Abstract
Purpose
The present study compared the ability of different berry types to prevent chemically-induced tumorigenesis in the rat esophagus. We also determined if berries influence the levels of inflammatory cytokines in the serum of carcinogen-treated rats.
Methods
Rats were treated with the carcinogen N-nitrosomethylbenzylamine (NMBA) for 5 weeks, then placed on diets containing 5% of either black or red raspberries, strawberries, blueberries, noni, açaí or wolfberry until the end of the study. The effects of the berries on tumor incidence, multiplicity and size were determined, as well as their effects on the levels of selected inflammatory cytokines in serum.
Results
All berry types were about equally effective in inhibiting NMBA-induced tumorigenesis in the rat esophagus. They also reduced the levels of the serum cytokines, interleukin 5 (IL-5) and GRO/KC, the rat homologue for human interleukin-8 (IL-8), and this was associated with increased serum antioxidant capacity.
Conclusions
Seven berry types were about equally capable of inhibiting tumor progression in the rat esophagus in spite of known differences in levels of anthocyanins and ellagitannins. Serum levels of IL-5 and GRO/KC (IL-8) may be predictive of the inhibitory effect of chemopreventive agents on rat esophageal carcinogenesis.
Keywords: berries, chemoprevention, esophagus, rat
INTRODUCTION
Research on the effects of berry intake on human cardiovascular and neurological diseases, diabetes and cancer has increased significantly in the past 10–15 years (1,2). Berry consumption has beneficial effects on all of these diseases, but the mechanisms of berry protection have not been fully elucidated. Some of the commonly produced and consumed berries in the United States include strawberry (Fragaria x ananassa), blueberry (Vaccinium corymbosum), cranberry (Vaccinium macrocarpon), blackberry (Rubus fruticosus L.), red raspberry (Rubus idaeus) and black raspberry (Rubus occidentalis). Recently, there has been a substantial increase in the U.S. consumption of more “exotic” berries, such as noni (Morinda citrifolia) from Polynesia and the Hawaiian Islands, wolfberry (also known as goji) (Lycium barbarum) from China, and açaí (Euterpe oleracea) from the flood plains of the Amazon River in Brazil. Some of these berry types are advertised as having remarkable antioxidant potential, exceeding that of other berry types, and this property is purported to give them superior potential for disease prevention. With respect to cancer, these claims have been difficult to confirm because there have been no parallel comparisons of the relative ability of different berry types to prevent cancer either in animals or in humans.
Our laboratory and others have conducted numerous studies on the cancer preventive potential of black raspberries (BRBs). BRBs were chosen for study because of their high concentrations of ellagitannins and anthocyanins, compounds known to exhibit chemopreventive potential (3,4). In addition, BRBs have strong antioxidant potential, presumably due to their high concentrations of anthocyanins (5). Dietary freeze-dried BRB powder inhibits carcinogen-induced tumors in the rat esophagus and colon (6,7), hamster cheek pouch (8), and estrogen-induced tumors in the rat mammary gland (9). The removal of water by freeze-drying results in concentrating the chemopreventive agents in berries approximately 10-fold because berries are composed of 85–90% water. A topically applied ethanol/water extract of BRBs inhibited ultraviolet-light-induced skin tumors in mice (10). BRBs inhibit tumorigenesis in the rat esophagus by a number of mechanisms, such as stimulating the detoxification of the esophageal carcinogen, N-nitrosomethylbenzylamine (NMBA); reducing inflammation, angiogenesis and the proliferation rate of premalignant cells; stimulating apoptosis and squamous differentiation (11); and inhibiting the expression of matrix metalloproteinases (unpublished data). On a molecular level, BRBs have been shown to influence the mRNA and protein expression levels of multiple genes associated with these cellular effects (12).
Relatively few studies have investigated the potential chemopreventive effects in vivo of berry types other than BRBs. Two investigations in our laboratory showed that freeze-dried strawberries and blackberries exhibit chemo-preventive potential in the rat esophagus, and both berry types were nearly as effective as BRBs (13,14). Multiple studies have shown that extracts from different berry types, such as blueberries, strawberries, red raspberries, blackberries, etc., exhibit chemopreventive effects in cultured cell lines, but there is only limited data on their effects in vivo (15). With respect to the “exotic” berries, the Kinghorn laboratory isolated several compounds from noni that exhibit potent antioxidant activity in vitro, and they are strong inducers of the phase II metabolizing enzyme, quinone reductase (16,17). An anthraquinone from noni had particularly potent quinone reductase-inducing activity, exceeding that of sulforaphane from broccoli by a factor of 40 (17). Noni fruit glycosides were shown to suppress activator protein-1 (AP-1) transactivation and 12-0-tetradecanolyphorbol-13-acetate (TPA)- and epidermal growth factor (EGF)-induced transformation of mouse epidermal JB6 cells (18). The Kinghorn laboratory also reported that lignans and other constituents of açaí fruit exhibit antioxidant and cytoprotective activities in vitro (19). Açaí polyphenolics also induce apoptosis in HL-60 human leukemia cells (20). Treatment of mice bearing sarcoma cell (S180) tumors with a polysaccharide-protein complex from goji resulted in reduced tumor weights (21).
The present study was conducted to compare the ability of four commonly consumed berries in the U.S.—strawberry, blueberry, red raspberry and black raspberry—and three other berry types—noni, goji and açaí—to inhibit N-nitrosomethylbenzylamine (NMBA)-induced tumors in the rat esophagus, a model for human esophageal squamous cell carcinoma (22). Because previous studies in our laboratory have shown that the anthocyanins are responsible for a substantial portion of the chemopreventive activity of BRBs in the rat esophagus (4) and that pure ellagic acid, a component of the ellagitannins, is also chemopreventive in rat esophagus (23), we predicted that those berry types with reportedly high levels of anthocyanins and ellagitannins were likely to exhibit the strongest chemopreventive potential in our study. A secondary goal of the study was to determine if we could identify inflammatory cytokines in the serum of NMBA-treated rats that might serve as biomarkers of the chemopreventive effects of berries.
MATERIALS AND METHODS
Source of Berries and Preparation of Berry Diets
Ripe BRBs of the Jewel variety and red raspberries (RRBs) designated as WGO2 were obtained from the Stokes Raspberry Farm in Wilmington, OH, washed and frozen on the farm, and shipped frozen to Van Drunen Farms in Momence, IL for freeze-drying as described (6). A second batch of RRBs of the Meeker variety provided by the Washington State Raspberry Commission, blueberries (BBs) of the Reveille variety provided by Watershed Foods, Gridley, IL, and strawberries (STRWs) of the Commander variety provided by Driscoll Farms, Watsonville, CA, were picked ripe, washed, frozen, and shipped frozen to Van Drunen Farms for freeze-drying. Freeze-dried BRBs, RRBs, BBs and STRWs were then shipped frozen in sealed plastic bags from Van Drunen Farms to the Ohio State University where they were kept frozen at −20°C until used in the bioassay. Freeze-dried noni fruit powder (lot numbers 0148857 and 0158130) from the Polynesian Pacific Islands was obtained from Nature’s Sunshine Products, Inc., Spanish Fork, UT. Ripe noni fruit, certified 100% pure, was harvested, washed, sliced, sun dried and milled into a powder in Polynesia and shipped to Nature’s Sunshine, Inc. The ratio of fresh fruit to powder was 10:1. Açaí flakes extract (lot number 0153045) from Brazil and wolfberry dried fruits (lot number S-1654) from Ninxia Province, People’s Republic of China, were also obtained from Nature’s Sunshine Products, Inc. Açaí flakes were prepared by taking freshly washed açaí berries and extracting the pulp using friction/sieve. The pulp was then pasteurized and dried. After drying, the pulp was ground and sieved, producing the dehydrated açaí flakes. The ratio of fresh pulp to dried flakes was 5:1. The dried wolfberry fruit was ground into a powder at the Ohio State University.
In vitro and in vivo studies suggest that among the most active chemopreventive agents in berries are the anthocyanins and ellagitannins (5). Table I provides data on the reported levels of anthocyanins and ellagitannins, as well as total phenolics and dietary fiber, in the berry types used in this study (24–33). Because the anthocyanins are responsible for the color of berries, in general, the darker the color, the higher the anthocyanin levels. BRBs contain the highest amount of ellagitannins, and BRBs and RRBs appear to contain the highest amounts of dietary fiber. It should be recognized that the levels of these berry components can vary considerably as a function of heredity, environmental conditions in which the berries are grown, and degree of ripeness of the berries (24). We have not measured the amounts of these components in the berry preparations used in this study.
Table I.
Reported Levels of Anthocyanins, Ellagitannins, Total Phenolics and Dietary Fiber in the Berry Types Used in This Study
| Type | Anthocyanins | Ellagitannins | Total phenolics | Dietary fiber | References |
|---|---|---|---|---|---|
| a BRBs | 197–589 | 85 | 98–267 | 6504 | (24,25) |
| BBs | 131–208 | 2 | 319–444 | 2400 | (26–28) |
| STRWs | 39 | 20 | 103–230 | 2000 | (24,29) |
| RRBs | 65–68 | 25 | 52–234 | 6500 | (24,25) |
| Noni | b NA | NA | NA | 500 | (30) |
| Wolfberry | NA | NA | NA | 1000 | (31) |
| Açaí | 32 | NA | 125 | 4420 | (32,33) |
Total phenolics is measured as gallic acid equivalents. Data are presented in mg/100 g fresh weight.
BRBs (black raspberries) and RRBs (red raspberries) were from Oregon and Maryland; BBs (blueberries) from Maryland and Arkansas; STRWs (strawberries) from Maryland and California; Noni from Hawaii; Wolfberry from China; and, Açaí from Brazil.
NA = data not available
Chemicals
NMBA, obtained from Ash Stevens (Detroit, MI), was greater than 98% pure as determined by HPLC. Dimethyl sulfoxide (DMSO) was purchased from Sigma (St Louis, MO).
Animals
Male F344 rats, 4–5 weeks old, were obtained from Harlan Sprague-Dawley (Indianapolis, IN). The animals were housed two per cage under standard conditions (20±2°C, 50±10% relative humidity, 12-hour light/dark cycles). Food and water were available ad libitum. Hygienic conditions were maintained by twice-weekly cage changes. The animals were fed a modified American Institute of Nutrition-76A (AIN-76A) synthetic diet (Dyets, Inc., Bethlehem, PA). Body weights and food intake were recorded weekly after administration of the various berry diets. The animals were housed and maintained according to the recommendations of the American Association of Laboratory Animal Care (AALAC).
Animal Bioassay
Two weeks after arrival in the animal facility, the rats were randomly assigned into ten groups of 15 animals each as shown in Table II. A post-initiation protocol (6) was used to assess the relative ability of the different berry types to influence the progression stage of rat esophageal tumorigenesis. One group (Grp 1) of 6–8-week-old rats was injected s.c. with 0.2 ml of a solution containing 20% DMSO in water (the vehicle for NMBA) three times per week for five weeks. The remaining nine groups (Grps 2–10) were injected s.c. with NMBA (0.3 mg/kg b.w) in 0.2 ml vehicle three times per week for five weeks after which they were fed either control AIN-76A diet or AIN-76A diet containing 5% berry powder/flakes until the end of the study (35 wks). Berries were tested at 5% of the diet because this concentration of BRBs has consistently produced a predictable inhibitory effect (about 50%) on NMBA-tumorigenesis in the rat esophagus when provided in the diet post-carcinogen-administration (4,6). To maintain an isocaloric diet, the starch in the diet of rats fed 5% berry powders was reduced by 5%. At 35 weeks, all animals were killed by CO2 asphyxiation, the esophagus of each animal was opened longitudinally, and the surface tumors were mapped, counted, and sized. Lesions greater than 0.5 mm in a single dimension (length, width and height) were considered to be tumors (6). Tumor volume was calculated using the formula for a prolate spheroid: length × width × height × p/6, and expressed in mm3; this can also be considered as an estimation of tumor size.
Table II.
Effect of Different Berry Types on NMBA-Induced Esophageal Tumors in F-344 Rats When Administered at 5% of the Diet
| Grp | Diet | NMBA (0.3mg/kg/inj) | Tumor incidence (%) | Tumor multiplicity (Mean±SE) | Tumor size (mm3) (Mean±SE) |
|---|---|---|---|---|---|
| 1 | AIN-76A control diet | − | 0 | 0 | 0 |
| 2 | AIN-76A | + | 95 | 2.15±0.41 | 11.69±5.07 |
| 3 | AIN-76A + 5% BRBs | + | 60a | 1.07±0.28b | 7.50±2.46 |
| 4 | AIN-76A + 5% BBs | + | 63a | 1.00±0.32b | 9.21±7.01 |
| 5 | AIN-76A + 5% STRWs | + | 75a | 1.25±0.32b | 8.58±3.46 |
| 6 | AIN-76A + 5% RRBs (WGO2) | + | 75a | 1.19±0.28b | 6.72±1.85 |
| 7 | AIN-76A + 5% RRBs (Meeker) | + | 63a | 0.88±0.27b | 9.07±3.86 |
| 8 | AIN-76A + 5% noni | + | 60a | 1.10±0.41b | 7.93±3.21 |
| 9 | AIN-76A + 5% wolfberry | + | 63a | 0.94±0.27b | 5.73±1.24 |
| 10 | AIN-76A + 5% açaí | + | 75a | 1.19±0.25b | 5.26±2.15 |
BRBs, black raspberries; BBs, blueberries; STRWs, strawberries; RRBs, red raspberries
Significantly lower than Group 2 (NMBA only) as determined by χ2 test (P<0.05)
Significantly lower than Group 2 (NMBA only) as determined by analysis of variance (P<0.05)
Serum Inflammatory Cytokines
Serum was prepared from ten rats per control and experimental group at the end of the 35-wk bioassay. Serum cytokine (IFN-γ, IL-1β, IL-13, IL-4, IL-5, GRO/KC and TNF-α) concentrations were measured using a MS6000 Rat Demonstration-7Base Kit on a Sector™ Imager 6000 according to the manufacturer’s recommendation (Meso Scale Diagnostics, Gaithersburg, MD). Cytokines in serum from ten rats per group were measured and values averaged. Standard calibration curves were run on each plate using standards supplied by Meso Scale Diagnostics with a linear range of 0 pg/mL to 10,000 pg/mL.
Serum Antioxidant Capacity
The serum samples used above for measurement of inflammatory cytokines were also used to measure the effect of the individual berry types on serum antioxidant capacity using an Antioxidant Assay Kit (Cayman Chemical Company, Ann Arbor, MI) and following the manufacturers’ instructions. The assay relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS® (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS®+ by metmyoglobin. The capacity of the antioxidants in the sample to prevent ABTS oxidation was compared with that of Trolox, a water-soluble tocopherol analogue, and is expressed as molar Trolox equivalents.
Statistical Analysis
Body weights, food consumption, tumor multiplicity (number of esophageal tumors per rat), tumor size (volume), and serum inflammatory markers in control and berry-treated animals were compared using ANOVA and an unpaired t test StatView® (SAS Institute). Tumor incidences (percent of rats with esophageal tumors) in control and berry-treated animals was compared using Chi-square analysis. P values ≤ 0.05 were considered to be significant.
RESULTS
Effects of the Various Berry Diets on Body Weights, Food Consumption, NMBA-Induced Esophageal Tumors, Serum Cytokine Levels and Serum Antioxidant Capacity
No significant differences in animal body weights and food consumption were found amongst any of the groups during the entire 35-week bioassay (data not shown). Interestingly, at 5% of the diet, all berry types were about equally effective in reducing esophageal tumor incidence and multiplicity, but had no significant effect on tumor size in any of the groups (Table II). Histological evaluation of an average of ten tumors per group indicated that all tumors exhibited the histopathological features of papillomas.
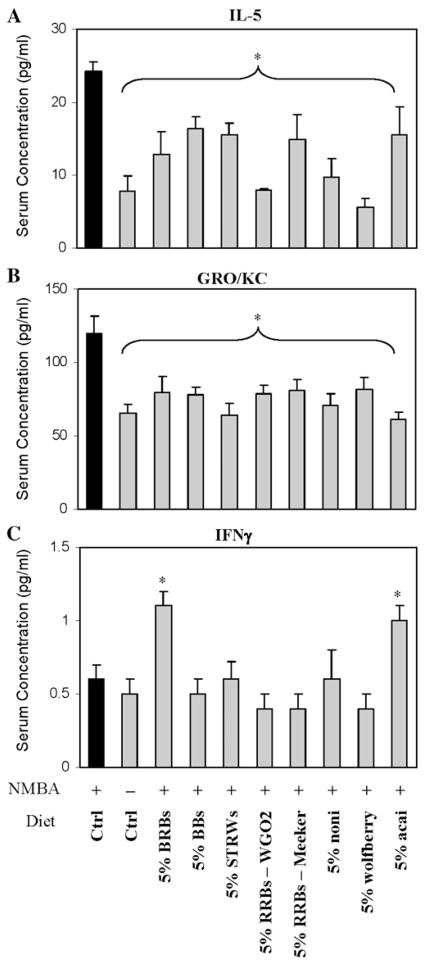
All berry diets (Grps 3–10) were equally effective in reducing serum interleukin 5 (IL-5) and GRO/KC levels (Fig. 1) compared with the NMBA control group (Grp 2). Serum levels of IL-1β, IL-13 and TNF-α were not significantly affected by any of the berry diets. Interestingly, 5% BRBs (Grp 3) and 5% açaí (Grp 10), but not other berry diets, significantly increased interferon gamma (IFNγ) serum levels (Fig. 1). RRBs from Ohio (Grp 5; WGO2) and Washington (Grp 6; Meeker) did not differ significantly either in their ability to reduce NMBA tumorigenesis in the esophagus or in influencing serum cytokine levels. The reduced serum cytokine levels were associated with increased serum antioxidant capacity in berry-fed animals (Fig. 2).
Fig. 1.
Effects of dietary administration of multiple berry types on serum inflammatory cytokines, IL-5 (A), GRO/KC (B) and IFNγ (C) in NMBA-treated F-344 rats. BRBs, black raspberries; BBs, blueberries; STRWs, strawberries; RRBs, red raspberries. Bar = Mean±S.E. aSignificantly lower than Group 2 (NMBA only) as determined by analysis of variance (P<0.05).
Fig. 2.
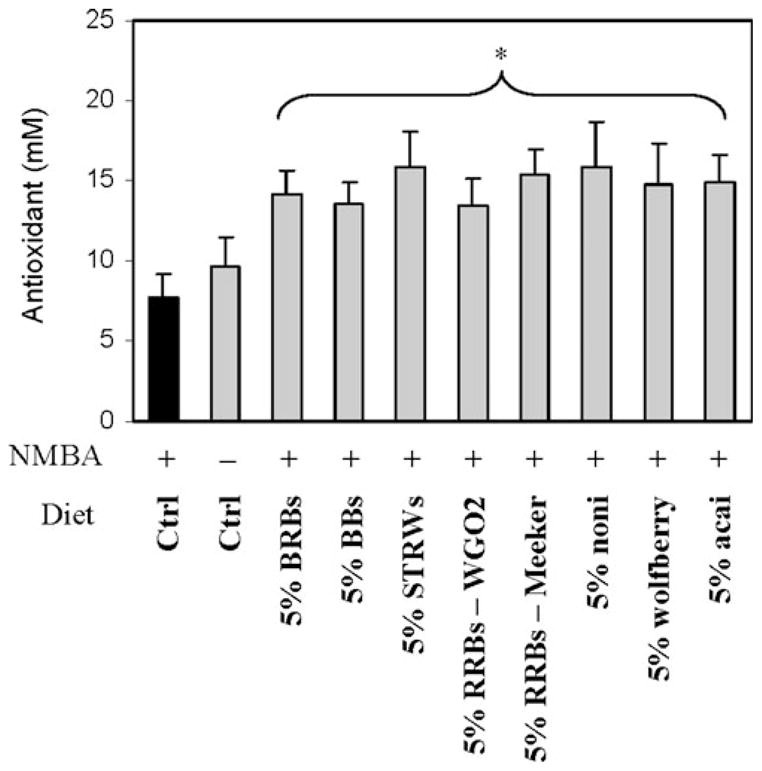
Effects of dietary administration of multiple berry types on serum antioxidant capacity in NMBA-treated F-344 rats. BRBs, black raspberries; BBs, blueberries; STRWs, strawberries; RRBs, red raspberries. Bar = Mean±S.E. aSignificantly lower than Group 2 (NMBA only) as determined by analysis of variance (P<0.05). The capacity of the antioxidants in the sample was compared with that of Trolox, a water-soluble tocopherol analogue, and is expressed as molar Trolox equivalents.
DISCUSSION
This is the first study in which a comparison is made between the ability of different berry types to influence chemically induced tumor development in an animal model system. The rat esophagus tumor model is particularly useful for making this comparison because the provision of berry powder in the diet ad libitum permits the localized absorption of berry compounds directly into the esophagus on a consistent basis. This is especially relevant for the anthocyanins and ellagitannins in berries because the uptake of these compounds into blood is generally less than 1% of the administered dose (34,35). Therefore, the localized absorption of these compounds is thought to be important for their chemopreventive potential (36).
Our results indicate that all seven berry types were effective in reducing esophageal tumor incidence and multiplicity when administered in the diet in a post-initiation protocol. This is important because this protocol permits an evaluation of whether the berries are capable of preventing the progression of NMBA-induced premalignant lesions (dysplastic lesions) to papillomas (6). This has relevance to the human situation in which chemopreventive agents are evaluated for their ability to inhibit the progression of dysplastic lesions in the esophagus (identified by endoscopy) to squamous cell carcinoma (22). The berry types were not significantly different in their ability to prevent esophageal tumorigenesis in this protocol; however, the data are preliminary in that the berries were tested at only a single dose level (5%) in the diet. The reason for selection of a 5% berry diet is that this dose level of BRBs has consistently produced an inhibitory effect on NMBA-tumorigenesis in the rat esophagus in multiple studies (4,6,11,14). Higher dose levels (10% and 20%) do not produce significantly greater inhibitory effects than the 5% BRB diet, and, at 2.5% of the diet, BRBs were not effective (data not published). It is conceivable, however, that differences in the ability of the various berry types to prevent esophageal tumorigenesis could be revealed if they were tested at doses that are both higher and lower than 5% of the diet.
The ellagitannins and anthocyanins in berries have been shown in multiple studies to produce chemopreventive effects in vitro and in vivo (5). In 1990, we reported that dietary ellagic acid, the chemopreventive component of the ellagitannins, inhibits NMBA-induced tumorigenesis in the rat esophagus (23). Recently, we reported that an anthocyanin-enriched fraction of BRBs was equally as effective as whole BRB powder itself in reducing NMBA-tumorigenesis in the rat esophagus (4). We predicted, therefore, that those berry types with high levels of anthocyanins and ellagitannins might be the most effective in inhibiting NMBA-tumorigenesis in the esophagus. Results from the present study suggest that this is not the case, at least when the berries are provided at 5% of the diet. In fact, the present results confirm a previous report from our laboratory in which STRWs were found to be nearly as effective as BRBs in preventing rat esophageal tumorigenesis, even though STRWs have lower levels of both anthocyanins and ellagitannins (13,24,29) (Table I). This is the first report of the ability of red raspberries (RRBs) to inhibit NMBA-induced tumorigenesis in the rat esophagus, and they appear to be quite effective. For comparative purposes, RRBs were obtained from growers in Ohio and Washington, and berries from both states appear to be about equally effective in preventing esophageal tumor progression. To our knowledge, this is the first attempt to compare the same berry type grown in different regions of the United States for its ability to inhibit tumorigenesis in vivo. Blueberries are also effective, and they have high levels of anthocyanins and very low levels of ellagitannins (26–28) (Table I). These results suggest, therefore, that additional studies of the chemopreventive potential of commercially produced strawberry, red raspberry and blueberry powders in preclinical animal model systems (and ultimately in humans) are warranted and desirable in that these berry types are readily available for consumption throughout the entire year.
Because of their recent popularity, and varying levels of anthocyanins and ellagitannins, we examined three “exotic” berries—wolfberry, noni and açaí—for their ability to inhibit tumor progression in the rat esophagus. Wolfberry contains some ellagitannins and low levels of anthocyanins; however, the inhibitory effect of these berries against esophageal cancer could well be due to their high level of carotenoids (31). Zeaxanthin, an antioxidant, is the most abundant carotenoid in wolfberry, and a human trial showed that the dietary intake of wolfberry increased plasma levels of zeaxanthin (37). In addition, as mentioned above, in sarcoma cell (S180) tumor-bearing mice, a wolfberry-derived polysaccharide-protein complex reduced tumor weight and increased interleukin-2 (IL-2) expression (21). Although the macromolecular structure of wolfberry polysaccharides has not been elucidated, preliminary structural studies indicate that they exist in the form of complex glycoconjugates (38). The chemopreventive effect of noni is likely due to a number of chemical constituents that exhibit strong antioxidant activity, including certain anthraquinones, flavonol glycosides, iridoid glycosides and triterpenoids (16,17). To our knowledge, anthocyanins and ellagitannins have not been detected in noni. Noni has been shown to inhibit tumor development and induce apoptosis of Ehrlich ascites tumors in Balb-c mice (39), and, as mentioned above, noni fruit glycosides suppress TPA- and EGF-induced transformation of mouse epidermal JB6 cells, an effect associated with reduced AP-1 expression (18).
Freeze-dried açaí fruit pulp and skin has been shown to contain about 3.19 mg/g of anthocyanins, principally cyanidin-3-glucoside and cyanidin-3-rutinoside (40). However, the anthocyanins are thought to contribute a relatively small percentage of the total antioxidant capacity of açaí fruit because açaí also contains appreciable quantities of proanthocyanidins (12.89 mg/g), which undoubtedly contribute substantially to their antioxidant potential (32,33). It seems likely that the anthocyanins and proanthocyanidins are responsible for at least a portion of the chemo-preventive activity of açaí in the present study. To our knowledge, there are no reports of the presence of ellagitannins in açaí.
The present study suggests that another mechanism by which berries inhibit NMBA-induced esophageal tumorigenesis is through the regulation of cytokine expression. All berry types reduced the levels of IL-5 and GRO/KC in the serum of NMBA-treated rats. Elevated expression of IL-5 and other interleukins has been reported in rat lung adenocarcinomas induced by N-Nitrosobis(2-hydroxypropyl)amine (41). GRO/KC is the rat analog for human Interleukin-8 (IL-8), and human IL-8 is a macrophage-derived mediator of angiogenesis (42). The ability of all berry types to reduce serum levels of IL-5 and GRO/KC may therefore be related to their inhibitory effect on NMBA-induced esophageal tumorigenesis. The BRB and açaí diets significantly upregulated serum levels of IFNγ and activated macrophage-released IFNγ induced apoptosis through the Fas/FasL pathway in glioma cells (43). Thus, it is possible that these two berry types influence the apoptotic rate in NMBA-treated rat esophagus as a mechanism of tumor inhibition. These observations on serum cytokine levels require confirmation in NMBA- and berry-treated esophageal tissues to determine if the changes in serum levels reflect tissue levels of these cytokines.
CONCLUSIONS
Our preliminary results suggest that multiple berry types have the capability of reducing NMBA-induced tumorigenesis in the rat esophagus. Therefore, it seems logical to determine if berry types other than BRBs are also effective in other organ sites. One advantage of extending these studies to other berry types is that most berry types are more readily available to the public throughout the year than BRBs. Our data also suggest that compounds other than anthocyanins and ellagitannins are responsible for the inhibitory activity of some berry types. For example, the carotenoids and complex polysaccharides may well be responsible for the chemopreventive activity of wolfberry. Finally, all berry types reduced serum levels of the cytokines, IL-5 and GRO/KC, and increased serum antioxidant capacity, suggesting that this may be a mechanism for inhibition of esophageal tumorigenesis. These results require confirmation in esophageal tissue itself.
Acknowledgments
We thank Mr. Dale Stokes of the Stokes Raspberry Farm, Wilmington, OH for provision of the Jewel variety of black raspberries and WGO2 variety of red raspberries; Erin Theony and the Washington State Raspberry Commission for provision of the Meeker variety of red raspberries; Watershed Foods, Gridley, IL for provision of the Reveille variety of blueberries; Driscoll Farms of Watsonville, CA for provision of the Commander variety of strawberries; and Dr. William J. Keller of Nature’s Sunshine, Inc., Spanish Fork, UT 84660 for the supply of noni, açaí and wolfberries. This study was supported by NCI R01 grant CA103180 and U.S.D.A. grant 38903-19245 through the Ohio Agricultural Research and Development Center.
ABBREVIATIONS
- AP-1
Activator protein-1
- AIN-76A
American Institute of Nutrition-76A
- BRBs
black raspberries
- BBs
blueberries
- DMSO
dimethylsulfoxide
- EGF
epidermal growth factor
- IL-8
GRO/KC
- IFNγ
interferon-gamma
- IL
interleukin
- NMBA
N-nitrosomethylbenzylamine
- RRBs
red raspberries
- STRWs
strawberries
- TPA
12-O-tetradecanolyphorbol-13-acetate
- TNF-α
tumor necrosis factor-alpha
Contributor Information
Gary D. Stoner, Email: gary.stoner@osumc.edu, Department of Internal Medicine and Comprehensive Cancer Center, College of Medicine, The Ohio State University, Columbus, Ohio 43210, USA. Department of Internal Medicine, Ohio State University Comprehensive Cancer Center, 2001 Polaris Pkwy, Columbus, Ohio 43240, USA
Li-Shu Wang, Department of Internal Medicine and Comprehensive Cancer Center, College of Medicine, The Ohio State University, Columbus, Ohio 43210, USA.
Claire Seguin, Department of Internal Medicine and Comprehensive Cancer Center, College of Medicine, The Ohio State University, Columbus, Ohio 43210, USA.
Claudio Rocha, Department of Internal Medicine and Comprehensive Cancer Center, College of Medicine, The Ohio State University, Columbus, Ohio 43210, USA.
Kristen Stoner, Department of Internal Medicine and Comprehensive Cancer Center, College of Medicine, The Ohio State University, Columbus, Ohio 43210, USA.
Steven Chiu, Department of Internal Medicine and Comprehensive Cancer Center, College of Medicine, The Ohio State University, Columbus, Ohio 43210, USA.
A. Douglas Kinghorn, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University, Columbus, Ohio 43210, USA.
References
- 1.Seeram NP. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem. 2008;56:627–9. doi: 10.1021/jf071988k. [DOI] [PubMed] [Google Scholar]
- 2.Seeram NP. Recent trends and advances in berry health benefits research. J Agric Food Chem. 2009 doi: 10.1021/jf902806j. [DOI] [PubMed] [Google Scholar]
- 3.Cerda B, Tomas-Barberan FA, Espin JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem. 2004;53:227–35. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 4.Wang L-S, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoner GD, Wang L-S, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–9. [PubMed] [Google Scholar]
- 7.Harris GK, Gupta A, Nines RG, Kresty LA, Habib SG, Frankel WL, et al. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr Cancer. 2001;40:125–33. doi: 10.1207/S15327914NC402_8. [DOI] [PubMed] [Google Scholar]
- 8.Casto BC, Kresty LA, Kraly CL, Pearl DK, Knobloch TJ, Schut HA, et al. Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002;22:4005–15. [PubMed] [Google Scholar]
- 9.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60:227–34. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 10.Duncan FJ, Martin JR, Wulff BC, Stoner GD, Tober KL, Oberyszyn TM, et al. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev Res. 2009;2:665–72. doi: 10.1158/1940-6207.CAPR-08-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoner GD, Wang L-S, Zikri N, Chen T, Hecht SS, Huang C, et al. Cancer prevention with freeze-dried berries and berry components. Sem Cancer Biol. 2007;17:403–10. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoner GD, Dombkowski AA, Reen RK, Cukovic D, Salagrama S, Wang L-S, et al. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both black raspberries and phenethyl isothiocyanate. Cancer Res. 2008;68:6460–7. doi: 10.1158/0008-5472.CAN-08-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlton PS, Kresty LA, Siglin JC, Morse MA, Lu J, Morgan C, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. 2001;22:441–6. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 14.Stoner GD, Chen T, Kresty LA, Aziz R, Reinemann MT, Nines R. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr Cancer. 2006;54:33–46. doi: 10.1207/s15327914nc5401_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L-S, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–90. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su B-N, Pawlus AD, Jung HD, Keller WJ, McLaughlin J-L, Kinghorn AD. Chemical constitutents of the fruits of Morinda citrifolia (Noni) and their antioxidant activity. J Nat Prod. 2005;68:592–5. doi: 10.1021/np0495985. [DOI] [PubMed] [Google Scholar]
- 17.Pawlus AD, Su B-N, Keller WJ, Kinghorn AD. An anthraquinone with potent quinine reductase –inducing activity and other constituents of the fruits of Morinda citrifolia. J Nat Prod. 2005;68:1720–2. doi: 10.1021/np050383k. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Bode A, Ma W-Y, Sang S, Ho C-T, Dong Z. Two novel glycosides from the fruits of Morinda Citrifolia (Noni) inhibit AP-1 transactivation and cell transformation in the mouse epidermal JB6 cell line. Cancer Res. 2001;61:5749–56. [PubMed] [Google Scholar]
- 19.Chin Y-W, Chai H-B, Keller WJ, Kinghorn AD. Lignans and other constituents of the fruits of Euterpe oleracea (Acai) with antioxidant and cytoprotective activities. J Agric Food Chem. 2008;56:7759–64. doi: 10.1021/jf801792n. [DOI] [PubMed] [Google Scholar]
- 20.Del Pozo-Insfran D, Percival SS, Talcott ST. Acai (Euterpe oleracea Mart.) Polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J Agric Food Chem. 2006;54:1222–9. doi: 10.1021/jf052132n. [DOI] [PubMed] [Google Scholar]
- 21.Gan L, Hua Zhang S, Liang Yang X, Bi Xu H. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int Immunopharmacol. 2004;4:563–9. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–46. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 23.Mandal S, Stoner GD. Inhibition of N-nitrosobenzylmethylamine induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Wang SY, Lin H-S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000;48:140–6. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- 25.Wada L, Ou B. Antioxidant activity and phenolic content of Oregon caneberries. J Agric Food Chem. 2002;50:3495–500. doi: 10.1021/jf011405l. [DOI] [PubMed] [Google Scholar]
- 26.Ehlenfeldt MK, Prior RL. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J Agric Food Chem. 2001;49:2222–7. doi: 10.1021/jf0013656. [DOI] [PubMed] [Google Scholar]
- 27.Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J Agric Food Chem. 2002;50:519–25. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- 28.Wang SY, Chen C-T, Sciarappa W, Wang CY, Camp MJ. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J Agric Food Chem. 2008;56:5788–94. doi: 10.1021/jf703775r. [DOI] [PubMed] [Google Scholar]
- 29.Asami DK, Hong Y-J, Barrett DM, Mitchell AE. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J Agric Food Chem. 2003;51:1237–41. doi: 10.1021/jf020635c. [DOI] [PubMed] [Google Scholar]
- 30.Chen-Blanco Y, Vaillant F, Perez AM, Reynes M, Brillouet J-M, Brat P. The noni fruit (Morinda citrifolia L.): a review of agricultural research, nutritional and therapeutic properties. J Food Comp Anal. 2006;19:645–54. [Google Scholar]
- 31.Gross PM, Zhang X, Zhang R. Wolfberry: nature’s bounty of nutrition and health. BookSurge Publishing; 2006. http://www.wolfberry.org. [Google Scholar]
- 32.Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, et al. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (Acai) J Agric Food Chem. 2006;54:8598–603. doi: 10.1021/jf060976g. [DOI] [PubMed] [Google Scholar]
- 33.Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.) J Agric Food Chem. 2008;56:4631–6. doi: 10.1021/jf800161u. [DOI] [PubMed] [Google Scholar]
- 34.He J, Magnuson BA, Guisti MM. Analysis of anthocyanins in rat intestinal contents-impact of anthocyanin chemical structure on fecal excretion. J Agric Food Chem. 2005;53:2859–66. doi: 10.1021/jf0479923. [DOI] [PubMed] [Google Scholar]
- 35.Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153–64. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 36.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res. 2009;2:187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng CY, Chung WY, Szeto YT, Benzie IFF. Fasting plasma zeaxanthin response to Fructus barbarum L. (wolfberry; Kei Tze) in a food-based human supplementation trial. Br J Nutr. 2005;93:123–30. doi: 10.1079/bjn20041284. [DOI] [PubMed] [Google Scholar]
- 38.Tian M, Wang M. Studies on extraction, isolation and composition of Lycium barbarum polysaccharides. Zhongguo Zhong Yao Za Zhi. 2006;31:1063–7. [PubMed] [Google Scholar]
- 39.Taşkin EI, Akgün-Dar K, Kapucu A, Osanç E, Doğruman H, Eraltan H, et al. Apoptosis-inducing effects of Morinda citrifolia L. and doxorubicin on the Ehrlich ascites tumor in Balb-c mice. Cell Biochem Funct. 2009;27:542–6. doi: 10.1002/cbf.1604. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenthäler R, Rodrigues R, Maia J, Papagiannopoulos M, Fabricius H, Marx F. Total oxidant scavenging capacities of Euterpe oleracea Mart. (Acaí) fruits. Int J Food Sci Nutr. 2005;56:53–64. doi: 10.1080/09637480500082082. [DOI] [PubMed] [Google Scholar]
- 41.Tsujiuchi T, Sasaki Y, Tsutsumi M, Konishi Y. Elevated expression of interleukins in lung adenocarcinomas induced by N-Nitrosobis(2-hydroxypropyl)amine in rats. Jpn J Cancer Res. 2000;91:955–9. doi: 10.1111/j.1349-7006.2000.tb00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch A, Polverini P, Kunkel S, Harlow L, DiPietro L, Elner V, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 43.Chen G, Chu Y, Chak E, Leung B, Poon W. Induction of apoptosis in glioma cells by molecules released from activated macrophages. J Neurooncol. 2002;57:179–86. doi: 10.1023/a:1015763916020. [DOI] [PubMed] [Google Scholar]