Abstract
Background
Several risk factors are associated with the incidence of human stillbirths. The prevention of stillbirths in women is a pressing clinical problem.
Methods
We reviewed 402 pathology records of fetal loss occurring in a large baboon (Papio spp.) colony during a 15-year period. Clinical histories of 565 female baboons with one or more fetal losses during a 20-year period were analyzed for weight, age, and reproductive history.
Results
Fetal loss was most common at term (35.57%) and preterm (28.61%) and less common in the first half of gestation (11.20%) and post-term (5.22%). Greater maternal weight, older age, history of stillbirth and higher parity were independent predictors for stillbirth. An exponential increase in the incidence of fetal loss was observed beginning at age 14 years in baboons.
Conclusion
Fetal loss and maternal risk factors associated with stillbirths in baboons were similar to those documented in women.
Keywords: fetal loss, reproduction, animal model, epidemiology, non-human primates
Introduction
Stillbirth in the women is defined as intrauterine fetal death occurring greater than 20 weeks of gestation and represents the majority of perinatal death. In the United States stillbirth occurs in about one in 200 of all births (or 6.4 per 1000 of live births). Approximately 50% of fetuses die of unknown causes. Several risk factors associated with the incidence of human stillbirths include maternal weight, age, race and parity [20]. The prevention of stillbirths is a pressing clinical problem, as evident by the on-going NIH-sponsored Stillbirth Collaborative Research Network [56].
The baboon (Papio hamadryas) is one of many nonhuman primates species used in biomedical research and is a well established model for studying reproductive function [26]. Stillbirths in this species has been reported to occur in 5.9 to 20% of pregnancies and varies depend on facility, housing condition, habitat [3, 5] with influence of seasonality and associated hormonal changes on fetal loss have been investigated intensively in this species [3, 19], the incidence and causes of fetal loss in a large baboon population has not been thoroughly studied [61].
The aims of this study were to evaluate the epidemiology and pathology associated with fetal loss and to analyze maternal risk factors for stillbirth in the baboon model.
Materials and Methods
Animal husbandry
The main breeding colony at the Southwest National Primate Research Center (SNPRC) consists of approximately 3800 baboons housed in either corrals or metal and concrete gang cages that usually contain between 16 and 20 animals. Two study sets were performed respectively: 1) pathology records of all fetal losses that occurred during the 15-year period from 1988 through 2002 (n=402) were analyzed for gross pathology and microscopic findings, main and secondary diagnoses and fetal sex), 2) female baboons with a history of stillbirth, recorded in the SNPRC animal database, were evaluated (n=565) for maternal age, parity, number of stillbirths, and weight. Maternal age was recorded at the time of stillbirth. Maternal weight was recorded at the non-pregnant stage during maximum one year prior to the pregnancy that ended as stillbirth and then calculated as average weight over this time period. Complete data sets (maternal age, weight, and parity) were available in 261 cases. All animal procedures were approved by the SNPRC Institutional Animal Care and Use Committee.
Calculation of gestational age and recording of fetal loss
Clinical and reproductive histories were retrieved from the computerized animal records database (Computerized Animal Management Program, CAMP). When the menstrual cycle records were available, pregnancies were scored as followed: the cessation of sexual cycling (>40 days) without evidence of menstruation (i.e., vaginal bleeding followed by sexual swelling within 1 week) and presence of pregnant color (pink) [27]. The estimated day of conception (first day of pregnancy) was then counted as the day that a female's sexual swelling began to reduce minus two days, as this is common practice for gestational age estimation for all pregnancies in this facility [27]. Sexual swelling has proven to correlate greatly with oocyte development and ovulation in the baboons [60].
Fetal loss was established in early gestation (0-89 days gestational age [dGA]) by a history of females who previously showed pregnant color and signs of vaginal bleeding after that or absence of fetus upon ultrasound examination. In addition to these criteria, at gestational age 90 dGA and above fetal loss was determined either by presence of a fetus (or its remnants) or placental tissue.
Pathological evaluation and pathological diagnosis
Gross examination of fetuses/placentas was generally performed within 12-18 hours of delivery. In several cases, the time between birth and necropsy was difficult to estimate because of logistic variations in time of the delivery. The primary criteria for intracranial trauma were hemorrhage in the brain and meninges, luxation and fracture of the bones of the skull and distortion of the face and head. Diagnosis of stillbirth was confirmed by negative floating test and microscopic lung evaluation. In all 402 cases of available pathology records histological evaluation was performed.
Statistical analysis
Baboons with fetal loss were divided into two major groups based on the definition of stillbirth in the human population [14, 56]. These two groups were: early pregnancy loss (first half of gestation, 0-89 dGA) and stillbirths (second half of gestation, 90dGA and above). The stillbirth group was comprised of three subgroups: 1) preterm stillbirths (90-163 dGA), 2) stillbirths at term (164-185 dGA), and 3) post-term stillbirths (186 dGA and above). These stillbirth subgroups were formed based on the published data for full-term pregnancy in the baboons at 175±11days of gestation [3, 39]. We used a Poisson regression for prediction of stillbirth number, adjusted by maternal age, weight, and parity. Poisson regression models are generalized linear models with a natural logarithm as the (canonical) link function. The Poisson distribution for the dependent variable is limited to positive values, and has a variance equal to its mean. Thus, in populations in which events are rare, the distribution is highly skewed to the right; as the mean of events rises, the distribution is increasingly normal. Fetal weight comparison was performed using one-way ANOVA. Values were considered to be statistically significant with p<0.05.
Results
The number of births in SPNRC colony ranged from 379 to 689 per year, with a total of 8,336 for the 15-year period. Recorded fetal loss during this same period ranged from 15-49 per year, with a total of 402 losses (4.82 % of all births). We established accurate gestational ages in 323 (80.34%) cases: 45 (11.20%) cases of fetal loss were in the first half of gestation, 115 (28.61%) were preterm, 143 (35.57%) were at term, and 21 (5.22%) were post-term (Table 1). The placenta was available for examination in 35 (8.71%) cases and 351 (87.31%) fetuses were necropsied. The etiology of stillbirth was undetermined in 93.5% of early fetal loss, 65.79% of preterm stillbirths, and 57.14% of post-term stillbirths. In pregnancies at term, the most common cause of fetal death was intracranial trauma or intracranial and intraabdomial hemorrhages (52.45%). Stillbirths of undetermined etiology comprised 25.87% of all diagnoses in this group. Table 2 lists the distribution of cause of death across different gestational ages.
Table 1. Number of the stillbirths and distribution of main pathological diagnosis.
| Gestational age | Total number of stillbirths/abortions registered | No. evaluated | Main pathological diagnosisa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fetuses | Placentas | Relative disproportion of fetal size and maternal pelvis | Undetermined etiology | Placental and umbilical cord pathology | Intrapartum trauma or intracranial heamorrhage | Pathology of fetusb | Maternal conditions | ||
| 0-89 | 45 (11.2%) | 12 (3.0%) | 2 (0.5%) | NDa | 42 (93.3%) | 2 (4.4%) | 1 (2.2%) | NA | NA |
| 90-163 | 114 (28.4%) | 103 (25.6%) | 13 (3.2%) | ND | 75 (65.8%) | 7 (6.1%) | 22 (19.3%) | 7 (6.1%) | 4 (3.5%) |
| 164-185 | 143 (35.5%) | 143 (35.2%) | 10 (2.5%) | 7 (4.9%) | 37 (25.9%) | 6 (4.2%) | 75 (52.4%) | 11 (7.7%) | 7 (4.9%) |
| 186 and more | 21 (5.2%) | 21 (5.2%) | 2 (0.5) | ND | 12 (57.1%) | 1 (4.8%) | 6 (28.6%) | 2 (9.5%) | NA |
| Unknown gestational age | 79 (19.7%) | 73 (18.2) | 8 (2.0%) | ND | 52 (65.8%) | 1 (1.3%) | 25 (31.6%) | ND | 1 (1.3%) |
| Sum | 402 (100%) | 351 (87.3%) | 35 (8.7%) | 219 (54.3%) | 17 (4.2%) | 128 (31.8%) | 20 (5.0%) | 12 (3.0%) | |
Percentage was calculated from total incidence of stillbirths at particular gestational age.
Pathology of fetus includes fetal infection, fetal malformation, hydrops, and growth retardation.
ND, not done.
Table 2. Distribution of main pathological diagnoses among the groups of different gestational ages (dGA – days of gestation).
| dGA | |||||
|---|---|---|---|---|---|
| 0-89 | 90-163 | 164-185 | 186 and over | Unknown gestational age | |
| Trauma and hemorrhages | 1 (2.2%) | 22 (19.3%) | 75 (52.4%) | 6 (28.6%) | 20 (25.3%) |
| Isolated intracranial trauma or intracranial hemorrhage | 1 (2.2%) | 12 (10.5%) | 33 (23.1%) | 2 (9.5%) | 10 (12.7%) |
| Liver trauma | 0 | 1 (0.9%) | 1 (0.7%) | 0 | 1 (1.3%) |
| Isolated intraabdominal hemorrhage | 0 | 0 | 12 (8.4%) | 0 | 3 (3.8%) |
| Combined intracranial trauma and intraabdominal hemorrhage | 3 (2.6%) | 22 (15.4%) | 3 (14.3%) | 5 (6.3%) | |
| Chest cavity trauma | 0 | 0 | 2 (1.4%) | 0 | 0 |
| Diagnosed dystocia | 0 | 0 | 19 (13.3%) | 0 | 3 (3.8%) |
| Breech presentation | 0 | 0 | 1 (0.7%) | 1 (4.8%) | 1 (1.3%) |
| Face presentation | 0 | 0 | 4 (2.8%) | 0 | 1 (1.3%) |
| Macrosomia and intrauterine growth retardation (IUGR). | 0 | 2 IUGR (1.80%) | 4 IUGR 3 fetuses with macrosomia (4.90%) | 1 IUGR (4.80%) | 1 IUGR (1.30%) |
| Placental pathology | 2 (4.4%) | 7 (6.1%) | 8 (5.6%) | 0 | 1 (1.3%) |
| Placentitis | 2 (4.4%) | 5 (4.3%) | 3 (2.1%) | 0 | 1 (1.3%) |
| Abruptio | 0 | 1 (0.9%) | 3 (2.1%) | 0 | 1 (1.3%) |
| Maternal pathology | 4 (8.8%) | 4 (3.5%) | 7 (4.9%) | 0 | 1 (1.3%) |
| Shock | 0 | 0 | 2 (1.4%) | 0 | 0 |
| Diabetes | 1 (2.2%) | 1 (0.9%) | 4 (2.8%) | 0 | 0 |
| Uterine rupture | 0 | 0 | 3 (2.1%) | 0 | 0 |
| Fetal pathology | 2 (4.4%) | 7 (6.1%) | 11 (7.7%) | 0 | 0 |
| Pneumonia | 0 | 2 (1.8%) | 4 (2.8%) | 0 | 0 |
| Malformation | 0 | 0 | 2 (1.4%) | 0 | 0 |
| Hydrops and hydrocephalus | 2 (4.4%) | 0 | 2 (1.4%) | 0 | 0 |
| Twins | 0 | 1 (0.9%) | 0 | 0 | 0 |
| Severe growth retardation | 0 | 0 | 0 | 0 | 0 |
| Other fetal infection | 0 | 2 (1.8%) | 1 (0.7%) | 0 | 0 |
| Total number of fetal loss in the subgroups | 45 | 114 | 143 | 21 | 79 |
In the group of stillbirths at term intracranial trauma and intracranial hemorrhage (n=33; 23.08%) were registered more often compared to abdominal hemorrhage (n=12; 8.39%), combined intracranial / abdominal hemorrhage (n=22; 15.38 %), liver trauma (n=1; 0.70%), and whole body trauma (n=1; 0.70%). Birth weight in the intracranial trauma and hemorrhages subgroup was not different than in other subgroups of pathological diagnoses (maternal, placental and fetal pathology) and the population of live-born fetuses, but it was higher than birth weight in cases of stillbirth of undetermined etiology (Fig. 1). Fetal infection with unknown etiology was diagnosed in 2 fetuses in this group, 2 fetuses had malformations, 4 were small for their gestational age (one had hydrocephalus), 2 had lung pathology (atelectasis and hyalinosis) and one fetus had confirmed encephalomyocarditis virus infection (EMCV). Five cases (6.41%) of infections in the stillborn term fetuses and placentas were of unknown etiologies. Maternal pathology included maternal diabetes, hemorrhagic shock, uterine rupture, and histoplasmosis. Three cases of placental abruption, three cases of placentitis, one case of massive placental necrosis associated with hydrops fetalis, and one case of placenta previa were diagnosed. One animal had intrauterine death of twins of undetermined cause. The functional discrepancies between fetal and maternal pelvic size involved face presentation (4 cases), breech presentation (1 case), and transverse position of fetus (1 case).
Figure 1.
Fetal weight distribution in control population of baboons (vaginal deliveries (CTR, n=6)), and stillbirths at term (due to intracranial trauma/hemorrhages (Intracr T/H, n=75), maternal, fetal and placental pathology (MFP, n=26) and stillbirths of undetermined etiology (UN, n= 37). “a” is statistically significant different from “b”. Data presented as mean ± SEM.
In the group of post-term stillbirths 1 fetus was small for its gestational age, 1 had congenital heart failure, and a true umbilical cord knot was diagnosed in 1 case.
In preterm stillbirths there were 7 cases of placental pathology: 5 were cases of placentitis (one positive culture for two microorganisms: Klebsiella pneumonia and Aeromonas hydrophila), one case with probable placenta abrupta was identified and one case with placental infarction and hemorrhage. In diagnosed cases of fetal pathology, 2 were small for the gestational age, and 3 had signs of infection (pneumonia and EMCV). The total number of cases with intrauterine infection was 8 (6.8% of all fetuses examined at this gestational age). When the cause of death could be determined, maternal conditions that led to stillbirth were maternal diabetes with ketoacidosis and maternal trauma.
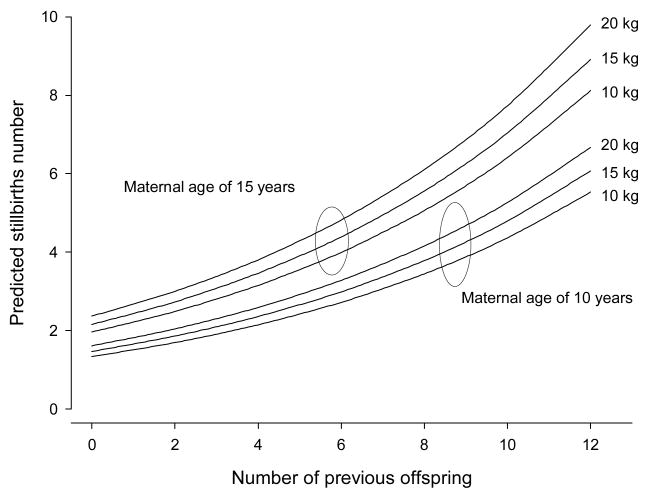
Of the 565 animals recorded with a history of pregnancy loss during all years of observation at SNPRC, data analysis revealed that 45 (7.96%) animals had no previous offspring; 159 (28.1%) had 1 to 4; 321 (56.8%) had 5 to 10; and 40 (7.1%) had more than 11 offspring. Of those animals with a history of pregnancy loss 21.2% had one stillbirth/abortion in the clinical history, 14.2% - two; 12.2% - three, and 44.3%- 4 or more stillbirths/abortions. The average age of the baboons at the time of stillbirth/abortion was 11.2±4.5 years, and weight was 17.3±3.0 kg (data presented as mean±SEM). Each factor studied (maternal age, weight and parity) showed a significant and independent prediction for the incidence of fetal loss (Table 3, Fig. 2). Exponential increase in the incidence of fetal loss was observed beginning at age 14 in this group (Fig. 3).
Table 3.
Independent variables that explain the incidence of stillbirths in captive baboons.
| Variable | Coefficient (SE)a | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Number of offspring | 0.118 (0.012) | <0.001 | 0.094, 0.142 |
| Maternal weight (kg) | 0.019 (0.010) | 0.051 | -0.001, 0.038 |
| Age (years) | 0.077 (0.008) | <0.001 | 0.061, 0.092 |
| Constant | -0.665 (0.189) | <0.001 | -1.036, -0.294 |
Coefficients were calculated for a general lineal model using Poisson process (n=565).
Figure 2.
Predicted fetal loss number using a Poisson process adjusted by the total offspring number. The graphic estimates numbers of stillbiths/abortions for a 10- and 15-year-old baboon weighing 10, 15, or 20 kg. As weight increases, the number of negative pregnancy outcome increases (n=565).
Figure 3.
Poisson process adjusted by maternal age, weight, and the number of offspring. Around 14 years of age the predicted number of stillbirths/abortions increases exponentially (n=261).
Discussion
Incidence of fetal loss in nonhuman primates and humans
The incidence of fetal loss in the SNPRC baboon colony remains within the range published by Hendrickx in 1966 [28] for the same facility (2.4-11.2%). The stillbirth rate in our study (35/1,000 live births) is higher than has been reported for human population (6.4/1000 live births). This difference is primarily due to pregnancy loss caused by intracranial trauma/hemorrhages (56.7%) that were much more frequent than in the human population (0-1%). Our data concerning distribution of fetal loss among a captive baboon population contrast with those of Packer et al. [45] who reported an equal distribution of stillbirths across the trimesters in a population of wild baboons. These differences may be explained by the data arrangement in the two studies. We did not use a trimester approach in our study as did Packer et al. [45]; rather, we divided the groups by gestational age according to the definition of stillbirth. The observation that peak pregnancy loss is approximately at 175 days (term stillbirths) in our study agrees with the data published for wild baboons [3].
The higher percentage of intracranial trauma and hemorrhages in nonhuman primates could be explained by differences in the birth mechanism in human and non-human primates, e.g., less degree of head rotation, possible natural delivery with face presentation, and absence of social contact at birth (assistance at birth) in baboons [50].
Infection as a cause of pregnancy loss in baboons and humans
Combined incidences of intrauterine infection as indicated by placentitis and fetal infection were very similar in the term and preterm stillbirth (6.8%) groups in this study. The incidence of infections as a cause of stillbirths in this baboon population was lower than those published for human populations (10-25%) [9, 23, 43]. This observation is remarkable, since most of the infectious agents causing stillbirths in humans [23] are also found in the baboon population with few exceptions (Table 4): Chlamydia trachomatis, varicella zoster virus, parvovirus (B19), Western equine encephalitis and Brucella spp. For example, Ureaplasma urealyticum and Mycoplasma hominis, which cause intrauterine infection in women [49], were detected in baboons in previous studies [10]. However, their role in spontaneous intrauterine infection in baboons is unknown. Only Ureaplasma urealyticum has been experimentally evaluated to determine its effects on fetal baboons [62]. Klebsiella spp. were reported to be natural infectious agents in baboons, but our study is the first to associate this agent with placentitis in Papio sp. Herpesvirus papio 2 (HVP2) and cytomegalovirus (CMV) infections among baboons are high (almost the same prevalence as in human population), with 95% seropositivity in the colony [6, 17]. Both herpes virus and CMV have been reported to be associated with pregnancy loss in human studies [12, 48].
Table 4.
Comparative incidences of infections in baboon and human populations associated with fetal loss in human population (selected publications).
| Infectious agent | Human population | References | Baboon population | References |
|---|---|---|---|---|
| Spirochetes | ||||
| Treponema pallidum | 2.4 cases per 1,000,000 population | 11 | Naturally occurring cases. 65-76% seropositivity in some areas of Africa | 21 |
| Protozoa | ||||
| Toxoplasma gondii | 15-80% of women of childbearing age | 36 | 32% of population | 41 |
| Trypanosoma cruzi | 0.6% of population of pregnant women in some regions in United States | 15 | 9.4-22.5% of whole population | 2 |
| Plasmodium falciparum | 25.3-30.5% slides positive in endemic areas | 57 | Not reported | N/A |
| Viruses | ||||
| Rubella | 0.13:100,000 per year among adult population | 47 | Experimental infection | 28 |
| Cytomegalovirus | antibodies present in 40-100% of adult population | 14 | antibodies present in more than 95% of population | 6 |
| Herpes simplex 2 | 40% of the population of young women | 8 | 93.5% of population seropositive for Herpesvirus papio 2 (HVP2) | 17 |
| Varicella zoster virus | 0.05% of pregnant women | 7 | Not reported | N/A |
| Lymphocytic choriomeningitis | 0.3-5.4% seropositivity among adult population | 46 | 3 of 22 captive baboons were seropositive | 38 |
| Parvovirus (B19) | 1-5% of pregnant women | 18 | Not reported | N/A |
| Bacterial and mycoplasmal infections | ||||
| Chlamydia trachomatis | Present in 25.4% of vaginal cultures of the women with preterm deliveries | 10 | Experimental infection | 35 |
| Klebsiella pneumoniae | Present in 41.7% of vaginal cultures of women with preterm deliveries | 10 | Bacteria were isolated from oral swabs of captive baboons | 37 |
| Ureaplasma urealyticium | Present in cervix or vagina of 40-80% of sexually mature asymptomatic women | 58 | 10% of baboon population | 62 |
| Escherichia coli | Present in 30.8% of vaginal cultures of women preterm deliveries | 10 | Pathogenic and atypic E. coli was isolated in 20 from 208 captive baboons (9.6%) | 37 |
| Mycoplasma hominis | Present in cervix or vagina of 21-53% of sexually mature asymptomatic women | 58 | Detected by PCR in 29% of baboon population | 55 |
The discrepancies in distribution of intrauterine infections in baboon and human pregnancy loss could be explained by absence of placental material for evaluation at necropsy for fetal loss occurring in the first half of gestation and a restricted number of placentas available for evaluation in the second half of gestation in our study baboons. In the population of wild baboons Beehner et al. [3] observed, most fetal loss was associated with fetal, and not placental, abnormalities as indicated by a decreasing estradiol, and not progesterone, level prior to stillbirths. We could not rule out that a natural placental barrier to infection is present in nonhuman primates. The relatively high incidence (4 cases) of pregnancy loss associated with ECMV in our study could be explained by an outbreak of this viral infection at SNPRC during the period of evaluation [32].
Maternal age, weight, and parity
The analysis of this baboon population found that an increased risk of a negative pregnancy outcome was associated with a higher number of offspring. Moreover, our observation that a history of stillbirths is correlated with a greater risk of a subsequent stillbirth parallels epidemiological studies in the human population [53].
The World Health Organization (WHO) has declared obesity to be one of the top 10 adverse health risk conditions in the world and one of the top 5 in developed nations (www.who.int/nut/obs.htm.). Between 20 and 34% of women of reproductive age are obese [9]. Maternal obesity is associated with a 5-fold increase in risk of stillbirth with placental dysfunction, an increased risk of pregnancy and delivery complications (gestational diabetes, pre-eclampsia, macrosomia, shoulder dystocia, higher rates of cesarean sections, and infections), neural tube defects, and fetal mortality in the human population [44]. In our study, increased maternal weight was associated with an increased incidence of fetal loss. Baboons spontaneously develop obesity and diabetes, and they have been used extensively as models of these conditions [13]. However, there are no previous reports of diabetes during gestation or development of gestational diabetes in this species. Our records demonstrate the presence of two documented cases (one with maternal ketoacidosis) of diabetes as the cause of fetal death. In the human population, the prevalence of gestational diabetes is 5-10% [51]. The low percentage of diabetes in our study baboons may be due, in part, to lack of maternal evaluation during pregnancy.
Mean perimenopausal age (based on menstrual cycle regularity) is 18.89 years of age in this baboon colony [40]. In our study, an increase in fetal loss was seen beginning at 14 years of age. In the observation of baboons in the wild, other authors have observed either a decrease in reproduction, and a higher rate of miscarriages beginning at 21 years of age [45], or no increase in the rate of fetal loss with age in this species [59]. Our data are consistent with data from the human population, where onset of decreased fecundity takes place before perimenopausal changes associated with irregularities of the menstrual cycle occur [24]. In humans, the association of advanced maternal age with an increased rate of chromosomal aneuploidy has been recognized for decades [25, 34]. Ten of 34 fertilized human ova recovered during the first 17 days of development were found to be abnormal [29, 30]. A high rate of chromosomal abnormalities has been found in oocytes (see reviews by Jacobs [34] and Hunt and Hassold [33]), in pre-implantation embryos [1, 4], and in embryonic/fetal or extra-embryonic tissues after spontaneous abortion [22]. The analysis of meiosis or abnormal live births in nonhuman primates has been sparse. The only study of aneuploidy in nonhuman primate oocytes involved five younger and seven older animals in which 4 out of 30 oocytes were aneuploid [54]. There are 15 cases of autosomal trisomies reported in nonhuman primates [16, 31, 42].
Conclusion
The data presented here have shown that the causes of fetal loss are similar in human and baboon populations. The incidence of fetal infection is lower and intracranial trauma/hemorrhages higher compared to those observed in humans. We present for the first time a documented case of Klebsiella infection associated with placentitis and two cases of maternal diabetes during pregnancy in the baboons. As seen in women, age, parity, and history of fetal loss are independent predictors of fetal loss in baboons, suggesting similar pathological mechanisms behind this phenomenon.
Acknowledgments
We would like to acknowledge the work of veterinarian and technical personal of SNPRC on animals care and records keeping. We appreciate the critical reading of the manuscript and help of Drs. Karen Rice and Michelle Leland. We further thank Mr. Jeremiah J. Gomez, M.S. and Ms. Catherine L. Weaver, B.S. for their work with data acquisition.
Financial Support: This research was supported in part by NIH-NCRR grant P51 RR013986 to the Southwest National Primate Research Center and conducted in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR014578 and C06 RR015456.
References
- 1.Angell RR, Templeton AA, Aitken RJ. Chromosome studies in human in vitro fertilization. Hum Genet. 1986;72:333–339. doi: 10.1007/BF00290960. [DOI] [PubMed] [Google Scholar]
- 2.Arganaraz ER, Hubbard GB, Ramos LA, Ford AL, Nitz N, Leland MM, VandeBerg JL, Teixeira AR. Blood-sucking lice may disseminate Trypanosoma cruzi infection in baboons. Rev Inst Med Trop Sao Paulo. 2001;43:271–276. doi: 10.1590/s0036-46652001000500007. [DOI] [PubMed] [Google Scholar]
- 3.Beehner JC, Nguyen N, Wango EO, Alberts SC, Altmann J. The endocrinology of pregnancy and fetal loss in wild baboons. Horm Behav. 2006;49:688–699. doi: 10.1016/j.yhbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Birrell AM, Hennessy A, Gillin A, Horvath J, Tiller D. Reproductive and neonatal outcomes in captive bred baboons (Papio hamadryas) J Med Primatol. 1996;25:287–293. doi: 10.1111/j.1600-0684.1996.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 5.Blewett EL, White G, Saliki JT, Eberle R. Isolation and characterization of an endogenous cytomegalovirus (BaCMV) from baboons. Arch Virol. 2001;146:1723–1738. doi: 10.1007/s007050170059. [DOI] [PubMed] [Google Scholar]
- 6.Bielanska M, Tan SL, Ao A. Chromosomal mosaicism throughout human preimplantation development in vitro: incidence, type, and relevance to embryo outcome. Hum Reprod. 2002;17:413–419. doi: 10.1093/humrep/17.2.413. [DOI] [PubMed] [Google Scholar]
- 7.Brunell PA. Varicella in pregnancy, the fetus, and the newborn: problems in management. J Infect Dis. 1992;166:S42–S47. doi: 10.1093/infdis/166.supplement_1.s42. [DOI] [PubMed] [Google Scholar]
- 8.Buchacz K, McFarland W, Hernandez M, Klausner JD, Page-Shafer K, Padian N, Molitor F, Ruiz JD, Bolan G, Morrow S, Katz MH. Prevalence and correlates of herpes simplex virus type 2 infection in a population-based survey of young women in low-income neighborhoods of Northern California. The Young Women's Survey Team. Sex Transm Dis. 2000;27:393–400. doi: 10.1097/00007435-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56–59. doi: 10.5694/j.1326-5377.2006.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 10.Carey JC, Klebanoff MA. Is a change in the vaginal flora associated with an increased risk of preterm birth? Am J Obstet Gynecol. 2005;192:1341–1346. doi: 10.1016/j.ajog.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Primary and secondary syphilis–United States, 2002. MMWR Morbid Mortal Weekly Report. 2003;52:1117–1120. [PubMed] [Google Scholar]
- 12.Chow SS, Craig ME, Jacques CF, Hall B, Catteau J, Munro SC, Scott GM, Camaris C, McIver CJ, Rawlinson WD. Correlates of placental infection with cytomegalovirus, parvovirus B19 or human herpes virus 7. J Med Virol. 2006;78:747–756. doi: 10.1002/jmv.20618. [DOI] [PubMed] [Google Scholar]
- 13.Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- 14.Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1991;13:315–329. doi: 10.1093/clinids/13.2.315. [DOI] [PubMed] [Google Scholar]
- 15.Di Pentima MC, Hwang LY, Skeeter CM, Edwards MS. Prevalence of antibody to Trypanosoma cruzi in pregnant Hispanic women in Houston. Clin Infect Dis. 1999;28:1281–1285. doi: 10.1086/514790. [DOI] [PubMed] [Google Scholar]
- 16.Dudley CJ, Hubbard GB, Moore CM, Dunn BG, Raveendran M, Rogers J, Nathanielsz PW, McCarrey JR, Schlabritz-Loutsevitch NE. A male baboon (Papio hamadryas) with a mosaic 43,XXy/42,XY karyotype. Am J Med Genet A. 2006;140A:94–97. doi: 10.1002/ajmg.a.31014. [DOI] [PubMed] [Google Scholar]
- 17.Eberle R, Black DH, Lehenbauer TW, White GL. Shedding and transmission of baboon Herpesvirus papio 2 (HVP2) in a breeding colony. Lab Anim Sci. 1998;48:23–28. [PubMed] [Google Scholar]
- 18.Ergaz Z, Ornoy A. Parvovirus B19 in pregnancy. Reprod Toxicol. 2006;21:421–435. doi: 10.1016/j.reprotox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Fortman JD, Herring JM, Miller JB, Hess DL, Verhage HG, Fazleabas AT. Chorionic gonadotropin, estradiol, and progesterone levels in baboons (Papio anubis) during early pregnancy and spontaneous abortion. Biol Reprod. 1993;49:737–742. doi: 10.1095/biolreprod49.4.737. [DOI] [PubMed] [Google Scholar]
- 20.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 21.Fribourg-Blanc A, Mollaret HH. Natural treponematosis of the African primate. Primates Med. 1969;3:113–121. [PubMed] [Google Scholar]
- 22.Fritz B, Aslan M, Kalscheuer V, Ramsing M, Saar K, Fuchs B, Rehder H. Low incidence of UPD in spontaneous abortions beyond the 5th gestational week. Eur J Hum Genet. 2001;9:910–916. doi: 10.1038/sj.ejhg.5200741. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 24.Gougeon A. The biological aspects of risks of infertility due to age: the female side. Rev Epidemiol Sante Publique. 2005;53:2S37–2S45. [PubMed] [Google Scholar]
- 25.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 26.Hendrickx AG. Proceedings of the Conference of Non-Human Primate Toxicology; Warrenton, Virginia. June 12-14, 1966; p. 120. [Google Scholar]
- 27.Hendrickx AG, Kraemer DC. Observations on the menstrual cycle, optimal mating time and pre-implantation embroyos of the baboon, Papio anubis and Papio cynocephalus. J Reprod Fertil. 1969;(Supplement):119–128. [Google Scholar]
- 28.Hendrickx AG, Peterson PE. Perspectives on the use of the baboon in embryology and teratology research. Hum Reprod Update. 1997;3:575–592. doi: 10.1093/humupd/3.6.575. [DOI] [PubMed] [Google Scholar]
- 29.Hertig AT, Rock J, Adams EC, Menkin MC. Thirty-four fertilized human ova, good, bad and indifferent, recovered from 210 women of known fertility; a study of biologic wastage in early human pregnancy. Pediatrics. 1959;23:202–211. [PubMed] [Google Scholar]
- 30.Hertig AT, Rock J. Searching for early fertilized human ova. Gynecol Investig. 1973;4:121–139. doi: 10.1159/000301716. [DOI] [PubMed] [Google Scholar]
- 31.Howell KH, Hubbard GB, Moore CM, Dunn BG, von Kap-Herr C, Raveendran M, Rogers JA, Leland MM, Brasky KM, Nathanielsz PW, Schlabritz-Loutsevitch NE. Trisomy of chromosome 18 in the baboon (Papio hamadryas anubis) Cytogenet Genome Res. 2006;112:76–81. doi: 10.1159/000087516. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard GB, Soike KF, Butler TM, Carey KD, Davis H, Butcher WI, Gauntt CJ. An encephalomyocarditis virus epizootic in a baboon colony. Lab Anim Sci. 1992;42:233–239. [PubMed] [Google Scholar]
- 33.Hunt PA, Hassold T. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs PA. The chromosome complement of human gametes. Oxford Rev Reprod Biol. 1992;14:47–72. [PubMed] [Google Scholar]
- 35.Johnson AP, Taylor-Robinson D. Chlamydial genital tract infections. Experimental infection of the primate genital tract with Chlamydia trachomatis. Am J Pathol. 1982;106:132–135. [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001;154:357–365. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- 37.Kalter SS. Bacteriology. Primates Med. 1973;8:15–33. [Google Scholar]
- 38.Kalter SS. Virology. Primates Med. 1973;8:106–145. [Google Scholar]
- 39.Kriewaldt FN, Hendrickx AG. Reproductive parameters in the baboon. Lab Anim Care. 1968;18:361–370. [PubMed] [Google Scholar]
- 40.Martin LJ, Carey KD, Comuzzie AG. Variation in menstrual cycle length and cessation of menstruation in captive raised baboons. Mech Ageing Dev. 2003;124:865–871. doi: 10.1016/s0047-6374(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 41.McConnell EE, Basson PA, Wolstenholme B, De Vos V, Malherbe HH. Toxoplasmosis in free-ranging chacma baboons (Papio ursinus) from the Kruger National Park. Trans R Soc Trop Med Hyg. 1973;67:851–855. doi: 10.1016/0035-9203(73)90014-x. [DOI] [PubMed] [Google Scholar]
- 42.Moore CM, Hubbard GB, Dick E, Dunn BG, Raveendran M, Rogers JA, Williams V, Gomez JJ, Butler SD, Leland MM, Schlabritz-Loutsevitch NE. Trisomy 17 in a baboon (Papio hamadryas) with polydactyly, patent foramen ovale and pyelectasis. Am J Primatol. 2007;69:1105–1118. doi: 10.1002/ajp.20424. [DOI] [PubMed] [Google Scholar]
- 43.Moyo SR, Hägerstrand I, Nyström L, Tswana SA, Blomberg J, Bergström S, Ljungh A. Stillbirths and intrauterine infection, histologic chorioamnionitis and microbiological findings. Int J Gynaecol Obstet. 1996;54:115–123. doi: 10.1016/0020-7292(96)02705-1. [DOI] [PubMed] [Google Scholar]
- 44.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol. 2005;106:250–259. doi: 10.1097/01.AOG.0000172422.81496.57. [DOI] [PubMed] [Google Scholar]
- 45.Packer C, Collins DA, Sindimwo A, Goodall J. Reproductive constraints on aggressive competition in female baboons. Nature. 1995;373:60–63. doi: 10.1038/373060a0. [DOI] [PubMed] [Google Scholar]
- 46.Park JY, Peters CJ, Rollin PE, Ksiazek TG, Katholi CR, Waites KB, Gray B, Maetz HM, Stephensen CB. Age distribution of lymphocytic choriomeningitis virus serum antibody in Birmingham, Alabama: evidence of a decreased risk of infection. Am J Trop Med Hyg. 1997;57:37–41. doi: 10.4269/ajtmh.1997.57.37. [DOI] [PubMed] [Google Scholar]
- 47.Reef SE, Frey TK, Theall K, Abernathy E, Burnett CL, Icenogle J, McCauley MM, Wharton M. The changing epidemiology of rubella in the 1990s: on the verge of elimination and new challenges for control and prevention. JAMA. 2002;287:464–472. doi: 10.1001/jama.287.4.464. [DOI] [PubMed] [Google Scholar]
- 48.Robb JA, Benirschke K, Barmeyer R. Intrauterine latent herpes simplex virus infection: I. Spontaneous abortion. Hum Pathol. 1986;17:1196–1209. doi: 10.1016/S0046-8177(86)80561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg KR, Trevathan WR. The evolution of human birth. Sci Am. 2001;285:72–77. doi: 10.1038/scientificamerican1101-72. [DOI] [PubMed] [Google Scholar]
- 51.Ross G. Gestational diabetes. Aust Fam Physician. 2006;35:392–396. [PubMed] [Google Scholar]
- 52.Ruppenthal GC, Moore CM, Best RG, Walker-Gelat CG, Delio PJ, Sackett GP. Trisomy 16 in a pigtailed macaque (M. nemestrina) with multiple anomalies and developmental delays. Am J Ment Retard. 2004;109:9–20. doi: 10.1352/0895-8017(2004)109<9:TIAPMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 53.Samueloff A, Xenakis EM, Berkus MD, Huff RW, Langer O. Recurrent stillbirth. Significance and characteristics. J Reprod Med. 1993;38:883–886. [PubMed] [Google Scholar]
- 54.Schramm RD, Paprocki AM, Bavister BD. Features associated with reproductive aging in female rhesus monkeys. Hum Reprod. 2002;17:1597–1603. doi: 10.1093/humrep/17.6.1597. [DOI] [PubMed] [Google Scholar]
- 55.Schoeb TR, Dybvig K, Keisling KF, Davidson MK, Davis JK. Detection of urogenital mycoplasmal infections in primates by use of polymerase chain reaction. Lab Anim Sci. 1997;47:468–471. [PubMed] [Google Scholar]
- 56.Silver RM, Varner MW, Reddy U, Goldenberg R, Pinar H, Conway D, Bukowski R, Carpenter M, Hogue C, Willinger M, Dudley D, Saade G, Stoll B. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196(5):433–44. doi: 10.1016/j.ajog.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syafruddin D, Asih PB, Coutrier FN, Trianty L, Noviyanti R, Luase Y, Sumarto W, Caley M, van der Ven AJ, Sauerwein RW. Malaria in Wanokaka and Loli sub-districts, West Sumba District, East Nusa Tenggara Province, Indonesia. Am J Trop Med Hyg. 2006;74:733–737. [PubMed] [Google Scholar]
- 58.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wasser SK, Norton GW, Rhine RJ, Klein N, Kleindorfer S. Ageing and social rank effects on the reproductive system of free-ranging yellow baboons (Papio cynocephalus) at Mikumi National Park, Tanzania. Hum Reprod Update. 1998;4:430–438. doi: 10.1093/humupd/4.4.430. [DOI] [PubMed] [Google Scholar]
- 60.Wildt DE, Doyle LL, Stone SC, Harrison RM. Correlation of perineal swelling with serum ovarian hormone levels, vaginal cytology, and ovarian follicular development during the baboon reproductive cycle. Primates. 1977;18:261–270. [Google Scholar]
- 61.Yeligulashvili IS. Anatomophysiological investigation (Hamadrayas) Moscow: 1955. Pregnancy and birth in monkeys; p. 158. [Google Scholar]
- 62.Yoder BA, Coalson JJ, Winter VT, Siler-Khodr T, Duffy LB, Cassell GH. Effects of antenatal colonization with Ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54:797–807. doi: 10.1203/01.PDR.0000091284.84322.16. [DOI] [PubMed] [Google Scholar]