Abstract
BACKGROUND
BAY 41-2272 (5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-pyrimidin-4-ylamine) relaxes mesenteric arteries (MA) in a synergistic fashion with nitric oxide (NO). We hypothesized that the relaxation to BAY 41-2272 is decreased in spontaneously hypertensive rats (SHR) because of the reduced NO bioavailability in this strain and that relaxation would be improved by inhibiting the oxidative stress. We aimed to evaluate the influence of oxidative stress in BAY 41-2272-induced vasorelaxation in isolated MA from SHR.
METHODS
MA function was evaluated by concentration-response curves to BAY 41-2272. We measured protein expression of endothelial NO synthase (eNOS), soluble guanylyl cyclase (sGC) and human-antigen R (HuR) (sGC mRNA-stabilizing protein), sGC activity and plasma levels of superoxide dismutase (SOD), and total antioxidant status (TAS).
RESULTS
Cyclic guanosine monophosphate (cGMP)-dependent and -independent relaxation induced by BAY 41-2272 (0.0001–1 μmol/l) was impaired in SHR compared with Wistar-Kyoto (WKY). We observed reduced expression of eNOS, sGC and HuR, and decreased sGC activity in SHR. Plasma levels of SOD and TAS were also diminished in SHR. Incubation with SOD or indomethacin increased relaxation to BAY 41-2272 in SHR. Furthermore, acetylcholine (ACh)-induced relaxation was increased in the presence of BAY 41-2272 or SOD, apocynin, or indomethacin.
CONCLUSION
Augmented oxidative stress in SHR impaired cGMP-dependent and -independent relaxation induced by BAY 41-2272, by decreasing NO bioavailability and sGC expression and by increasing contractile activity. Inhibiton of oxidative stress improved the relaxation of BAY 41-2272 in SHR. BAY 41-2272 might be an alternative therapeutic tool for hypertension if administrated with antioxidant compounds.
As endothelium-derived nitric oxide (NO) was described as the major vasodilator molecule regulating the smooth muscle tone,1,2 its target, the soluble guanylyl cyclase (sGC), became a therapeutic target for treating hypertension and cardiovascular diseases associated with endothelial dysfunction. Activated sGC catalyses the conversion of guanosine triphosphate to the intracellular second messenger cyclic guanosine monophosphate (cGMP) that activates different effector proteins, such as the cGMP-dependent protein kinases, leading to relaxation.3-5
Because of the tolerance observed after prolonged use of organic nitrates (which mimic endogenous NO),6 non-NO-based drugs activating sGC are being developed in order to find an effective vasodilator drug that could replace organic nitrates.
BAY 41-2272 (5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-pyrimidin-4-ylamine) is a non-NO-based pyrazolopyridine that potently stimulates recombinant sGC in the cysteine 238 and cysteine 243 spanning region of the α-subunit of the enzyme,7,8 binding to the enzyme by a haem-depedent but NO-independent mechanism. We showed recently that BAY 41-2272 relaxes different vascular beds by increasing cGMP levels. BAY 41-2272 acts synergistically with NO, as its relaxation is reduced after endothelium removal or NO synthase inhibition. In addition, in the presence of the sGC inhibitor 1H-[1,2,4] oxadiazolo [4,3,-a]quinoxalin-1-one (ODQ), it has been reported a remaining relaxation produced by BAY 41-2272, although the increased cGMP levels observed after stimulation with BAY 41-2272 are markedly inhibited in the presence of ODQ.9-11 Moreover, it has been reported that BAY 41-2272, in the presence of ODQ, reduces CaCl2-induced contractions, indicating that the relaxation induced by BAY 41-2272 in the presence of ODQ is independent of cGMP formation and is due to its calcium influx-blocking properties.11
In spontaneously hypertensive rats (SHR), endothelium-dependent and -independent relaxation are reported to be impaired,12,13 and sGC expression is decreased as well.13,14 Recently, it was described that reactive oxygen species modulates sGC expression.15 As BAY 41-2272 relaxes the mesenteric arteries (MA) in a synergistic fashion with NO,9,10,16 we hypothesized that the relaxation of BAY 41-2272 might be decreased in the SHR because of the reduced NO bioavailability. Furthermore, we hypothesized that the relaxation of BAY 41-2272 might be improved by inhibiting oxidative stress. Our aim was to evaluate the involvement of oxidative stress in the vasorelaxation mediated by BAY 41-2272 in isolated MA from SHR.
METHODS
Experiments were conducted in accordance with institutional guidelines and approved by the Medical College of Georgia Institutional Animal Care and Use Committee. Experiments were performed on male Wistar-Kyoto (WKY) and SHR (12–16 weeks old) obtained from Harlan Laboratories (Indianapolis, IN). Animals were housed four per cage on a 12h light–dark cycle, fed with standard chow diet and water ad libitum.
Vascular reactivity studies
Animals were stunned by inhalation of CO2, killed by decapitation, and exsanguinated. Superior MA was quickly removed and placed in chilled physiological buffer with the following composition (mmol/l): NaCl, 130; NaHCO3, 14.9; dextrose, 5.5; KCl, 4.7; KH2PO4, 1.18; MgSO47H2O, 1.17; and CaCl22H2O, 1.6. The MA was cleaned of connective tissue and divided into rings of 3 mm in length. Each ring was mounted in a wire myograph for isometric force recording (Danish Myograph Technology, Aarhus, Denmark) coupled to a PowerLab 8/SP data acquisition system (software Chart 5.0, ADInstruments, Colorado Springs, CO). The bathing solution was maintained at 37 °C, gassed with 95% O2 and 5% CO2. Tissues were allowed to equilibrate for 45 min under a resting tension of 10mN. In some experiments, the endothelium was removed by gently rubbing the intimal surface.
After equilibration, preparations were contracted with phenylephrine (PE; 1 μmol/l). Thereafter, BAY 41-2272 (0.0001–1 μmol/l), acetylcholine (ACh; 0.01–10 μmol/l), or sodium nitroprusside (SNP; 0.0001–1 μmol/l) were added cumulatively to endothelium-intact mesenteric rings. In parallel rings, the effects of BAY 41-2272 were investigated in the presence of either NG-nitro-L-arginine methyl ester (L-NAME; 100 μmol/l), ODQ (1 μmol/l), superoxide dismutase (SOD) (150 U/ml), apocynin (100 μmol/l), or indomethacin (10 μmol/l). Concentration-response curves to BAY 41-2272 were also obtained in endothelium denuded rings. Endothelium denudation was verified by the lack of relaxation to ACh (1 μmol/l) in vessels contracted with PE. In another set of experiments, the magnitude of the relaxation induced by ACh (1 μmol/l) was monitored minute-to-minute during 15 min in the absence or in the presence of 10, 30, and 100 nmol/l of BAY 41-2272 or in the presence of SOD (150 U/ml), indomethacin (10 μmol/l), apocynin (100 μmol/l), or the thromboxane A2 antagonist SQ 29,648 (10 nmol/l).
Western blotting analysis
Frozen vessel segments of MA were homogenized in a lysis buffer containing 40 mmol/l HEPES, 1% Triton X-100, 10% glycerol, 1 mmol/l Na3VO4, and 1 mmol/l phenylmethylsulphonyl fluoride. The tissue lysate was centrifuged at 10,000g and the supernatant collected. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce Chemical, Rockford, IL). An aliquot of 40 μg protein from each sample was loaded per lane and resolved by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Proteins were subsequently transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked by treatment with 5% milk in Tris-buffered saline containing 0.05% Tween 20, probed with antibodies against endothelial NO synthase (eNOS) (1:1000), sGCα1 (1:100), sGCβ1 (1:200), and human-antigen R (HuR) (1:200) and incubated with a horseradish peroxidase-conjugated second antibody. Immunoreactivity was detected by enhanced chemiluminescence autoradiography.
sGC activity
sGC activity was determined in the precleared supernatant fractions of the tissue samples by the conversion of guanosine triphosphate to cGMP in the presence of four different doses of BAY 41-2272. Briefly, 30 μg of each protein sample were incubated for 10 min at 37 °C in a total volume of 100 μl containing the following: 50 mmol/l Tris-HCl (pH 7.4), 1 mmol/l 3-isobutyl-1-methylxanthine, 3 mmol/l MgCl2, 0.5 mmol/l guanosine triphosphate, 3 mmol/l DTT, 5 mmol/l phosphocreatine, and 0.25 mg/ml creatine kinase. The stimulation of enzyme activity was measured in the presence of BAY 41-2272 (0.001–1 μmol/l). The reaction was terminated by inactivating sGC at 95 °C for 10 min. cGMP was measured by using a commercially available cGMP enzyme-linked immunoassay according to the manufacturer's instructions (Cayman Chemical Cyclic GMP EIA kit, Ann Arbor, MI).
SOD activity and total antioxidant status (TAS) assay
For both assay, immediately after arterial blood collection, samples were centrifuged (1,000g) for 10 min at 4 °C. The top yellow plasma layer was collected without disturbing the white buffy layer and frozen at −80 °C. The assays were performed in duplicates using different sample dilutions.
SOD activity was measured using a SOD Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. To perform the SOD activity assay, erythrocytes were lysed in four times its volume of ice-cold, high-performance liquid chromatography-grade water and centrifuged at 10,000g for 15 min at 4 °C. Supernatant was collected and kept in ice for assaying. SOD activity is assessed by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine.
To perform TAS assay, plasma sample was diluted with Assay Buffer (1:20). TAS was measured using an Antioxidant Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. It measures the overall antioxidant capacity and gives relevant biological information, considering the cumulative effect of all antioxidants present in plasma. The assay relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS· + by metmyoglobin. The capacity of the antioxidants in the sample to prevent ABTS oxidation is compared with that of Trolox, a water-soluble tocopherol analogue, and is quantified as molar Trolox equivalents.
Drugs and chemicals
Acetylcholine, indomethacin, L-NAME, ODQ, PE, SNP, and BAY 41-2272 were purchased from Sigma Chemical (St Louis, MO). The antibody used to probe for eNOS was obtained from BD Biosciences (San Diego, CA) and for sGCα1, sGCβ1, and HuR were obtained from Chemicon (Temecula, CA). All other reagents used were of analytical grade. Stock solutions of ACh, PE, and SNP were prepared in deionized water and stored in aliquots at −20 °C; dilutions were made immediately before use. Indomethacin were prepared as stock solutions in ethanol. BAY 41-2272 and ODQ was prepared in dimethyl sulphoxide. Solutions were stored at −20 °C and further diluted in deionized water just before use. The final concentration of dimethyl sulphoxide/ethanol did not exceed 0.1%. Preliminary experiments ascertained the lack of response of MA rings to these vehicles at the concentrations used.
Statistical analysis
Relaxations were calculated as percentages of the contraction induced by PE in each tissue, which was taken as 100%. The pEC50 values for BAY 41-2272 were determined as –log of the molar concentration to produce 50% of the maximal relaxation in contracted rings. Data are shown as the percentage of relaxation of n experiments, expressed as the mean ± SEM. Analysis of variance and Student's t-test were used to evaluate the results. P < 0.05 was considered to indicate significance. A program package was used for the statistical analysis of all data (GraphPAD Software, version 3.00; San Diego, CA).
RESULTS
Relaxation induced by ACh and SNP
ACh and SNP concentration dependently relaxed MA of both strains. There were no changes in the pEC50 of ACh or SNP of SHR (7.56 ± 0.08 and 8.30 p 0.03, respectively) compared to WKY (7.54 ± 0.06 and 8.18 ± 0.02, respectively). However, a significant decrease in the maximal responses (EMAX) evoked by ACh and SNP was observed in SHR (76 ± 4% and 46 ± 3%, respectively) compared to WKY (97 ± 1% and 77 ± 2%, respectively; data not shown).
Relaxation induced by BAY 41-2272
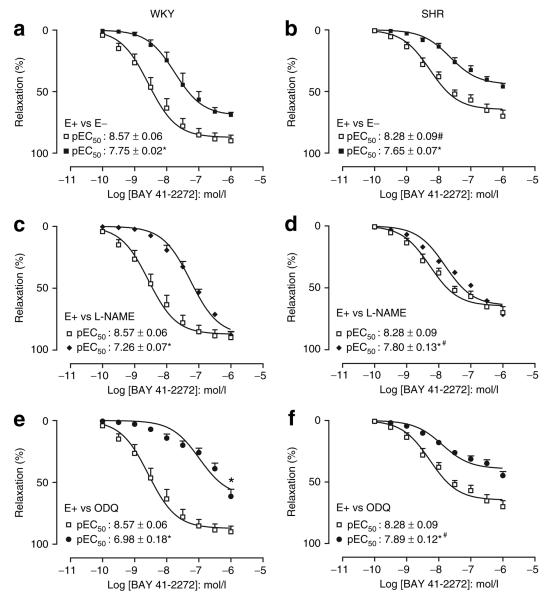
In MA, BAY 41-2272 produced concentration-dependent relaxations in both strains. The potency and EMAX to BAY 41-2272 were higher in WKY (EMAX: 90 ± 5%) compared to SHR (EMAX: 70 ± 5%). Endothelium denudation decreased significantly EMAX (WKY: 69 ± 2%; SHR: 46 ± 3%) and the potency of BAY 41-2272 in both strains (Figure 1a,b).
Figure 1.
BAY 41-2242-induced relaxations in the superior mesenteric arteries from (a,c,e) WKY and (b,d,f) SHR. The relaxation to BAY 41-2272 in endothelium-intact (E+, open squares) mesenteric rings were compared to its effects in endothelium denuded (E−, closed squares) preparations (a,b), in endothelium-intact rings in the presence of the NOS inhibitor L-NAME (closed lozenge) (c,d), or in endothelium-intact rings in the presence of the sGC inhibitor ODQ (closed circles) (e,f). Results are expressed as mean ± SEM of five animals. *P < 0.05, compared with the respective control. #P < 0.05, SHR compared with WKY in the same condition. BAY 41-2272, 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-pyrimidin-4-ylamine; L-NAME, NG-nitro-L-arginine methyl ester; NOS, nitric oxide synthase; ODQ, 1H-[1,2,4] oxadiazolo [4,3,-a]quinoxalin-1-one; sGC, soluble guanylyl cyclise; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto.
Addition of L-NAME produced a rightward shift in the curves to BAY 41-2272 in both strains with no changes in the EMAX (WKY: 88 ± 3%; SHR: 72 ± 5%; Figure 1c,d). In the presence of ODQ, there was a reduction in the potency and EMAX (WKY: 61 ± 6%; SHR: 45 ± 3%) to BAY 41-2272. Nevertheless, the rightward shift observed in the presence of either L-NAME or ODQ was much greater in WKY compared to SHR (Figure 1e,f).
sGC activity assay
It was observed a concentration-dependent increase in the sGC activity after stimulation with BAY 41-2272 which was significantly higher in WKY compared to SHR (Table 1).
Table 1.
BAY 41-2272-induced soluble guanylyl cyclase activity in rat mesenteric artery
| BAY 41-2272 (μmol/l) |
sGC activity (pmol/mg/min) | |
|---|---|---|
| WKY | SHR | |
| Basal | 2.64 ± 0.13 | 2.28 ± 0.17 |
| 0.001 | 4.94 ± 1.29 | 3.13 ± 1.05 |
| 0.01 | 10.39 ± 2.77 | 4.05 ± 1.15* |
| 0.1 | 18.37 ± 3.68 | 6.72 ± 2.36** |
| 1 | 23.49 ± 4.88 | 8.33 ± 3.31** |
BAY 41-2272, 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-pyrimidin-4-ylamine; sGC, soluble guanylyl cyclise; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto.
P < 0.05
P < 0.01, WKY vs. SHR.
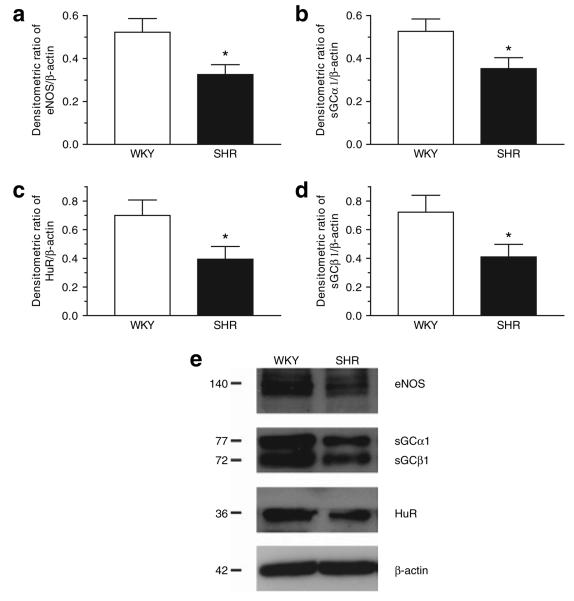
Expression of eNOS, sGC, and HuR
Protein expression of eNOS, sGCα1 and sGCβ1, and HuR were smaller in MA of SHR compared with WKY (Figure 2).
Figure 2.
(a,b,c,d) Densitometric ratio and representative blotting (e) of eNOS, sGCα1, sGCβ1, and HuR expression in the homogenates of superior mesenteric artery from WKY and SHR detected by western blotting. *P < 0.05, WKY vs. SHR. eNOS, endothelial nitric oxide synthase; HuR, human-antigen R; sGC, soluble guanylyl cyclise; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto.
Oxidative stress measurements
Oxidative stress was measured by assaying SOD activity and TAS and both were significantly decreased in SHR compared with WKY (Table 2).
Table 2.
Plasma SOD activity and total antioxidant status (TAS) of WKY and SHR
SHR, spontaneously hypertensive rats; SOD, superoxide dismutase; WKY, Wistar-Kyoto.
P < 0.05, WKY vs. SHR.
Role of superoxide anion, NADPH oxidase, and COX products in the relaxation of BAY 41-2272
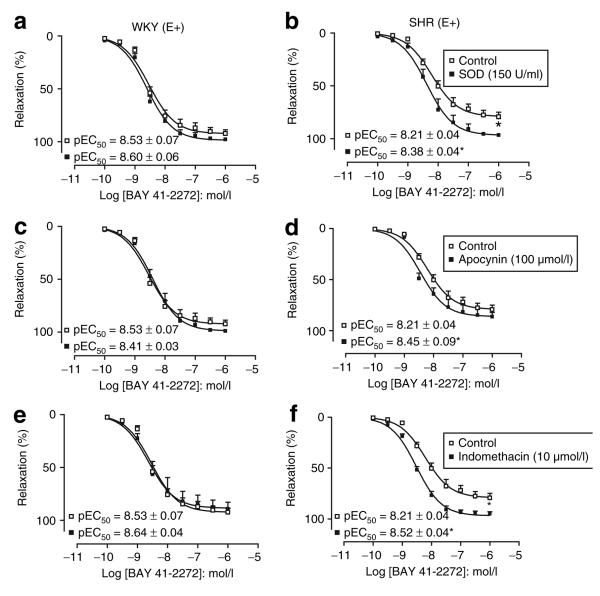
In MA of SHR, incubation with SOD, apocynin, or indomethacin significantly enhanced the potency of BAY 41-2272, whereas the EMAX was significantly increased in the presence of SOD (EMAX: 97 ± 1%) and indomethacin (EMAX: 95 ± 2%) but not in the presence of apocynin (EMAX: 86 ± 4%; Figure 3).
Figure 3.
Concentration-response curves to BAY 41-2242 in rings of the superior mesenteric artery from WKY (a,c,e) and SHR (b,d,f) in the absence (open squares) or in the presence (closed squares) of SOD, Apocynin, or indomethacin. Results are expressed as mean ± SEM of five animals. P < 0.05, compared to the relaxation of BAY 41-2272 in the absence of any agent. BAY 41-2272, 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-pyrimidin-4-ylamine; SHR, spontaneously hypertensive rats; SOD, superoxide dismutase; WKY, Wistar-Kyoto.
Effects of BAY 41-2272, SOD, indomethacin, and apocynin on the relaxation induced by ACh in MA of SHR
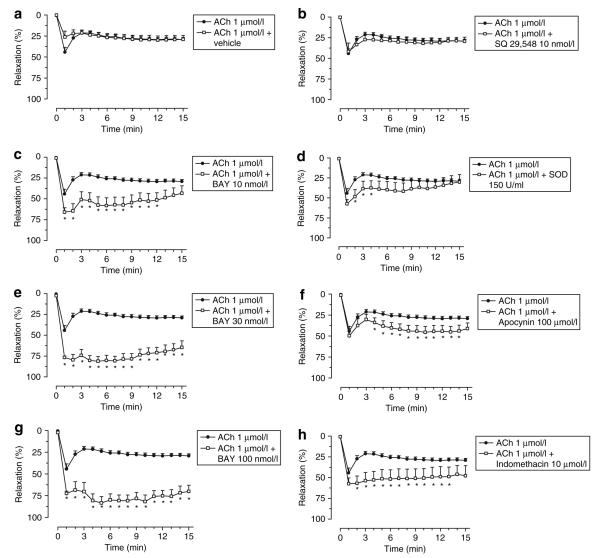
In control conditions, the magnitude of the relaxation induced by ACh was about 45%, reached in the 1st min and then decreasing to about 30% after the 2nd min and lasting till the 15th min. In the presence of BAY 41-2272, the magnitude of the relaxation of ACh was increased to 66%, 81%, and 83% (for 10, 30, and 100 nmol/l of BAY 41-2272, respectively). These magnitudes lasted from the 1st through the 12th min for 10 nmol/l and from the 1st through the 15th min for 30 and 100 nmol/l. The magnitude of the relaxation induced by ACh was significantly changed by the addition of SOD, indomethacin, and apocynin and the maximum effect of ACh lasted till the 4th, 13th, and 14th min, in the presence of SOD, indomethacin, and apocynin, respectively. Neither the magnitude nor the duration of the response of ACh was changed in the presence of SQ 29,648 (Figure 4).
Figure 4.
Effects of (a) vehicle, (c,e,g) BAY 41-2272, (b) SQ 29,548, (d) SOD, (f) apcynin and (h) indomethacin in the magnitude and duration of the response induced by a single dose of ACh in mesenteric arteries of SHR. *P < 0.05, compared to the relaxation to ACh in the absence of any agent.
DISCUSSION
In the present study, we showed that both cGMP-dependent and -independent relaxation induced by BAY 41-2272 were impaired in MA of SHR. It was accompanied by decreased eNOS, sGC, and HuR expression; decreased sGC activity; and increased oxidative stress as well. As arterial hypertension is associated with endothelial dysfunction and people develop tolerance to NO donors after prolonged use, endothelium-independent and non-NO-based sGC activators have been developed as alternative tools for treating hypertension.
BAY 41-2272 is a potent sGC stimulator that binds to the regulatory site of this enzyme by an haem-dependent but NO-independent mechanism and its action is blunted by ODQ, which oxidizes the haem portion of the enzyme.7 However, in in vitro studies, ODQ failed to abolish the relaxation induced by BAY 41-2272 in vessels and corpus cavernosum, suggesting that BAY 41-2272, additionally, relaxes smooth muscle by mechanisms independent of the sGC/cGMP pathway.9-11,16 Recently, we showed that BAY 41-2272 inhibits calcium influx through calcium channels by a mechanism independent of cGMP formation, as BAY 41-2272, in the presence of ODQ, did not increase cGMP levels and decreased calcium-induced contractions.11
BAY 41-2272-induced cGMP-dependent relaxation in MA of SHR
BAY 41-2272- induced relaxation (as well as SNP and ACh) were greater in MA from WKY compared with SHR. Indeed, both endothelium-dependent and -independent relaxation were reported to be impaired in SHR,12,13 likely related to reduced NO bioavailability.17 In agreement with these findings, we showed decreased eNOS protein expression and increased oxidative stress, suggesting deficient NO availability in SHR. It might explain the smaller relaxation of BAY 41-2272 in SHR, as BAY 41-2272 relaxes smooth muscle in a synergistic fashion with NO.9-11 In SHR, this synergism was confirmed by the relaxation elicited by a single dose of ACh, which was increased in magnitude and duration by addition of BAY 41-2272. In addition, in SHR the further decrease observed in the relaxation of BAY 41-2272 after blunting endogenous NO by endothelium removal or L-NAME addition is predictive of NO involvement in this response and indicates that the remaining NO available in the vasculature of SHR contributes to BAY 41-2272-induced relaxation.
BAY 41-2272-induced cGMP-independent relaxation in MA of SHR
In vascular smooth muscle, the relaxations induced by sGC activators such as SNP and GTN are dependent of cGMP formation and are abolished by ODQ.16,18 Herein, the relaxation induced by BAY 41-2272 was reduced (but not abolished) by ODQ, indicating that BAY 41-2272 relaxes MA of both strains by a cGMP-independent mechanism. Moreover, the smaller remaining relaxation of BAY 41-2272 in the presence of ODQ in SHR indicates impairment of the cGMP-independent relaxation in SHR. The physiological effects associated to this observation will be discussed below.
Increased oxidative stress impairs both cGMP-dependent and -independent relaxation response of BAY 41-2272
Increased oxidative stress observed in SHR (represented by diminished levels of SOD and TAS) might be affecting both cGMP-dependent and -independent relaxation.
Oxidative stress, expression of eNOS, sGC, HuR, and sGC activity and cGMP-dependent relaxation
Whether oxidative stress affects the relaxation of BAY 41-2272 in SHR was assessed by using antioxidant agents. The potency of BAY 41-2272 and the duration of the response of a single dose of ACh were enhanced by apocynin, suggesting that NADPH oxidase contributes to diminished relaxation in SHR. It was previously reported that reduced relaxation induced by ACh and SNP was associated with increased activity of NADPH oxidase in SHR, which was reversed by antioxidant vitamins.19 In addition, in SHR, BAY 41-2272-induced relaxation and the magnitude of ACh-induced relaxation were enhanced by SOD or indomethacin, indicating, respectively, that superoxide anion and constrictor prostanoids formation are increased in SHR. Indeed, in vascular tissues of hypertensive animals, higher levels of oxidative stress markers such as superoxide anion, malondialdehyde, diminished TAS, and increased cyclooxygenase-2 products have been demonstrated.20
Corroborating a previous study,21 we observed decreased protein expression of sGC (both subunits) and HuR in MA of SHR. In addition to the reduced sGC activity and eNOS expression, it might be responsible by the impaired cGMP-dependent relaxation of BAY 41-2272. Increased oxidative stress might be downregulating the sGC expression in SHR, as reactive oxygen species regulate the sGC expression.15 However, it is unlikely that the short incubation period with antioxidants is changing the sGC expression. In SHR, SOD and apocynin might improve the relaxing responses by decreasing oxidative stress and restoring NO bioavailability.
Oxidative stress, prostanoids formation, and cGMP-independent relaxation
Increased prostanoid formation is a secondary effect of the enhanced oxidative stress, as the superoxide anion activates endothelial enzymes, such as COX, producing endothelium-derived contracting factors.22 These prostanoids are unrelated to thromboxane A2, as its antagonist SQ 29,548 was ineffective in improving either the relaxation of BAY 41-2272 or the magnitude and duration of ACh-induced relaxation in the MA of SHR. The enhanced contractile forces in the vasculature of SHR impairs BAY 41-2272-induced cGMP-independent relaxation, as the mechanism involved in this effect of BAY 41-2272 is through inhibition of calcium entry through the calcium channels. In SHR, indomethacin is probably enhancing the relaxing responses by inhibiting the release of contractile agents that counteract to relaxing agents.
Taken together, these data suggest that increased oxidative stress in SHR causes diminished NO bioavailability and leads to a downregulation of the sGC expression and activity. On the other hand, oxidative stress increases the contracting prostanoids release, causing an imbalance between contractile and relaxing endogenous agents, in favor of the contractile activity. Although BAY 41-2272 potently relaxes MA, the augmented oxidative stress in SHR impairs both the cGMP-dependent and -independent relaxation of BAY 41-2272. Oxidative stress impaired the cGMP-dependent mechanisms by decreasing NO bioavailability and sGC expression and activity, whereas the cGMP-independent mechanisms are impaired by increased contractile activity, probably because of the enhanced release of constrictor prostanoids, which counteracts the relaxation of BAY 41-2272. In conclusion, our data suggest that BAY 41-2272 might be an alternative therapeutic tool for hypertension if administrated with antioxidant compounds.
Acknowledgments
This study was supported by grants from the National Institutes of Health (HL-71138 and HL-74167). F.B.M.P. is supported by a postdoctoral fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo, Brazil).
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Rees DD, Schulz R, Palmer RM. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci USA. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylate cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 4.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113:1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 5.Juilfs DM, Soderling S, Burns F, Beavo JA. Cyclic GMP as substrate and regulator of cyclic nucleotide phosphodiesterases (PDEs) Rev Physiol Biochem Pharmacol. 1999;135:67–104. doi: 10.1007/BFb0033670. [DOI] [PubMed] [Google Scholar]
- 6.Parker JO. Nitrate tolerance in angina pectoris. Cardiovasc Drugs Ther. 1989;2:823–829. doi: 10.1007/BF00133214. [DOI] [PubMed] [Google Scholar]
- 7.Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schröder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M. NO-independent regulatory site on soluble guanylate cyclase. Nature (London) 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- 8.Becker EM, Schmidt P, Schramm M, Schroder H, Walter U, Hoenicka M, Gerzer R, Stasch JP. The vasodilator-stimulated phosphoprotein (VASP): target of YC-1 and nitric oxide effects in human and rat platelets. J Cardiovasc Pharmacol. 2000;35:390–397. doi: 10.1097/00005344-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Priviero FBM, Baracat JS, Teixeira CE, Claudino MA, De Nucci G, Antunes E. Mechanisms underlying rabbit aorta relaxation by BAY 41-2272, a nitric oxide independent soluble guanylate cyclase activator. Clin Exp Pharmacol Physiol. 2005;32:728–734. doi: 10.1111/j.1440-1681.2005.04262.x. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira CE, Priviero FB, Webb RC. Molecular mechanisms underlying rat mesenteric artery vasorelaxation induced by the nitric oxide-independent soluble guanylyl cyclase stimulators BAY 41-2272 [5-cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-4-ylamine] and YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzyl Indazole] J Pharmacol Exp Ther. 2006;317:258–266. doi: 10.1124/jpet.105.095752. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira CE, Priviero FB, Todd J, Jr, Webb RC. Vasorelaxing effect of BAY 41-2272 in rat basilar artery: involvement of cGMP-dependent and independent mechanisms. Hypertension. 2006;47:596–602. doi: 10.1161/01.HYP.0000199914.36936.1b. [DOI] [PubMed] [Google Scholar]
- 12.Sekiguchi F, Adachi T, Matsubara H, Matsuda K, Kita K, Shimamura K, Sunano S. Spontaneous and agonist-induced contractions and endothelium-dependent relaxation in aortae from SHRSP and WKY rats under various levels of passive force. Clin Exp Pharmacol Physiol. 1996;23:483–489. doi: 10.1111/j.1440-1681.1996.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 13.Ruetten H, Zabel U, Linz W, Schmidt HH. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res. 1999;85:534–541. doi: 10.1161/01.res.85.6.534. [DOI] [PubMed] [Google Scholar]
- 14.Kagota S, Tamashiro A, Yamaguchi Y, Sugiura R, Kuno T, Nakamura K, Kunitomo M. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. Br J Pharmacol. 2001;134:737–744. doi: 10.1038/sj.bjp.0704300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerassimou C, Kotanidou A, Zhou Z, Simoes DC, Roussos C, Papapetropoulos A. Regulation of the expression of soluble guanylyl cyclase by reactive oxygen species. Br J Pharmacol. 2007;150:1084–1091. doi: 10.1038/sj.bjp.0707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baracat JS, Teixeira CE, Okuyama CE, Priviero FB, Faro R, Antunes E, De Nucci G. Relaxing effects induced by the soluble guanylyl cyclase stimulator BAY 41-2272 in human and rabbit corpus cavernosum. Eur J Pharmacol. 2003;477:163–169. doi: 10.1016/j.ejphar.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Schäfer A, Widder J, Eigenthaler M, Ertl G, Bauersachs J. Reduced basal nitric oxide bioavailability and platelet activation in young spontaneously hypertensive rats. Biochem Pharmacol. 2004;67:2273–2279. doi: 10.1016/j.bcp.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Cogolludo AL, Pérez-Vizcaíno F, Zaragozá-Arnáez F, Ibarra M, López-López G, López-Miranda V, Tamargo J. Mechanisms involved in SNP-induced relaxation and [Ca+]i reduction in piglet pulmonary and systemic arteries. Br J Pharmacol. 2001;132:959–967. doi: 10.1038/sj.bjp.0703894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003;41:534–539. doi: 10.1161/01.HYP.0000057421.28533.37. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez Y, Pérez-Girón JV, Hernanz R, Briones AM, García-Redondo A, Beltrán A, Alonso MJ, Salaices M. Losartan reduces the increased participation of cyclooxygenase-2-derived products in vascular responses of hypertensive rats. J Pharmacol Exp Ther. 2007;321:381–388. doi: 10.1124/jpet.106.115287. [DOI] [PubMed] [Google Scholar]
- 21.Klöss S, Rodenbach D, Bordel R, Mülsch A. Human-antigen R (HuR) expression in hypertension: downregulation of the mRNA stabilizing protein HuR in genetic hypertension. Hypertension. 2005;45:1200–1206. doi: 10.1161/01.HYP.0000165674.58470.8f. [DOI] [PubMed] [Google Scholar]
- 22.Vanhoutte PM, Félétou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]