Abstract
AIM: To compare the long-term outcome of percutaneous vs surgical radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) in dangerous locations.
METHODS: One hundred and sixty-two patients with HCC in dangerous locations treated with percutaneous or surgical RFA were enrolled in this study. The patients were divided into percutaneous RFA group and surgical RFA group. After the patients were regularly followed up for a long time, their curative rate, hospital stay time, postoperative complications and 5-year local tumor progression were compared and analyzed.
RESULTS: No significant difference was observed in curative rate between the two groups (91.3% vs 96.8%, P = 0.841). The hospital stay time was longer and more analgesics were required while the incidence of bile duct injury and RFA-related hemorrhage was lower in surgical RFA group than in percutaneous RFA group (P < 0.05). The local progression rate of HCC in dangerous locations was significantly lower in surgical RFA group than in percutaneous RFA group (P = 0.05). The relative risk of local tumor progression was 14.315 in percutaneous RFA group.
CONCLUSION: The incidence of severe postoperative complications and local tumor progression is lower after surgical RFA than after percutaneous RFA.
Keywords: Hepatocellular carcinoma, Radiofrequency ablation, Liver cirrhosis, Recurrence, Local therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is currently the fifth most common malignant neoplasm in the world[1], causing more than 500 000 deaths every year[2]. HCC is prevalent in Asia and Africa and its incidence has steadily increased in European and American populations[3,4]. Theoretically, the best treatment of HCC is orthotopic liver transplantation (OLT) which provides the opportunity for its cure[5], but the scarcity of donors limits this treatment.
In the last two decades, local ablative therapy has become a safe and effective procedure for small HCC, of which radiofrequency ablation (RFA) is considered the most promising one[6]. It was reported that RFA for small HCC provides a comparable survival time and local tumor control after surgical resection[7,8], and may also be used as a bridge therapy for liver transplantation[9-11]. RFA is minimally invasive with a lower complication rate and a shorter hospital stay time than hepatectomy[12,13].
Although the indication of RFA is much wider than that of surgical resection for HCC, tumors in some circumstances are reported[14-18] not quit e suitable for RFA, such as a central nodule near the porta hepatis due to the risk of injuring major bile ducts, a nodule near large vessels due to a heat sink effect-induced incomplete ablation, a peripheral nodule near extrahepatic organs due to the risk of alimentary tract perforation or pleural effusion caused by heat injury.
In our institute, tumor location is not simply regarded as a contraindication of RFA. This retrospective study was designed to compare the long-term outcome of percutaneous and surgical RFA for HCC in these so-called dangerous locations. To the best of our knowledge, it is the first study comparing the efficacy of surgical and percutaneous RFA for HCC in dangerous locations of the liver.
MATERIALS AND METHODS
Diagnostic criteria
Diagnosis of HCC was made according to the diagnostic criteria for HCC recommended by the European Association for the Study of the Liver[19], which was based on ultrasound-guided biopsy, or the concordant classical dynamic radiological features of HCC in two radiologic techniques, or one radiologic technique showing typical features of HCC together with an elevated α fetoprotein (AFP) level over 400 ng/mL.
Definition
Tumor in dangerous locations[18] was defined as a lesion (≤ 0.5 cm in diameter) near large vessels such as a primary or secondary branch of the portal vein, the base of hepatic veins, or the inferior vena cava (IVC), or as a lesion (less than 0.5 cm in diameter) near extrahepatic organs measured on radiological imagines.
A curative treatment[19] was defined as no residual viable tumor tissue within the treatment zone confirmed by a 4-wk-afterward-performed spiral triphasic enhanced CT after a complete ablation of the lesion assessed by intraoperative ultrasonography (IOUS).
Local tumor progression[20] was defined as the appearance of viable tumor tissue that was contiguous with the area completely ablated during follow-up.
Inclusion criteria and enrollment
In our institute, a curative RFA is usually expected for patients conforming to the Milan criteria for liver transplantation. The inclusion criteria in this study included patients with a confirmed diagnosis of HCC or a solitary HCC (≤ 5 cm in diameter) or up to 3 nodules (< 3 cm in diameter), liver function of Child-Pugh class A or B, a prothrombin time of less than 5 s, a HBV-DNA-PCR quantitation of less than 105 copies/mL, but without extrahepatic metastasis or obvious vascular invasion, previous or simultaneous malignancies or evident bleeding tendency (a platelet count > 50 × 109/L or correctable by transfusion, no previous treatment of HCC, and those suitable and willing to be treated with RFA.
The study was performed according to the guidelines of the Helsinki Declaration. A written informed consent was obtained from each patient before intervention. Between February 2003 and February 2007, RFA was performed for 794 consecutive HCC patients in West China Hospital. Of these patients, 513 were diagnosed as primary HCC, 484 of them met the inclusion criteria. Of these 484 patients, 162 had at least one nodule in dangerous locations.
Follow-up
Patients were followed up at a three month interval after treatment. Abdominal ultrasonography and helical computer tomography (CT), serum AFP measurement and liver function tests were performed during each visit. When intrahepatic recurrence was suspected, spiral CT or magnetic resonance imaging (MRI) was performed. When extrahepatic metastases were suspected, thoracic CT and bone scintigraphy were performed. Local tumor progression was specifically noticed as the endpoint in this study.
Statistical analysis
Differences in the surgical and percutaneous RFA groups were analyzed by the unpaired t test for continuous variables and by the χ2 test or continuity correction method for categorical variables. Local tumor progression curves were plotted with the Kaplan-Meier method and compared by the log-rank test. Relative prognostic significance of the variables in predicting local tumor progression was assessed with univariate and multivariate Cox proportional hazards regression models. All variables with their P < 0.05 by univariate comparison were subjected to multivariate analysis. Results of multivariate analysis were presented as relative risk (RR) with corresponding 95% confidence intervals (CI). Statistical analysis was performed using the SPSS 13.0 statistical software (SPSS Company, Chicago, Illinois, USA). All statistical tests were two-sided and differences were considered when P < 0.05.
RFA procedure
Equipments: All RFA procedures were performed on an inpatient basis by surgeons from the Department of Hepato-biliary-pancreatic Surgery using a commercially available system (Radionics, Cool-Tip System, Burlington, MA, USA), single/clustered needle electrode(s) with a 2 cm or 3 cm exposed tip and ultrasound guidance (Vivid4, GE, USA; iU22, Philips, USA). Clustered electrodes were used systematically for lesions (> 3 cm in diameter).
Percutaneous RFA: General anesthesia was employed, 2-4 grounding pads were attached to the thighs of patients and the electrode was inserted into the lesion according to a route assessment via ultrasound. The needle tip was inserted to the bottom of the tumor (i.e. the most distal border from the skin puncture site) in the first session to avoid gas formation between non-ablated lesion and ultrasound transducer. At the time of subsequent RFA sessions, the electrode position was determined via IOUS scrutiny. The ablation subsequence was always from “bottom” to “top” to provide a clear, real-time ultrasound image. The electrode was inserted at different sites and overlapping ablations were performed until the entire lesion was ablated as determined by IOUS.
Assessment of ablation: After measurement of the baseline impedance, generator output power was gradually increased from 80 W to 200 W, with a peristaltic pump infusing cold saline into the electrode lumen to maintain the tip temperature below 20°C. The timer was usually set to 12 min for each session. Impedance was synchronously monitored with the system. Session in the same site was repeated until the impedance increased at least 10 Ohms over baseline and became stable. The electrode was heated to 90-100°C before it was drawn back in order to eliminate seeding cancer cells and prevent bleeding. Treatment was continued until complete ablation features were achieved in IOUS.
RFA in dangerous locations
Percutaneous RFA: The route of electrode insertion should be carefully considered on ultrasound scrutiny. When the tumor was in segment VII, close to the diaphragm, the electrode was inserted through the right pleural cavity of patients. Saline was infused into the right pleural cavity to compress the right lobe of the lung, then the electrode reached the target under the ultrasound-guidance and percutaneous RFA was achieved through an artificial serothorax. A thoracic close drainage was needed for 2 d after therapy.
Surgical RFA: A right subcostal incision with a midline extension was chosen. Extensive dissociation of the liver was usually performed from the ligaments and adhesions to other organs, such stomach, colon or kidneys and large vessels. The route of surgical RFA was assessed by IOUS on the liver surface. The distance between the tumor and other vulnerable organs or vessels could be enlarged when the operator rotated the liver.
Ablation timing: The time of RFA was usually irregular in the dangerous locations, RFA was stopped as soon as the ultrasound detected microbubbles generated by RFA reaching the distal border of the assumed area. An experienced operator managed most injures to adjacent organs and structures as well as the heat sink effect from large vessels with RFA.
Assessment of response
Response was assessed according to the modified European Association for the Study of the Liver criteria[19]. Spiral triphasic enhanced CT was performed one month after RFA. Residual viable tumor was diagnosed if an enhanced area was noted within the treatment zone. If RFA was repeated, another CT was performed four weeks later to assess the response to RFA. If residual viable tissue of the tumor still existed, RFA was considered a failure and the patient was treated with transcatheter hepatic arterial chemoembolization (TACE).
Potential conflict of interest
This study did not receive any support from industry or private corporations.
RESULTS
Of the 482 patients, 162 had at least one nodule in the dangerous locations (156 had a lesion and 6 had 2 lesions in the dangerous locations) and 320 had HCC in the ordinary location. Of the 162 patients with HCC in dangerous locations, 34 had their diagnosis made by biopsy and 128 were diagnosed non-invasively, 69 received percutaneous RFA and 93 underwent surgical RFA. The demographic parameters of patients who underwent percutaneous and surgical RFA are listed in Table 1. A significant difference was found in HBV/HCV-infection and serum AFP level (P < 0.05). The tumor locations and adjacent vessels and organs in patients who underwent percutaneous and surgical RFA are shown in Table 2. The mean follow-up time of patients who underwent percutaneous and surgical RFA was 28.4 ± 14.7 mo (range 3-81 mo) and 31.6 ± 24.1 mo (range 6-78 mo), respectively (P > 0.05). Censored patients included 17 out of the 69 patients who underwent percutaneous RFA and 22 out of the 93 patients who underwent surgical RFA (P = 0.885).
Table 1.
Dempgraphic parameters of patients undergoing percutaneous and surgical radiofrequency ablation
| Parameters | Patients undergoing percutaneous RFA (n = 63) | Patients undergoing surgical RFA (n = 93) | P value |
| Age (yr) | 57.8 ± 16.1 | 52.4 ± 11.7 | 0.531 |
| Gender (M/F) | 55/14 | 81/12 | 0.205 |
| HBV infected | 58 | 91 | 0.006 |
| HCV infected | 1 | 0 | |
| None-HBV&HCV | 10 | 2 | |
| Liver cirrhosis | 46 | 71 | 0.174 |
| AST (IU/L) | 47.3 ± 36.2 | 44.6 ± 33.8 | 0.311 |
| ALT (IU/L) | 42.3 ± 31.4 | 44.1 ± 19.6 | 0.354 |
| TB (μmol/L) | 15.4 ± 3.4 | 14.2 ± 5.6 | 0.601 |
| ALB (g/L) | 39.1 ± 9.8 | 41.7 ± 5.4 | 0.852 |
| Child A/B | 61/8 | 93/0 | 0.001 |
| PLT (< 1011) | 13 | 9 | 0.092 |
| PT (> 15’) | 6 | 14 | 0.224 |
| Tumor number, 1/2/3 | 51/16/2 | 79/13/1 | 0.095 |
| Tumor size (cm), > 3/≤ 3 | 11/78 | 23/85 | 0.099 |
| Solitary HCC (cm), ≤ 3 | 39 | 62 | 0.188 |
| Tumor in dangerous locations | 73 | 95 | 0.242 |
| AFP (ng/mL), ≤ 400/> 400/> 1210 | 13/41/15 | 17/60/16 | 0.000 |
Non-hepatitis B virus (HBV) & hepatitis C virus (HCV): Patients who were negative for HBV and HCV antibody but not for anti-HBs. RFA: Radiofrequency ablation; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AFP: α fetoprotein; TB: Total bilirubin; PLT: Platelet; PT: Prothrombin time; ALB: Albumin.
Table 2.
Locations of lesions in patients undergoing percataneous and surgical radiofrequency ablation
|
RFA |
||
| Percataneous (n = 89) | Surgical (n = 108) | |
| Segment location | ||
| I | 0 | 0 |
| II | 7 | 12 |
| III | 13 | 17 |
| IV | 9 | 12 |
| V | 10 | 14 |
| VI | 6 | 11 |
| VII | 12 | 20 |
| VIII | 12 | 7 |
| Adjacent vessels or organs | ||
| PH | 8 | 12 |
| RHV | 17 | 15 |
| MHV | 8 | 12 |
| LHV | 11 | 16 |
| IVC | 6 | 8 |
| Heart | 3 | 5 |
| Stomach | 9 | 17 |
| Lung | 15 | 2 |
| R.Kidney | 4 | 7 |
| Colon | 5 | 12 |
| GB | 3 | 2 |
P = 0.640 by Pearson χ2 test for segment location and P = 0.054 by Pearson χ2 test for adjacent vessels or organs. Lesion between segments was registered at the major location. n: Lesion number; RFA: Radiofrequency ablation; PH: Porta hepatis; RHV: Right hepatic vein; MHV: Middle hepatic vein; LHV: Left hepatic vein; IVC: Inferior vena cava; GB: Gall bladder.
Patients who underwent percutaneous RFA
Eighty-nine lesions were found in 69 patients who underwent percutaneous RFA (Table 1). The mean treatment session was 2.0 ± 1.2/lesion for the 78 nodules (≤ 3 cm in diameter) and 3.4 ± 0.8/lesion for the 7 nodules (larger than 3 cm but smaller than 5 cm in diameter). Of the 89 lesions, 73 nodules were found in the dangerous locations, the mean tumor size was 1.7 ± 1.1 cm, and the mean treatment session was 3.7 ± 2.1/lesion. The complete RFA rate was 98.6% (68/69) assessed intraoperatively, and the curative rate was 91.3% (63/69) assessed by CT 4 wk thereafter. The RFA failure rate was 4.3% (3/69). Two patients failed to achieve a curative outcome after 2 times of percutaneous RFA. The last patient had one nodule (1 cm in diameter) in 2 tumors very close to the pericardium. RFA was aborted due to the concern of malpositioning the electrode by IOUS. These three patients were later treated with TACE.
Patients who underwent surgical RFA
One hundred and eight lesions were found in 93 patients who underwent surgical RFA (Table 1). The mean treatment session was 1.2 ± 0.5/lesion for the 85 nodules (≤ 3 cm in diameter) and 2.8 ± 0.9/lesion for the 23 nodules (larger than 3 cm but smaller than 5 cm in diameter). The mean tumor size and mean treatment session were 1.8 ± 1.0 cm and 2.9 ± 2.0/lesion, respectively, for the 95 nodules in the dangerous locations. The complete RFA rate was 100% (93/93) assessed intraoperatively, and the curative rate was 96.8% (90/93) assessed by CT 4 wk afterward. The RFA failure rate was 1.1% (1/93). A nodule (4 cm in diameter) in a patient who failed to RFA compressed the right hepatic duct. To avoid the bile duct injury, a stent was inserted into the compressed bile duct, and ethanol was injected into the adjacent tumor border to the bile duct before RFA. Unfortunately, bile fistula still occurred on day 25 after operation, and CT showed an incomplete ablation of the tumor. A T-tube drainage was placed via laparotomy later. Tumor encroaching on the right hepatic duct wall was highly suspected, and treated with palliative therapy due to poor liver function.
Hospital stay time and mortality of patients, and complications of RFA
The hospital stay time of HCC patients was significantly longer after surgical RFA than after percutaneous RFA (6.1 ± 3.1 d vs 3.5 ± 2.9 d, P < 0.001).
No patient died within 30 d after surgical and percutaneous RFA with a mortality of 0%.
According to the accordion severity grading system of surgical complications[21], the complications of percutaneous and surgical RFA are shown in Table 3. The number of patients requiring analgesics was significantly greater after surgical RFA than after percutaneous RFA (P < 0.001). However, the incidence of bile duct injury and RFA-related hemorrhage was higher in patients after percutaneous RFA than after surgical RFA (P = 0.05).
Table 3.
Major complications of radiofrequency ablation
| Classification of complications | Percutaneous RFA (n = 69) | Surgical RFA (n = 93) | P value |
| Grade I | |||
| Analgesics requirement | 17 | 58 | 0.000 |
| Fever above 38.5°C | 23 | 45 | 0.055 |
| Grade II | |||
| Ascites | 4 | 11 | 0.190 |
| Persistent jaundice | 2 | 0 | 0.315 |
| Gastric hemorrhage | 0 | 3 | 0.132 |
| Grade III | |||
| Hydrothorax requiring drainage | 5 | 9 | 0.586 |
| Skin burn | 1 | 0 | 0.244 |
| Encapsulated effusion needing drainage | 3 | 1 | 0.184 |
| Grade IV | |||
| Partial hepatic infarction | 1 | 3 | 0.471 |
| Gastric perforation | 1 | 0 | 0.244 |
| Bile duct injury | 5 | 1 | 0.040 |
| Procedure-related hemorrhage | 6 | 1 | 0.018 |
| Malignant seeding | 2 | 0 | 0.315 |
RFA: Radiofrequency ablation.
Local tumor progression
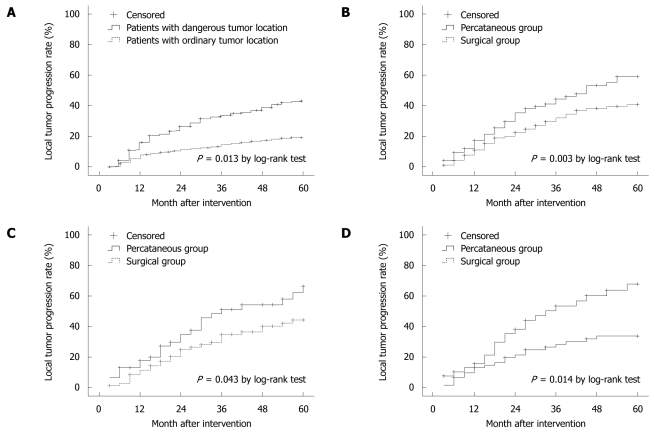
During the 5-year study period after treatment, the local tumor progression was observed in 75 (46.3%) out of the 162 patients with HCC in the dangerous locations and in 71 (22.2%) out of the 320 patients with HCC in the general locations. The local tumor progression was more severe in patients with HCC in the dangerous locations than in those with HCC in the general locations (P < 0.015, Figure 1A).
Figure 1.
Local tumor progression in patients with hepatocellular carcinoma at common and dangerous locations (A), and hepatocellular carcinoma (B), cirrhosis (C), solitary hepatocellular carcinoma with its diameter ≥ 3 cm (D) after percutaneous and surgical radiofrequency ablation.
Of the 162 patients with HCC in the dangerous locations, 69 and 93 were treated with percutaneous RFA and surgical RFA, respectively. Local tumor progression was observed in 40 out of the 69 patients with HCC after percutaneous RFA and in 35 out of the 93 patients after surgical RFA. The 1-, 2-, 3-, 4-, 5-year local tumor progression rate was 17.4%, 36.2%, 46.4%, 53.6%, 57.6%, respectively, for patients after percutaneous RFA, and 9.9%, 21.5%, 30.1%, 35.5%, 37.6%, respectively, for those after surgical RFA. The local tumor progression was more severe in patients after percutaneous RFA than after surgical RFA (P < 0.003, Figure 1B).
Forty-six out of the 63 cirrhotic patients underwent percutaneous RFA and 71 out of the 93 cirrhotic patients underwent surgical RFA (Table 1). The local tumor progression was more severe in patients after percutaneous RFA than after surgical RFA (P < 0.05, Figure 1C).
Thirty-nine out of the 69 patients with solitary HCC (≤ 3 cm in diameter) underwent percutaneous RFA and 62 out of the 93 patients with HCC underwent surgical RFA (Table 1). The local tumor progression was more severe in patients after percutaneous RFA than after surgical RFA (P < 0.05, Figure 1D).
Univariate analysis revealed that 5 out of the 10 variables (RFA approach, Child-Pugh class, total bilirubin level, serum AFP level and tumor size) were related to local tumor progression. Multivariate Cox proportional hazards regression analysis showed that percutaneous RFA, total bilirubin level > 10 ng/L and tumor size > 3 cm were the related risk factors for HCC. The corresponding relative risks were 14.315 (95% CI: 4.857-25.412), 8.124 (95% CI: 2.325-101.587), and 11.741(95% CI: 3.754-21.665), respectively (Table 4).
Table 4.
Univarite and multivariate analysis of relative risks for local tumor progression
| Variable | Univariate analysis |
Multivariate analysis |
|
| P value | Relative risk (95% CI) | P value | |
| Percutaneous vs surgical RFA | 0.000 | 14.315 (4.857-25.412) | 0.000 |
| Age (yr) (> 60 vs ≤ 60) | 0.402 | ||
| HBV- infected (Y vs N) | 0.455 | ||
| Child-Pugh (B vs A) | 0.038 | ||
| Albumin (IU/L), ≤ 35 vs > 35 | 0.233 | ||
| Total bilirubin (mg/L), > 10 vs ≤ 10 | 0.010 | 8.124 (2.325-101.587) | 0.012 |
| Serum AFP (ng/mL), ≥ 400 vs < 400 | 0.019 | ||
| Prothrombin time, ≤ 15’ vs > 15’ | 0.512 | ||
| Tumor size (cm), > 3 vs ≤ 3 | 0.003 | 11.741 (3.754-21.665) | 0.005 |
| Tumor number, multiple vs single | 0.111 | ||
RFA: Radiofrequency ablation; HBV: Hepatitis B virus; AFP: α fetoprotein.
DISCUSSION
One important advantage of RFA for liver tumors is micro-invasive when compared with partial hepatectomy[12,13,22-25]. Some institutes have reported RFA on an out-patients basis[26]. However, even though the morbidity of malignant seeding in the needle tract is low, it is hard to avoid[27,28]. Moreover, hemorrhage after the electrode is drawn out appears undetectable in a short time by ultrasonography.
In this study, the hospital stay time of patients with HCC was significantly longer with more analgesics required after surgical RFA than after percutaneous RFA. Surgical RFA seemed more invasive than percutaneous RFA. However, the incidence of more severe complications, such as bile duct injury and procedure-relate hemorrhage, was lower in patients after surgical RFA than after percutaneous RFA.
It was reported that a lesion in dangerous locations of the liver is treated with artificial hydrothorax and ascites to achieve percutaneous RFA[18]. A curative RFA was achieved with rtificial hydrothorax in 3 patients in this study. However, artificial acsites was not applied when HCC near extrahepatic organs was treated, because the local acsites was not always capable of dividing a safety zone, the fluidity of liquid made the ascites lack of tension to support a safety zone, membrane adhesions unusually existed between organs and liver, the lesion was often located very close to the surface of the liver when the artificial ascites was needed, and ascites decreased the temperature at the outer part of the lesion when RFA was performed. Thus viable tumor cells could survive.
Compared with surgical RFA, IOUS of percutaneous RFA is indirect (through abdominal wall), and the choice of route to the lesion is restricted. Injury of important structures, such as bile ducts or extrahepatic organs, should be avoided and RFA should eliminate the viable tumor cells in the assumed area as complete as possible. RFA should be stopped as soon as the ultrasonography shows microbubbles generated by RFA reaching the assumed distal border. In this study, the mean session for each lesion in the dangerous locations was 3.7 ± 2.1/lesion in percutaneous RFA group and 2.9 ± 2.0/lesion in surgical RFA group. The patients undergoing percutaneous RFA needed significantly more sessions than those undergoing surgical RFA to ablate a lesion (P < 0.05). The effect of percutaneous RFA mainly depends on the experience of operators. On the contrary, surgical RFA may provide a direct ultrasonography monitoring the liver surface, even a visual contact during the procedure. A mobilized liver could offer more choices of route for the electrode and a reliable safety zone in extrahepatic organs or IVC. Thus more attention should be paid to tumor elimination, even the routine impedance-depended assessment technique can be applied in some surgical RFA procedures and in evaluation by IOUS, which may be more accurate than that used in percutaneous RFA procedures and can at least in part explain why more severe local tumor progression was found in patients after percutaneous RFA than after surgical RFA.
This study has the following limitations. First, it was a retrospective study and therefore had inherent defects due to the nature of the method. Second, the rate of censor patients was relatively high in patients undergoing percutaneous and surgical RFA. Third, physicians with diverse experiences might achieve different outcomes of percutaneous RFA. Finally, there was a significant difference in proportion of HBV/HCV-infection and serum AFP level between the patients who underwent surgical or percutaneous RFA.
In conclusion, surgical RFA seems more invasive than percutaneous RFA and the incidence of severe postoperative complications and local tumor progression is lower after surgical RFA than after percutaneous RFA for HCC in dangerous locations.
COMMENTS
Background
The efficacy of radiofrequency ablation (RFA) on hepatocellular carcinoma (HCC) has been debated for a long time. Whether RFA is effective against HCC both in dangerous location and in common location remains controversial.
Research frontiers
The corona of micro-invasion makes lots of colleagues concentrate on percataneous RFA. Althrough laparoscopic RFA has been applied recently in some major institutes, laparoscopic ultrasonograghy needs a long time to be evaluated. Surgical RFA seems more suitable to be employed in most hospitals when difficult circumstances are encountered.
Innovations and breakthroughs
This study was a retrospective study assessing the value of surgical and percutaneous RFA for local or regional HCC in difficult anatomical positions with a large number of patients and a long follow-up time.
Applications
This study may help clinicians to chose RFA when encountering HCC in difficult anatomical positions.
Terminology
Tumor in the dangerous location is defined as a lesion (≤ 0.5 cm in diameter) near large vessels, such as a primary or secondary branch of the portal vein, the base of hepatic veins, or the inferior vena cava (IVC), or a lesion near extrahepatic organs (less than 0.5 cm in diameter) measured on radiological imagines.
Peer review
This article is a multinational collaborative study, assessing the value of percutaneous vs surgical RFA for local or regional HCC in difficult anatomical positions. It is a very interesting and clinically useful study with a large number of patients who were followed up for a long time, thus permitting evaluation of the final outcome of respective treatment modalities.
Footnotes
Peer reviewer: Emanuel K Manesis, MD, Professor of Medicine, Athens University School of Medicine, Liver Unit, Euroclinic, 19 Mavromateon Street, Athens 10 34, Greece
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
References
- 1.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619–634. doi: 10.1002/jso.21364. [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142–1143. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 5.Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 6.Duffy JP, Hiatt JR, Busuttil RW. Surgical resection of hepatocellular carcinoma. Cancer J. 2008;14:100–110. doi: 10.1097/PPO.0b013e31816a5c1f. [DOI] [PubMed] [Google Scholar]
- 7.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 9.Fontana RJ, Hamidullah H, Nghiem H, Greenson JK, Hussain H, Marrero J, Rudich S, McClure LA, Arenas J. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–1174. doi: 10.1053/jlts.2002.36394. [DOI] [PubMed] [Google Scholar]
- 10.Yamashiki N, Tateishi R, Yoshida H, Shiina S, Teratani T, Sato S, Mine N, Kondo Y, Kawabe T, Omata M. Ablation therapy in containing extension of hepatocellular carcinoma: a simulative analysis of dropout from the waiting list for liver transplantation. Liver Transpl. 2005;11:508–514. doi: 10.1002/lt.20392. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–179. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–1483. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 15.McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 16.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 17.Meloni MF, Goldberg SN, Moser V, Piazza G, Livraghi T. Colonic perforation and abscess following radiofrequency ablation treatment of hepatoma. Eur J Ultrasound. 2002;15:73–76. doi: 10.1016/s0929-8266(01)00171-9. [DOI] [PubMed] [Google Scholar]
- 18.Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108. doi: 10.1002/hep.21164. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg SN, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT Jr, Livraghi T, McGahan JP, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–345. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 21.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 22.Jiang HC, Liu LX, Piao DX, Xu J, Zheng M, Zhu AL, Qi SY, Zhang WH, Wu LF. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002;8:624–630. doi: 10.3748/wjg.v8.i4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guglielmi A, Ruzzenente A, Battocchia A, Tonon A, Fracastoro G, Cordiano C. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2003;50:480–484. [PubMed] [Google Scholar]
- 24.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 25.Liang HH, Chen MS, Peng ZW, Zhang YJ, Zhang YQ, Li JQ, Lau WY. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15:3484–3493. doi: 10.1245/s10434-008-0076-y. [DOI] [PubMed] [Google Scholar]
- 26.Nazir B, Chaturvedi AK, Rao A. Image-guided radiofrequency ablation of tumors: Current status. Indian J Radiol Imaging. 2003;13:315–322. [Google Scholar]
- 27.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Al-Leswas D, O'Reilly DA, Poston GJ. Biopsy of solid liver tumors: adverse consequences. Hepatobiliary Pancreat Dis Int. 2008;7:325–327. [PubMed] [Google Scholar]