Abstract
The skin is the outer layer of protection against the environment. The development and formation of the skin is regulated by several genetic cascades including the bone morphogenetic protein (BMP) signaling pathway, which has been suggested to play an important role during embryonic organ development. Several skin defects and diseases are caused by genetic mutations or disorders. Ichthyosis is a common genetic skin disorder characterized by dry scaly skin. Loss-of-function mutations in the filaggrin (FLG) gene have been identified as the cause of the ichthyosis vulgaris (IV) phenotype; however, the direct regulation of filaggrin expression in vivo is unknown. We present evidence that BMP signaling regulates filaggrin expression in the epidermis. Mice expressing a constitutively active form of BMP-receptor-IB in the developing epidermis exhibit a phenotype resembling IV in humans, including dry flaky skin, compact hyperkeratosis, and an attenuated granular layer associated with a significantly downregulated expression of filaggrin. Regulation of filaggrin expression by BMP signaling has been further confirmed by the application of exogenous BMP2 in skin explants and by a transgenic model overexpressing Noggin in the epidermis. Our results demonstrate that aberrant BMP signaling in the epidermis causes overproliferation and hyperkeratinization, leading to an IV-like skin disease.
Keywords: BMP signaling, Ichthyosis, Filaggrin, Skin, Mouse
Introduction
Skin epidermis represents the outer-most layer of protection; it functions as a barrier against external elements and the environment. This barrier provides at least two types of protection: (1) to prevent water loss, and (2) to protect the organism from external hazards, such as infections, radiation, and toxic chemicals (Segre 2006). The skin epidermis is composed of five cell layers or strata, depending on keratinocyte differentiation stage. In ascending order, they are the stratum basale, spinosum, granulossum, lucidum, and corneum. The barrier function is primarily provided by the external layer of epidermis, viz., the cornified layer (stratum corneum), which is formed by the terminal differentiation of keratinocytes derived from the basal layer (stratum basale). As keratinocytes develop, the skin protein expression profile changes as they enter distinct differentiation stages. Keratins K5 and K14 are expressed in the basal layer keratinocytes (Lersch and Fuchs 1988; Nelson and Sun 1983). When keratinocytes exit from the cell cycle and detach from the basal membrane, keratins K1 and K10 begin to be expressed (Fuchs and Green 1980). These two keratins are considered to be the first molecular markers of keratinocyte differentiation. In the granular layer (stratum granulosum), the expression of filaggrin and loricrin is activated; these two proteins are aggregated to form keratohyalin granules. Filaggrin is the processed product of profilaggrin and acts to aggregate keratin filaments. This condensed cytoskeleton functions as a scaffold for the formation of a cornified envelope (Candi et al. 2005). Filaggrin is finally degraded by proteases, such as caspase14 (Denecker et al. 2007), thereby releasing free amino acids that provide essential epidermal hydration (Candi et al. 2005).
Recent studies have identified loss-of-function mutations in the filaggrin (FLG) gene as the cause of ichthyosis vulgaris (IV; McGrath and Uitto 2008; Smith et al. 2006). IV (OMIM no. 146700) is an inherited skin disorder characterized by dry scaly skin, absent or attenuated keratohyalin granules, and compact uniform hyperkeratosis (McGrath and Uitto 2008). The expression of filaggrin is reduced in the skin of IV patients and is also frequently associated with atopic dermatitis (McGrath and Uitto 2008). However, the absence of filaggrin granules in the stratum granulosum of the skin is also related to harlequin ichthyosis (HI), the most severe form of scaly skin. Mutations in the ABCA12 gene in humans and knock-out mouse models have revealed the importance of this gene in membrane lipid transport and homeostasis and in membrane permeability (Zuo et al. 2008; Smyth et al. 2008; Kelsell et al. 2005; Thomas et al. 2008). HI is usually postnatally lethal unless proper treatment is administered (Kelsell et al. 2005).
Bone morphogenetic proteins (BMPs) are a family of crucial signaling molecules that have been implicated in many aspects of biological processes, including embryonic induction, pattern formation, cell proliferation, apoptosis, and differentiation. Transduction of BMP signals requires type I (BMPR-I) and type II receptors (BMPR-II) and Smads (Hogan 1996). In vertebrates, two type I BMP-receptors (BMPR-IA and BMPR-IB) and an activin receptor type IA (ActRIa or Alk2) were originally identified that, upon ligand binding, are phosphorylated by a Type II receptor and then transduce the signal into the cell by phosphorylating Smad proteins (Kawabata et al. 1998; Nohe et al. 2004). A single amino acid substitution in the GS activation domain of the type I receptor can generate a constitutively active form of BMPR-I that can signal in the absence of BMP ligand and type II receptor (Wieser et al. 1995; Hoodless et al. 1996; Kretzschmar et al. 1997). Significant insight into the role of BMP signaling in hair development has been obtained during the last decade (Botchkarev and Sharov 2004). However, limited information is available regarding BMP signaling in epidermis development and homeostasis. In the mouse, although Bmp2 and Bmp4 are expressed in the developing hair follicles (Lyons et al. 1989, 1990; Bitgood and McMahon 1995; Botchkarev and Sharov 2004), Bmp6 and Bmp7 have been detected in the suprabasal layer and basal layer of the epidermis, respectively (Lyons et al. 1989; Wall et al. 1993; Takahashi and Ikeda 1996). Concomitantly, BmprIA and BmprIB are also expressed in the epidermis; BmprIA is expressed in the basal layer, whereas BmprIB is expressed in the suprabasal layer (Botchkarev and Sharov 2004; Botchkarev et al. 1999). Overexpression of Bmp6 in the suprabasal layer causes psoriatic lesions associated with aberrant proliferation and differentiation (Blessing et al. 1996).
In an effort to study BMP signaling in skin organogenesis, we have established a conditional transgenic mouse model that overexpresses a constitutively active form of BMP-receptor-IB (caBmprIB) on being crossed with a Cre mouse line. Here, we report that mice carrying the K14-Cre and caBmprIB transgenic alleles exhibit a skin phenotype that resembles human IV. Elevated BMP signaling in the epidermis leads to enhanced K6 expression and an elevated level of cell proliferation. Most importantly, we have observed a downregulation of filaggrin expression in the granular layer. This observation is strengthened by the findings that the application of exogenous BMP2 protein inhibits filaggrin expression in skin explants, and that mice overexpressing Noggin in the epidermis show enhanced filaggrin expression. BMP signaling thus appears to function upstream of filaggrin as a negative regulator to control filaggrin expression.
Materials and methods
Generation of transgenic mice
A constitutively active form of the chick Bmpr-IB (caBmprIB) with a Gln-203 to Asp mutation in the GS domain (Zou et al. 1997) was cloned into the pMES-IRES2-Egfp vector in front of the IRES2-Egfp sequence under the control of the chick β-actin promoter. A STOP cassette flanked by LoxP sequences was inserted between the actin promoter and the caBmprIB gene (Fig. 1a). Pronuclear injection was carried out at the Research Institute at the Nationwide Children’s Hospital Transgenic Core Facility. Genotyping was performed by using the following primers: 5′-ATTGCCCACCGTGATCTAAAAAGT-3′; 5′-AAAGACGGCAATATGGTGGAAAAT-3′. The same strategy was used to generate Noggin conditional transgenic mice as described previously (Xiong et al. 2009). K14-Cre mice were obtained from the Jackson Laboratories (Bar Harbor, Me., USA).
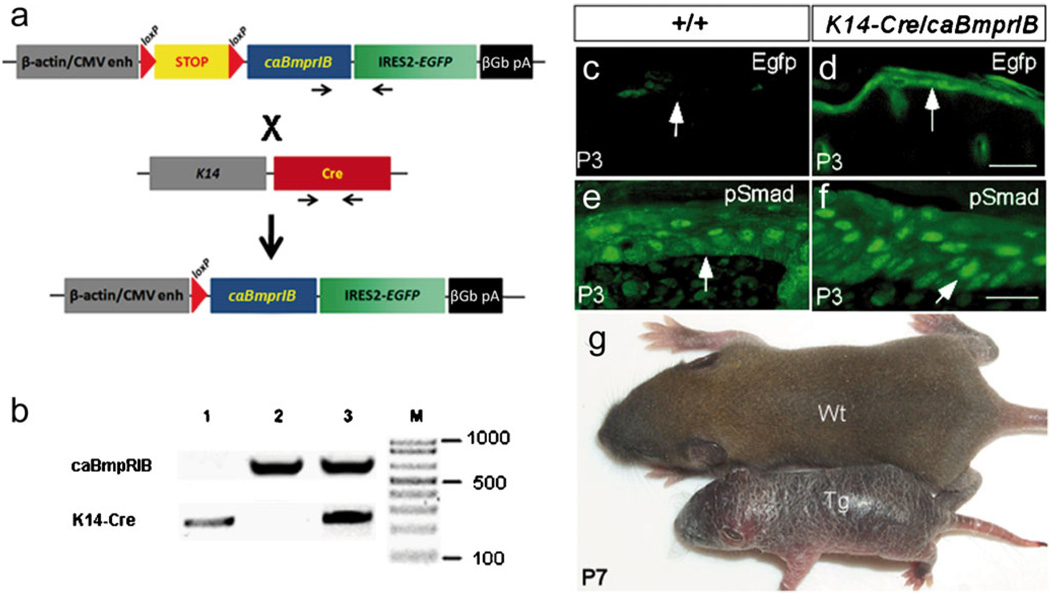
Fig. 1.
Transgenic construct and phenotype of K14-Cre/caBmprIB mice. a Representation of the conditional caBmprIB transgenic construct (paired arrows primers for genotyping). b Polymerase chain reaction (PCR)-based genotyping of transgenic mice (lane 1 K14-Cre mouse, lane 2 caBmprIB conditional transgenic mouse, lane 3 K14-Cre/caBmprIB double-transgenic mouse. c, d Egfp expression was detected in the epidermis (arrows) of the K14-Cre/caBmprIB mouse (d) but not in the wild-type control (c). e, f Immunostaining of pSmad1/5/8 in the epidermis (arrows) of postnatal day 3 (P3) wild-type (e) and transgenic mice (f). Stronger and larger numbers of pSmad1/5/8-positive cells were detected in K14-Cre/caBmprIB epidermis (f) than that in wild-type control (e). g A P7 K14-Cre/caBmprIB mouse (Tg) has a dry and flaky skin phenotype, as compared with a control littermate (Wt). Bar 100 µm (c, d), 50 µm (e, f)
Histology, immunohistochemistry, and Western blot
Dorsal skin samples were dissected from freshly killed mice at postnatal day 0 (P0), P3, and P7, fixed in 4% paraformaldehyde at 4°C overnight, followed by graded ethanol dehydration, and paraffin-embedding. Samples were sectioned at 5 µm for immunostaining and at 10 µm for histological analysis. Hematoxylin-eosin staining was performed according to the standard protocol. Immunohistochemical staining was also carried out following the standard protocol. Briefly, paraffin sections were de-waxed and rehydrated to phosphate-buffered saline (PBS). Antigen retrieval was performed by incubating slides in 10 mM sodium citrate buffer at 95°C for 10 min. After a blocking step with 10% goat serum, sections were incubated with the primary antibodies at 4°C overnight. Samples were washed extensively with TRIS-buffered saline prior to being incubated with goat fluorescein-isothiocyanate-conjugated secondary antibodies (1:100; Sigma-Aldrich, St. Louis, Mo., USA). The following antibodies were used at the concentrations recommended by the manufacturers: pSmad1/5/8 (Cell Signaling Technology, Danvers, Mass., USA), keratin 6 (Covance, Trenton, N.J., USA), keratin 10 (Covance), keratin 14 (Covance), filaggrin (Covance), and loricrin (Covance). Staining for proliferating cell nuclear antigen (PCNA) was performed according to manufacturer’s instructions (Invitrogen, Carlsbad, Calif., USA). Western blotting was carried out as described previously (Wang et al. 2004).
Real-time PCR
Freshly isolated skin samples were incubated in 1 U/ml dispase at 37°C for 1.5 h. Dermis was carefully removed. Total RNA was extracted from the separated epidermis by using an RNAqueous-4PCR kit (Ambion) or Trizol reagent (Invitrogen). Reverse transcription (RT) was carried out with a Supercript III kit (Invitrogen). Real-time PCR was performed on a ABI 7500 Sequence Detector (Applied Biosystems) or a Bio-Rad iCycler (BioRad) with SYBR Green (QPCR SYBR Green Mix, Applied Biosystems). Real-time PCR assays were performed twice with two different batches of cDNA. The following primer pairs were used: D-glyceraldehyde-3-phosphate dehydrogenase (5′-GACCCCTTCATTGACCTCA-3′; 5′-GCTCCTGGAAGATGGTGA-3′); Filaggrin (5′-GCAAGTGGTCAGGGAGGATA-3′; 5′-TCACCCAAATGGAAAAACCT-3′), and Abca12 (5′-AAGATGCTGACTGGAGACATAATTC-3′; 5′-GAAATACAAGTGCTCTTCCACAGTT-3′).
Organ culture and bead implantation
In vitro organ culture was performed as described previously (Zhang et al. 1999). Briefly, skin tissue was dissected from mouse embryo at embryonic day 18 (E18.5) and placed on Millipore filter paper in Trowell-type organ culture dishes. Samples were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. For bead implantation, Affi-Gel beads soaked in BMP2 (100 ng/µl; R&D Systems Minneapolis, Minn., USA) or bovine serum albumin (BSA) were inserted into dermis subjacent to epidermis. Samples were cultured for 24 h and then harvested and processed for immunostaining.
Skin permeability staining
Permeability staining was performed as previously described (Hardman et al. 1998). Briefly, neonatal mice were rinsed twice for 10 min with PBS and immersed in a 0.4% hematoxylin solution at 4°C overnight. Finally, mice were rinsed twice for 10 min with PBS at room temperature prior to photography.
Results
Generation of transgenic mice overexpressing caBmprIB in the epidermis
The construct used to generate conditional caBmprIB transgenic mice is shown in Fig. 1a; PCR results for the identification of the mouse genotype is also shown (Fig. 1b) The construct contains the hCMV-IE enh/chicken β-actin promoter, which is spaced by a LoxP-flanked STOP cassette from a constitutively active form of the chick BmprIB coding sequence followed by the IRES-Egfp sequence. This constitutively active BMP-receptor-IB has been shown to signal in the absence of the BMP ligand and type II receptor, mimicking the effects of overexpression of the BMP ligand (Zou et al. 1997; Zhang et al. 2000).
Eight transgenic founders were produced. All transgenic offspring from these founders appeared normal. Upon crossing to the K14-Cre transgenic mouse, three lines gave similar skin phenotypes. We chose one line for subsequent studies. The double-transgenic animals (named K14-Cre/caBmprIB) were born with barely visible skin abnormalities but developed an evidently dry and flaky skin and exhibited retarded growth by P3 (Fig. 1g). Annular tail lesion was also observed starting at P5 (data not shown). Most of K14-Cre/caBmprIB mice died between P3 to P10. Any survivors, from P15 on, appeared normal including hair growth, except for the annular tail and smaller body size. These survivors probably had a lower level of transgene expression, most likely because of an inconsistent expression pattern of the K14-Cre transgenic allele.
The transgene expression in the epidermis was confirmed by Egfp expression and the elevated level of phosphorylated Smad in the epidermis of double-transgenic animals (Fig. 1c, d). Although K14 was expressed in basal layer keratinocytes, removal of the STOP cassette from the caBmprIB transgenic allele allowed the continuous expression of the transgene in keratinocytes throughout their differentiation process because of the activity of the hCMV-IE enh/β-actin promoter. As shown in Fig. 1d, Egfp expression was detected in the epidermis and hair follicles, indicating the correctly targeted expression of the transgene. Smad1/5/8, once phosphorylated by the activated type I BMP receptor, transduced the BMP signal and served as a BMP activity indicator. We examined the phosphorylation status of Smad1/5/8 in epidermal cells by immunostaining. In the epidermis of K14-Cre/caBmprIB mice, we found larger numbers of pSmad1/5/8 positive cells, as compared with those in wild-type controls (Fig. 1e, f). Taken together, these results demonstrated that caBmprIB was successfully overexpressed in the epidermis of K14-Cre/caBmprIB mice.
Hyperproliferation results in an aberrant epidermis in transgenic skin
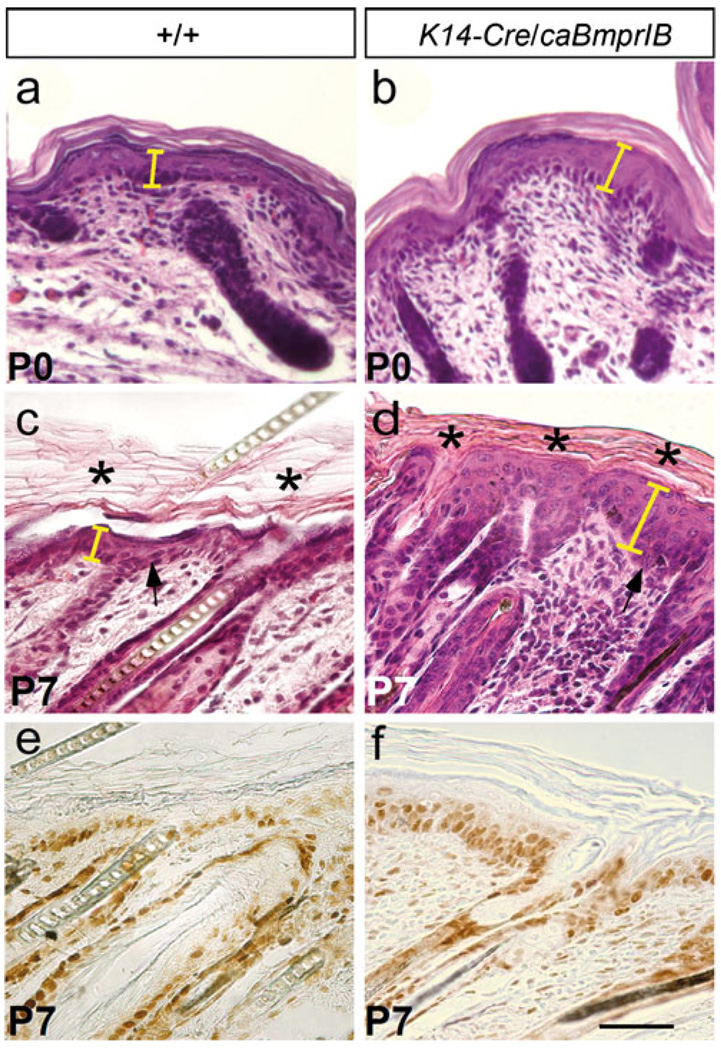
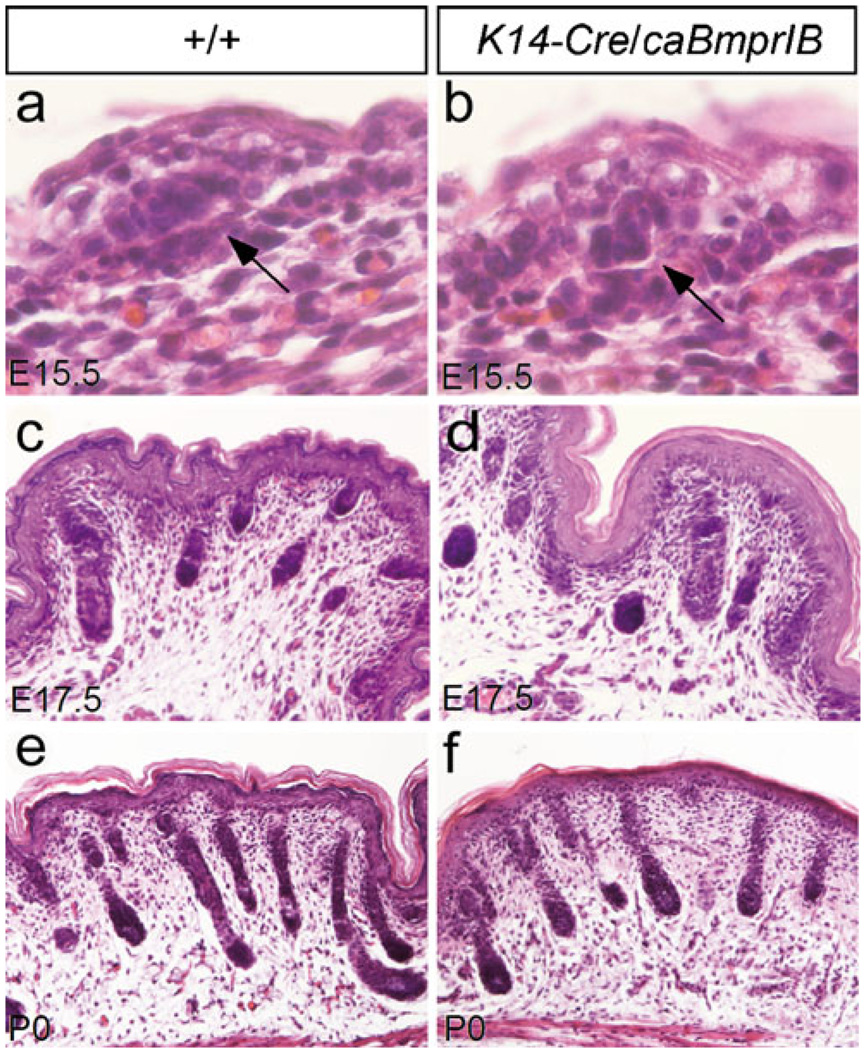
Histological analyses of the skin from P0 and P7 K14-Cre/caBmprIB mice revealed severe abnormalities in skin structures. At P0, transgenic mice began to exhibit epidermis thickening, and an obviously thickened epidermis, compact hyperkeratosis, and hypogranulosis were observed at P3 and P7 (Fig. 2a–d). The epidermis of K14-Cre/caBmprIB mice displayed focal minimal parakeratosis (data not shown). Since the BMP signaling pathway has been implicated in cell proliferation in many developing organs, we wondered whether the thickened epidermis was attributable to an elevated level of cell proliferation in the transgenic animals. As evidenced by PCNA staining, proliferating cells were found not only in the basal layer, as in the wild-type controls (Fig. 2e), but also in the suprabasal layers (Fig. 2f). Thus, hyperproliferation apparently accounted for the thickening of epidermis in the K14-Cre/caBmprIB mice. Interestingly, although no obvious developmental abnormalities were found in hair follicles (Fig. 3), the transgenic mice seldom grew hairs (Fig. 1g). The formation of a compact stratum corneum might act as a barrier to prevent hair growth.
Fig. 2.
Histology of K14-Cre/caBmprIB skin. a–d Hematoxylin-eosin staining of wild-type skin (a, c) and K14-Cre/caBmprIB skin (b, d). Note the thickened epidermis (arrows) in the transgenic sample (see also yellow bars). Compact hyperkeratosis (asterisks in d) was present in the transgenic sample (asterisks in c stratum corneum). e, f PCNA staining showed an increased number of proliferative cells in K14-Cre/caBmprIB (f) compared with the wild-type control (e). PCNA-positive cells were not limited to the basal layer in the transgenic sample. Bar 50 µm
Fig. 3.
Development of hair follicle in K14-Cre/caBmprIB mice. a, b Mesenchymal condensation (arrow) of the hair follicle is similar in E15.5 wild-type control (a) and K14-Cre/caBmprIB embryo (b). ×200. c–f Comparable hair follicle development is observed at E17.5 and P0 in wild-type controls (c, e) and K14-Cre/caBmprIB mice (d, f). ×50
Keratinocyte terminal differentiation is not affected in transgenic epidermis
Keratinocyte differentiation is a tightly controlled process. Distinct keratin proteins are expressed at different differentiation stages. Keratins K5 and K14 are mainly expressed in the basal layer of the epidermis, where keratinocytes are in a proliferating state. Keratins K1 and K10, two molecular markers of keratinocyte differentiation, are expressed in the suprabasal layer (Candi et al. 2005). Although K6 is not expressed in the interfollicular basal layer of normal epidermis, it is activated in the basal layer of epidermis under hyperproliferative conditions, such as wound healing (McGowan and Coulombe 1998).
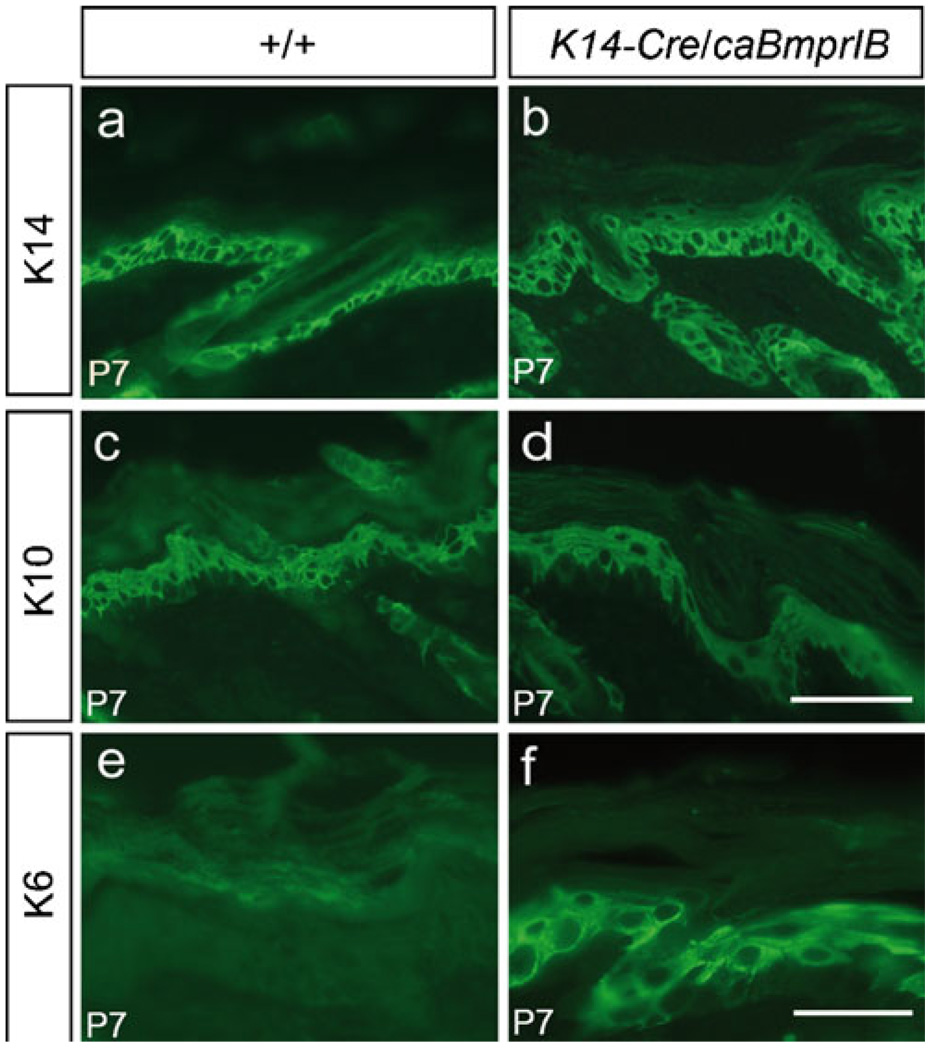
Immnohistochemical studies were performed to determine the keratinocyte differentiation status in the transgenic epidermis. The results revealed comparable expression patterns and levels of both K14 and K10 in the transgenic skin and the wild-type controls (Fig. 4a–d), suggesting that keratinocyte differentiation was not affected in the K14-Cre/caBmprIB mice. In contrast, we detected abundant K6 expression in the basal layer of epidermis in transgenic mice (Fig. 4e, f). This observation confirmed the PCNA staining results and further supported the notion that the epidermal cells in K14-Cre/caBmprIB transgenic skin were indeed in a hyperproliferative condition.
Fig. 4.
Expression of keratins in transgenic mice. Skin from P7 wild-type controls and K14-Cre/caBmprIB mice were assayed for the expression of K14 (a, b), K10 (c, d), and K6 (e, f). Whereas K14 and K10 exhibited comparable expression levels and patterns in both wild-type and transgenic samples, K6 expression was ectopically activated in the basal layer of the transgenic skin. Bars 100 µm (a–d), 50 µm (e, f)
Filaggrin expression is downregulated in transgenic epidermis
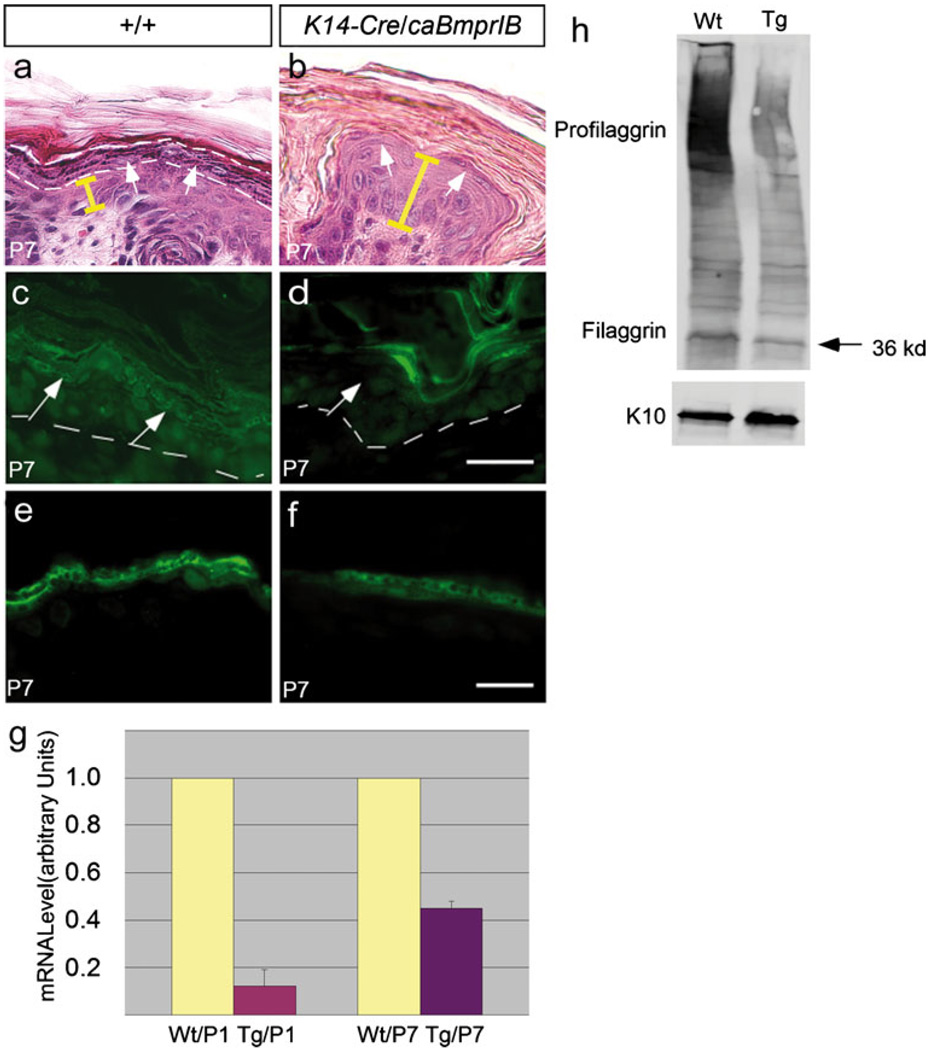
As described above, hypogranulosis represents one of the major histological abnormalities in the K14-Cre/caBmprIB epidermis. A closer examination reveals a significantly reduced amount of keratohyalin granules in the P7 transgenic epidermis (Fig. 5a, b). Keratohyalin granules contain primarily profilaggrin/filaggrin and loricrin. Our immunostaining results show a significantly reduced profilaggrin/filaggrin expression in the transgenic epidermis (Fig. 5c, d). Whereas the expression of filaggrin is barely detectable, loricrin expression is only slightly reduced in the granular layer of the transgenic epidermis, as compared with the wild-type controls (Fig. 5e, f). Subsequently, we performed qRT-PCR and Western blot assays and obtained evidence further confirming the downregulation of filaggrin expression in the transgenic epidermis. As shown in Fig. 5g, quantitative RT-PCR (qRT-PCT) assays demonstrated a greatly reduced amount of filaggrin transcripts in P1 and P7 K14-Cre/caBmprIB epidermis. Filaggrin is initially synthesized as profilaggrin, which normally contains 10–12 tandemly arranged filaggrin and is processed gradually into the final filaggrin polypeptides (McGrath and Uitto 2008). Western blot assay on proteins isolated from skin samples of P7 wild-type control and transgenic mice detected profilaggrin (500 kDa) and filaggrin (36 kDa) plus partially processed profilaggrins (Fig. 5h). However, transgenic skin contained a much smaller amount of profilaggrin/filaggrin. K10 protein, which was expressed at comparable levels in the epidermis of both wild-type and transgenic mice, was included as a loading control (Fig. 5h). These results demonstrate a significant downregulation of profilaggrin/filaggrin expression at the transcriptional level in K14-Cre/caBmprIB epidermis; this might account for the ichthyosis phenotype.
Fig. 5.
Expression of filaggrin is downregulated in the K14-Cre/caBmprIB epidermis. a, b The granular layer, outlined by dashed lines and indicated by arrows in the control skin (a), was greatly attenuated in P7 K14-Cre/caBmprIB epidermis (b). Note the extremely thin granular layer (arrows in b). The depth of the epidermis is shown by yellow bars. c, d Immunostaining showed profilaggrin/filaggrin expression in the epidermis of P7 wild-type control (c) and transgenic mice (d). Significant downregulation of profilaggrin/filaggrin was observed in the transgenic epidermis (arrows granular layer, dashed lines boundary of basal layer). e, f Loricrin expression was slightly downregulated in the epidermis of a P7 transgenic skin sample (f), as compared with its expression in the wild-type control (e). g Real-time RT-PCR demonstrated decreased profilaggrin mRNA expression in the epidermis of P1 and P7 K14-Cre/caBmprIB (Tg) mice, as compared with wild-types (Wt). h Profilaggrin and filaggrin (36 kDa) expression was equally decreased in transgenic mice. K10 expression was used as a loading control. Bars 50 µm (a–d), 80 µm (e, f)
BMP negatively regulates filaggrin expression in the epidermis
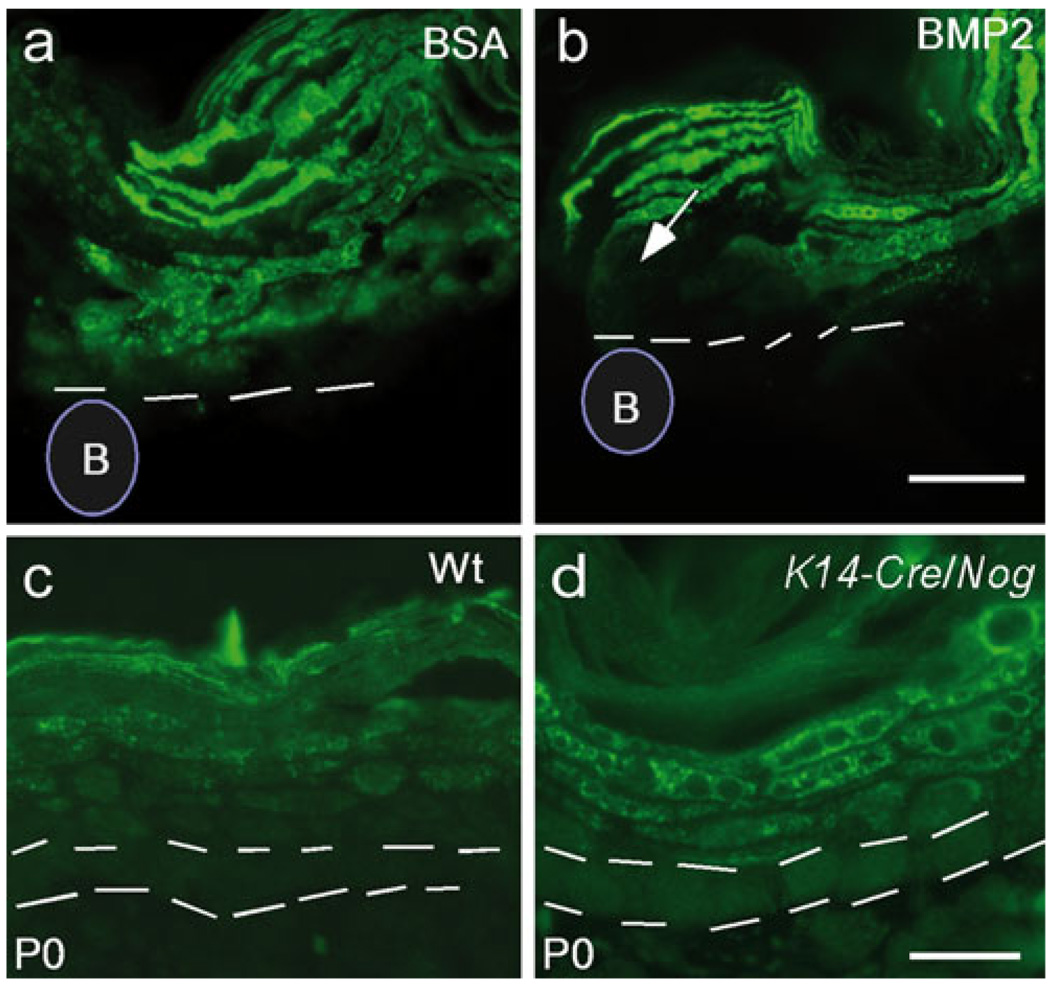
The downregulation of filaggrin expression in the epidermis expressing caBmprIB indicates a repressive role for BMP signaling in the regulation of filaggrin expression. To validate this genetic regulatory pathway further, we used additional in vitro and in vivo gain-of- and loss-of-function approaches. We first applied BMP2 protein-soaked beads or BSA-soaked control beads to explanted skin samples from E18.5 wild-type embryos. At 24 h after bead implantation, we observed a dramatically reduced level of filaggrin protein in the epidermis associated with BMP2 beads (Fig. 6a, b). Loss-of-function studies were performed by overexpressing Noggin, an antagonist of BMP signaling, to the mouse skin. We generated a conditional transgenic mouse model, by using the same strategy as that described in Fig. 1a, in order to overexpress Noggin. Upon compounding the K14-Cre allele with the conditional Noggin transgenic allele, driven by the chicken β-actin promoter, Noggin should be the first to be activated in the basal layer of epidermis and continues to be expressed in differentiating keratinocytes. In K14-Cre/Noggin mice, an enhanced and expanded expression of filaggrin was observed (Fig. 6c, d). Cells immediately adjacent to the basal layer also expressed filaggrin (Fig. 6d). However, ectopic filaggrin expression was not detected in basal layer cells. Based on these observations, we conclude that BMP signaling functions as a negative regulator of filaggrin expression in the epidermis.
Fig. 6.
BMP signaling pathway is a negative regulator of filaggrin expression. a, b A BMP2-soaked bead (B) was able to inhibit profilaggrin/filaggrin expression (arrow) in the epidermis of a skin explant from an E18.5 embryo (b), as compared with the BSA-soaked bead (B) control (a). Dashed lines mark the boundary of the basal layer in a, b. c, d A skin sample from a P0 K14-Cre/Nog transgenic mouse showed profilaggrin/filaggrin expression was enhanced and expanded in the epidermis (d), as compared with the age-matched wild-type (Wt) control (c). Double dashed lines inicate the position of the basal layer. Bars 100 µm (a, b), 50 µm (c, d)
K14-Cre/caBmprIB epidermis shows reduced permeability
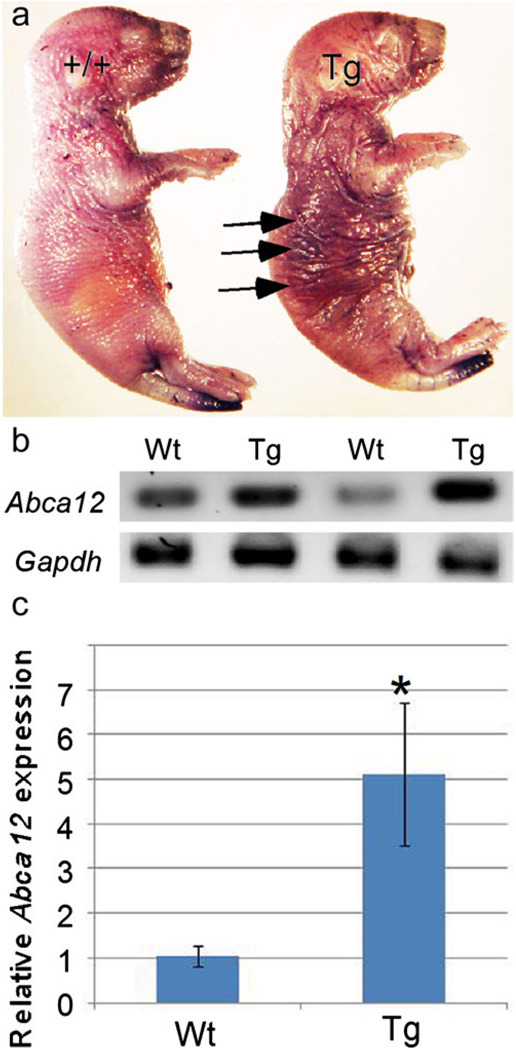
The cellular and molecular aberrations in our transgenic model suggest a functional defect in the skin. The ichthyosis phenotype is characterized by a reduced permeability of the skin; thus, skin permeability staining by using the hematoxylin dye was performed in order to assay for the barrier properties of the skin. The results showed a higher retention of the dye after rinses in the double-transgenic specimens, as compared with wild-type controls (Fig. 7a).
Fig. 7.
Skin permeability test and expression of Abca12 (a transmembrane protein). a Skin permeability staining results in a higher retention of dye (arrows) in the transgenic (Tg) neonatal mouse compared with the wild-type (+/+) littermate. b, c Expression of Abca12 in transgenic skin is upregulated as shown by RT-PCR (b) and qRT-PCR (c), which indicates a six-fold increase of Abca12 mRNA in transgenic (Tg) skin samples compared to wild-type (Wt). *P<0.05 (Gapdh D-glyceraldehyde-3-phosphate dehydrogenase)
Upregulation of Abca12 expression in transgenic epidermis
The ATP-binding cassette, sub-family A (ABC1), member 12 (ABCA12), a transmembrane protein, has been reported to play an important role in lipid transport in the skin (Zuo et al. 2008). Null mutations in the ABCA12 gene in humans have been shown to be a direct cause of HI, an autosomal recessive disease, which presents hyperkeratinosis and dry skin (Kelsell et al. 2005; Smyth et al. 2008). Because of the external skin characteristics, similar histology, and postnatal lethality evidenced by the overexpression of the BMP signaling pathway, we analyzed Abca12 mRNA expression levels in wild-type controls and double-transgenic pups. Both conventional RT-PCR and qRT-PCR analyses revealed an increased level of Abca12 expression in the double-transgenic specimens (Fig. 7b, c). These results rule out the possibility that the ichthyosis defect observed in our transgenic mice is an HI phenotype, which exhibits a reduced level or lack of expression of Abca12.
Discussion
The role of the BMP signaling has been studied extensively in many organs and tissues. However, although several BMP ligands and their receptors including both BmprIA and BmprIB are expressed in the developing epidermis (Botchkarev and Sharov 2004), little is known about BMP signaling in epidermis development and homeostasis. In this report, we present evidence, for the first time, that aberrant BMP signaling causes an ichthyosis-like phenotype. The epidermis expressing elevated BMP signaling is hyperproliferative, thus becoming thickened. We further demonstrate that elevated BMP signaling downregulates the expression of the filaggrin gene whose mutations have been identified as the cause of IV (McGrath and Uitto 2008).
IV is a common skin disorder, occurring in around 1 out of 250 in European populations (Wells and Kerr 1966). Mutations in the filaggrin gene cause IV and have also been implicated in other skin disorders including atopic dermatitis (McGrath and Uitto 2008). Moreover, the reduced expression of filaggrin has been reported also to be the result of a processing defect. A reduction of filaggrin is also related to other types of ichthyosis, i.e., HI. In HI, the underlying cause for the reduction of filaggrin in the skin is the mutation in the ABCA12 gene, which encodes a lipid transporter (Kelsell et al. 2005; Smyth et al. 2008). Lack of a functional ABCA12 transporter inhibits the processing of profilaggrin to filaggrin, leading to an absence of granules in the stratum granulosum. So far, all mutations that result in HI and that have been found in the ABCA12 gene in humans render a non-functional protein. A less severe phenotype related to mutations in the ABCA12 gene is a result of missense mutations (Lefevre et al. 2003). However, whether these missense mutations render an overactive protein or a defective one is unknown. In our studies, we have found a reduction in filaggrin granules in the skin. In order to identify further the type of ichthyosis observed in our double-transgenic mice, expression of the Abca12 gene has been assessed by RT-PCR and qRT-PCR. Surprisingly Abca12 expression is significantly higher in transgenic samples compared with their wild-type counterparts. Moreover, the amount of total pro-filaggrin protein found in the skin is reduced in transgenic samples, indicating that the phenotype observed does not correspond to HI.
Whereas cell culture studies have demonstrated the regulation of filaggrin by AP1, Ets, and POU domain factors (Andreoli et al. 1997; Jang et al. 1996, 2000), little is known about the genetic regulation of filaggrin expression in vivo. In the current study, we present evidence that BMP signaling is a negative regulator of profilaggrin expression. The exclusive expression patterns of BMP receptors and filaggrin in the epidermis in which filaggrin expression is activated in the cells of the granular layer, with BmprIA and BmprIB in the basal and suprabasal layers, respectively, support a repressive role of BMP signaling in the regulation of filaggrin expression. We propose that BMP signaling functions to prevent premature filaggrin expression in the basal and suprabasal layers. Once keratinocytes mature into their terminal differentiation status in the granular layer, the expression of BMP receptors turns off, allowing for the activation of filaggrin. This hypothesis is further supported by the evidence that the overexpression of Noggin in the epidermis leads to a premature expression of filaggrin in cells immediately adjacent to the basal layer (suprabasal layer; Fig. 6). Interestingly, we have not observed a premature expression of filaggrin in basal layer cells in mice overexpressing Noggin. The restricted expression of BmprIB in the suprabasal layer might attribute to this limited premature expression, suggesting a functional specificity of BmprIB-mediated signaling. Consistent with the notion that filaggrin gene expression is regulated primarily at the transcriptional level (Jang et al. 1996), a significantly reduced amount of filaggrin mRNA transcripts has been found in skin expressing caBmprIB. Intriguingly, a genetic locus on chromosome 10q22.3-q24.3 has recently been identified for two families with autosomal-dominant IV (Liu et al. 2007). The BMPR-IA gene is located within this locus. Both BMPR-IA and BMPR-IB show high affinity binding to BMP2 and BMP4 and transduce similar intracellular signals in cell cultures (Wozney et al. 1988; ten Dijke et al. 1994; Hoodless et al. 1996; Kretzschmar et al. 1997; Sieber et al. 2009). Many studies have demonstrated that these two receptors have redundant but distinct functions during embryonic development. In our studies presented here, ectopic expression of caBmprIb in the epidermis does not impair hair follicle development. However, the ectopic expression of BmprIA in the epidermis has previously been reported to promote premature hair follicle differentiation (Kobielak et al. 2007). Therefore, BmprIA and BmprIB possibly share a similar function, but also have distinct roles, in skin and hair follicle development.
Taken together, the overexpression of the BMP signaling pathway results in the hyperproliferation of the basal layer of the skin, hyperkeratinazation, a reduction of filaggrin expression, and the overexpression of the Abca12 gene.
Acknowledgments
This work was supported by grants from the NIH to Y.-P.C.
Contributor Information
Xueyan Yu, Section of Oral Biology, The Ohio State University College of Dentistry, Columbus OH 43210, USA.
Ramón A. Espinoza-Lewis, Section of Oral Biology, The Ohio State University College of Dentistry, Columbus OH 43210, USA Department of Cell and Molecular Biology, Tulane University, New Orleans LA 70118, USA.
Cheng Sun, Section of Oral Biology, The Ohio State University College of Dentistry, Columbus OH 43210, USA; Department of Cell and Molecular Biology, Tulane University, New Orleans LA 70118, USA.
Lisong Lin, Section of Oral Biology, The Ohio State University College of Dentistry, Columbus OH 43210, USA.
Fenglei He, Section of Oral Biology, The Ohio State University College of Dentistry, Columbus OH 43210, USA; Department of Cell and Molecular Biology, Tulane University, New Orleans LA 70118, USA.
Wei Xiong, Section of Oral Biology, The Ohio State University College of Dentistry, Columbus OH 43210, USA; Department of Cell and Molecular Biology, Tulane University, New Orleans LA 70118, USA.
Jing Yang, Center for Cell & Developmental Biology, The Research Institute at Nationwide Children’s Hospital, Columbus OH 43205, USA.
Alun Wang, Department of Pathology, Tulane University Health Sciences Center, New Orleans LA 70112, USA.
YiPing Chen, Email: ychen@tulane.edu, Section of Oral Biology, The Ohio State University College of Dentistry, Columbus OH 43210, USA; Department of Cell and Molecular Biology, Tulane University, New Orleans LA 70118, USA.
References
- Andreoli JM, Jang SI, Chung E, Coticchia CM, Steinert PM, Markova NG. The expression of a novel, epithelium-specific ETS transcription factor is restricted to the most differentiated layers in the epidermis. Nucleic Acids Res. 1997;25:4287–4295. doi: 10.1093/nar/25.21.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Blessing M, Schirmacher P, Kaiser S. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J Cell Biol. 1996;135:227–239. doi: 10.1083/jcb.135.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–526. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, MaMahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, Van den Broecke C, Van Damme P, D’Herde K, Hachem JP, Borgonie G, Presland RB, Schoonjans L, Libert C, Vandekerckhove J, Gevaert K, Vandenabeele P, Declercq W. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Dijke P ten, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DI, Ichijo H, Heldin C-H, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O’Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Jang SI, Steinert PM, Markova NG. Activator protein 1 activity is involved in the regulation of the cell type-specific expression from the proximal promoter of the human profilaggrin gene. J Biol Chem. 1996;271:24105–24114. doi: 10.1074/jbc.271.39.24105. [DOI] [PubMed] [Google Scholar]
- Jang SI, Karaman-Jurukovska N, Morasso MI, Steinert PM, Markova NG. Complex interactions between epidermal POU domain and activator protein 1 transcription factors regulate the expression of the profilaggrin gene in normal human epidermal keratinocytes. J Biol Chem. 2000;275:15295–15304. doi: 10.1074/jbc.275.20.15295. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Norgett EE, Unsworth H, Teh M-T, Cullup T, Mein CA, Dopping-Hepenstal PJ, Dale BA, Tadini G, Fleckman P, Stephens KG, Sybert VP, Mallory SB, North BV, Witt DR, Sprecher E, Taylor AE, Ilchyshyn A, Kennedy CT, Goodyear H, Moss C, Paige D, Harper JI, Young BD, Leigh IM, Eady RA, O’Toole EA. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet. 2005;76:794–803. doi: 10.1086/429844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci USA. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Liu F, Hata A, Doody J, Massague J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11:984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, Blanchet-Bardon C, Heilig R, Foglio M, Weissenbach J, Lathrop M, Prud’homme JF, Fischer J. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet. 2003;12:2369–2378. doi: 10.1093/hmg/ddg235. [DOI] [PubMed] [Google Scholar]
- Lersch R, Fuchs E. Sequence and expression of a type II keratin, K5, in human epidermal cells. Mol Cell Biol. 1988;8:486–493. doi: 10.1128/mcb.8.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Yang Q, Wang X, Feng A, Yang T, Yang R, Wang P, Yuang M, Liu M, Liu JY, Wang QK. Identification of a genetic locus for ichthyosis vulgaris on chromosome 10q22.3-q24.2. J Invest Dermatol. 2007;128:1418–1422. doi: 10.1038/sj.jid.5701191. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- McGowan K, Coulombe PA. The wound repair-associated keratins 6, 16, and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell Biochem. 1998;31:173–204. [PubMed] [Google Scholar]
- McGrath JA, Uitto J. The filaggrin story: novel insights into skin-barrier function and disease. Trends Mol Med. 2008;14:20–27. doi: 10.1016/j.molmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Nelson WG, Sun TT. The 50- and 58-kDalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J Cell Biol. 1983;47:244–251. doi: 10.1083/jcb.97.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- Smyth I, Hacking DF, Hilton AA, Mukhamedova N, Meikle PJ, Ellis S, Satterley A, Collinge JE, Graaf CAde, Bahlo M, Sviridov D, Kile BT, Hilton DJ. A mouse model of harlequin ichthyosis delineates a key role for Abca12 in lipid homeostasis. PLoS Genet. 2008;4:e1000192. doi: 10.1371/journal.pgen.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ikeda T. Transcripts for two members of the transforming growth factor-beta superfamily BMP-3 and BMP-7 are expressed in developing rat embryos. Dev Dyn. 1996;207:439–449. doi: 10.1002/(SICI)1097-0177(199612)207:4<439::AID-AJA8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Thomas AC, Sinclair C, Mahmud N, Cullup T, Mellerio JE, Harper J, Dale BA, Turc-Carel C, Hohl D, McGrath JA, Vahlquist A, Hellstrom-Pigg M, Ganemo A, Metcalfe K, Mein CA, O’Toole EA, Kelsell DP. Novel and recurring ABCA12 mutations associated with harlequin ichthyosis: implications for prenatal diagnosis. Br J Dermatol. 2008;158:611–613. doi: 10.1111/j.1365-2133.2007.08277.x. [DOI] [PubMed] [Google Scholar]
- Wall NA, Blessing M, Wright CV, Hogan BL. Biosynthesis and in vivo localization of the decapentaplegic-Vg-related protein, DVR-6 (bone morphogenetic protein-6) J Cell Biol. 1993;120:493–502. doi: 10.1083/jcb.120.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Yu XY, Zhang T, Zhang XY, Zhang ZY, Chen YP. Chick Pc12 regulates the left-right asymmetry by repressing Shh expression in Hensen’s node. Development. 2004;131:4381–4391. doi: 10.1242/dev.01269. [DOI] [PubMed] [Google Scholar]
- Wells RS, Kerr CB. Clinical features of autosomal dominant and sex-linked ichthyosis in an English population. BMJ. 1966;1:947–950. doi: 10.1136/bmj.1.5493.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massague J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsok LM, Whitters MJ, Kris RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Xiong W, He F, Morikawa Y, Yu X, Zhang Z, Lan Y, Jiang R, Cserjesi P, Chen YP. Hand2 is required in the epithelium for palatogenesis in mice. Dev Biol. 2009;330:131–141. doi: 10.1016/j.ydbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YD, Zhao X, Hu YP, St Amand T, Zhang MF, Ramamurthy R. Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev Dyn. 1999;215:45–53. doi: 10.1002/(SICI)1097-0177(199905)215:1<45::AID-DVDY5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Yu XY, Zhang YD, Geronimo B, Lovlie A, Fromm SH, Chen YP. Targeted misexpression of constitutively active BMP receptor-IB causes bifurcation, duplication, and posterior transformation of digit in mouse limb. Dev Biol. 2000;220:154–167. doi: 10.1006/dbio.2000.9637. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massague J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Zhuang DZ, Han R, Isaac G, Tobin JJ, McKee M, Welti R, Brissette JL, Fitzgerald ML, Freeman MW. ABCA12 maintains the epidermal lipid permeability barrier by facilitating formation of ceramide linoleic esters. J Biol Chem. 2008;283:36624–36635. doi: 10.1074/jbc.M807377200. [DOI] [PMC free article] [PubMed] [Google Scholar]