Abstract
Peroral cholangioscopy was first described in 1970s and has recently gained popularity. Peroral cholangioscopy is appealing to therapeutic endoscopists because a direct intraluminal view of the biliary duct system offers possibilities for diagnosis and interventions beyond that which other imaging or endoscopic modalities can provide. As the image quality of cholangioscopies improves, so too does their diagnostic capability, and as their durability and maneuverability increases, so too does their potential use for therapeutic applications. This editorial is intended to provide a brief review of recent developments in peroral cholangioscopy and current indications for its use.
Keywords: Endoscopic retrograde cholangiopancreatography, Cholangioscopy, Peroral cholangioscopy, Cholangiocarcinoma, Biliary stricture, Pancreatic cancer, Biopsy, Brush cytology
INTRODUCTION
Diseases of the biliary system are frequently encountered in clinical practice[1]. An examination of the bile ducts is often required for the appropriate diagnosis and management of patients with biliary diseases. The dramatic technical advances of flexible endoscopy during the last four decades have resulted in endoscopic retrograde cholangiopancreatography (ERCP) being used as a primary method of diagnosing and treating many biliary diseases[1]. In the United States alone, approximately half a million ERCP procedures are performed annually. ERCP can demonstrate the anatomy of the biliary tract and reveal anatomical abnormalities, strictures and intraductal filling defects. However, this technique does not always differentiate the biological nature of bile duct lesions and can fail to determine their intraluminal extension. Furthermore, it is unable to provide information about biliary mucosal lesions that do not project into the biliary lumen. Peroral cholangioscopy as an adjunct to ERCP is a promising procedure that provides direct visualization of the biliary tree. It has been shown to have value in treating difficult-to-remove biliary stones[2], assessing indeterminate biliary strictures[3], and distinguishing between different intraductal lesions of the biliary tree[4]. In recent years, cholangioscopy has gained popularity in the United States and is being performed in increasing numbers not only in academic institutions and large tertiary care referral centers, but also in smaller hospitals and private practices. In this paper, clinical applications of peroral cholangioscopy and its role in diagnosis and management of biliary disorders are reviewed.
HISTORICAL PERSPECTIVES
The first peroral cholangioscopy was performed in 1975 using a prototype cholangioscope that was thin enough to pass through the accessory channel of a duodenoscope[5]. The concept of passing a thinner endoscope through a larger one later became known as the “mother-baby” or “mother-daughter” concept. Even today, almost all cholangioscopy systems are based on this concept. The initial prototype cholangioscope had poor image quality, no instrumentation or irrigation capability, and no tip deflection. Despite all its shortcomings, it proved that peroral cholangioscopy is feasible. In the mid-1980s second generation cholanigoscopes were introduced[5]. These cholangioscopies had added tip deflection and an accessory channel that could be used either for irrigation or instrumentation. In the late 1990s and early in the new millennium, advances in imaging technology led to the introduction of video cholangioscopies with improved image quality that enabled satisfactory views of the biliary mucosa (Figure 1). Addition of narrow band imaging (NBI) capability led to further improvements in detection of abnormal vascularization of biliary mucosa, which is of importance for diagnosis of certain biliary malignancies[6]. The first semi- disposable single-operator cholangioscopy system was developed in 2005 and made it possible for a single endoscopist to operate both the baby and mother endoscopes.
Figure 1.
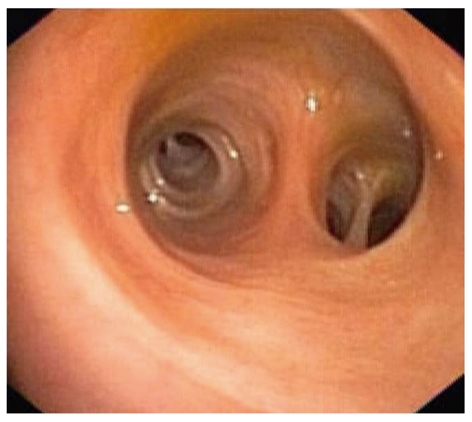
Cholangioscopic view of normal intrahepatic biliary mucosa (image by a prototype video cholangioscope, CHF type Y0002, Olympus Corporation, Tokyo, Japan).
SINGLE AND DUAL OPERATOR CHOLANGIOSCOPY SYSTEMS
In cholangioscopy, the terms “single operator” and “dual operator” refer to the number of endoscopists required to perform the procedure. As a general rule, dual operator cholangioscopy systems require two endoscopists, while single operator cholangioscopy systems require only one endoscopist for performance. There are, however, reports of use of dual operator cholangioscopy systems by a single operator with the help of appropriate accessory equipment[7].
Currently, most cholangioscopy systems are dual operator. Dual operator cholangioscopies of varying length, diameter and image quality are available. Most dual operator cholangioscopies have fiberoptic image quality. There is limited commercial availability of video cholangioscopies with enhanced image quality. At present, all video cholangioscopies with NBI capability are prototypes and not commercially available.
The only single operator cholangioscopy system currently available is the SpyGlass direct visualization system (Boston Scientific, Natick, MA, USA). This system is fiberoptic-based and has single and multi-use components.
Some of the advantages and disadvantages of the currently available single and dual operator cholangioscopies are summarized in Table 1.
Table 1.
Comparison of currently available single and dual operator cholangioscopies
| Endoscopists needed | Image quality | Tip deflection | Simultaneous irrigation and instrumentation | Fragility | |
| Single operator (SpyGlass) | One | Moderate - good | 4 way (up/down, left/right) | Yes | No1 |
| Dual operator | |||||
| Fiberoptic cholangioscopies | Two | Moderate - good | 2 way (up/down) | No | Yes |
| Video cholangioscopies | Two | Excellent | 2 way (up/down) | No | Yes |
All components of the spyglass system are single-use with the exception of the spyprobe (the light and image conveyor of the system) which is multi-use. The spyprobe is fragile and has to be handled with care.
CLINICAL APPLICATIONS
Several clinical applications for peroral cholangioscopy have been described. With expanded use, additional indications are expected to be reported. Clinical applications of cholangioscopy can be divided into common, uncommon and rare applications. Common applications include stone therapy and diagnosis of indeterminate biliary strictures. Uncommon applications include guidewire placement during ERCP, assessment of post-liver-transplantation biliary strictures, and evaluation of indeterminate intraductal filling defects or irregularities of the bile duct wall seen on imaging studies such as computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS) or ERCP. Rare applications include staging and ablation of biliary neoplasms, investigation of recurrent pancreatitis, and evaluation of hemobilia.
Common applications
Currently, most peroral cholangioscopy procedures are performed for two indications: biliary stones and indeterminate biliary strictures.
Biliary stones
Difficult to remove stones: Gallstone disease or cholelithiasis continues to be a major health problem throughout the world, and affects 10%-20% of the Caucasian population[8-13]. It has been estimated that 15%-20% of patients with gallstone disease also have stones in their bile ducts (choledocholithiasis)[13]. Stones in the bile ducts have to be removed because of their potential to cause jaundice, cholangitis, and pancreatitis[14-16]. This is accomplished in close to 95% of the cases during ERCP by conventional methods such as sphincterotomy with or without sphincter dilatation, use of extraction balloons or retrieval baskets, mechanical lithotripsy, or a combination of these methods[17]. At times, however, stone extraction by standard methods is not possible. There are a number of reasons as to why some stones cannot be removed by conventional means; some of the most common of which are presented in Table 2.
Table 2.
Common factors associated with failed biliary stone removal during endoscopic retrograde cholangiopancreatography
| Patient factors |
| Abnormal anatomy |
| Prior surgery |
| Extremely J-shaped stomach |
| Large hernias |
| Malrotations |
| Unstable or difficult endoscope position |
| Short duodenal bulb |
| Abnormal anatomy |
| Long duodenoscope position |
| Bile duct abnormalities |
| Presence of ductal strictures |
| Severely dilated ducts |
| Stone factors |
| Size |
| Large size |
| Location |
| Intrahepatic |
| Cystic duct |
| Proximal to strictures |
| Impacted stones |
A variety of methods have been devised for endoscopic extraction of stones that are not removable by conventional means during ERCP. As a general rule, these methods involve using shock waves to crush or fragment the stones inside the bile duct, with subsequent removal of the fragments (Figure 2). The shock waves for fragmentation of biliary stones are usually generated using electric spark (electrohydraulic lithotripsy) or laser light (laser lithotripsy). Probes that pass through the accessory channels of cholangioscopies for laser or electrohydraulic lithotripsy are commercially available. Although use of these probes through an extraction balloon under fluoroscopic guidance has been reported[18,19], in our institution, we use them under direct visualization by utilizing a cholangioscope. These probes have to be precisely positioned on the stone to increase effectiveness and reduce complications. Direct visualization ensures that the shock waves are aimed at the stone and not the bile duct wall, because shock waves delivered to the bile duct wall can cause bleeding and perforation. Direct visualization by cholangioscopy also allows distinction between stone fragments, air bubbles or blood clots, which can be indistinguishable on contrast cholangiography[20].
Figure 2.
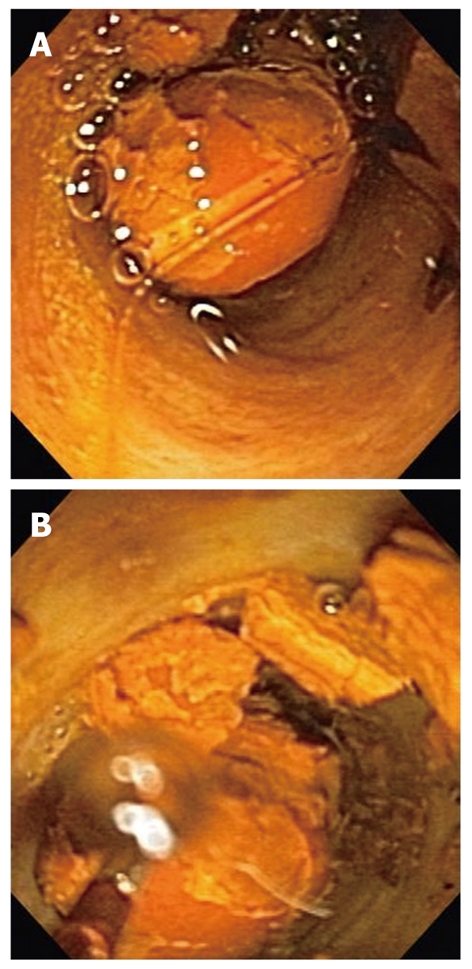
Cholangioscopic views of a bile duct stone prior to (A) and after (B) electrohydraulic lithotripsy. The lithotripsy probe is visible in the left lower corner of (B).
Laser or electrohydraulic lithotripsy has been used for fragmentation and subsequent extraction of difficult to remove stones for many years, and both techniques have been shown to be safe and effective[21,22]. In a recent multicenter study, cholangioscopy-guided laser or electrohydraulic lithotripsy were effective in > 90% of the cases[2].
There are currently no randomized studies that have compared the effectiveness of laser and electrohydraulic lithotripsy for fragmentation and subsequent extraction of difficult-to-remove biliary stones.
Missed stones: Cholangioscopy allows detection of stones that might have been missed during cholangiography. Small stones can be “drowned” in contrast and be missed, and larger stones can block a duct, thus preventing passage of contrast, and evade detection during ERCP (Figure 3). In a study of patients with primary sclerosing cholangitis, stones were not detectable on cholangiography in seven of 23 patients (30%)[23]. In a more recent multicenter study, stones were missed in 29% of patients who presented for ERCP for different indications. In that study, ERCP was immediately followed by peroral cholangioscopy, which led to detection of the stones[24].
Figure 3.
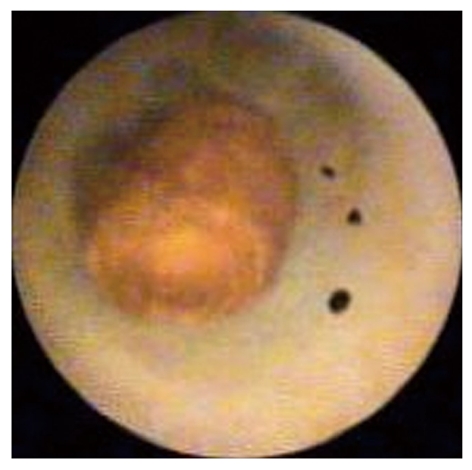
Cholangioscopic view of a small stone surrounded by contrast in an intrahepatic duct. The stone was missed during endoscopic retrograde cholangiopancreatography (image by Spyglass Direct Visualization System, Boston Scientific, Natick, USA).
Indeterminate biliary strictures
Biliary strictures can be benign or malignant. Accurate diagnosis of biliary strictures is essential for treatment planning and the correct choice of treatment, such as surgical resection or endoscopic stenting. However, differentiation of malignant from benign ductal lesions remains a challenge[25]. Brush cytology during ERCP or fine needle aspiration by EUS has become the preferred initial method of pursuing a diagnosis in many patients with pancreatobiliary malignancies[25-27]. These techniques allow easy and convenient sampling and have a low complication rate[25,27,28]. The diagnostic specificity of biliary brush cytology or fine needle aspiration is very high and few false-positive diagnoses have been reported[25,29]. The major limitation of these techniques has been the relatively modest diagnostic sensitivity, ranging from 10% to 50% in most series[25,29].
There have been attempts to improve the sensitivity of brush cytology obtained during ERCP. Physical changes to the brushing device itself, such as use of longer and stiffer brushes, have not been shown to improve sensitivity[30]. Balloon dilatation of strictures, to expose underlying tissue, prior to obtaining brush samples has been tried but not shown to be of any benefit[31]. Mutation analysis of the cells obtained by brushing does not seem to improve diagnostic accuracy[32], and DNA methylation analysis of ERCP brush specimens has shown only small benefit[25].
It has been suggested that peroral cholangioscopy can improve diagnosis of indeterminate biliary strictures by visualization of the mucosa at the site of the stricture, and by targeted biopsy.
Visualization of the mucosa at the site of the stricture: It is well known that the presence of irregularly dilated and tortuous blood vessels (so-called tumor vessels) due to neovascularization at the site of pancreatic or biliary strictures is indicative of malignancy[33]. Tumor vessels can be detected by direct visualization using a cholangioscope. Intraductal nodules or masses can also be indicative of malignancy and be easily detected by cholangioscopy. However, tumor vessels and intraductal masses can be appreciated only in a fraction of malignant strictures; probably those with more advanced disease. Certain types of cholangiocarcinoma involve submucosal layers of the bile duct wall and cannot be detected by cholangioscopy, which visualizes the superficial layers. Biliary strictures caused by extraluminal compression, such as those associated with pancreatic cancer, cannot be detected by cholangioscopy, unless at later stages when the tumor has infiltrated and penetrated the bile duct wall.
Studies to assess the value of stricture visualization by cholangioscopy have reported high sensitivity for detection of malignant lesions[4,34]. The reported sensitivity in some of these studies has approached 100%[4]. In these studies, however, the criteria used for labeling a stricture as malignant have been somewhat lax. As an example, irregular biliary mucosa has been used to label a stricture as malignant. It is well known that irregular biliary mucosa on cholangioscopy can also be seen in benign lesions such as primary sclerosing cholangitis, or chronic inflammation associated with choledocholithiasis or recurrent cholangitis[35]. Therefore, the high sensitivity in such studies is often achieved at the cost of lower specificity. This is alarming, because false-positive results can have a devastating impact on the affected patients’ lives.
Although, undoubtedly, direct visualization of indeterminate biliary strictures can aid in their diagnosis, the true value of peroral cholangioscopy for this purpose has not been vigorously studied.
Targeted biopsy: Targeted biopsy is defined as biopsy of the sites that are clearly affected by disease under direct visualization. Theoretically, targeted biopsy should improve cancer detection rate in malignant biliary strictures by allowing sampling of the sites that appear suspicious. In a recent multicenter study that assessed the role of cholangioscopy-guided targeted biopsy for diagnosis of indeterminate biliary strictures, initial observations suggested a large improvement in sensitivity[3]. However, later observations at conclusion of the study have indicated a somewhat more modest benefit[24]. Well-designed studies are needed to assess better the value of cholangioscopy-guided targeted biopsy for evaluation of indeterminate biliary strictures.
Uncommon applications: In our institution, 10%-20% of peroral cholangioscopy procedures are performed for indications other than stone disease and stricture diagnosis. Some of these indications are discussed below.
Characterization of indeterminate intraductal lesions or filling defects
Increased use of imaging studies such as CT, MRI and EUS has led to an increase in incidental findings such as intraductal biliary lesions or filling defects. Although, most often these findings are real, they can also be due to artifacts.
Direct visualization of the intraluminal biliary tree is the most appropriate way to investigate further the nature of these findings. Cholangioscopy has been shown to be effective for this purpose[4,36].
Assessing post-liver-transplantation anastomotic strictures
Various refinements in surgical techniques and postoperative and immunosuppressive management have reduced the incidence of complications after liver transplantation. Biliary complications, however, continue to be a significant cause of morbidity after liver transplantation[37,38].
In selected cases, cholangioscopy can prove beneficial in diagnosis and treatment of biliary complications after liver transplantation. In a study of 20 liver transplant patients, cholangioscopy helped diagnose ischemia, ulcerations, scar tissue, intraductal clots, and retained suture material, which otherwise might have been missed by ERCP alone[39]. The role of cholangioscopy in assessment of anastomotic strictures after liver transplantation is evolving.
Assistance in guidewire placement
ERCP has attained a primary role in the treatment of biliary strictures and biliary stones. Success of ERCP in these cases, however, depends on the ability to traverse the stricture or the stone with a guidewire that is then used to direct instruments such as dilating balloons or lithotripsy baskets[40]. In the vast majority of cases, this is accomplished easily. With severe strictures or impacted stones, however, it can represent a time-consuming challenge, and in some studies, a failure rate of up to 20% has been reported[41]. In such cases, cholangioscopy can facilitate guidewire placement and prevent more invasive procedures such as transhepatic access or surgery. Several studies have highlighted the value of cholangioscopy in such instances[40,42].
Rare applications: We define rare applications as those responsible for ≤ 1% of our peroral cholangioscopy volume. For obvious reasons, these indications have been reported only in one or two case reports and no studies have assessed the true value of peroral cholangioscopy in these settings.
Evaluation of recurrent pancreatitis
Peroral cholangioscopy was used in a 62-year-old post-cholecystectomy patient with recurrent acute pancreatitis of undetermined etiology. It revealed a T-tube remnant in the cystic duct stump, which served as a nidus for biliary sludge and stone formation. The T-tube remnant had evaded detection by ERCP, CT and magnetic resonance cholangiopancreatography. Removal of the T-tube remnant prevented further episodes of pancreatitis[43].
Determination of source of bleeding in hemobilia
A 54-year-old man was reported to have bleeding from arteriovenous malformations of the bile duct, which was detected by peroral cholangioscopy, with subsequent successful treatment by endovascular intervention[44]. In another study, the cause of hemobilia in a 57-year-old-man could not be identified by ERCP, CT or angiography. Peroral cholangioscopy revealed multiple biliary ulcers. Biopsies were consistent with cytomegalovirus cholangiopathy that responded to antiviral therapy, with subsequent cessation of bleeding[45].
Staging and ablation of biliary neoplasms
Peroral cholangioscopy was used in a 78-year-old man to determine the extent of a biliary neoplasm. Use of a video cholangioscope with NBI capability allowed precise determination of the margins of the lesion. Successful ablation of the neoplasm with brachytherapy was confirmed by repeat peroral cholangioscopy at 1 mo follow-up[46].
CONCLUSION
Recent advances such as introduction of a single operator cholangioscopy system or video cholangioscopies with high image quality have led to renewed interest in cholangioscopy, with subsequent expanded use. Currently, the most common indications for cholangioscopy are stone therapy and evaluation of indeterminate biliary strictures. Several other clinical applications have been described. As this technology is gaining more popularity and use, other indications are certain to be described.
Footnotes
Peer reviewer: Richard A Kozarek, MD, Executive Director, Digestive Disease Institute, Virginia Mason Medical Center 1100 Ninth Avenue, PO Box 900, Seattle, WA 98111-0900, United States
S- Editor Sun H L- Editor Kerr C E- Editor Lin YP
References
- 1.Cohen S, Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ Sr, McNair AE Jr, Mulholland M, Norton NJ, Rabeneck L, et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002;56:803–809. doi: 10.1067/mge.2002.129875. [DOI] [PubMed] [Google Scholar]
- 2.Parsi MA, Neuhaus H, Pleskow D, Binmoeller KF, Hawes RH, Petersen BT, Sherman S, Stevens PD, Deviere J, Haluszka O, et al. Peroral cholangioscopy guided stone therapy - report of an international multicenter registry. Gastrointest Endosc. 2008;67:AB102. [Google Scholar]
- 3.Pleskow D, Parsi MA, Chen YK, Neuhaus H, Slivka A, Haluszka O, Petersen BT, Deviere J, Sherman S, Meisner S, et al. Biopsy of indeterminate biliary strictures - does direct visualization help? - A multicener experience. Gastrointest Endosc. 2008;67:AB103. [Google Scholar]
- 4.Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374–382. doi: 10.1016/j.gie.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima M, Mukai H, Kawai K. Peroral cholangioscopy and pancreatoscopy. In: Sivak MV, editor. Gastrointestinal Endoscopy. 2nd ed. Philadelphia: WB Saunders; 2000. pp. 1055–1068. [Google Scholar]
- 6.Itoi T, Neuhaus H, Chen YK. Diagnostic value of image-enhanced video cholangiopancreatoscopy. Gastrointest Endosc Clin N Am. 2009;19:557–566. doi: 10.1016/j.giec.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Farrell JJ, Bounds BC, Al-Shalabi S, Jacobson BC, Brugge WR, Schapiro RH, Kelsey PB. Single-operator duodenoscope-assisted cholangioscopy is an effective alternative in the management of choledocholithiasis not removed by conventional methods, including mechanical lithotripsy. Endoscopy. 2005;37:542–547. doi: 10.1055/s-2005-861306. [DOI] [PubMed] [Google Scholar]
- 8.Steiner CA, Bass EB, Talamini MA, Pitt HA, Steinberg EP. Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med. 1994;330:403–408. doi: 10.1056/NEJM199402103300607. [DOI] [PubMed] [Google Scholar]
- 9.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 10.Everhart JE, Yeh F, Lee ET, Hill MC, Fabsitz R, Howard BV, Welty TK. Prevalence of gallbladder disease in American Indian populations: findings from the Strong Heart Study. Hepatology. 2002;35:1507–1512. doi: 10.1053/jhep.2002.33336. [DOI] [PubMed] [Google Scholar]
- 11.Aerts R, Penninckx F. The burden of gallstone disease in Europe. Aliment Pharmacol Ther. 2003;18 Suppl 3:49–53. doi: 10.1046/j.0953-0673.2003.01721.x. [DOI] [PubMed] [Google Scholar]
- 12.Festi D, Dormi A, Capodicasa S, Staniscia T, Attili AF, Loria P, Pazzi P, Mazzella G, Sama C, Roda E, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project) World J Gastroenterol. 2008;14:5282–5289. doi: 10.3748/wjg.14.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic) Best Pract Res Clin Gastroenterol. 2006;20:1075–1083. doi: 10.1016/j.bpg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Caddy GR, Tham TC. Gallstone disease: Symptoms, diagnosis and endoscopic management of common bile duct stones. Best Pract Res Clin Gastroenterol. 2006;20:1085–1101. doi: 10.1016/j.bpg.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 16.Moreau JA, Zinsmeister AR, Melton LJ 3rd, DiMagno EP. Gallstone pancreatitis and the effect of cholecystectomy: a population-based cohort study. Mayo Clin Proc. 1988;63:466–473. doi: 10.1016/s0025-6196(12)65644-4. [DOI] [PubMed] [Google Scholar]
- 17.Van Dam J, Sivak MV Jr. Mechanical lithotripsy of large common bile duct stones. Cleve Clin J Med. 1993;60:38–42. doi: 10.3949/ccjm.60.1.38. [DOI] [PubMed] [Google Scholar]
- 18.Cho YD, Cheon YK, Moon JH, Jeong SW, Jang JY, Lee JS, Shim CS. Clinical role of frequency-doubled double-pulsed yttrium aluminum garnet laser technology for removing difficult bile duct stones (with videos) Gastrointest Endosc. 2009;70:684–689. doi: 10.1016/j.gie.2009.03.1170. [DOI] [PubMed] [Google Scholar]
- 19.Moon JH, Cha SW, Ryu CB, Kim YS, Hong SJ, Cheon YK, Cho YD, Kim YS, Lee JS, Lee MS, et al. Endoscopic treatment of retained bile-duct stones by using a balloon catheter for electrohydraulic lithotripsy without cholangioscopy. Gastrointest Endosc. 2004;60:562–566. doi: 10.1016/s0016-5107(04)02012-7. [DOI] [PubMed] [Google Scholar]
- 20.Darcy M, Picus D. Cholangioscopy. Tech Vasc Interv Radiol. 2008;11:133–142. doi: 10.1053/j.tvir.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Bratcher J, Kasmin F. Choledochoscopy-assisted intraductal shock wave lithotripsy. Gastrointest Endosc Clin N Am. 2009;19:587–595. doi: 10.1016/j.giec.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Piraka C, Shah RJ, Awadallah NS, Langer DA, Chen YK. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin Gastroenterol Hepatol. 2007;5:1333–1338. doi: 10.1016/j.cgh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Awadallah NS, Chen YK, Piraka C, Antillon MR, Shah RJ. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis? Am J Gastroenterol. 2006;101:284–291. doi: 10.1111/j.1572-0241.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow D, Slivka A, Haluszka O, Petersen BT, Sherman S, Deviere J, et al. Peroral cholangioscopy (POC) using a disposable steerable single operator catheter for biliary stone therapy and assessment of indeterminate strictures - A multicenter experience using Spyglass. Gastrointest Endosc. 2009;69:AB264–AB265. [Google Scholar]
- 25.Parsi MA, Li A, Li CP, Goggins M. DNA methylation alterations in endoscopic retrograde cholangiopancreatography brush samples of patients with suspected pancreaticobiliary disease. Clin Gastroenterol Hepatol. 2008;6:1270–1278. doi: 10.1016/j.cgh.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671–677. doi: 10.1136/gut.40.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvaggi SM. Biliary brushing cytology. Cytopathology. 2004;15:74–79. doi: 10.1111/j.1365-2303.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 28.Govil H, Reddy V, Kluskens L, Treaba D, Massarani-Wafai R, Selvaggi S, Gattuso P. Brush cytology of the biliary tract: retrospective study of 278 cases with histopathologic correlation. Diagn Cytopathol. 2002;26:273–277. doi: 10.1002/dc.10098. [DOI] [PubMed] [Google Scholar]
- 29.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogel EL, deBellis M, McHenry L, Watkins JL, Chappo J, Cramer H, Schmidt S, Lazzell-Pannell L, Sherman S, Lehman GA. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc. 2006;63:71–77. doi: 10.1016/j.gie.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Ornellas LC, Santos Gda C, Nakao FS, Ferrari AP. Comparison between endoscopic brush cytology performed before and after biliary stricture dilation for cancer detection. Arq Gastroenterol. 2006;43:20–23. doi: 10.1590/s0004-28032006000100007. [DOI] [PubMed] [Google Scholar]
- 32.Stewart CJ, Burke GM. Value of p53 immunostaining in pancreatico-biliary brush cytology specimens. Diagn Cytopathol. 2000;23:308–313. doi: 10.1002/1097-0339(200011)23:5<308::aid-dc4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635–638. doi: 10.1067/mge.2000.108969. [DOI] [PubMed] [Google Scholar]
- 34.Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Moriyasu F, Gotoda T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos) Gastrointest Endosc. 2007;66:730–736. doi: 10.1016/j.gie.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 35.Seo DW, Lee SK, Kim MH, Min YI. Benign lesions of bile ducts and gallbladder. In: Seo DW, Lee SK, Kim MH, Min YI, eds , et al., editors. Cholangioscopy. Seoul: Koonja Publishing; 2002. pp. 57–83. [Google Scholar]
- 36.Abdel Aziz AM, Sherman S, Binmoeller KF, Deviere J, Hawes RH, Haluszka O, Neuhaus H, Pleskow D, Raijman I. SpyGlass cholangioscopy - Impact on patients with bile duct filling defect(s) of uncertain etiology. Gastrointestinal endoscopy. 2008;67:AB325. [Google Scholar]
- 37.Shah SA, Grant DR, McGilvray ID, Greig PD, Selzner M, Lilly LB, Girgrah N, Levy GA, Cattral MS. Biliary strictures in 130 consecutive right lobe living donor liver transplant recipients: results of a Western center. Am J Transplant. 2007;7:161–167. doi: 10.1111/j.1600-6143.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- 38.Tashiro H, Itamoto T, Sasaki T, Ohdan H, Fudaba Y, Amano H, Fukuda S, Nakahara H, Ishiyama K, Ohshita A, et al. Biliary complications after duct-to-duct biliary reconstruction in living-donor liver transplantation: causes and treatment. World J Surg. 2007;31:2222–2229. doi: 10.1007/s00268-007-9217-x. [DOI] [PubMed] [Google Scholar]
- 39.Siddique I, Galati J, Ankoma-Sey V, Wood RP, Ozaki C, Monsour H, Raijman I. The role of choledochoscopy in the diagnosis and management of biliary tract diseases. Gastrointest Endosc. 1999;50:67–73. doi: 10.1016/s0016-5107(99)70347-0. [DOI] [PubMed] [Google Scholar]
- 40.Parsi MA, Guardino J, Vargo JJ. Peroral cholangioscopy-guided stricture therapy in living donor liver transplantation. Liver Transpl. 2009;15:263–265. doi: 10.1002/lt.21584. [DOI] [PubMed] [Google Scholar]
- 41.Hisatsune H, Yazumi S, Egawa H, Asada M, Hasegawa K, Kodama Y, Okazaki K, Itoh K, Takakuwa H, Tanaka K, et al. Endoscopic management of biliary strictures after duct-to-duct biliary reconstruction in right-lobe living-donor liver transplantation. Transplantation. 2003;76:810–815. doi: 10.1097/01.TP.0000083224.00756.8F. [DOI] [PubMed] [Google Scholar]
- 42.Parsi MA. Peroral cholangioscopy-assisted guidewire placement for removal of impacted stones in the cystic duct remnant. World J Gastrointest Surg. 2009;1:59–61. doi: 10.4240/wjgs.v1.i1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsi MA, Sanaka MR, Dumot JA. Iatrogenic recurrent pancreatitis. Pancreatology. 2007;7:539. doi: 10.1159/000108972. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi S, Baba Y, Ueno K, Nakajo M. Small arteriovenous malformation of the common bile duct causing hemobilia in a patient with hereditary hemorrhagic telangiectasia. Cardiovasc Intervent Radiol. 2008;31 Suppl 2:S131–S134. doi: 10.1007/s00270-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 45.Prasad GA, Abraham SC, Baron TH, Topazian MD. Hemobilia caused by cytomegalovirus cholangiopathy. Am J Gastroenterol. 2005;100:2592–2595. doi: 10.1111/j.1572-0241.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 46.Lu XL, Itoi T, Kubota K. Cholangioscopy by using narrow-band imaging and transpapillary radiotherapy for mucin-producing bile duct tumor. Clin Gastroenterol Hepatol. 2009;7:e34–e35. doi: 10.1016/j.cgh.2008.11.001. [DOI] [PubMed] [Google Scholar]


