): Firstly, high metabolic rates might lead to elevated body temperatures and impaired milk flow. Alternatively, to increase thermal conductance via vasodilatation, blood flow might be directed away from the mammary glands, thereby decreasing milk production. Secondly, lactating females might terminate suckling events earlier if they are confronted with hyperthermia and thus, pups raised at thermoneutral conditions might grow considerably slower (Krol et al., 2007; Speakman, 2008). By breeding laboratory mice at 21° C (i.e. moderate cold exposure) but dorsally shaving them to increase rates of heat loss, Krol et al., 2007 demonstrated that shaved mice showed in fact a significantly enhanced reproductive performance. These results confirm, and functionally explain, earlier observations that milk yield is reduced in domesticated livestock when ambient temperatures are high (Forbes, 2007). These findings include data on lagomorphs. For instance, Marai et al., 2001 showed that domesticated rabbits have impaired reproductive performance under hot weather conditions in Egypt. Essentially, the heat dissipation hypothesis is a central limitation concept, but it is related to the thermoregulatory capacity of an animal instead of its ability to absorb nutrients (Speakman, 2008).
Most previous studies on SusMR during peak lactation have used small rodents, such as laboratory mice, as animal models. Laboratory mice efficiently raise large litters and give birth to very fast growing offspring. It is easy to envision that heat dissipation is a problem for small rodents like mice. Firstly, they have high weight specific metabolic rates and produce massive amounts of new tissue during pregnancy (relative to their body weight), which requires a proportional increase in metabolism. Secondly, they typically face small body to environmental temperature gradients under which they routinely raise young in laboratory environments. These problems may not be as relevant for other mammals. Here, we test whether heat dissipation also imposes a limit for a medium sized mammal, the European hare (Lepus europaeus, Pallas).
European hares raise relatively few (litter size 1-5) but highly precocial young which are fully furred at birth with eyes open and capable of thermoregulation from their day of birth (Zörner, 1996). Hares lack protective burrows and, in contrast to rodents, nurse their offspring only once a day for a few minutes (Broekhuizen and Maaskamp, 1982). Yet, they start taking up solid food only at the end of the second week of lactation and solely depend on maternal milk early in life (Hackländer et al., 2002a; Valencak et al., 2009). From previous studies we knew that energy intake and milk transfer levels off with increasing litter size, and that most females reach SusMR at a litter size of 3 young during weeks 3-4 of lactation (Hackländer et al., 2002b; Valencak et al., 2009; Valencak and Ruf, 2009). Due to the once per day suckling period it is possible to accurately and directly assess daily milk transfer to the juveniles (by weighing them before and after suckling). In addition, hares allow manipulation of the female’s thermoregulatory demands independently from their young. This can be achieved by keeping females and offspring separately for most of the time either at thermoneutrality or in the cold.
We hypothesised that, if heat dissipation was limiting, mothers kept at thermoneutrality should be unable to match any increased demand of cold-exposed pups, while cold-exposed mothers would be able to increase energy intake and milk output. If alternatively, females exposed to thermoneutral conditions with their juveniles kept at cold conditions increase rates of energy assimilation, we would reject the hypothesis that heat dissipation is limiting for hares. Further, if females were unable to increase energy assimilation when exposed to cold, we would attribute this to a central limitation caused by the animals’ maximal capacity to absorb nutrients. Finally, the peripheral limitation hypothesis would be supported if cold exposed females increased their energy intake rates but at the same time could not provide their young with more milk. In the present study we therefore quantified sustained energy intake and the amount of milk transferred to the pups in lactating hares raising size-manipulated litters of three juveniles each under different temperature conditions: two groups of females were either kept at 5° C or at thermoneutrality (22° C), while juveniles of both groups were cold exposed (5° C). In a third control group, both females and young were maintained at thermoneutrality.
MATERIALS AND METHODS
Animals and housing
European hares were born and kept in our outbred breeding colony at the Research Institute of Wildlife Ecology, University of Veterinary Medicine Vienna, Austria (48° 14′ N 16° 20′ E) . Hares were housed individually in cages as outlined in Hackländer et al., 2002 a, b. As in the wild (Broekhuizen and Maaskamp, 1980), females were kept separately from their young except for a short nursing period in the morning (8-9 am). All females and their young were provided with water and food ad libitum. Animals were fed standard hare pellets (Raiffeisen, Salzburg, Austria) produced to match the mean chemical composition of stomach contents from free-ranging hares (Brüll 1976; Onderscheka and Tataruch, 1982). The mean gross energy content of the hare diet over the whole study period was 16.7 ± 0.02 kJ g-1 (dry weight) with 16.6 ± 0.05% protein, 70.7 ± 0.09% fibre, and 3.0 ± 0.06% fat.
Data were sampled between 2004 and 2008. All experimental animals were aged between 1 and 5 years and were in good health and condition. Hares were exposed to natural photoperiod in all three experimental groups. Females were paired with males for two days three times per year, i.e. in February-March (spring), May-June (summer) and late July-August (autumn) as outlined in Valencak et al., 2009. All litters were size manipulated to match a constant litter size of three young to keep energy demands for all females equally high. As outlined in Valencak et al., 2009, we did not fully cross-foster litters but mostly added one pup from another female which was then left without pups until the next mating. To allow litter size manipulations, all matings took place synchronously. Litter sizes were manipulated immediately after birth of the young (within 26 hours). All females readily accepted and nursed additional young and thus had similar energy demands throughout the entire lactation period.
Females and their offspring were assigned to three experimental groups. Group WW consisted of females and their offspring continuously kept at 22 ± 2° C over the study period. This temperature range well matches the thermoneutral zone of European hares (18 – 25° C; (Hackländer et al., 2002a; Valencak and Ruf, 2009). All animals (mothers and offspring) assigned to group CC were transferred to a climate chamber on the day after parturition and were maintained there at 5 ± 2° C throughout lactation until weaning. In the third experimental group, WC, mothers were kept at room temperature conditions whereas juveniles were maintained at 5 ± 2° C in the climate chamber except for the short daily nursing period during which young were transferred to their mother’s cage. In all groups, juveniles of one litter were kept together in one cage.
Measurements of energy intake and milk output
Body weight of all animals was determined weekly to the nearest 1g (Sartorius, Germany). Food intake was determined during bi-weekly feeding trials (over three and four day intervals) by weighing offered and uneaten food of all females. Food items spilled from the racks were dried, weighed, and subtracted from food consumption. To minimize effects of changes in humidity on food mass, food pellets were stored next to the cages prior to their usage.
Total faeces produced by the females were also collected bi-weekly over three and four day intervals dried at 60° C in a drying oven (Heraeus, Germany) for 48 hours and then weighed to the nearest 0.1g (Ohaus, Germany). Gross energy content of faeces was determined by near infrared spectroscopy (NIRS) as outlined in detail in Valencak et al., 2009. The following parameters were determined: dry matter, protein, fat, ash, acid detergent fiber (ADF) and lignin. For calibration of the NIRS analysis, 80 samples were chemically analyzed using standardized methods for crude protein, crude fat, crude ash and dry matter (Nehring, 1960). ADF and lignin were determined by the van Soest detergent analyses (Otzelberger, 1983). The NIR calibration results were evaluated by cross validation. Coefficients of determination for fat, protein, ash, lignin and dry matter were 0.93, 0.93, 0.83, 0.87, 0.87, and 0.96, respectively.
Milk intake of young was measured daily by weighing the juveniles before and after the 1 h suckling period to the nearest 1g (Sartorius, Germany). Milk output (MO) of mothers (g d-1) is given as the sum of milk intake from all 3 juveniles per kg body weight of mothers. Initial trials showed that weight losses during the nursing period due to faecal and urinary losses in juveniles were negligible (<2g per juvenile) compared to the milk intake (~60g) (T. G. Valencak, unpublished). Therefore, faecal and urinary losses during these periods were not determined. During suckling, juveniles had no access to other food sources. Otherwise, all juveniles had ad libitum access to high-fat pellets whose composition is outlined in Valencak et al., 2009. Food intake of each litter was determined at weekly intervals, using the same methods as for adult females.
The energy content of solid food and faeces was calculated by using energetic values given in Livesey (Livesey, 1984) and Livesey and Marinos (Livesey and Marinos, 1988). Thus, the gross energy content of protein, fat and fibre/nitrogen-free extract (NFE) was 23.3kJg−1, 39.6kJg−1 and 17.5kJg−1, respectively. Gross energy intake (GEI) was computed from the amount of food consumed per day multiplied by its energy content. Metabolisable energy intake (MEI) was calculated by (i) correcting GEI for urinary energy losses due to nitrogen excretion by using a metabolisable protein energy content of 19.3kJg−1 (Livesey, 1984) and (ii) computing the difference between this corrected, utilizable GEI and the energy content of the daily amount of faeces excreted. Assimilation efficiency (AE) was computed as MEI as percent of GEI. To allow comparison with published data from other species, multiples of mass-specific resting metabolic rate (RMR) were computed by dividing both mass-specific GEI and mass-specific MEI by 172.3kJ kg−1 day−1, the RMR of non-reproducing hares at thermoneutrality (20°C) measured for >=4 h with open-flow respirometry, as outlined elsewhere (Hackländer et al., 2002a).
As obtaining milk samples require anesthetizing females which may affect lactational performance, we did not milk hares in this study. Thus, milk energy output (MEO) was only estimated by multiplying milk intake of young (g d−1) by an average milk energy content of 12.62kJ g−1 during mid-lactation as obtained previously. Details on the determination of milk energy content are given in Valencak et al., 2009 and Valencak and Ruf, 2009.
Statistical analyses
Data analysis was restricted to a total of 23 mothers and their 29 litters (N= 12, 8, and 9 for the groups WW, CC and WC respectively) which weaned three young after four weeks (28 days) of lactation. The mean age of females in these groups was 2.23 ± 0.17, 2.10 ± 0.12, and 2.77 ± 0.49 years in groups WW, CC, and WC, respectively. Since energy assimilation tended to decrease with age (see results), age (in years) was included in all statistical tests to adjust for slight differences in the age-composition of groups. All statistical analyses were computed in R (2.9.0.; R Development Core Team 2008). MEI, AE, and milk transfer in females, as well as growth of young (weight from birth to weaning) and solid food intake of litters were analysed with a repeated measures design, as data from within, and partly between separate lactation periods, were sampled from the same animals. Therefore, we fitted linear mixed effect models with separate intercepts for each female included as the random factor, using function lme from the R-package nlme (Pinheiro et al., 2008).
Fixed effects in full multiple regression models were always treatment (WW, CC, WC), time interval (bi-weekly intervals 1-8), body weight, and female age (1-3 years). As most variables changed in a nonlinear manner over the lactation period (e.g. Fig. 1), lactation interval was treated as a factor, rather than a continuous variable. Since season is known to affect lactation energetics in hares, with higher levels of food intake and MEI in autumn, compared to spring and summer (Valencak et al., 2009), we initially included season as an additional fixed factor in all models. However, our current data set contained only two lactation periods from autumn, and thus season had no detectable effect on MEI (P>0.38) and was removed from final models. In repeated measurement models testing for differences in juvenile growth, we used “litter ID” as the random factor. Solid food intake of juveniles (determined for entire litters only) was compared between treatment groups using simple linear (lm) models, using the sum of food consumed over all four lactation weeks.
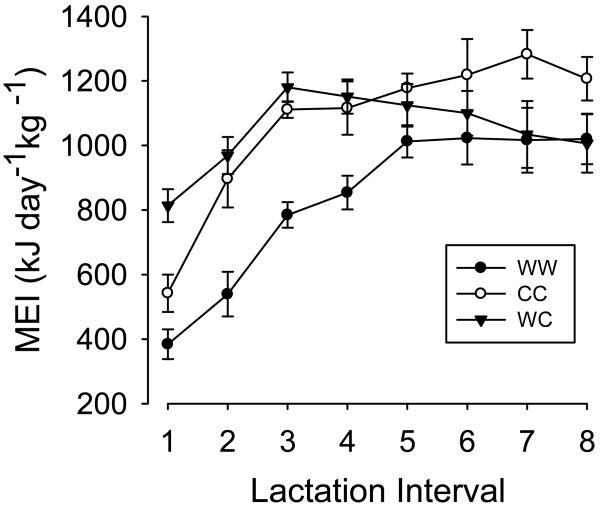
Fig. 1.
Metabolisable energy intake of all three experimental groups in the course of lactation. Means ± s.e.m. from a total of 29 lactation periods in 23 females.
ANOVAs were computed using marginal (Type III) sums of squares. Residuals from all models were normally distributed and showed no evidence for heterogeneity of variances. To test for specific differences between treatments, we used Tukey- type post hoc comparisons (R-package multcomp; Hothorn et al., 2008). These comparisons are based on z and t statistics for lme and lm models, respectively, which are given in the text.
Body mass slightly differed between experimental groups (3.578 ± 0.054, 3.438 ± 0.034, 3.763 ± 0.051kg, for groups WW, CC, and WC, respectively). In the text and the figures, we give weight-specific data. However, in all statistical analyses possible effects of body mass differences were eliminated by entering body weight as a fixed covariate. All data are presented as mean ± s.e.m.
RESULTS
Energy intake in experimental groups
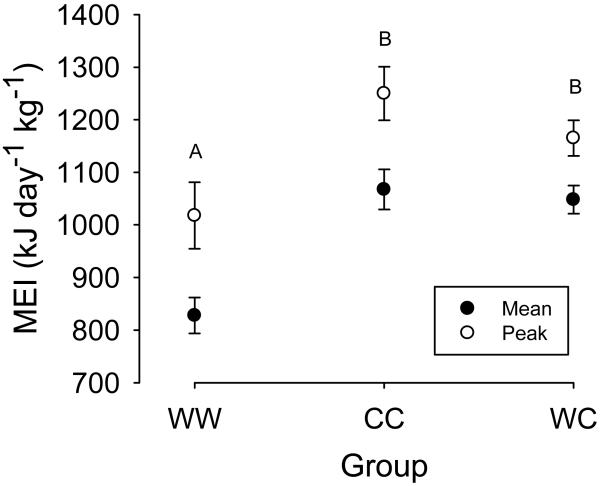
Metabolisable energy intake (MEI) increased with body mass (F 1,184 = 10.74; P= 0.0013) but slightly decreased with increasing age of the mothers (by 43.3kJ day−1 kg−1 per year; F 1,184 = 4.5; P= 0.036). Mean MEI and its time course over lactation significantly differed between the three experimental groups WW, CC and WC (experimental group: F 2,184= 19.3; P <0.0001; interval: F 7,184= 24.17; P <0.0001; Table 1, Fig. 1). Specifically, mean MEI was about 20% higher in both the WC and the CC group compared to the WW group (z= 5.35; P <0.0001 post hoc; Fig. 2, Table 1). Mean MEI was similar in the WC and CC group (z=−0.01; P >0.05 post hoc comparison).
Table 1.
Energy intake of lactating hares in the three experimental groups
| WW | CC | WC | |
|---|---|---|---|
| GEI (kJ kg −1 day−1) | 1325.5 ± 42.03 | 1680.03 ± 54.01 | 1572.9 ± 37.07 |
| MEI (kJ kg −1 day−1) over intervals 1-3 (lactation onset) |
568.09 ± 42.44 | 863.353 ± 60.37 | 987.55 ± 41.13 |
| Mean MEI (kJ kg −1 day−1) | 827.96 ± 34.12 | 1067.32 ± 37.96 | 1048.2 ± 26.6 |
| Peak MEI (kJ kg −1 day−1) | 1018.13 ± 62.9 | 1250.1 ± 50.8 | 1165.9 ± 34.3 |
| AE (%) | 61.34 ± 1.2 | 63.14 ± 0.8 | 67.13 ± 1.08 |
|
| |||
| Mean MEO (kJ kg −1 day−1) | 261.5 ± 12.6 | 317.3 ± 19.6 | 359.6 ± 14.1 |
Data are means ± s.e.m for n= 12, 8 and 9 litters from groups WW, CC and WC, respectively.
WW: females and juveniles at room temperature conditions (22 ± 2° C)
CC: females and juveniles exposed to 5° ± 2° C during lactation
WC: females maintained at room temperature conditions (22 ± 2° C) while juveniles kept at 5° ± 2° C
Fig. 2.
Mean (solid dot) and peak metabolisable energy intake (open dot) in the experimental groups. Different upper case letters indicate significant changes between groups. Means ± s.e.m.
Among females in groups WW and CC, peak rates of MEI were observed during week 4 of lactation (intervals 7 and 8) but already during week 2 (intervals 3 and 4) in group WC (Fig. 1, Table 1). Thus, at the onset of lactation (intervals 1-3), MEI was significantly higher in the WC group than in the two other groups WW and CC (z= 2.96; P= 0.009; Table 1). After week 2, MEI decreased in group WC, with the difference to week 2 becoming significant in week 4 (z= −2.583; P= 0.048).
Therefore, statistical differences between groups depended on the time period considered. Only in the last week (4) of lactation was MEI significantly higher in group CC than in both other groups (z>=2.58; P<=0.026). Comparing the second half of lactation (weeks 3-4) we found that MEI in was statistically different only between groups CC and WW (z= 2.50; P=0.033), but indistinguishable between groups CC and WC (z= 1.905; P= 0.137) as well as between groups WW and WC (z=0.435; P=0.901). The same was true for the comparison of lactation weeks 2- 4.
Importantly, the maximum rate of MEI (reached weeks 4, 4, and 2 in groups WW, CC, and WC, respectively) differed between the experimental groups (ANOVA: F 2,34 = 10.3; P= 0.0003, Fig. 2, Table 1). Peak MEI in groups CC and WC was significantly higher than in WW (z ≥ 3.69, P< 0.0001) but did not differ between each other (z= −0.576, P= 0.833).
Similar to MEI, gross energy intake (GEI) was affected by body mass (F 1,185= 16.49; P= 0.0001) and slightly decreased with age (F 1,185= 4.4; P= 0.037). GEI increased in the course of lactation (F 1,185= 42.07; P< 0.0001) in all three groups, but mean GEI differed between groups WW, CC and WC (F 2,185= 17.65; P< 0.0001, Table 1) with higher intake rates found in groups CC and WC than in group WW (z ≥ 4.73, P< 0.0001).
Higher rates of MEI in the groups CC and WC were caused not only by increased rates of energy intake (GEI) but also by increased energy assimilation efficiency (AE) (Table 1). Mean AE differed between experimental groups (F 2,186= 6.7; P= 0.0012) and was highest in group WC (Table 1). For this variable, only the difference between groups WC and WW was statistically significant (z=3.81, P<0.001). AE was unaffected by body mass and age and remained constant over the entire lactation period (F 7,186= 0.7; P=0.7).
In all experimental groups body weight slightly increased over the lactation period, on average by 4.2% (F 7,186 = 5.6872, P <.0001).
Milk output
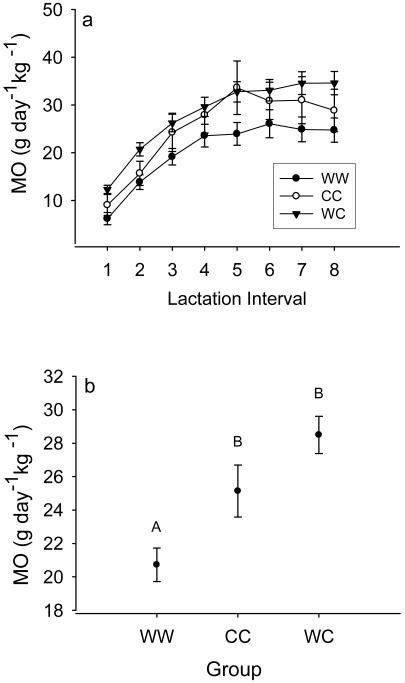
Daily milk energy output (MO) significantly differed between experimental groups (F 2,185=20.74; P< 0.0001; Fig. 3a, b). MO was higher in both groups WC and CC compared to WW (z ≥ 5.54; P< 0.0001) but indistinguishable between groups WC and CC (z= 1.18; P= 0.46, Fig. 3b). Expectedly, MO was strongly affected by lactation interval (F 7,185= 34.25; P<0.0001, Fig. 3a) and also by body mass (F 1,185= 9.03; P= 0.003). Also, MO slightly decreased with the female’s age (F 1,185= 8.01; P= 0.005).
Fig. 3.
Quantitative milk output in the course of lactation (a) and mean milk output of all three experimental groups (b). Different upper case letters indicate significant changes between groups. Means ± s.e.m.
Interestingly, at the onset of lactation (intervals 1-3, Fig. 3a, b) MO in females of the WC group was significantly higher than in both groups WW (z=4.49, P<0.001) and CC (z=2.47; P=0.036).
Juvenile growth and solid food intake
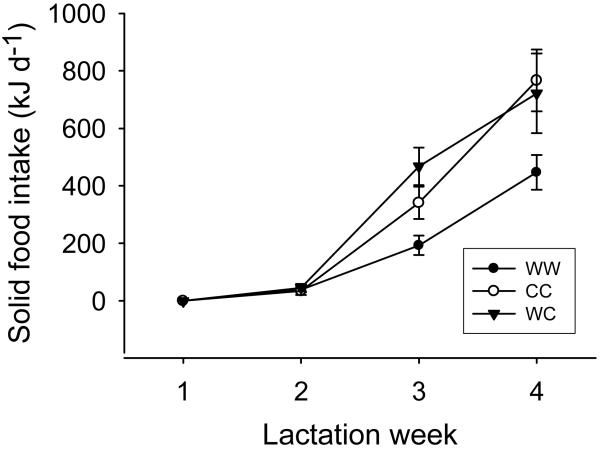
Mean solid food intake of young differed between groups (F 1,27 = 11.5; P= 0.002; Fig. 4). Food intake in group CC was somewhat higher than in WW (t=2.48; P=0.050), and in group WC it was significantly higher than in WW (t=3.09; P=0.012). Mean birth weights of young were 124.3 ± 3.4g (n=36), 112 ± 4.4g (n=24), and 127.6 ± 3.2g (n=27) for groups WW, CC and WC respectively. Weaning weights were 657.6 ± 23.7g, 643 ± 30.4g and 768.4 ± 31.5g for groups WW, CC and WC respectively. The differences in birth weights were statistically significant (F 2, 66 = 5.33; P= 0.007) while differences in weaning weights were not (F 2, 66= 2.32; P= 0.106). The weight gains between birth and weaning amounted to 533.3, 546.4 and 640.8 for groups WW, CC and WC respectively but were not significantly different from each other (F 2, 66 = 1.99; P= 0.14). Thus, cold exposure did not affect juvenile growth under our experimental conditions of ad libitum food availability.
Fig. 4.
Solid food intake of litters (3 young) in the course of lactation. Means ± s.e.m. from 29 litters.
DISCUSSION
Energy turnover in females
Does heat dissipation set the limit?
Our study showed that exposure of lactating hares to temperature conditions of 5° C led to a massive increase in GEI, MEI and milk transfer to young (Figs. 2, 3). If we had restricted our measurements to the last (4th) week of lactation only they would seem to support the heat dissipation limit hypothesis. At that time, MEI in both groups of warm-exposed females (WW and WC) was indistinguishable from each other and significantly lower than in cold exposed females. This outcome would have been predicted by the HDL hypothesis, indicating that females of group WC were unable to increase their MEI further, due to the associated heat generation. However, females of group WC, despite being kept at thermoneutrality, were in fact able to reach higher rates of MEI, although earlier than expected, with a peak occurring already in week 2 of lactation. The fact that this maximum rate of MEI was statistically indistinguishable from that in CC females (but significantly higher than in group WW) demonstrates that rates of heat dissipation apparently did not set the limit. This conclusion also holds if lactation weeks 2-4 (or only 3-4) are considered, during which MEI in group WC was intermediate, but not significantly lower than in group CC. However, since MEI in group WC was also not statistically distinguishable from that in group WW, pooled data from this second half of lactation (weeks 3-4) are inconclusive. Still, the absolute maxima of MEI reached in each group suggest that, as long as increased MEI indicates increased heat production, females in group WC were apparently physiologically capable to dissipate as much heat as cold-exposed (CC) females (Fig. 1). Thus, while heat load seems to be a crucial factor determining lactational performance in laboratory MF1 mice (Krol et al., 2003a, b; Krol et al., 2003; Speakman and Krol, 2005; Krol et al., 2007), rats (Croskerry et al., 1978) and hamsters (Scribner and Wynne-Edwards, 1994), and also in livestock animals (Forbes, 2007), we have to conclude that this physiological constraint was not decisive for European hares in our study.
The early peak of MEI in group WC was likely caused by the unnatural experimental conditions of keeping females and young at different ambient temperatures (Ta’s). It seems that WC females initially responded to the high energy demands of their cold-exposed young by rapidly increasing MEI and milk transfer (Figs. 1, 3). We know that hares, like other mammals, respond to raised energy requirements, e.g. during lactation, by increasing size and capacity of the gastrointestinal tract (T. G. Valencak, unpublished; Hammond and Diamond, 1994). Arguably, WC females with low own costs for thermoregulation could allocate more energy to elevate digestive capacity than CC females, which is supported by the observation that they had significantly increased rates of AE (Table 1). This explains why WC females reached maximum energy turnover when milk transfer was not yet at its maximum. The heat limitation hypothesis states that the limits to SusEI are imposed by the capacity of the animal to dissipate body heat generated as a by-product of processing food and producing milk (Krol et al., 2007). Our experimental conditions apparently created a situation where the early peak SusEI in WC females was caused to a relatively large degree by an up-regulated function of alimentary organs and the processing of food, whilst they reached maximum milk production only in week 4 of lactation. We see, however, no reason to assume that MEI allocated to milk synthesis should generate more heat than energy used for nutrient digestion and assimilation, maintenance, or tissue growth. Thus, as long as peak MEI in all groups was associated with approximately the same amount of heat production, these results clearly challenge the hypothesis that heat dissipation plays a limiting role in this species, irrespective of the temporal patterns of MEI.
This last statement includes a caveat to this conclusion, however. It rests on the assumption that peak MEI in all experimental groups directly reflected heat production. This is strictly true only in the absence of energy retention, i.e., deposition of fat or glycogen stores. Theoretically, the earlier peak of MEI in females in group WC could have been associated with deposition of energy stores and hence, lower heat production. There are, however, several lines of evidence which render this scenario very unlikely. Firstly, using a fatty acid marker, we have previously shown that hares slightly increase milk fat content by depleting body fat stores during lactation (without notable effects on body mass; Valencak et al., 2009). Thus, lactating hares typically release stored body energy rather than retain it. Secondly, at peak MEI in week 2 of lactation, females in group WC already had reached 85% of their final MO in weeks 3-4, indicating a very high energetic burden. Thirdly, and most importantly, the elevation of MEI in group WC females above group WW during the first 2 weeks of lactation (+60%) was paralleled by a similar increase of MO (+51% Figs. 1, 3), indicating that energy from food was indeed largely transferred to milk. Together, this evidence argues against significant energy retention and suggests that MEI reflected heat production at all times. This leaves the rejection of a limiting role of heat dissipation in European hares the most parsimonious interpretation of our results.
Is energy turnover determined by pup demand?
Pup demand, i.e., differences in suckling frequency and/or duration should be a powerful mechanism by which females sense the energy requirements conditions, and the demand of young may well explain several of the differences in the time course of MEI between our experimental groups, especially during early lactation (Fig. 1). Arguably however, later in lactation, particularly around peak lactation, the system is no more driven by pup demand alone. As demonstrated by Hackländer et al. 2002a, both MEI and MO do not rise further but level off as litter size exceeds 2 juveniles. Thus, SusMR and MO are capped despite increasing pup demand.
In our current experiment, juveniles in group WC grew fasted and had significantly higher rates of solid food intake. This higher proportion of juvenile energy intake gained from solid food may explain why females in group WC were able to significantly reduce MEI in the second half of lactation (Fig. 1). This reduction of MEI underlines that peak energy turnover was apparently not simply governed by pup demand. This finding likely represents another case of active limitation of energy turnover by females, at a time when juveniles have grown out of the most critical, thermoregulatory demanding early period (Hackländer et al, 2002a). In this context, it is interesting that despite the decline of MEI in WC females, their MO further increased up to week 4 of lactation. This suggests that females may down-regulate their energy transfer to young by decreasing milk energy content, rather than its volume. Note that generally, MEI and MO in our experiment showed only a moderate correlation (r=0.61), which is suggestive of changes in milk quality. We have previously shown that this type of down-regulation of milk energy content occurs on a seasonal basis in European hares (Valencak et al., 2009). This mechanism may be particularly effective in species, such as hares, that nurse their young only once a day. However, it is clear that measurements of milk quality over the lactation period would be required to confirm this method of limiting reproductive investments.
Is energy turnover constrained by physiological limits at all?
If neither heat dissipation nor pup demand explain maximum rates of energy expenditure in European hares, further, possibly limiting factors should be considered. Our results do not, however, support the central limitation hypothesis, because females of both groups WC and CC were capable of assimilating substantially more energy when energetically challenged. Cold exposed females in this study reached MEIs corresponding to 7.3 times RMR at peak lactation whereas in females at room temperature only rates of 6 times RMR were observed. Thus, at least for females and young living under thermoneutral conditions, gut capacity did not set the limit. Equally, we did not find any evidence that hares might have been peripherally limited. Mothers of cold exposed young provided their offspring with more milk throughout lactation, indicating that females of the control group (WW) were not limited by the capacity of their mammary glands. Thus, although females in our study reached peak energy expenditures, relative to their RMR, that were very similar to the levels of SusMR in other mammals (e.g. Hammond and Diamond, 1997), our results give no evidence that lactating females of any experimental group ever encountered an actual physiological limitation.
Responses of juveniles
Surprisingly, the large thermoregulatory burden to cold-exposed juveniles did not affect growth of young (see also Valencak et al., 2009). We attribute this observation to compensatory solid food intake by cold-exposed juveniles from their second week of life on (Fig. 4). In free-living juveniles however, such compensation could not be achieved without consequences. High energy demands early in life likely would cause increased predation risk due to increased rates of foraging and hence conspicuousness of juveniles. In addition, young would have to find solid food in the nutritional quality of milk. Also, differences in milk intake may well affect the qualities of offspring, e.g. immune function, that does not translate into differences in growth rate.
Recently, Zhao and Cao (2009) reported a lack of differences in MEI translating into growth of young, similar to our results. Their study repeated an experiment by Krol et al., 2007, testing the effects of shaving mothers at the onset of lactation, but in Swiss instead of MF1 mice. Shaving led to significantly increased MEI of mothers but unexpectedly, it did not affect the growth of pups. These results differ from the study of Krol et al., 2007 and also from observations in livestock, for which high ambient temperatures are known to impair growth rates of e.g. piglets and calves (Forbes, 2007). Clearly these differences ask for further experiments on different species and strains to find out more about the consequences of differences in energy intake of mothers on their offspring.
Synopsis – The role of life histories
Our results indicate that the capacity of heat dissipation was not limiting for European hares during lactation. Thus, rates of heat dissipation may be clearly limiting for certain species and environments, but not for mammals in general. Also, the present and previous experiments on European hares gave no evidence for central or peripheral limitation of SusMR during peak lactation (Valencak et al., 2009; Valencak and Ruf, 2009). These observations reinforce our view (Valencak et al., 2009) that female hares may routinely restrict their energy turnover and investment to their current offspring in order to maximise lifetime reproductive success (e.g., Williams, 1966; Drent and Daan, 1980). Thus, they may approach peak energy turnover only if the reproductive value of the offspring is particularly high (Stearns, 1989) and seek to avoid reaching SusMR as this might invoke both direct and indirect costs (recently reviewed in Speakman, 2008).
This still leaves the question why European hares apparently actively avoid reaching maximal rates of energy turnover, while a purely physiological limitation, namely by maximum heat dissipation, can clearly be found in some other mammals. We suggest that key factors to understand these apparently conflicting results are differences in the (either natural or artificial) selection for maximum reproductive effort between species. Small mammals, such as mice, are typically selected for early onset of reproduction, large litter size, and high effort during each reproductive event. This is primarily a consequence of high predation risk and low survival rates due to small size, which drives them to the “fast” end of the “fast-slow” continuum of life histories (Gaillard et al., 1989; Promislow and Harvey, 1990; Oli, 2004). Consequently, mammals such as mice and voles can be expected to routinely approach the physiologically possible limit of energy turnover, which indeed seems to be set by heat dissipation (Krol and Speakman 2003a, b; Krol et al., 2007; Wu et al., 2009). Medium sized mammals such as hares (or larger wild animals) have much higher life expectancy, and will be selected for a ‘prudent parent’ strategy (Drent and Daan, 1980) which limits investment in the current litter in favour of future reproduction. In line with this, European hares restrict investment into litters born late in the season, which have lower reproductive value, as discussed previously (Valencak et al., 2009). Therefore, in outbred strains of these species, such as our colony of European hares, it may well be impossible to create environmental conditions in which lactating females actually approach physiologically possible rates of SusMR. Arguably, the only way to overcome genetically determined mechanisms that limit energy allocation during reproduction, specifically maximum energy allocation to the current litter, is artificial selection. One product of artificial selection for high reproductive performance are laboratory mice, which have more than twice the average litter size than wild-type house mice, even at higher birth weights (e.g., Meikle and Drickamer, 1986). This may also explain why effects of heat dissipation limits are more pronounced in laboratory mice (Krol and Speakman 2003a, b; Krol et al., 2007) than in similar-sized, but outbred Brandt’s voles where differences in MEI were smaller and occurred only at large litter sizes (Wu et al., 2009). Further, there is little doubt that selection for high reproductive output was one of the aims and consequences of domestication of livestock animals. In dairy cows, for instance, selection for maximum milk output has created strains that invest substantial amounts of body energy reserves in the current calf, leading to delayed subsequent pregnancy (Forbes, 2007; Rauw, 2009). Not surprisingly then, heat dissipation limits are also detectable in domesticated livestock animals, despite their large size (Forbes, 2007). Up to now there is no evidence, however, that SusMR is also limited by heat dissipation, or any other physiological constraint, in wild, free-living ancestral species of these livestock animals.
Thus, it seems that differences in either natural or artificial selection for or against high investment in reproduction per unit time explain much of the observed variation between species concerning their susceptibility to encounter physiological limits. However, our current knowledge on these differences is restricted to experimental evidence from very few mammals. Clearly, more studies on other species, with a focus on outbred populations, or ideally on free-living animals without food supply constraints, are needed to clarify the role of physiological limits for reproductive success of animals.
ACKNOWLEDGMENTS
This work was funded by grant P17794-B06 from the Austrian Science Fund (FWF) to T.R. and a habilitation grant from the Deutsche Wildtierstiftung to K.H., by the Austrian Federal Ministry of Education, Science and Culture, the Austrian hunting associations, the City of Vienna, and the Department of Science and Culture of the Government of Lower Austria. We are grateful to the animal house facility staff (Michaela Salaba, Peter Steiger) and to numerous students who greatly helped with hare welfare and data sampling. We would like to thank Eva Steiger who performed the chemical analyses under direction of Frieda Tataruch. We would also like to thank Christopher Turbill for extensive discussions on the topic and previous versions of the MS. All experiments described here comply with the current laws in Austria, where the experiments were performed.
LIST OF ABBREVIATIONS
- AE
Assimilation Efficiency
- ADF
Acid Detergent Fibre
- ANOVA
Analysis of Variance
- GEI
Gross Energy Intake
- HDL
Heat dissipation limitation
- MEI
Metabolisable Energy Intake
- MO
Milk Output
- MEO
Milk Energy Output
- NIRS
Near Infrared Spectroscopy
- NFE
Nitrogen Free Extracts
- RMR
Resting Metabolic Rate
- SusEI
Sustained Energy Intake
- SusMR
Sustained Metabolic Rate
REFERENCES
- Broekhuizen S, Maaskamp F. Behaviour of does and leverets of the European hare (Lepus europaeus) whilst nursing. J. Zool. Lond. 1980;191:487–501. [Google Scholar]
- Brüll U. Nahrungsbiologische Studien am Feldhasen in Schleswig- Holstein: ein Beitrag zur Äsungsverbesserung. In: Pielowski Z, Pucek Z, editors. Ecology and management of the European hare populations. Polish Hunting Association; Warszawa: 1976. pp. 93–99. [Google Scholar]
- Croskerry PG, Smith GK, Leon M. Thermoregulation and the maternal behavior of the rat. Nature. 1978;273:299–300. doi: 10.1038/273299a0. [DOI] [PubMed] [Google Scholar]
- Drent RH, Daan S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Gaillard JM, Pontier D, Allaine D, Lebreton JD, Trouvilliez J, Clobert J. An analysis of demographic tactics in mammals and birds. Oikos. 1989;56:59–76. [Google Scholar]
- Hackländer K, Arnold W, Ruf T. Postnatal development and thermoregulation in the precocial european hare (Lepus europaeus) J. Comp. Biochem. Physiol. B. 2002a;172:183–190. doi: 10.1007/s00360-001-0243-y. [DOI] [PubMed] [Google Scholar]
- Hackländer K, Tataruch F, Ruf T. The effect of dietary fat content on lactation energetics in the European hare (Lepus europaeus) Physiol. Biochem. Zool. 2002b;75:19–28. doi: 10.1086/324770. [DOI] [PubMed] [Google Scholar]
- Forbes JM. Reproduction and lactation. In: Forbes JM, editor. Voluntary food intake and diet selection in farm animals. 2nd edition Cab International; London: 2007. pp. 341–364. [Google Scholar]
- Hammond KA, Diamond J. An experimental test for a ceiling on sustained metabolic rate in lactating mice. Physiol. Zool. 1992;65:952–977. [Google Scholar]
- Hammond KA, Diamond J. Limits to dietary nutrient intakes and intestinal nutrient uptakes in lactating mice. Physiol. Zool. 1994;67:282–303. [Google Scholar]
- Hammond KA, Lloyd KCC, Diamond J. Is mammary output capacity limiting to lactational performance in mice? J. Exp. Biol. 1996;199:337–349. doi: 10.1242/jeb.199.2.337. [DOI] [PubMed] [Google Scholar]
- Hammond KA, Diamond J. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. [DOI] [PubMed] [Google Scholar]
- Hammond KA, Kristan D. Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus) Physiol. Biochem. Zool. 2000;73:547–556. doi: 10.1086/317757. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simulataneous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Thomson SC, Speakman JR. Limits to sustained energy intake: I. Lactation in the laboratory mouse, Mus musculus. J. Exp. Biol. 2001a;204:1925–1935. doi: 10.1242/jeb.204.11.1925. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Thomson SC, Speakman JR. Limits to sustained energy intake: II. Inter-relationships between resting metabolic rate, life-history traits and morphology in Mus musculus. J. Exp. Biol. 2001b;204:1937–1946. doi: 10.1242/jeb.204.11.1937. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Thomson SC, Speakman JR. Limits to sustained energy intake: III. Effects of concurrent pregnancy and lactation in Mus musculus. J. Exp. Biol. 2001c;204:1947–1956. doi: 10.1242/jeb.204.11.1947. [DOI] [PubMed] [Google Scholar]
- Koteja P. Limits to the energy budget in a rodent, Peromyscus maniculatus: The central limitation hypothesis. Physiol. Biochem. Zool. 1996;69:981–993. [Google Scholar]
- Krol E, Speakman JR. Limits to sustained energy intake. VI. Energetics of lactation in laboratory mice at thermoneutrality. J. Exp. Biol. 2003a;206:4255–4266. doi: 10.1242/jeb.00674. [DOI] [PubMed] [Google Scholar]
- Krol E, Speakman JR. Limits to sustained energy intake VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 2003b;206:4267–4281. doi: 10.1242/jeb.00675. [DOI] [PubMed] [Google Scholar]
- Krol E, Johnson MS, Speakman JR. Limits to sustained energy intake. VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 2003;206:4283–4291. doi: 10.1242/jeb.00676. [DOI] [PubMed] [Google Scholar]
- Krol E, Murphy M, Speakman JR. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J. Exp. Biol. 2007;207:4233–4244. doi: 10.1242/jeb.009779. [DOI] [PubMed] [Google Scholar]
- Livesey G. The energy equivalents of ATP and the energy values of food proteins and fats. Brit. J. Nutr. 1984;51:15–28. doi: 10.1079/bjn19840005. [DOI] [PubMed] [Google Scholar]
- Livesey G, Marinos E. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am. J. Cli. Nutr. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- Marai IFM, Ayyat MS, Abd El-Monem UM. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 2001;33:451–462. doi: 10.1023/a:1012772311177. [DOI] [PubMed] [Google Scholar]
- Meikle DB, Drickamer LC. Food availability and secondary sex ratio variation in wild and laboratory house mice (Mus musculus) J. Reprod. Fertil. 1986;78:587–591. doi: 10.1530/jrf.0.0780587. [DOI] [PubMed] [Google Scholar]
- Nehring K. Agrikulturchemische Untersuchungsmethoden für Dünge- und Futtermittel, Böden und Milch. Parey; Hamburg: 1960. [Google Scholar]
- Oli MK. The fast-slow continuum and mammalian life-history patterns: an empirical evaluation. Basic and Applied Ecology. 2004;5:449–463. [Google Scholar]
- Onderscheka K, Tataruch F. Ein Versuch zur Erstellung von Normalwerten wildlebender Tiere und die Anwendung dieser Daten in der Wildbiologie. Wien. Tierärztl. Monschr. 1982;69:274–279. [Google Scholar]
- Otzelberger K. Österreichisches Methodenbuch für die Untersuchung von Futtermitteln, Futterzusatzstoffen und Schadstoffen. Arbeitsgemeinschaft der Landwirtschaftlichen Versuchsanstalten in Österreich; Vienna: 1983. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Core team . Linear and Nonlinear Mixed Effects Models. R package version 3.1-88. 2008. [Google Scholar]
- Promislow DEL, Harvey PH. Living fast and dying young: A comparative analysis of life-history variation among mammals. J. Zool. 1990;220:417–437. [Google Scholar]
- Rauw WM. Resource allocation theory applied to farm animal production. Cab International; London: 2009. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Scribner SJ, Wynne-Edwards KE. Thermal constraints on maternal behavior during reproduction in dwarf hamsters (Phodopus) Physiol. Behav. 1994;55:897–903. doi: 10.1016/0031-9384(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Stearns SC. Trade-offs in life history evolution. Funct. Ecol. 1989;3:259–268. [Google Scholar]
- Speakman JR, Gidney A, Bett J, Mitchell IP, Johnson MS. Limits to sustained energy intake. IV. Effect of variation in food quality on lactating mice Mus musculus. J. Exp. Biol. 2001;204:1957–1965. doi: 10.1242/jeb.204.11.1957. [DOI] [PubMed] [Google Scholar]
- Speakman JR. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Krol E. Limits to sustained energy intake. IX. A review of hypotheses. J. Comp. Physiol. B. 2005;175:375–394. doi: 10.1007/s00360-005-0013-3. [DOI] [PubMed] [Google Scholar]
- Valencak TG, Tataruch F, Ruf T. Peak energy turnover in lactating European hares: the role of fat reserves. J. Exp. Biol. 2009;212:231–237. doi: 10.1242/jeb.022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencak TG, Ruf T. Energy turnover in European hares is centrally limited during early, but not during peak lactation. J. Comp. Biochem. Physiol. B. 2009;179:933–943. doi: 10.1007/s00360-009-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am. Nat. 1966;100:687–6901. [Google Scholar]
- Wu SH, Zhang LN, Speakman JR, Wang DH. Limits to sustained energy intake XI. A test of the heat dissipation limitation hypothesis in lactating Brandt’s vole (Lasiopodomys brandtii) J. Exp. Biol. 2009;212:3455–3465. doi: 10.1242/jeb.030338. [DOI] [PubMed] [Google Scholar]
- Zhao ZJ, Cao J. Effect of fur removal on the thermal conductance and energy budget in lactating Swiss mice. J. Exp. Biol. 2009;212:2541–2549. doi: 10.1242/jeb.029603. [DOI] [PubMed] [Google Scholar]
- Zörner E. Der Feldhase. 2nd ed. Spektrum Akademischer Verlag; Heidelberg: 1996. [Google Scholar]