Abstract
Gastrointestinal cancers represent a major cause of morbidity and mortality, with incomplete response to chemotherapy in the advanced stages and poor prognosis. Angiogenesis plays a crucial part in tumor growth and metastasis, with most gastrointestinal cancers depending strictly on the development of a new and devoted capillary network. Confocal laser endomicroscopy is a new technology which allows in vivo microscopic analysis of the gastrointestinal mucosa and its microvascularization during ongoing endoscopy by using topically or systemically administered contrast agents. Targeting markers of angiogenesis in association with confocal laser endomicroscopic examination (immunoendoscopy), as a future challenge, will add functional analysis to the morphological aspect of the neoplastic process. This review describes previous experience in endomicroscopic examination of the upper and lower digestive tract with emphasis on vascularization, resulting in a broad spectrum of potential clinical applications, and also preclinical research that could be translated to human studies.
Keywords: Confocal laser endomicroscopy, Immunoendoscopy, Fluoresceine, Acriflavine, Cancer
INTRODUCTION
Confocal laser endomicroscopy (CLE) is an emerging technology which allows in vivo imaging of cellular and subcellular details of the gut mucosa and vessels during ongoing endoscopy. With real-time microscopic analysis of the mucosal layer at high resolution, an immediate diagnosis is possible for different diseases and types of tissues. A magnification of about 1000 × enables evaluation of the epithelial cells, connective tissue and changes in vascular patterns. By using intravenously administered fluorescein sodium, images of vessel architecture are readily available, and early recognition of vascular changes in the gastrointestinal (GI) neoplasia is possible[1]. Furthermore, if real-time diagnosis cannot be obtained, the same technique allows targeted sampling of relevant areas (“smart biopsies”) with fewer specimens sent to the pathologist but with significantly higher diagnostic yield compared to random biopsies[2].
The potential role of CLE has been explored in different pathologic conditions of the GI tract, the possibility of diagnosing premalignant and malignant lesions of the GI tract being particularly important considering the prognostic implications. Gastroenterologists have shown significant interest in this technique, looking for further potential applications in molecular imaging.
Cancers of the digestive tract nowadays represent a major cause of morbidity and mortality. Tumor growth and metastatic potential are strictly dependent on the development of a new and devoted capillary network, which will supply oxygen and nutrients to the newly formed tumor and will allow malignant cells to access the systemic circulation[3]. Following intravenous administration of contrast agent, CLE allows in vivo real-time visualization of the tumor vasculature which is structurally and functionally altered by comparison with the pre-existing vessel network. Furthermore, pre-clinical trials have shown that quantitative characterization of microvessels in vivo is possible with confocal mini probes and the additional image processing software[4]. Such analysis of the microvascular architecture is fundamental for early detection of neoplastic transformation and assessment of the tumor’s metastatic potential. The complex, multistep process of neovascularization is the consequence of a dynamic balance between pro- and anti-angiogenic factors[5]. Targeting markers of angiogenesis, such as vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR), in association with endomicroscopic examination, as a future challenge, will add to the morphological aspect functional analysis of the neoplastic process. First steps towards such molecular endomicroscopic imaging have been made by recent studies on animal models.
PRINCIPLES AND TECHNIQUE
The newly developed endomicroscope integrates a miniature confocal microscope into the distal tip of a conventional endoscope enabling simultaneous standard video imaging and confocal microscopy of the mucosal layer. During CLE, an argon ion laser delivers an excitation beam of 488 nm wavelength at the surface of the tissue allowing targeted endomicroscopic images to be captured[6]. By placing the distal tip of the endomicroscope in intimate contact with the mucosa, multiple grayscale optical sections are recorded at different depths within a range of 0-250 μm. The optical slices are parallel with the mucosal surface with a 7 μm thickness and a lateral resolution of 0.7 μm, the field of view being 475 μm × 475 μm[7,8].
Apart from this dedicated endomicroscope, CLE can be performed with a miniprobe. This stand-alone confocal probe can be passed through the working channel of most endoscopes for examination of both the upper and the lower digestive tract. The entire probe-based endomicroscopy system consists of a flexible catheter probe representing a bundle of optical fibers linked to a micro-objective, a laser scanning unit (excitation wavelength 488 nm) and control and acquisition software. A range of mini probes were designed with various optical parameters for different endoscopic procedures[8,9]. The advanced image processing software allows real-time sequence display for an immediate morphologic diagnosis and also post-procedural analysis and editing. It enables vessel detection and quantitative microvascular measurements, features that make the system suitable for angiogenesis studies[4].
Contrast agents
High-resolution confocal imaging is achieved by using an exogenous fluorescence technique. Of the potentially suitable contrast agents in humans, the most commonly used are intravenous fluorescein sodium (10%) and topically applied acriflavine (0.2%)[10]. Acriflavine hydrochloride passes the cell membrane and strongly labels acidic constituents, providing clear visualization of the nuclei and cytoplasm, but only in the superficial layers of the mucosa (0-100 μm). Thus, it is particularly important in depicting intraepithelial neoplasia and cancer of the GI tract[11]. Fluorescein is a slightly acidic, hydrophilic dye with nonspecific staining properties. Within seconds of intravenous administration it strongly binds to serum albumin and makes the vascular pattern easily detectable. The remaining unbound dye diffuses across the capillaries and, by entering the tissue, it highlights the extracellular matrix[10,11].
With increasing interest by gastroenterologists in molecular imaging, novel fluorescent contrast agents have been developed targeting disease-specific biomarkers. These include labeled peptides which are easy to deliver to the target structure due to their low molecular weight, but they have variable affinity[12]. A heptapeptide conjugated with fluorescein has been topically administered for specific in vivo imaging of human colorectal neoplasia[13]. Fluorescently labeled antibodies, on the other hand, are highly selective, binding to their defined target, but may induce immune reactions. Already approved therapeutic antibodies could be labeled for imaging tumors, thus deciding the choice of targeted chemotherapy and potentially predicting the response to treatment[12]. CLE was able to accurately classify human xenograft tumors in mice and human tissue specimens based on their EGFR expression using fluorescently labeled antibodies against EGFR[14]. Anti-VEGF antibodies were also used as fluorescent contrast agents for in vivo molecular imaging in animal models[15]. Extensive pharmacokinetic and safety studies are needed to define clinical applications for these molecular probes.
CLINICAL APPLICATIONS IN GI CANCERS
The spectrum of potential indications for CLE is broad and includes pathology of both the upper and lower GI tract, most importantly screening and surveillance for cancer based on the cellular and vascular changes. Microscopic imaging of the mucosa is performed after previous detection by standard or optically enhanced endoscopy (autofluorescence imaging, narrow band imaging, i-scan, etc.) of “areas of interest”[10].
Esophagus
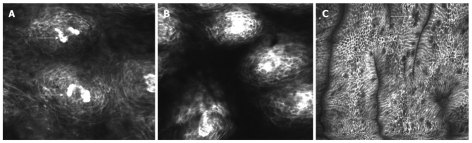
The normal endomicroscopic view of the esophageal surface includes polygonal cells within the non-keratinized squamous epithelium and the regular capillary loops of the esophageal papillae directed towards the luminal surface (Figure 1A)[7,11].
Figure 1.
Esophageal surface epithelium visualized using intravenous fluorescein. A: Capillary loops (arrows) of the esophageal papillae; B: Dilatation of intercellular spaces and increased vasculature off the papillae in reflux esophagitis; C: Presence of cylindrical epithelial cell and goblet cells (arrows) in the distal esophagus suggesting Barrett’s epithelium.
In patients with gastroesophageal reflux disease (Figure 1B), Barrett’s epithelium is a well defined premalignant condition associated with adenocarcinoma of the lower esophagus. Although the main diagnostic feature of Barrett’s esophagus is the presence of goblet cells, easily detectable with CLE, the vascular pattern, highlighted by fluorescein staining, can also to be considered. The subepithelial capillaries are still regular in shape beneath a specialized columnar epithelium (Figure 1C)[7]. Significant alterations of the capillary loops are notable in neoplastic tissue with irregular, dilated vessels of increased permeability recognized in the lamina propria due to the brighter signal intensity. Thus CLE is able to detect early neoplastic transformation based on changes in the vascular architecture[1,16]. In the progression of cancers associated with Barrett’s esophagus, angiogenesis represents an essential step. Confocal endomicroscopy can detect this newly developed vascular network and also it enables quantitative measurements such as microvessel density (MVD). Becker et al[17] evaluated confocal microscopy images acquired from 20 patients, which showed that, in neoplastic Barrett’s esophagus, MVD was significantly higher compared to benign conditions.
Stomach
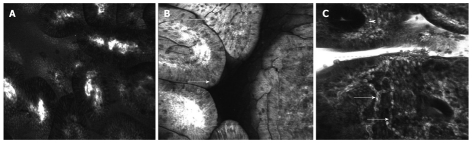
In imaging the stomach, confocal diagnostic criteria have been established in clinical studies for normal gastric mucosa (Figure 2A) as well as for chronic gastritis with intestinal metaplasia (Figure 2B) and cancer by correlation with histopathological examination as the gold standard[18-22]. While most of these studies classified lesions based mainly on the changes in cells and pit patterns, Liu et al[23] described endomicroscopic aspects of vascular architecture in both normal and malignant mucosa of the upper GI tract. Contrast enhanced CLE (fluorescein sodium 10%, 5 mL) highlights the vascular network of the normal gastric mucosa revealing different aspects between parts of the stomach. The subepithelial capillaries of the gastric body show a honeycomb-like pattern surrounding the gastric pits while in the antrum they are typically coil-shaped. Differentiated early gastric cancer appears to be hypervascular with tortuous, dilated vessels, irregular in shape and size (Figure 2C). In contrast, a hypovascular aspect was described for undifferentiated cancer with isolated, short branch vessels that did not interconnect. In one case report, probe-based endomicroscopy made it possible to diagnose angiodysplasia as a cause of chronic anemia by real-time imaging of a dilated blood vessel with moving red blood cells[24], adding proof to CLE’s feasibility for vascular imaging.
Figure 2.
Confocal laser endomicroscopy of the stomach using intravenous fluorescein. A: Columnar epithelium of the antral gastric mucosa and regulated microvascular matrix; B: Atrophic gastritis with intestinal metaplasia and presence of goblet cells (arrows); C: Early gastric cancer with disorganized tissue architecture, few regular crypts (arrowhead) and very tortuous, dilated, irregular vessels (arrows).
Colon
Clinical trials have delineated potential applications for endomicroscopic examination of the colon. Following intravenous administration of fluorescein the capillaries of the normal colonic mucosa are clearly visualized in the deeper layers of the lamina propria as bright structures (Figure 3A) with moving dark shadows in the lumen representing the red blood cells which are not labeled by fluorescein (Figure 3B). This network of capillaries circumscribing the regular round mucosal glands results in a typical honeycomb pattern in CLE sequences[6,11]. In the proximal colon the vasculature appears more dense than in the distal colon, an aspect which correlates with the physiological profile of water absorption along the large intestine[25].
Figure 3.
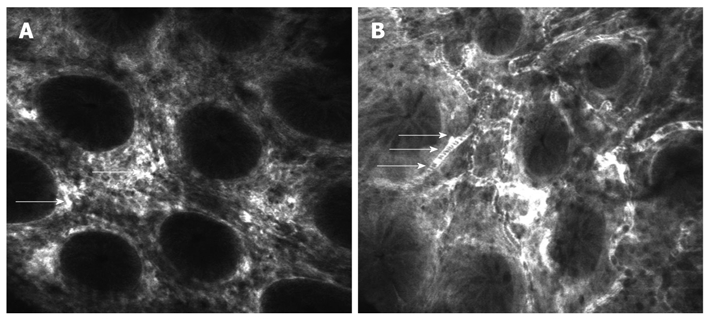
Confocal laser endomicroscopy of the normal colon using intravenous fluorescein. A: Normal aspect of colonic mucosa showing regular architecture of crypts and capillaries of lamina propria (arrows); B: Dark shadows in the lumen of the vessels representing the red blood cells (arrows).
Confocal patterns based on changes in vessel and crypt architecture have been defined to differentiate among neoplastic and non-neoplastic tissue[26,27]. In contrast to the regular vascular pattern of the normal mucosa, in colonic cancer the vessels are dilated, distorted with little or no orientation to the adjacent tissue. Increased permeability of the new tumoral vessels is proved by fluorescein passing from the lumen into the interstice which appears intensely bright (Figure 4A)[11,26]. Colon cancer, with 655 000 deaths worldwide per year, is the second leading cause of cancer-related death in the Western world[28]. In order to improve the prognosis, colorectal cancer should be detected in the early stages or even precursor stages, when it is possible to cure the selected cases by immediate endoscopic resection. Flat lesions are difficult to detect using standard endoscopic methods and conventional colonoscopy has a significant false-negative rate for intraepithelial neoplasia. CLE is able to predict intraepithelial neoplasia with high accuracy (99.1%) based on the morphological changes in vascularization mentioned above and the irregular cell architecture[27].
Figure 4.
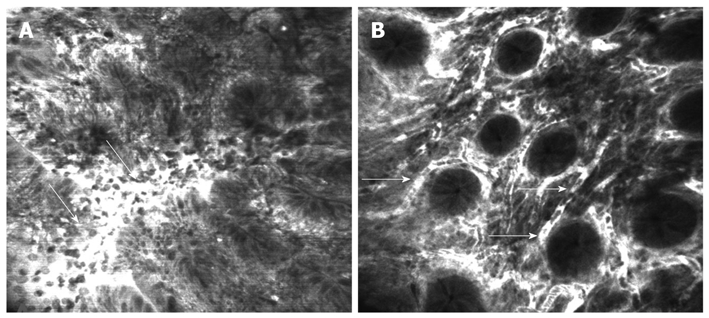
Confocal laser endomicroscopy of the colon using intravenous fluorescein. A: Colon carcinoma with total disorganization of cell architecture, invasion and destruction of the vessels with leakage of fluorescein (arrows); B: Severe inflammatory changes in ulcerative colitis with cellular infiltrate causing an increase in the distance between crypts and excessive vascularity (arrows).
Precursor neoplastic lesions are also detected by endomicroscopic examination, such as adenomatous polyps, the most important risk factor for colon cancer[29]. They can be diagnosed microscopically in situ with high accuracy, taking into account the altered vascular pattern and also the lack of epithelial surface maturation, crypt budding, and loss of cell polarity[30].
Patients with inflammatory bowel diseases (IBD), both ulcerative colitis (UC) and Crohn’s disease (CD) have a higher risk of developing colorectal cancer as a result of persistent inflammation of the colon. In long-standing ulcerative colitis, CLE is a feasible solution for detecting neoplastic changes in suspect areas. When preceded by a wide-field examination technique, such as chromoendoscopy, CLE has been shown to increase the diagnostic yield of intraepithelial neoplasia while reducing the number of biopsy samples[31,32]. Inflammatory changes are also depicted by endomicroscopic examination. Increased vasculature of the mucosa (Figure 4B) together with the chronic inflammatory infiltrate result in an enlarged distance between crypts, which show different shapes and sizes[11].
A recent published study aimed to evaluate the role of CLE in the assessment of inflammatory activity in UC[33]. On CLE images microvascular alterations and fluorescein leakage as well as crypt architecture were analyzed, and showed good correlation with the histological findings.
While the reported studies have considered mainly descriptive features of the vascular architecture for diagnosis purposes, quantitative measurements of the normal and tumoral vessels could be possible by translating results from preclinical research on small animal models to clinical trials. Laemmel et al[4] demonstrated in a murine model that is possible to make in vivo microvascular observation with a miniprobe confocal fluorescence microscope. They used fluorescein-isothiocyanate-labeled dextran (FITC-dextran) or FITC-albumin injected intra-arterially for clear visualization of the microvascular network. Confocal microscopy enabled observations and measurements usually provided by intravital microscopy: functional capillary density, capillary permeability, vasoconstriction and dilation effects in a minimally invasive procedure. Thus, confocal miscroscopy has shown potential in the field of microcirculation that should be explored for clinical translation.
IMMUNOENDOSCOPY
The association of endomicroscopic examination with tagged markers of inflammation and proliferation represents an evolutionary leap in GI endoscopy. Thus “immunoendoscopy” aims at the detection and characterization of lesions based on the molecular changes found in inflammation and neoplasia, with potential major impact on current diagnostic and therapeutic algorithms. The exogenous contrast agents for molecular imaging include fluorescently labeled antibodies and peptides which directly bind to their targets, and “smart” probes with tumor-specific activation[12].
Goetz et al[34] approached in vivo molecular imaging in a study of human inflammatory and neoplastic diseases in rodent models. They aimed to evaluate a newly developed, handheld confocal miniprobe for in vivo subsurface morphological, functional and molecular imaging. Octreotate was labeled with 5-carboxyfluorescein for targeted imaging of somatostatin receptors. After systemic application of developed tracer, neuroendocrine tumors and somatostatin-receptor-expressing pancreatic islet cells were visualized with excellent correlation with immunohistochemistry.
In a pilot in vivo human study, a phage library was screened to isolate a peptide that specifically bound colonic dysplasia. The identified heptapeptide sequence was then synthesized and conjugated with fluorescein for use in patients undergoing colonoscopy. After its topical administration, endomicroscopic examination visualized preferential binding of the labeled peptide to dysplastic colonocytes over normal mucosa with high sensitivity and specificity (81% and 82%, respectively), although the molecular target of the sequence was unknown[13].
With constant interest in understanding angiogenesis and recent advances in antiangiogenic therapies for oncologic patients came the need for imaging this complex process in vivo. Targeting angiogenic regulators has been already performed on animal models with promising results for future clinical trials.
One trial evaluated the feasibility of CLE for real-time molecular imaging of EGFR expression, an already established therapeutic target for colorectal cancer[14]. EGFR is overexpressed in many tumors, playing a central role in proliferation, angiogenesis, invasion, and metastasis[35,36]. In this trial it was possible to visualize and differentiate EGFR expression patterns in human xenograft tumors in mice with a handheld CLE probe after injection of fluorescently labeled antibodies. Additionally, topical application of fluorescently labeled antibodies provided adequate contrast, enabling distinction of neoplastic from non-neoplastic human colorectal tissue samples based on their EGFR expression[14]. The same team specifically targeted VEGF for imaging its expression in murine tumors, human xenografts and human tissue samples of colorectal cancer[15]. VEGF has been intensely studied in basic and clinical research and is already a therapeutic target in metastatic colorectal cancer[5]. CLE images showed a VEGF-specific signal which correlated well with immunohistochemistry. These studies provide evidence that “immunoendoscopy” is feasible, and results from basic research could be technically translated into clinical practice. In vivo molecular imaging could enable selection of patients who would benefit from targeted therapies and could monitor the response to treatment.
Chick embryo chorioallantoic membrane (CAM) is a very convenient experimental model for studying angiogenesis of grafted tumors[37]. The feasibility of gastric and colonic biopsy implantation, both normal and neoplastic, on the chick CAM for the study of angiogenesis was recently demonstrated[38]. Fragments of human gastric and colonic mucosa were obtained through endoscopic biopsy, immersed in saline and implanted within 60 min on the chick CAM. Next, the implanted tissue fragments were examined using the confocal laser microscope after previous intravascular administration of 10% fluorescein. Confocal microscopic examination managed to identify both the initial vascularization (Figure 5A) and the newly formed vessels of the grafted tissue (Figure 5B), including intravascular blood flow, representing a starting point for future immunoendoscopy studies in humans, with great therapeutic implications for angiogenic inhibitors.
Figure 5.
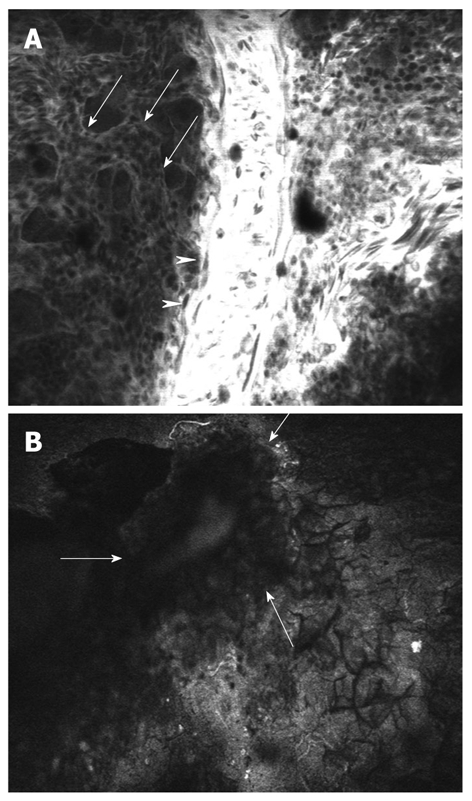
Confocal laser microscopy of the chick embryo chorioallantoic membrane. A: Chick normal chorioallantoic membrane with visualization of large vessels (arrowheads), medium vessels (arrows) and circulating nucleated erythrocytes; B: Fragment of viable human colon cancer tissue (arrows) implanted on chick embryo chorioallantoic membrane.
In conclusion, CLE has led GI endoscopy into a new era. CLE is certainly no longer considered just another endoscopic technique, but a crucial and revolutionary imaging method for real-time assessment of changes in the vascularization pattern of GI structures. Furthermore, in vivo molecular imaging, combining CLE with targeted staining, will have a significant impact on both basic research and clinical practice.
Footnotes
Supported by Research Grant, No. 239/2007, entitled OCTEUS, financed by the CNCSIS Romania and by the Sectoral Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/89/1.5/S/64109
Peer reviewer: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan
S- Editor Sun H L- Editor Cant MR E- Editor Ma WH
References
- 1.Deinert K, Kiesslich R, Vieth M, Neurath MF, Neuhaus H. In-vivo microvascular imaging of early squamous-cell cancer of the esophagus by confocal laser endomicroscopy. Endoscopy. 2007;39:366–368. doi: 10.1055/s-2007-966217. [DOI] [PubMed] [Google Scholar]
- 2.Kiesslich R, Neurath MF. Endomicroscopy is born--do we still need the pathologist? Gastrointest Endosc. 2007;66:150–153. doi: 10.1016/j.gie.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 4.Laemmel E, Genet M, Le Goualher G, Perchant A, Le Gargasson JF, Vicaut E. Fibered confocal fluorescence microscopy (Cell-viZio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy. J Vasc Res. 2004;41:400–411. doi: 10.1159/000081209. [DOI] [PubMed] [Google Scholar]
- 5.de Castro Junior G, Puglisi F, de Azambuja E, El Saghir NS, Awada A. Angiogenesis and cancer: A cross-talk between basic science and clinical trials (the “do ut des” paradigm) Crit Rev Oncol Hematol. 2006;59:40–50. doi: 10.1016/j.critrevonc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275–1283. doi: 10.1055/s-2006-944813. [DOI] [PubMed] [Google Scholar]
- 7.Kiesslich R, Goetz M, Neurath MF. Virtual histology. Best Pract Res Clin Gastroenterol. 2008;22:883–897. doi: 10.1016/j.bpg.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Meining A, Saur D, Bajbouj M, Becker V, Peltier E, Höfler H, von Weyhern CH, Schmid RM, Prinz C. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis. Clin Gastroenterol Hepatol. 2007;5:1261–1267. doi: 10.1016/j.cgh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology. 2009;136:1509–1513. doi: 10.1053/j.gastro.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 10.De Palma GD. Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009;15:5770–5775. doi: 10.3748/wjg.15.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiesslich R, Galle PR, Neurath MF. 2007. Atlas of Endomicroscopy. Springer Medizin Verlag Heidelberg; pp. 55–79. [Google Scholar]
- 12.Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology. 2010;138:828–833.e1. doi: 10.1053/j.gastro.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454–458. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz M, Ziebart A, Foersch S, Vieth M, Waldner MJ, Delaney P, Galle PR, Neurath MF, Kiesslich R. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology. 2010;138:435–446. doi: 10.1053/j.gastro.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Foersch S, Kiesslich R, Waldner MJ, Delaney P, Galle PR, Neurath MF, Goetz M. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut. 2010;59:1046–1055. doi: 10.1136/gut.2009.202986. [DOI] [PubMed] [Google Scholar]
- 16.Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Becker V, Vieth M, Bajbouj M, Schmid RM, Meining A. Confocal laser scanning fluorescence microscopy for in vivo determination of microvessel density in Barrett's esophagus. Endoscopy. 2008;40:888–891. doi: 10.1055/s-2008-1077718. [DOI] [PubMed] [Google Scholar]
- 18.Li WB, Zuo XL, Zuo F, Gu XM, Yu T, Zhao YA, Zhang TG, Zhang JP, Li YQ. Characterization and identification of gastric hyperplastic polyps and adenomas by confocal laser endomicroscopy. Surg Endosc. 2010;24:517–524. doi: 10.1007/s00464-009-0608-y. [DOI] [PubMed] [Google Scholar]
- 19.Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, Liu FG, Xie XJ, Zhu Q, Zhao YA. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy. 2008;40:547–553. doi: 10.1055/s-2007-995633. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JN, Li YQ, Zhao YA, Yu T, Zhang JP, Guo YT, Liu H. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc. 2008;67:843–853. doi: 10.1016/j.gie.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, Iguchi Y, Shimoyama Y, Nagasaka T, Maeda O, Ando T, et al. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. 2006;38:1110–1114. doi: 10.1055/s-2006-944855. [DOI] [PubMed] [Google Scholar]
- 22.Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y, et al. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy. 2006;38:886–890. doi: 10.1055/s-2006-944735. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, Zhang JN, Guo YT, Xie XJ, Zhang TG, Desmond PV. Confocal endomicroscopy for in vivo detection of microvascular architecture in normal and malignant lesions of upper gastrointestinal tract. J Gastroenterol Hepatol. 2008;23:56–61. doi: 10.1111/j.1440-1746.2007.05221.x. [DOI] [PubMed] [Google Scholar]
- 24.Meining A, Bajbouj M, Schmid RM. Confocal fluorescence microscopy for detection of gastric angiodysplasia. Endoscopy. 2007;39 Suppl 1:E145. doi: 10.1055/s-2007-966109. [DOI] [PubMed] [Google Scholar]
- 25.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686–695. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 27.Hurlstone DP, Baraza W, Brown S, Thomson M, Tiffin N, Cross SS. In vivo real-time confocal laser scanning endomicroscopic colonoscopy for the detection and characterization of colorectal neoplasia. Br J Surg. 2008;95:636–645. doi: 10.1002/bjs.5988. [DOI] [PubMed] [Google Scholar]
- 28.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 29.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847–1857. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Sanduleanu S, Driessen A, Gomez-Garcia E, Hameeteman W, de Bruïne A, Masclee A. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2010;8:371–378. doi: 10.1016/j.cgh.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874–882. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 32.Hurlstone DP, Kiesslich R, Thomson M, Atkinson R, Cross SS. Confocal chromoscopic endomicroscopy is superior to chromoscopy alone for the detection and characterisation of intraepithelial neoplasia in chronic ulcerative colitis. Gut. 2008;57:196–204. doi: 10.1136/gut.2007.131359. [DOI] [PubMed] [Google Scholar]
- 33.Li CQ, Xie XJ, Yu T, Gu XM, Zuo XL, Zhou CJ, Huang WQ, Chen H, Li YQ. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol. 2010;105:1391–1396. doi: 10.1038/ajg.2009.664. [DOI] [PubMed] [Google Scholar]
- 34.Goetz M, Fottner C, Schirrmacher E, Delaney P, Gregor S, Schneider C, Strand D, Kanzler S, Memadathil B, Weyand E, et al. In-vivo confocal real-time mini-microscopy in animal models of human inflammatory and neoplastic diseases. Endoscopy. 2007;39:350–356. doi: 10.1055/s-2007-966262. [DOI] [PubMed] [Google Scholar]
- 35.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Dutta PR, Maity A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 2007;254:165–177. doi: 10.1016/j.canlet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deryugina EI, Quigley JP. Chapter 2. Chick embryo chorioallantoic membrane models to quantify angiogenesis induced by inflammatory and tumor cells or purified effector molecules. Methods Enzymol. 2008;444:21–41. doi: 10.1016/S0076-6879(08)02802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gheonea DI, Săftoiu A, Cârţână T, Mândrilă I, Iordache S, Filip M, Ciurea T. Imaging angiogenesis by confocal laser microscopy on digestive tumors grafted on the chick chorioallantoic membrane. Gut. 2010;59:A241. [Google Scholar]